Abstract
Rationale: The conducting of clinical trials in infants with cystic fibrosis (CF) has been hindered by lack of sensitive outcome measures.
Objectives: To evaluate safety, feasibility, and ability to detect abnormalities in lung function of serial pulmonary function tests (PFTs) in infants with CF.
Methods: Multicenter observational study using a commercial device, rigorous training, ongoing quality control, and over-reading of data by an independent panel. Raised volume rapid thoracoabdominal compression technique and plethysmography were performed at enrollment and at 6 and 12 months, with an additional 1-month reproducibility visit.
Measurements and Main Results: A total of 342 procedures were performed in 100 infants with CF at 10 centers. FRC measurements were acceptable at a higher proportion of study visits (89%) than raised volume (72%) or fractional lung volume (68%) measurements. Average Z scores for many parameters differed significantly from historical control values. Mean (95% confidence interval) Z scores were: −0.52 (−0.78 to −0.25) for forced expiratory flow at 75% (FEF75) for FVC; 1.92 (1.39–2.45) for FRC; 1.22 (0.68–1.76) for residual volume; 0.87 (0.60–1.13) for FRC/total lung capacity; and 0.66 (0.27–1.06) for residual volume/total lung capacity. For future multicenter clinical trials using infant PFTs as primary endpoints, minimum detectable treatment effects are presented for several sample sizes.
Conclusions: In this 10-center study, key PFT measures were significantly different in infants with CF than in historical control subjects. However, infant PFTs do not yet appear ready as primary efficacy endpoints for multicenter clinical trials, particularly at inexperienced sites, based on acceptability rates, variability, and potentially large sample sizes required to detect reasonable treatment effects.
Keywords: plethysmography, longitudinal studies, FEV, forced expiratory flow rates, outcome assessment
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
Conducting clinical trials in infants with cystic fibrosis (CF) has been hindered by the lack of sensitive outcome measures. Single-center studies have demonstrated diminished lung function measures in infants with CF; however, the safety, feasibility, and sensitivity of these measures to distinguish disease in this population has not been studied in the multicenter setting.
What This Study Adds to the Field
In this 10-center, 100-subject study, diminished forced expiratory flows and elevated lung volumes were noted in infants with CF compared with historical control subjects. However, measurement acceptability rates, variability, and potentially large sample sizes required to detect reasonable treatment effects preclude use of infant lung function tests as a primary efficacy endpoint, especially at inexperienced sites.
Cystic fibrosis (CF) is characterized by progressive obstructive lung disease that ultimately results in irreversible structural airway damage and bronchiectasis. Studies using infant pulmonary function testing (PFT), computed tomography scanning, and bronchoalveolar lavage have demonstrated that CF lung disease often begins in infancy (1–14), providing a rationale for early treatment strategies aimed at delaying or preventing irreversible damage and improving prognosis. However, the conducting of clinical trials in infants with CF has been hindered by a lack of appropriate outcome measures in this age range (3). In older children and adults with CF, spirometry is a standard clinical trial endpoint. The raised volume rapid thoracoabdominal compression technique (RVRTC) allows the measurement of adult-type spirometry in sedated infants (15, 16). Through the use of this technique, investigators have demonstrated that forced expiratory flows (FEFs) are significantly reduced in infants with CF (2, 17), even in the absence of symptoms (1) and in those diagnosed by newborn screening (4). Fractional lung volumes measured by plethysmography coupled with the RVRTC technique have also been shown to be elevated in infants with CF, indicating hyperinflation (18). These prior studies have been performed at one or two centers, in specialized laboratories, and generally with homemade devices. There is also a paucity of published longitudinal infant PFT data (2, 4).
Because of their ability to identify early disease, infant PFTs may serve as useful clinical trial endpoints in infants with CF. Commercial equipment is now available, and standardized procedures for measurement and interpretation have been published (16, 19). However, infant PFTs require sedation, expensive equipment, and extensive training of personnel. Thus, their safety and feasibility in a multicenter setting must be evaluated before incorporating them as clinical trial endpoints.
We conducted a prospective, longitudinal, observational study of infant PFTs (raised volume FEFs and fractional lung volumes) at 10 U.S. centers, with a commercial device, rigorous training, quality control, and independent reading of all data by an expert panel. The aims of this study were to evaluate: (1) the ability of these measures to detect abnormalities in infants with CF; (2) variability relevant to clinical trial design; and (3) the safety and feasibility of these techniques in a multicenter setting. We hypothesized that infant PFTs performed at multiple centers would be safe, feasible, and able to detect abnormalities in lung function in infants with CF. Some of the results have previously been reported in the form of abstracts (20–22).
METHODS
Participants
Inclusion criteria included: (1) age 17 weeks after full-term birth to 24 months or younger at enrollment; (2) confirmed diagnosis of CF (23); and (3) informed consent. Exclusion criteria included: (1) acute intercurrent respiratory infection, defined as an increase in cough, wheezing, or respiratory rate in the preceding 3 weeks; (2) currently hospitalized for a pulmonary exacerbation, unless completion of intravenous antibiotics anticipated within 2 days; (3) oxyhemoglobin saturation less than 90% on room air; (4) contraindications to sedation, such as upper airway obstruction or severe gastroesphageal reflux (detailed in the online supplement). We aimed to enroll a total of 90 participants from 10 U.S. CF centers that are part of the CF Foundation Therapeutics Development Network (see author institutions). Human participant approval was obtained at all participating sites.
PFT
After sedation with 75–125 mg/kg of oral or rectal chloral hydrate, testing was performed with the nSpire Infant Pulmonary Lab (nSpire, Inc., Longmont, CO) following a standard operating procedure based on published guidelines (15, 16, 19, 24). The order of testing was specified as plethysmography followed by the RVRTC technique. The measures analyzed for this study were: FRC obtained from plethysmography, FVC, FEV0.5, FEF between 25% and 75% of FVC (FEF25–75), and FEF at 75% of FVC (FEF75), obtained by RVRTC; and residual volume (RV), total lung capacity (TLC), and the ratios RV:TLC and FRC:TLC obtained from a combined analysis of plethysmography and RVRTC measures, as previously described (24).
Site Training
Three of the sites had prior experience with the infant PFT device and techniques used in the study. Personnel from each of these sites had performed the RVRTC technique and/or plethysmography in a minimum of 100 infants before participating in the current study. The seven remaining sites received the nSpire infant PFT device as a condition of study participation, and had no prior experience with it. Personnel at each site underwent rigorous training and certification in infant PFTs, including a 1-week training session in the use of the Infant Pulmonary Lab device. Each site was required to send deidentified data from a minimum of five clinical testing sessions for review and approval by the Therapeutics Development Network Infant PFT Resource Center at the University of North Carolina before enrolling participants. Initial tests were monitored by live video conferencing between the site and the Infant PFT Resource Center.
Study Visits
Study visits occurred at enrollment, and at 6 and 12 months, with an additional 1-month reproducibility visit within a 28- to 35-day window of one of these visits. At each visit, infants participated in sedated infant PFTs, and clinical data were collected (see online supplement). Adverse events classified as possibly or probably related to the PFTs or sedation were recorded during each study visit and for 2 days after (by telephone follow up).
Independent Over-Reading and Quality Control
All PFT data were reviewed by an expert panel (S.D.D., R.C.J., G.S.K., and S.L.W.) for selection of acceptable measurements based on published guidelines (16, 19, 24). Only acceptable measurements were included in the final analysis dataset. Ongoing quality control feedback was provided to the sites, indicating why unacceptable measurements were classified as such. Additional training was conducted at regular meetings attended by study personnel; at these sessions, software use, common errors, and acceptability criteria were reviewed.
