Abstract
Pulmonary hypertension is a vascular proliferative disease characterized by pulmonary artery remodeling because of dysregulated endothelial and smooth muscle cell proliferation. Although the role of inflammation in the development of the disease is not well-defined, plexogenic lesions in human disease are characterized by perivascular inflammation composed, in part, of T cells. We explored the role of T-cell infiltration on pulmonary vascular remodeling after endothelial cell damage. We induced endothelial cell damage using monocrotaline and isolated the role of T cells by using Rag1tm1Mom mice and performing adoptive T-cell transfer. We found that monocrotaline causes pulmonary vascular endothelial cell injury followed by a perivascular inflammatory response. The infiltration of inflammatory cells primarily involves CD4+ T cells and leads to the progressive muscularization of small (<30 μm) arterioles. Pulmonary vascular proliferative changes were accompanied by progressive and persistent elevations in right ventricular pressure and right ventricular hypertrophy. Supporting the central role of CD4+ T cells in the inflammatory response, Rag1tm1Mom (Rag1−/−) mice, which are devoid of T and B cells, were protected from the development of vascular injury when exposed to monocrotaline. The introduction of T cells from control mice into Rag1−/− mice reproduced the vascular injury phenotype. These data indicate that after endothelial cell damage, CD4+ T-cell infiltration participates in pulmonary vascular remodeling. This finding suggests that a CD4+ T-cell immune response may contribute to the pathogenesis of inflammatory vascular lesions seen in some forms of pulmonary hypertension.
Keywords: pulmonary vascular remodeling, inflammation, CD4+ T cells, Rag1tm1Mom mice
CLINICAL RELEVANCE.
This study explored the role of inflammation in the development of pulmonary vascular disease. We showed an important role for CD4+ T cells in vascular remodeling after endothelial cell injury. Understanding the role of inflammation in pulmonary vascular injury and repair may lead to novel therapies in diseases such as pulmonary hypertension.
Pulmonary arterial hypertension (PAH) is a rare, debilitating, progressive, and fatal disease for which no cure yet exists. PAH is a proliferative disease of the pulmonary vasculature that leads to a persistent rise in pulmonary artery pressure. This eventually leads to right ventricular failure and death. Histologically, PAH is characterized by pulmonary artery smooth muscle and endothelial cell proliferation and the formation of hallmark plexogenic lesions in the pulmonary vasculature (1). Inflammation is thought to play a role in the pathogenesis of pulmonary hypertension, and evidence exists that markers of inflammation can predict poor outcomes in PAH (2, 3). An increased incidence of PAH occurs in autoimmune inflammatory diseases such as scleroderma and lupus (4, 5). T and B lymphocytes have been described to surround and penetrate plexiform lesions (6–8). The specific role of these cells in the onset and propagation of the disease has not been elucidated.
Vasculitides are characterized by the inflammation of blood vessels and commonly have both cutaneous and systemic features (9). Many diseases associated with the onset of PAH, such as connective tissue diseases, HIV infection, and sarcoidosis, are also associated with vasculitis syndromes (10–13). The best-described animal model of pulmonary hypertension (i.e., the rat monocrotaline model) is characterized by early-onset mononuclear cell vasculitis of the pulmonary arteries (14). Systemic vasculitides predispose patients to vascular remodeling and thrombosis. Both processes are thought to play a role in the progression of PAH (15). The mechanisms through which vasculitis leads to vessel remodeling and clinical manifestations of the disease are not fully understood. Some forms of PAH, such as lupus-related PAH, may respond to immunosuppressive therapies, whereas other forms, such as scleroderma-related PAH, do not (16). Understanding the interactions between the immune system and the pulmonary vasculature may exert a profound impact on the treatment of pulmonary vascular diseases.
Here, we demonstrate that the administration of monocrotaline causes endothelial cell injury, associated with a CD4+ T cell–mediated inflammatory response in mice. We further demonstrate that this T cell–mediated inflammation predates the development of vascular remodeling, characterized by arteriolar muscularization, sustained elevations in right ventricular pressure, and right ventricular hypertrophy. This study provides insights into the role of inflammation in pulmonary vascular remodeling. Further studies are needed to evaluate whether the inhibition of T-cell recruitment would prevent or reverse pulmonary vascular remodeling.