Historical Control Subjects
Human subject concerns precluded any of the sites from performing sedated infant lung function testing in normal infants, so that prospective normative control data could not be collected. Thus, to evaluate the ability of PFTs to detect lung function abnormalities in infants with CF, data from the current study were compared with previously published data from normal infants (15, 24). Measurements of FVC, FEF25–75, and FEF75 were available from 155 and FEV0.5 measurements from 153 historical control subjects from the study by Jones and colleagues (15). A total of 33 measurements of FRC, RV, and TLC were available from 22 historical control subjects from the study by Castile and colleagues (24). These authors kindly provided the original data from these studies.
Statistical Analysis
Lung function measures were expressed as observed values and as Z scores, calculated as the difference between the observed value and the predicted value (based on length and/or age), divided by the SD. The predicted values and SDs were derived from regression equations based on the historical control data (15, 24) (see online supplement).
The average trend in Z score with age among participants with CF was estimated by mixed-effects models to account for the correlation of repeated measures within subjects; the model included fixed and random effects for the intercept and slope (age), and a fixed effect for a quadratic function of age when it was statistically significant at the 0.05 level. The average Z scores (overall and by each year of age) were also estimated with models containing only intercepts. The reproducibility of observed measures obtained approximately 1 month apart was summarized by the intraclass correlation coefficient (ICC) with 95% confidence intervals (CIs) (25). Repeatability of these observed measures 1 month apart were also summarized by 95% limits of agreement.
RESULTS
Participants
A total of 100 participants was enrolled between 2003 and 2006. The number of participants enrolled at each site ranged from 3 to 19. Demographic and clinical characteristics at enrollment are described in Table 1. Participants generally had height and weight below the 50th percentile; their CF genotype distribution was similar to that of the U.S. CF population (26). A total of 18 infants had respiratory abnormalities on physical examination at any visit, including crackles (n = 6), rhonchi (n = 6), tachypnea with retractions or coarse breath sounds (n = 2), increased anterior–posterior diameter (n = 2), wheeze (n = 1), and retractions and prolonged expiratory phase (n = 1). PFTs were performed in one participant near the end of an inpatient admission for newly diagnosed CF; otherwise, no study visits took place during inpatient stays.
TABLE 1.
CHARACTERISTICS OF PARTICIPANTS WITH CYSTIC FIBROSIS AT THE ENROLLMENT VISIT (N = 100)
| Characteristic | Mean (SD) or % |
|---|---|
| Age, mo | 14.0 (6.2) |
| Length | |
| cm | 74.1 (7.2) |
| Percentile | 32.7 (26.4) |
| Weight | |
| kg | 9.2 (2.0) |
| Percentile | 27.7 (26.3) |
| Male, % | 55 |
| Race, % | |
| White | 87 |
| Hispanic | 9 |
| African American | 2 |
| Asian or Pacific Islander | 1 |
| Other | 1 |
| Genotype, % | |
| ΔF508 homozygous | 55 |
| ΔF508 compound heterozygous | 37 |
| Other | 5 |
| Unknown/not done | 3 |
| Diagnosis suggested by newborn or prenatal screening, %* | 21 |
| Exposed to cigarette smoke, %† | 11 |
| Family history of asthma, %‡ | 30 |
| Pseudomonas isolated from respiratory culture, %§ | 12 |
| Shwachman score (41) | 69.9 (6.3) |
Newborn screening for CF was being performed at only two of the participating centers at the time of study enrollment: the University of Colorado and Children's Hospital Boston.
Defined as either mother smoked during pregnancy or child exposed to smoke after birth, by parent report.
By parent report.
A total of 99 out of 100 participants were cultured at time of enrollment.
Feasibility of Infant PFTs in the Multicenter Setting
Table 2 displays the number of acceptable measurements obtained at each study visit. Participants were considered as having attempted a PFT if they attended the scheduled study visit. Acceptable plethysmographic measurements (FRC) were obtained at a higher proportion of study visits at which PFTs were attempted (89%) than acceptable RVRTC measurements (72%) or fractional lung volume measurements (68%) (which require both acceptable plethysmographic and RVRTC measurements). There was no evidence of improved acceptability rates with progressive visits.
TABLE 2.
FEASIBILITY OF PERFORMING PULMONARY FUNCTION TEST PROCEDURES
| Enrollment | 6-Mo Visit | 12-Mo Visit | 1-Mo Reproducibility | Total | |
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | n (%) | |
| No. of PFTs attempted* | 100 (—) | 91 (—) | 77 (—) | 74 (—) | 342 (—) |
| Plethysmography† | |||||
| Acceptable | 91 (91) | 78 (86) | 67 (87)* | 67 (91) | 303 (89) |
| Not acceptable | 8 (8) | 10 (11) | 3 (4) | 3 (4) | 24 (7) |
| Data not obtained‡ | 1 (1) | 3 (3) | 7 (9) | 4 (5) | 15 (4) |
| RVRTC measures§ | |||||
| Acceptable | 76 (76) | 64 (70) | 55 (71) | 51 (69) | 246 (72) |
| Not acceptable | 19 (19) | 11 (12) | 15 (19) | 9 (12) | 54 (16) |
| Data not obtained‡ | 5 (5) | 16 (18) | 7 (9) | 14 (19) | 42 (12) |
| Fractional lung volumes‖ | |||||
| Acceptable | 72 (72) | 60 (66) | 50 (65)‡ | 50 (68) | 232 (68) |
| Not acceptable | 24 (24) | 20 (22) | 17 (22) | 11 (15) | 72 (21) |
| Data not obtained‡ | 4 (4) | 11 (12) | 10 (13) | 13 (18) | 38 (11) |
Definition of abbreviations: FEF = forced expiratory flow; PFT = pulmonary function test; RVRTC = raised volume rapid thoracoabdominal compression.
Number of subjects attending the study visit.
FRC.
FVC, FEV0.5, FEF25–75.
Residual volume, total lung capacity.
Subjects in whom PFTs were attempted, but were unable to produce data for this measurement due to sedation difficulties (failure to sedate adequately or premature awakening).
Considerable between-site variability in the proportion of acceptable measurements among PFT attempts at each site was apparent. Across sites, the proportion of acceptable measurements ranged from 58 to 97% for FRC, 40 to 95% for RVRTC measurements, and 25 to 92% for fractional lung volumes. Of the 342 study visits at which PFTs were attempted, 169 were conducted at the 3 experienced sites, and 173 at the 7 inexperienced sites.
Acceptability rates were significantly higher at the experienced than at the inexperienced sites: for FRC, 96 versus 85% (P < 0.0001); for RVRTC measurements, 85 versus 59% (P < 0.0001); and for fractional lung volumes, 83 versus 53% (P < 0.0001). Figures E1 and E2 in the online supplement display the proportion of acceptable FEF75 and FRC measurements, and the mean Z score for these parameters, at the enrollment visit by site. These figures show the higher measurement acceptability rates at the experienced sites. In addition, marked intercenter variability is appreciated. For example, for FRC (Figure E2), even limiting the comparison to the three experienced sites, the median Z score ranges from 0 to 3.
Of the 91 participants who attempted PFTs at enrollment and the 6-month visit, 73 (80%) had acceptable FRC measurements at both visits, 53 (58%) had acceptable RVRTC measurements at both visits, and 48 (53%) had acceptable fractional lung volume measurements at both visits. Of the 77 participants who attempted PFTs at enrollment and the 12-month visit, 63 (82%) had acceptable FRC measurements at both visits, 46 (60%) had acceptable RVRTC measurements at both visits, and 39 (51%) had acceptable fractional lung volume measurements at both visits. Of the 63 participants who attempted PFTs at all four visits, 46 (73%) had acceptable FRC, 25 (40%) had acceptable RVRTC measurements, and 23 (36%) had acceptable fractional lung volume measurements at all visits.
The most common reasons for lack of acceptable data were: (1) flow limitation not demonstrated; (2) insufficient measurement reproducibility; (3) insufficient data to obtain expiratory reserve volume data (stable tidal breathing after the RVRTC maneuver); (4) insufficient number of acceptable FRC maneuvers; and (5) inability to sedate throughout the entire procedure.
Reasons for failure to attempt all four PFTs included: sedation difficulties (n = 12); scheduling or transportation difficulties (n = 8); loss to follow up or moving (n = 7); no reason noted (n = 6); infant too large for body box (n = 3); noncompliance (n = 1); tachypnea precluded ability to achieve acceptable data (n = 1); broken clavicle unrelated to procedure (n = 1); and acquisition of Burkholderia cepacia, thus unable to participate based on infection control policy (n = 1).
Safety of Infant PFTs
Out of the 342 study procedures performed, there were a total of 44 adverse events among 26 participants possibly or probably related to study procedures (Table 3). Vomiting was the most common adverse event, with 21 participants experiencing 29 events. The three serious adverse events (from one participant at one visit at an experienced site) were tachycardia, wheezing, and hypoxemia, leading to an overnight hospital stay. In retrospect, this subject had an early upper respiratory infection with rhinorrhea that, at the time of the visit, was believed to be allergy related.
TABLE 3.
ADVERSE EVENTS POSSIBLY OR PROBABLY RELATED TO LUNG FUNCTION TESTING OR SEDATION AMONG 342 PULMONARY FUNCTION TEST PROCEDURES IN 100 SUBJECTS
| Not Serious | Serious | |
|---|---|---|
| Adverse Event | N (n) | N (n) |
| Vomiting | 29 (21) | 0 |
| Irritability | 2 (2) | 0 |
| Diarrhea | 3 (3) | 0 |
| Obstructive airways disorder | 1 (1) | 0 |
| Fever | 2 (2) | 0 |
| Rhonchi | 1 (1) | 0 |
| Cough | 2 (2) | 0 |
| Somnolence | 1 (1) | 0 |
| Decreased oxygen saturation | 0 | 1 (1)* |
| Wheezing | 0 | 1 (1)* |
| Tachycardia | 0 | 1 (1)* |
| Total | 41 (25) | 3 (1) |
Definition of abbreviations: N = number of observations; n = number of participants.
These events all occurred in the same participant at the same visit.
Short-Term Reproducibility
The intraclass correlations between measurements obtained approximately 1 month apart (Table 4) were relatively high for RVRTC measurements and FRC, RV, and TLC (point estimates, 0.85–0.94). The reproducibility of FRC:TLC and RV:TLC were lower (point estimates, 0.70 and 0.77, respectively). The 95% limits of agreement for measurements expressed as raw values are shown in Table 4, and for measurements expressed as Z scores in Figure E3 (Bland-Altman plots). The raw values are intended to be helpful for planning future clinical trials, whereas the Z score limits of agreement may aid in evaluating the clinical significance of a Z score change for an individual patient.
TABLE 4.
WITHIN-SUBJECT REPRODUCIBILITY OF REPEATED MEASUREMENTS APPROXIMATELY 1 MONTH APART
| n | Intraclass Correlation Coefficient (95% CI) | Mean Difference (Limits of Agreement)* | |
|---|---|---|---|
| FEF25–75 | 41 | 0.85 (0.73–0.92) | −3.8 ml/sec (−253.6, 246.1) |
| FEV0.5 | 41 | 0.90 (0.82–0.95) | 8.3 ml (85.4, 102.0) |
| FEF75 | 41 | 0.85 (0.74–0.92) | −9.2 ml/sec (−166.4, 148.1) |
| FVC | 41 | 0.93 (0.87–0.96) | 25.0 ml (−82.5, 132.4) |
| FRC | 62 | 0.93 (0.89–0.96) | 7.5 ml (−62.7, 77.7) |
| RV | 40 | 0.85 (0.73–0.92) | −5.5 ml (−91.4, 80.4) |
| TLC | 40 | 0.94 (0.90–0.97) | 21.0 ml (−117.4, 159.4) |
| FRC:TLC | 40 | 0.77 (0.61–0.87) | −0.8 (−8.6, 6.9) |
| RV:TLC | 40 | 0.70 (0.50–0.83) | −0.02 (−0.12, 0.09) |
Definition of abbreviations: CI = confidence interval; FEF25–75 = 25–75% of forced expiratory flow; FEF75, 75% of FEF; RV = residual volume; TLC = total lung capacity.
Limits of agreement = 1.96 × SD of the difference.
Comparison of Infant PFT Measurements from Participants with CF and Historical Control Subjects
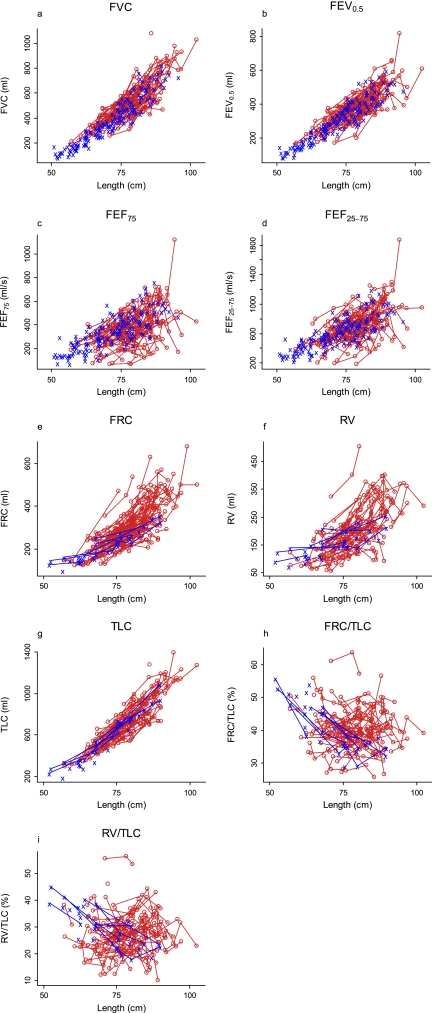
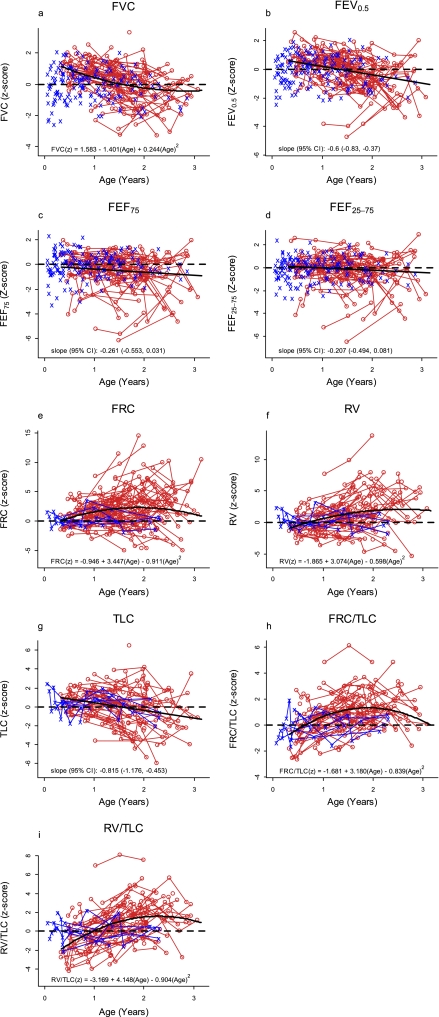
Plots of the observed values of the infant PFT measures versus length are shown in Figure 1. Plots of infant PFT Z scores versus age for the participants with CF and the historical control subjects are displayed in Figure 2, with the average trend in Z scores by age among the participants with CF superimposed (thick, solid lines). The average Z score was best described by a quadratic function of age for FRC, RV, FRC:TLC, RV:TLC, and FVC, whereas a linear function of age was sufficient for the other parameters. Z scores for FEV0.5, FEF75, FEF25–75, and FVC that are below zero, and Z scores for FRC, RV, TLC, FRC:TLC, and RV:TLC that are above zero, indicate lung function worse than that of the average control subject. The average slopes of Z scores for FEV0.5 (−0.60/yr; 95% CI, −0.83 to −0.37) and TLC (−0.815/yr; 95% CI, −1.176 to −0.453) among the participants with CF were significantly negative, indicating that these measures declined with age.