MATERIALS AND METHODS
Animal Handling and Experimental Design
Five-week-old C57BL/6J and Rag1tm1Mom mice on a C57BL/6J genetic background (Jackson Laboratory, Bar Harbor, ME), were subjected to weekly subcutaneous injections of different body weight–adjusted doses of monocrotaline (MCT; Sigma, St. Louis, MO) for 1, 2, 4, and 8 weeks. Based on dose–response data, 300 mg/kg/week was determined to be the optimal dose for inducing perivascular inflammation, and was used in all subsequent experiments. Mice injected with PBS served as controls. The mice were analyzed at the end of the various time points. Bromodeoxyuridine (BrdU) (25 mg/kg) was injected 24 hours and 1 hour before tissue harvest, to label actively proliferating cells. All animal experiments were approved by and conducted according to the guidelines of the Animal Care and Use Committee of the National Heart, Lung, and Blood Institute.
Measurements of Right Ventricular Pressure and Assessment of Right Ventricular Hypertrophy
Right and left ventricular pressures were measured using a Millar catheter transducer (Millar Instruments, Houston, TX). Internal jugular vein and carotid artery approaches were used. Right and left ventricular systolic pressures are reported. Further information is available in the online supplement.
The hearts were isolated, and divided into right ventricular (RV) and left ventricular (LV) with the interventricular septum by the same investigator in all experiments. The wet weights of the RV and LV plus septum were measured. The Fulton index was calculated as RV/LV + septum.
Immunostaining
For histology, lungs were flushed through the RV with PBS solution. The left lungs were isolated and perfused via the trachea with either 10% buffered formalin or 20% sucrose, fixed for 24 hours in the same fixative, and embedded in paraffin or optimum cutting temperature, respectively. Lung sections were stained with hematoxylin and eosin. Immunohistochemistry was performed using an ABC immunoperoxidase protocol or immunofluorescence, using antibodies against smooth muscle actin (Fluor 594 donkey anti-rat IgG, smooth muscle cy3 sigma), CD45 (CD45R, catalogue number 550286 clone RA3–6B2 rat monoclonal; BD, Franklin Lakes, NJ), T cells (CD3 T cells, catalogue number A 0452, anti-CD4 and anti-CD8; DAKO, Carpinteria, CA), B cells (CD79a B-cells, ab5691, rabbit polyclonal; Abcam, Cambridge, MA), macrophages (F4/80 AdD, catalogue number MCA497GA, Clone CIA3-1, rat monoclonal; Serotec, Oxford, UK), neutrophils (mouse neutrophils, catalogue number CL 8993B, antigen 7/4, clone 7/4, rat monoclonal; Cedarlane, Burlington, NC), and Bromodeoxyuridine (catalogue number 6326-250; Abcam), as described elsewhere (17).
Assessment of Pulmonary Arteriolar Muscularization and Vessel Wall Thickness
Cross-sectional areas of the media were measured in smooth muscle actin–stained sections by the same investigator, blinded to the genotype, using Image-Pro Plus software (Media Cybernetics, Betehsda, MD). At least 10 vessels were examined from each lung section. For analysis, vessels were divided into two groups according to vessel diameter (i.e., <30 μm and 30–100 μm).
Cross-sectional areas of pulmonary arterioles in hematoxylin and eosin–stained sections were analyzed by the same investigator, using Image-Pro Plus software (Media Cybernetics). Measurements of vessel wall and vessel lumen were taken from at least 10 vessels in each section, and the vessel wall/vessel lumen ratio was calculated.
Measurements of Cytokine and Serum Angiotensin-Converting Enzyme Concentrations
Concentrations of cytokines were measured in the supernatant of lung homogenates, using a murine SearchLight proteome array (Pierce Biotechnology, Rockford, IL). Further details are available in the online supplement.
The serum activity of angiotensin-converting enzyme (ACE) was measured using an ACE colorimetric assay according to the manufacturer's protocol (Alpco, Salem, NH). A detailed description of methods is available in the online supplement.
Adoptive T-Cell Transfer
Five-week-old C57BL/6J mice were treated weekly with 300 mg/kg monocrotaline for 8 weeks, and were killed after the last treatment. Untreated, age-matched C57BL/6J mice served as control donors. Splenocytes were harvested, and red blood cells were lysed. CD4+ T cells were stained with CD4 microbeads and separated using the AutoMACS cell separation system, according to the manufacturer's protocol (Miltenyi Biotech, Auburn, CA). The positive cell fraction consisted of at least 90% CD4+ cells, as measured by flow cytometry. Five to 10 × 106 CD4+ cells derived from monocrotaline-treated or control C57BL/6J mice were injected into the tail veins of 5-week-old Rag1−/− mice. Rag1−/− mice were allowed to recover without further treatment, or received weekly treatments of 300 mg/kg monocrotaline (only when receiving CD4+ cells from control donors). Blood was drawn 1 and 8 weeks after T-cell transfer, to confirm the presence of CD4+ T cells. Hemodynamic and histologic assessments were performed 8 weeks after T-cell transfer, as already described.