Figure 1.
Plots of the observed values of the infant pulmonary function test measures versus length. Measurements for historical control data are in blue. The measurements for the infants with cystic fibrosis are in red, and repeated measures are connected by the red segments. FEF25–75 = 25–75% of forced expiratory flow; FEF75, 75% of FEF; RV = residual volume; TLC = total lung capacity.
Figure 2.
Plots of infant pulmonary function test Z scores versus age for the cystic fibrosis (CF) participants and the historical control subjects, with the average trend in Z scores by age among the participants with CF superimposed (thick, solid lines). Measurements for historical control data are in blue. The measurements for the infants with CF are in red, and repeated measures are connected by the red segments. Dashed lines indicate a z score of zero. FEF25–75 = 25–75% of forced expiratory flow; FEF75, 75% of FEF; RV = residual volume; TLC = total lung capacity.
Table 5 displays mean infant PFT Z scores and 95% CIs (from the models without age) for the participants with CF for all ages combined and for each year of age. For FEF75, FRC, RV, FRC:TLC, and RV:TLC, the average Z score for participants with CF overall was worse than for the average control. Taken together, Figure 2 and Table 5 suggest subtle abnormalities in FEFs, and greater abnormalities in fractional lung volumes (gas trapping), with many parameters worsening with age.
TABLE 5.
AVERAGE Z SCORES AMONG CYSTIC FIBROSIS PARTICIPANTS, OVERALL AND BY AGE CATEGORY
| N (n)* | Mean (95% CI) | |
|---|---|---|
| FEV0.5 | 246 (90) | 0.06 (−0.15 to 0.26) |
| 0–1 yr | 64 (37) | 0.66 (0.39 to 0.93) |
| 1–2 yr | 128 (82) | 0.04 (−0.20 to 0.27) |
| 2–3 yr | 54 (34) | −0.50 (−0.85 to −0.14) |
| FEF75 | 246 (90) | −0.52 (−0.78 to −0.25) |
| 0–1 yr | 64 (37) | −0.29 (−0.67 to 0.09) |
| 1–2 yr | 128 (82) | −0.56 (−0.87 to −0.24) |
| 2–3 yr | 54 (34) | −0.82 (−1.32 to −0.32) |
| FEF25–75 | 246 (90) | −0.15 (−0.40 to 0.11) |
| 0–1 yr | 64 (37) | 0.00 (−0.36 to 0.37) |
| 1–2 yr | 128 (82) | −0.13 (−0.44 to 0.17) |
| 2–3 yr | 54 (34) | −0.39 (−0.87 to 0.09) |
| FVC | 246 (90) | 0.20 (−0.01 to 0.41) |
| 0–1 yr | 64 (37) | 0.93 (0.70 to 1.16) |
| 1–2 yr | 128 (82) | 0.14 (−0.11 to 0.38) |
| 2–3 yr | 54 (34) | −0.36 (−0.69 to −0.03) |
| FRC | 303 (96) | 1.92 (1.39 to 2.45) |
| 0–1 yr | 71 (41) | 0.99 (0.44 to 1.55) |
| 1–2 yr | 156 (88) | 2.17 (1.56 to 2.78) |
| 2–3 yr | 76 (45) | 2.16 (1.18 to 3.14) |
| RV | 232 (88) | 1.22 (0.68 to 1.76) |
| 0–1 yr | 62 (37) | −0.06 (−0.61 to 0.49) |
| 1–2 yr | 119 (76) | 1.41 (0.81 to 2.01) |
| 2–3 yr | 51 (32) | 2.07 (1.00 to 3.15) |
| TLC | 232 (88) | 0.04 (−0.32 to 0.40) |
| 0–1 yr | 62 (37) | 0.67 (0.32 to 1.01) |
| 1–2 yr | 119 (76) | 0.06 (−0.36 to 0.49) |
| 2–3 yr | 51 (32) | −0.69 (−1.44 to 0.05) |
| FRC:TLC | 232 (88) | 0.87 (0.60 to 1.13) |
| 0–1 yr | 62 (37) | −0.04 (−0.45 to 0.37) |
| 1–2 yr | 119 (76) | 1.11 (0.84 to 1.39) |
| 2–3 yr | 51 (32) | 1.23 (0.85 to 1.60) |
| RV:TLC | 232 (88) | 0.66 (0.27 to 1.06) |
| 0–1 yr | 62 (37) | −0.83 (−1.38 to −0.28) |
| 1–2 yr | 119 (76) | 1.00 (0.58 to 1.42) |
| 2–3 yr | 51 (32) | 1.44 (0.86 to 2.02) |
Definition of abbreviations: CI = confidence interval; FEF25–75 = 25–75% of forced expiratory flow; FEF75, 75% of FEF; N = number of observations; n = number of participants; RV = residual volume; TLC = total lung capacity.
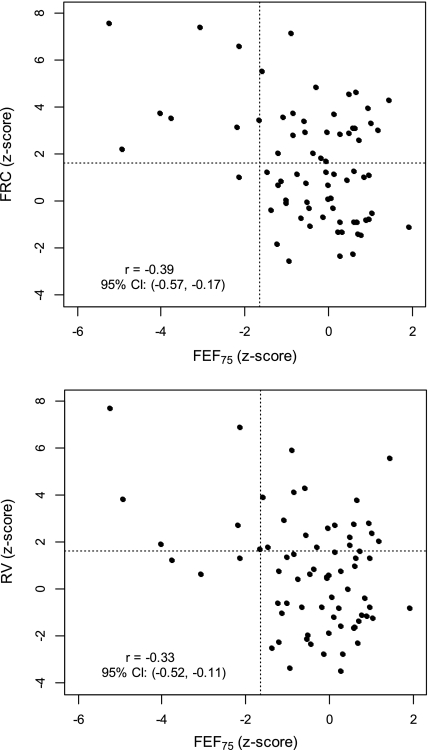
To further explore the observation of smaller abnormalities in FEFs than in indices of gas trapping, we plotted FRC Z score and RV Z score against FEF75 at enrollment (Figure 3). There was a statistically significant but relatively poor correlation between FEF75 and FRC or RV (for FRC, r = −0.39 [95% CI, −0.57 to −0.17]; for RV, r = −0.33 [95% CI, −0.52 to −0.11]). FRC and RV appeared to be more sensitive to abnormalities in lung function, as evidenced by: (1) the greater number of individuals with abnormal FRC or RV (points above dotted line at Z = 1.64) than FEF75 (points to left of dotted line at Z = −1.64); and (2) only approximately One-quarter of those with abnormal FRC or RV also had an abnormal FEF75 (compare left upper quadrant [abnormal FRC/RV and abnormal FEF75] to right upper quadrant [abnormal FRC/RV but normal FEF75]).
Figure 3.
Plots of FRC Z score (top) and residual volume (RV) Z score (bottom) against forced expiratory flow at the 75th percent of vital capacity (FEF75) z score at enrollment. Dotted lines are drawn at 1.64 and −1.64, the upper and lower limits of normal for FRC or RV, and FEF75, respectively.
The distribution of lung function measures in participants with CF was also compared with historical control subjects by evaluating the proportion of participants with CF who had at least one “abnormal” measurement, defined as a Z score greater than 1.64 for FRC, FRC:TLC, and RV:TLC, or less than −1.64 for FVC, FEV0.5, FEF75, and FEF25–75 (Table 6). A Z score of 1.64 (−1.64) indicates that the observed value is greater (less) than 95% of observed values from control subjects (of the same length and/or age). Thus, 5% of healthy control infants should have a Z score greater than 1.64, and 5% should have a Z score less than −1.64. For all measurements, more than 5% of participants with CF had abnormal measurements. The percentage of participants with CF with at least one abnormal value ranged from 14.4% for FVC to 67.7% for FRC.
TABLE 6.
PERCENTAGE OF CYSTIC FIBROSIS PARTICIPANTS WITH AT LEAST ONE ABNORMAL LUNG FUNCTION MEASUREMENT
| Parameter | N* | Percentage |
|---|---|---|
| FEV0.5 | 90 | (17.8) |
| FEF25–75 | 90 | (21.1) |
| FEF75 | 90 | (25.6) |
| FVC | 90 | (14.4) |
| FRC | 96 | (67.7) |
| FRC:TLC | 88 | (36.4) |
| RV:TLC | 88 | (44.3) |
| TLC | 88 | (31.8) |
| RV | 88 | (54.5) |
Definition of abbreviations: FEF25–75 = 25–75% of forced expiratory flow; FEF75, 75% of FEF; RV = residual volume; TLC = total lung capacity.
Abnormal lung function measurement defined as a Z score of less than −1.64 for FEV0.5, FEF25–75, FEF75, and FVC, and a Z score greater than 1.64 for FRC, FRC:TLC, and RV:TLC (5% of the reference population would be expected to have a Z score < −1.64 and 5% > 1.64).
N = number of cystic fibrosis participants with at least one acceptable measure.
Detectable Treatment Effects
Table 7 presents the minimum detectable treatment effect for selected lung function parameters as clinical trial endpoints at several sample sizes. To put the treatment effects in context, they are also expressed as a percentage of the mean lung function value for the infants with CF at two representative lengths (79 and 90 cm), corresponding to the average lengths of our study population at 18 and 30 months of age. Note that the stated sample sizes do not take into account missing data due to unacceptable measures or dropout. For the planning of future trials, the anticipated measurement acceptability rates and subject dropout at the participating sites would have to be factored in when determining proper sample size.
TABLE 7.
MINIMAL DETECTABLE TREATMENT EFFECTS
| Detectable Effect§ (%) |
|||||
|---|---|---|---|---|---|
| Parameter | Sample Size per Arm* | SD† | Minimum Detectable Treatment Effect‡ | Age 18 Mo (Length, 79 cm) | Age 30 Mo (Length, 90 cm) |
| FEF75, ml/s | 75 | (125.8) | 57.9 | 16.6 | 13.0 |
| 100 | (125.8) | 50.1 | 14.4 | 11.2 | |
| 150 | (125.8) | 40.8 | 11.7 | 9.1 | |
| FRC, ml | 75 | (84.5) | 38.9 | 13.0 | 9.7 |
| 100 | (84.5) | 33.7 | 11.2 | 8.4 | |
| 150 | (84.5) | 27.4 | 9.2 | 6.9 | |
| RV, ml | 75 | (71.6) | 33 | 16.1 | 12.0 |
| 100 | (71.6) | 28.5 | 13.9 | 10.4 | |
| 150 | (71.6) | 23.2 | 11.4 | 8.4 | |
| RV:TLC, % | 75 | (7.7) | 3.5 | 12.8 | 12.7 |
| 100 | (7.7) | 3.1 | 11.1 | 11.0 | |
| 150 | (7.7) | 2.5 | 9.0 | 9.0 | |
| FRC:TLC, % | 75 | (6.5) | 3 | 7.4 | 7.6 |
| 100 | (6.5) | 2.6 | 6.4 | 6.5 | |
| 150 | (6.5) | 2.1 | 5.2 | 5.3 | |
Definition of abbreviations: FEF75 = 75% of forced expiratory flow; RV = residual volume; TLC = total lung capacity.
n per arm assumes no dropout and 100% acceptable measures.
Estimated SD among the subjects with CF at enrollment.
The smallest detectable difference between treatment arms in a trial of total sample size 2N assuming a standard deviation of SD, a two-sided significance level of 0.05, and a power of 0.80.
Detectable effect as a percentage of the mean lung function value for infants with CF at a given age. To put the treatment effects in context, they are expressed as a percentage of the mean lung function value for the infants with CF at two representative lengths (79 and 90 cm), corresponding to the average lengths of our study population at 18 and 30 months of age. To calculate the mean values of each parameter, the lung function data from the subjects with CF were fit with models of the same form as the published reference equations (see online supplement).
Detectable treatment effects are displayed in Table 7 only for FEF75, FRC, RV:TLC, RV, and FRC:TLC. For the parameters not displayed, it would be difficult to detect a treatment effect due to the substantial overlap in the lung function of infants with CF and control subjects (Table 5, Figures 1 and 2), suggesting that even an effective intervention would not be able to substantially improve those measures in infants with CF. Similarly, based on the overlap in the lung function of infants with CF and historical control subjects, it would be difficult to detect a treatment effect with any lung function parameter below 12 months of age (Figure 2); thus, only ages 18 and 30 months are displayed in Table 7.
DISCUSSION
We report the results of the first study to evaluate the utility of infant PFTs as potential clinical trial endpoints in the multicenter setting. Our results reinforce that PFTs (FEF75, FRC, RV, RV:TLC, and FRC:TLC) are, on average, abnormal in infants with CF compared with healthy historical control subjects. We also found that prior site experience with the infant lung function testing device and procedures used in this study significantly affected the ability to generate acceptable measurements.
Based on our results, measures of infant lung function do not yet appear appropriate as the primary efficacy endpoint for a multicenter clinical trial, particularly involving relatively inexperienced sites. However, they may be useful as safety endpoints (27, 28). In addition, infant PFTs may serve as useful endpoints to evaluate the short-term response to an intervention, given the fact that acute treatment effects may be larger than those sustained over longer time periods.
We identified two issues that must be addressed before using infant PFTs as a primary endpoint in multicenter trials, particularly involving inexperienced sites. First, acceptable measurements could not be obtained in a substantial proportion of participants. FRC was acceptable in 89% of attempts (range, 58–97% across individual sites), and RVRTC measurements in 72% (site range, 40–95%). Second, although the adverse event rate was as expected for chloral hydrate sedation (29), 12% of participants withdrew due to side effects of sedation.
Because lung function has never been used as a primary endpoint in a clinical trial in infants, it is unknown whether the detectable treatment effects (Table 7) are achievable. In clinical trials using conventional spirometry in older patients with CF with established lung disease, tobramycin solution for inhalation improved mean FEV1 by 10% at Week 20 compared with enrollment (30), but it is unknown whether this magnitude of effect size could be achieved in infants with relatively mild lung disease. FRC:TLC may be able to detect a relatively small percent improvement in lung function (Table 7), but has never been tested as a clinical trial endpoint.
It should be noted that the treatment effect estimates from our multicenter study conducted at mostly relatively inexperienced centers might not apply to specialized, experienced centers. Indeed, collaborations between a limited number of experienced centers can be very effective, as demonstrated from the reports from the Australian Respiratory Early Surveillance Team for Cystic Fibrosis (AREST-CF) study in Australia (4) and the Extracorporeal Membrane Oxygenation (ECMO) study in the United Kingdom (31, 32). Sample size estimates for specific future trials should be based on lung function data from the anticipated participating centers.
We acknowledge that prospectively collected data from a greater number of control subjects would have substantially strengthened our findings. However, ethical concerns precluded sedating healthy infants for lung function testing at all participating sites. We therefore were limited to historical control data obtained with similar equipment and techniques. These data are, in fact, the basis for the only published or available reference equations for the nSpire device, which is in relatively widespread use in clinical laboratories across the United States (15, 24).
Reference values from a large cohort of healthy infants obtained with the nSpire device are critically needed for clinical as well as research purposes. The current reference equations are based on measurements in a limited number of children, with a preponderance of very young infants, conducted at one or two specialized centers, and lack serial measurements. Limitations of these data may explain some of our apparent findings, such as the magnitude of elevation of FRC and RV in some subjects, the apparent rate of decline in FEFs over the observation period, and the better-than-expected FVC, FEV0.5, and TLC in the subjects with CF in the first year of life. Fortunately, the ongoing Canadian Healthy Infant Longitudinal Development Study (principle investigators, M. Sears, P. Subbarao, A. Becker, P. Mandhane, and S. Turvey) is currently enrolling 750 normal infants who will have serial lung function testing with the nSpire device from 3 to 18 months of age. Results from this study will provide much-needed robust reference data for infant lung function with the nSpire device.
In a prior, large, multicenter study with infant PFTs (partial FEFs) to evaluate lung disease in infants born to mothers with human immunodeficiency virus infection, uniformity of equipment and training was implemented; however, significant variability in results from individual centers was found, and was ascribed, in part, to insufficient central supervision. The conclusion from that study was to conduct site visits as a form of centralized supervision to secure uniformity (33). In the current study, despite rigorous training and certification, standard operating procedures, and ongoing centralized quality control and feedback, our average rates of acceptable measurements were substantially lower than those reported from specialized, single or two-center studies (2, 4). It should be emphasized that this study was performed at sites relatively naive to infant lung function testing: of the 10 participating centers, only 3 had prior experience with the infant PFT device and procedures used in this study. The sites with prior experience performed the highest percentage of acceptable maneuvers. Thus, it appears that using sites with prior experience will yield the highest proportion of acceptable data in future studies.
Infant PFTs are now used routinely in clinical care at most of the participating sites, so these sites are significantly more experienced with these procedures than was true at the initiation of this study. For future trials using infant lung function testing as outcome measures, we suggest that the participating sites should have performed a minimum number of infant lung function tests, have a dedicated physician and team with at least 2 years of expertise in the procedures, and demonstrate the ability to perform acceptable maneuvers as criteria for study participation. A core infant lung function coordinating center that provides training, independently reads all site data, and provides ongoing quality control will help to ensure that only data with acceptable quality are analyzed. To further assess the ability of infant PFTs to serve as clinical trial endpoints, they are currently serving as an exploratory endpoint in a multicenter trial of an inhaled agent in infants with CF. The results of this trial will contribute valuable information regarding detectable treatment effects and feasibility of infant PFTs in the current era.
Similar to our findings, an elevated FRC measured by plethysmography or gas dilution has been reported in infants with CF in several small studies (18, 34–36). Only one small prior study has evaluated fractional lung volumes in infants with CF; our findings are consistent with that study (18). Of note, the majority of those infants with elevated RV or FRC values had diminished FEF75, consistent with airways obstruction occurring concomitantly with gas trapping.
Our results for FVC, FEV0.5, and FEF75 agree well, qualitatively, with prior studies (2, 4, 18, 37). Among 37 infants with CF evaluated by the London CF Collaborative (2), the average FVC, FEV0.5, and FEF75 was significantly lower than in concurrent control subjects at two measurements 6 months apart (median age at first measurement, 28 wk). A recent Australian study in 68 infants identified through newborn screening demonstrated that the average FVC, FEV0.5, and FEF75 were similar in CF and control infants younger than 6 months of age, but significantly lower in the infants with CF older than 6 months of age (4). In our study, the average FEF75 was lower among our infants with CF than the historical control subjects for all sampled ages. Although, across all ages, the average FVC and FEV0.5 Z scores among our infants with CF were not significantly different from zero, there was a significant decline in FVC and FEV0.5 Z scores with advancing age, similar to the Australian study. Our apparent finding of better average FVC and FEV0.5 among infants with CF than in the control subjects during the first 18 months of life may, in part, be explained by the small sample of historical control subjects, and the fact that the historical control subjects were, on average, younger than our infants with CF (Jones and colleagues [15]: mean age, 48 wk; range 3–149 wk; Castile and colleagues [24]: mean age, 45 wk; range 3–120 wk).
The magnitude of the differences between CF and control measurements in our study was not as dramatic as in these previous publications. In the London Collaborative Study (2), at a median age of 59 weeks, the mean Z score among the infants with CF for FVC, FEV0.5, and FEF75 were −1.5, −2.0, and −1.0, respectively. In the Australian infants identified by newborn screening, the corresponding mean Z scores at study visits after 6 months of age were −0.58, −1.13, and −1.07, respectively (4). In our study, between 1 and 2 years of age, the corresponding mean Z scores were 0.14, 0.04, and −0.56 (Table 5). The difference in our findings compared with these prior studies may be due to measurement techniques, differences between the control infants from which the Z score equations were derived, or true differences in respiratory health among the participants with CF at the time of testing, perhaps due to different treatment practices or exposures. There is some indication that the infants enrolled in our study were healthier than those in the London CF Collaborative study. For example, in the London study (2), the mean weight Z score at the first visit was −1.8 (equivalent to fourth percentile [38]), whereas the mean weight percentile (39) at enrollment in our study was 27.7. Infants in the London study also had a higher rate of Pseudomonas aeruginosa isolation and more chest exam abnormalities than the infants in our study.
Similar to the observations of Linnane and colleagues (4), we found FEFs and volumes to decline in infants at an apparently greater rate than those observed in older patients with CF who can cooperate with standard spirometry. Several explanations are possible for the apparent steeper decline in lung function in infancy. First, there may truly be loss of lung function in infancy not appreciated during later childhood. Second, the measurement of FEFs is performed differently in these two age ranges; therefore, they may not be directly comparable. We are completing a follow-up study of the majority of participants in this cohort, in which preschool spirometry measurements and clinical characteristics were obtained serially between 3 and 5 years of age. Future studies with the same measurement across all age ranges, such as the lung clearance index, may also help to elucidate age-related trends in lung function. Alternatively, the observed magnitude of the decline in lung function in the patients with CF may, in part, be attributed to the fact that the cohort of control infants to which they were compared was, on average, younger, and lacked serial measurements. Longitudinal changes in lung function in healthy control subjects deserves further study to better understand comparable changes in infants with chronic respiratory conditions.
There is rapidly growing interest in conducting clinical trials of established and novel therapies in infants with CF, in an attempt to delay or prevent irreversible structural airway damage. Therapies aimed at correcting the basic defect, such as CF transmembrane conductance regulator potentiators and correctors, may be most effective if initiated soon after diagnosis. CF newborn screening now affords the opportunity to intervene in the presymptomatic period. However, clinical trials in very youngest patients with CF are still hindered by the lack of appropriate outcome measures in this age range. Potential endpoints include infant PFTs, lung clearance assessed by multiple breath washout (5, 40), controlled ventilation computed tomography scans (6, 7, 12, 13), as well as parental reports of infant respiratory symptoms, pulmonary exacerbations, resting respiratory rate, oximetry, and objective monitoring of cough. All these measures have pros and cons, and are in varying stages of development. It is likely that no single endpoint will be the “holy grail” or fit the needs of all clinical trials. With appropriate site training and experience, infant PFTs may come to play a role in the armamentarium of infant clinical trial endpoints.
Acknowledgments
The authors acknowledge Dr. Mary Ellen Wohl, who was instrumental during study startup and served as a mentor for countless pediatric pulmonologists. They also acknowledge Dr. Bonnie Ramsey, Director of the Cystic Fibrosis (CF) Foundation Therapeutic Development Network, and Dr. Preston Campbell, III Executive Vice President for Medical Affairs of the CF Foundation, for their advice and support. They also acknowledge Steven Knudsen for assisting with data management of the Infant Pulmonary Lab database, and thank Drs. Robert Tepper, Marcus Jones, and Robert Castile for sharing their normative data. Finally, they thank all the families who participated in this project.
Supported by Cystic Fibrosis Foundation Therapeutics Inc. grants ROSENF03AO (M.R.) and DAVIS08Y2 (S.D.D.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200908-1236OC on July 9, 2010
Author Disclosure: S.D.D. received up to $1,000 from Inspire in consultancy fees, $1,001–$5,000 from MedImmune in advisory board fees, and more than $100,001 from the National Heart, Lung, and Blood Institute, and more than $100,001 from the Cystic Fibrosis (CF) Foundation in sponsored grants; M.R. received $1,001–$5,000 from the Genentech Epidemiology Study of CF in advisory board fees, and more than $100,001 from the National Institutes of Health (NIH) and more than $100,001 from the CF Foundation in sponsored grants; G.S.K. received $10,001–$50,000 from the CF Foundation as a grant for a study being published; L.B. received $50,001–$100,000 from the CF Foundation, and more than $100,001 from NIH in sponsored grants; M.H.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; J.D.A. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; A.A.C. received $1,001–$5,000 from Digestive Care, Inc. for consults about pancreatic enzymes, $5,001–$10,000 from MedImmune for serving on a scientific advisory board concerning lung disease in prematurity, and $5,001–$10,000 from Merck, Inc. for sponsored talks on lung development and asthma; C.K.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; M.A.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; P.W.H. received more than $100,001 from NIH in sponsored grants for basic science research (institutional), and more than $100,001 from the CF Foundation for research study grants (institutional); P.J.M. received more than $100,001 from the CF Foundation as a CF Center grant and research grants, and $50,001–$100,001 from the CF Foundation in advisory board fees for committee chairmanships; R.C.J. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; S.L.W. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; R.G.C. received up to $1,000 from MedImmune in consultancy fees, $10,001–$50,000 from nSpire Health, Inc. in royalties to Nationwide Children's Hospital, and $10,001–$50,000 from the CF Foundation in sponsored grants.
References
- 1.Ranganathan SC, Dezateux C, Bush A, Carr SB, Castle RA, Madge S, Price J, Stroobant J, Wade A, Wallis C, et al. for the, Group LCCF. Airway function in infants newly diagnosed with cystic fibrosis. Lancet 2001;358:1964–1965. [DOI] [PubMed] [Google Scholar]
- 2.Ranganathan SC, Stocks J, Dezateux C, Bush A, Wade A, Carr S, Castle R, Dinwiddie R, Hoo AF, Lum S, et al. The evolution of airway function in early childhood following clinical diagnosis of cystic fibrosis. Am J Respir Crit Care Med 2004;169:928–933. [DOI] [PubMed] [Google Scholar]
- 3.Davis SD, Brody AS, Emond MJ, Brumback LC, Rosenfeld M. Endpoints for clinical trials in young children with cystic fibrosis. Proc Am Thorac Soc 2007;4:418–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Linnane BM, Hall GL, Nolan G, Brennan S, Stick SM, Sly PD, Robertson CF, Robinson PJ, Franklin PJ, Turner SW, et al. Lung function in infants with cystic fibrosis diagnosed by newborn screening. Am J Respir Crit Care Med 2008;178:1238–1244. [DOI] [PubMed] [Google Scholar]
- 5.Lum S, Gustafsson P, Ljungberg H, Hulskamp G, Bush A, Carr SB, Castle R, Hoo AF, Price J, Ranganathan S, et al. Early detection of cystic fibrosis lung disease: multiple-breath washout versus raised volume tests. Thorax 2007;62:341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Long F, Williams R, Castile R. Structural airway abnormalities in infants and young children with cystic fibrosis. J Pediatr 2004;144:154–161. [DOI] [PubMed] [Google Scholar]
- 7.Martinez TM, Llapur CJ, Williams TH, Coates C, Gunderman R, Cohen MD, Howenstine MS, Saba O, Coxson HO, Tepper RS. High-resolution computed tomography imaging of airway disease in infants with cystic fibrosis. Am J Respir Crit Care Med 2005;172:1133–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dakin C, Numa A, Wang H, Morton J, Vertzysa C, Henry R. Inflammation, infection and pulmonary function in infants and young children with cystic fibrosis. Am J Respir Crit Care Med 2002;165:904–910. [DOI] [PubMed] [Google Scholar]
- 9.Peterson-Carmichael S HW, Goel R, Noah T, Johnson R, Leigh M, Davis S. The association of lower airway inflammation with physiologic findings in young children with cystic fibrosis. Pediatr Pulmonol 2009;44:503–511. [DOI] [PubMed] [Google Scholar]
- 10.Rosenfeld M, Gibson RL, McNamara S, Emerson J, Burns J, Castile R, Hiatt P, McCoy K, Wilson C, Inglis A, et al. Early pulmonary infection, inflammation, and clinical outcomes in infants with cystic fibrosis. Pediatr Pulmonol 2001;32:356–366. [DOI] [PubMed] [Google Scholar]
- 11.Khan T. JS W, Boat T, Martinez J, Accurso F, Riches D. Early pulmonary inflammation in infants with cystic fibrosis. Am J Respir Crit Care Med 1995;151:1075–1082. [DOI] [PubMed] [Google Scholar]
- 12.Davis SD, Fordham LA, Brody AS, Noah TL, Retsch-Bogart GZ, Qaqish BF, Yankaskas BC, Johnson RC, Leigh MW. Computed tomography reflects lower airway inflammation and tracks changes in early cystic fibrosis. Am J Respir Crit Care Med 2007;175:943–950. [DOI] [PubMed] [Google Scholar]
- 13.Stick SM, Brennan S, Murray C, Douglas T, von Ungern-Sternberg BS, Garratt LW, Gangell CL, De Klerk N, Linnane B, Ranganathan S, et al. Bronchiectasis in infants and preschool children diagnosed with cystic fibrosis after newborn screening. J Pediatr 2009;155:623–628. [DOI] [PubMed] [Google Scholar]
- 14.Sly PD, Brennan S, Gangell C, de Klerk N, Murray C, Mott L, Stick SM, Robinson PJ, Robertson CF, Ranganathan SC. Lung disease at diagnosis in infants with cystic fibrosis detected by newborn screening. Am J Respir Crit Care Med 2009;180:146–152. [DOI] [PubMed] [Google Scholar]
- 15.Jones M, Castile R, Davis S, Kisling J, Filbrun D, Flucke R, Goldstein A, Emsley C, Ambrosius W, Tepper R. Forced expiratory flows and volumes in infants: normative data and lung growth. Am J Respir Crit Care Med 2000;161:353–359. [DOI] [PubMed] [Google Scholar]
- 16.ATS/ERS statement: raised volume forced expirations in infants. Am J Respir Crit Care Med 2005;172:1463–1471. [DOI] [PubMed] [Google Scholar]
- 17.Davis S, Jones M, Kisling J, Howard J, Tepper RS. Comparison of normal infants and infants with cystic fibrosis using forced expiratory flows breathing air and heliox. Pediatr Pulmonol 2001;31:17–23. [DOI] [PubMed] [Google Scholar]
- 18.Castile R, Iram D, McCoy K. Gas trapping in normal infants and in infants with cystic fibrosis. Pediatr Pulmonol 2004;37:461–469. [DOI] [PubMed] [Google Scholar]
- 19.Stocks J, Godfrey S, Beardsmore C, Bar-Yishay E, Castile R. Plethysmographic measurements of lung volume and airway resistance: ERS/ATS task force on standards for infant respiratory function testing. European Respiratory Society/American Thoracic Society. Eur Respir J 2001;17:302–312. [DOI] [PubMed] [Google Scholar]
- 20.Davis S, Kerby G, Acton J, Castile R, Colin A, Conrad C, Hart M, Hiatt P, Mogayzel P, Johnson R, et al. Feasibility, sensitivity and variability of adult-type pulmonary function tests in infants with CF in a multicenter, longitudinal trial. Pediatr Pulmonol 2006;Suppl 29:360.
- 21.Davis SD, Kerby G, Acton J, Castile R, Colin A, Conrad C, Hart M, Hiatt P, Mogayzel P, Brumback L, Johnson R, et al. Creating an infant lung function testing network. Pediatr Pulmonol 2004;Suppl 27:329.
- 22.Rosenfeld M, Brumback L, Knutzen S, Acton J, Castile R, Colin A, Conrad C, Hart M, Hiatt P, Kerby G, Mogayzel P, et al. Multicenter longitudinal evaluation of raised volume forced expiratory flows in infants with CF is feasible. Pediatr Pulmonol 2005;Suppl 28:354.
- 23.Farrell PM, Rosenstein BJ, White TB, Accurso FJ, Castellani C, Cutting GR, Durie PR, Legrys VA, Massie J, Parad RB, et al. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation consensus report. J Pediatr 2008;153:S4–S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castile R, Filbrun D, Flucke R, Franklin W, McCoy K. Adult-type pulmonary function tests in infants without respiratory disease. Pediatr Pulmonol 2000;30:215–227. [DOI] [PubMed] [Google Scholar]
- 25.McGraw KOWS. Forming inferences about some intraclass correlations. Psychol Methods 1996;1:30–46. [Google Scholar]
- 26.Cystic Fibrosis Foundation. Cystic Fibrosis Foundation National Patient Registry annual data report 2007. Bethesda, MD: Cystic Fibrosis Foundation; 2008
- 27.Dellon EP, Donaldson SH, Johnson R, Davis SD. Safety and tolerability of inhaled hypertonic saline in young children with cystic fibrosis. Pediatr Pulmonol 2008;43:1100–1106. [DOI] [PubMed] [Google Scholar]
- 28.Subbarao P, Balkovec S, Solomon M, Ratjen F. Pilot study of safety and tolerability of inhaled hypertonic saline in infants with cystic fibrosis. Pediatr Pulmonol 2007;42:471–476. [DOI] [PubMed] [Google Scholar]
- 29.Mallol J, Sly PD. Effect of chloral hydrate on arterial oxygen saturation in wheezy infants. Pediatr Pulmonol 1988;5:96–99. [DOI] [PubMed] [Google Scholar]
- 30.Ramsey BW, Pepe MS, Quan JM, Otto KL, Montgomery AB, Williams-Warren J, Vasiljev KM, Borowitz D, Bowman CM, Marshall BC, et al. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis: cystic fibrosis inhaled tobramycin study group. N Engl J Med 1999;340:23–30. [DOI] [PubMed] [Google Scholar]
- 31.Beardsmore C, Dundas I, Poole K, Enock K, Stocks J. Respiratory function in survivors of the UK extracorporeal membrane oxygenation trial. Am J Respir Crit Care Med 2000;161:1129–1135. [DOI] [PubMed] [Google Scholar]
- 32.Dundas I, Beardsmore C, Wellman T, Stocks J. A collaborative study of infant respiratory function testing. Eur Respir J 1998;12:944–953. [DOI] [PubMed] [Google Scholar]
- 33.Colin AA, Sunil Rao J, Chen XC, Hunter JM, Hanrahan J, Hiatt P, Kattan M, Koumbourlis A, Mellins RB, Peavy HH, et al.; Pediatric Pulmonary and Cardiovascular Complications of Vertically Transmitted Human Immunodeficiency Virus Study Group, National Heart, Lung, and Blood Institute. Forced expiratory flow in uninfected infants and children born to HIV-infected mothers. Am J Respir Crit Care Med 2001;163:865–873. [DOI] [PubMed] [Google Scholar]
- 34.Beardsmore CS, Bar-Yishay E, Maayan C, Yahav Y, Katznelson D, Godfrey S. Lung function in infants with cystic fibrosis. Thorax 1988;43:545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Godfrey S, Mearns M, Howlett G. Serial lung function studies in cystic fibrosis in the first 5 years of life. Arch Dis Child 1978;53:83–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phelan PDGM, Williams HE, Anderson CM. Ventilatory function in infants with cystic fibrosis: physiological assessment of halation therapy. Arch Dis Child 1969;44:393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gappa M, Ranganathan S, Stocks J. Lung function testing in infants with cystic fibrosis: lessons from the past and future directions. Pediatr Pulmonol 2001;32:228–245. [DOI] [PubMed] [Google Scholar]
- 38.Freeman JVCT, Chinn S, Jones PRM, White EM, Preece MA. Cross sectional stature and weight reference curves for the UK, 1990. Arch Dis Child 1995;73:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Centers for Disease Control and Prevention (CDC). . National Health and Nutrition Examination Survey data. Hyattsville, MD: National Center for Health Statistics.
- 40.Aurora P, Bush A, Gustafsson P, Oliver C, Wallis C, Price J, Stroobant J, Carr S, Stocks J. Multiple-breath washout as a marker of lung disease in preschool children with cystic fibrosis. Am J Respir Crit Care Med 2005;171:249–256. [DOI] [PubMed] [Google Scholar]
- 41.Shwachman H, Kulczycki LL. Long-term study of one hundred five patients with cystic fibrosis; studies made over a five- to fourteen-year period. AMA J Dis Child 1958;96:6–15. [DOI] [PubMed] [Google Scholar]