Data Analysis
Statistical analysis was performed using Kruskal-Wallis one-way ANOVA with Dunn's post hoc comparison, when applicable. Results are expressed as mean ± SD, and are considered significant at P < 0.05.
RESULTS
Pulmonary Vascular Endothelial Cell Injury in a Murine Model of Monocrotaline-Induced Pulmonary Artery Remodeling
Endothelial cell damage is considered important in the initiation of vascular remodeling (18, 19). Therefore, we developed a model to induce endothelial cell damage in the pulmonary vasculature. We tested various doses of intraperitoneal monocrotaline injections. Based on our dose–response data, 300 mg/kg/week was determined to be the optimal dose for initiating the perivascular inflammatory response, and all further experiments were performed at this dose. To localize and confirm that the site of injury was primarily perivascular, staining with CD45 antibody was performed. Staining was localized to the perivascular area, with no evidence of CD45 staining in the bronchial tree (Figure 1A).
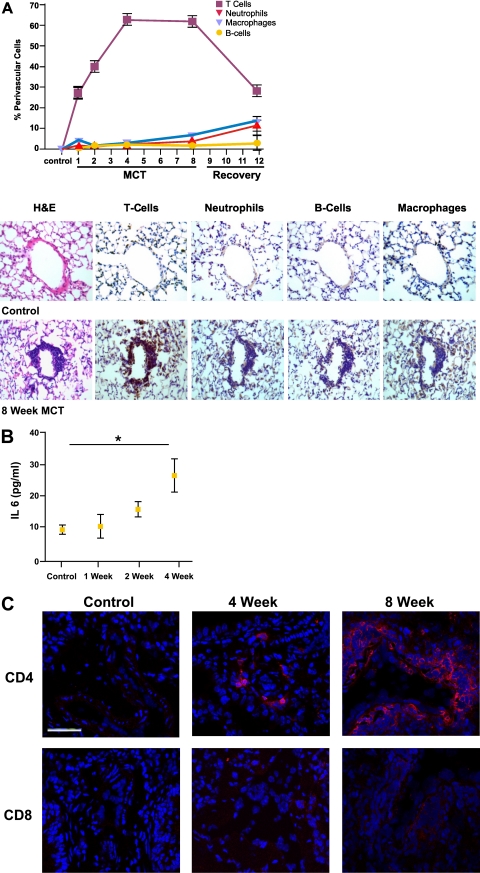
Figure 1.
Evidence of perivascular inflammation and endothelial cell damage induced by injections with monocrotaline. (A) CD45 staining of representative pulmonary vasculature in control mice (left) and in mice after 8 weeks of monocrotaline (MCT) injections (right) shows localization of inflammatory infiltrate surrounding the vasculature (solid arrows) and sparing airways (open arrows). (B) Hematoxylin and eosin staining of small (< 50 μm) pulmonary vessels in control mice (a) and in mice after 1 week (b) and 2 weeks (c) of MCT injections. Arrows indicate beading of endothelial cells. (C) Serum angiotensin-converting enzyme (ACE) concentrations in control mice and mice receiving injections of monocrotaline at 1-week and 2-week time points. Control mice (n = 5), 259.4 ± 5.8 μmol/L/minute; 1 week (n = 5), 279.4 ± 3.1 μmol/L/minute (P < 0.05), 2 weeks (n = 5), 278.7 ± 3.7 μmol/L/minute (P < 0.05). (D) vascular endothelial growth factor (VEGF) concentrations in lung homogenates in control mice and at 1-week and 2-week time points. Control mice (n = 5), 42.2 ± 8.7 pg/ml; 1 week (n = 5), 34.9 ± 4.1 pg/ml (P > 0.05); 2 weeks (n = 5), 21.8 ± 6.9 pg/ml (P < 0.05). Results are expressed as mean ± SD.
In mice receiving monocrotaline, evidence occurred of progressive, monocrotaline-induced endothelial cell (EC) injury at 1 and 2 weeks after initiating treatment. This injury was characterized histologically by the beading of ECs in treated mice. The normally flat EC monolayer had become raised and rounded compared with control samples (Figure 1B). Serum ACE concentrations, which were shown to be elevated after EC injury, were significantly elevated in mice treated at 1-week and 2-week time points compared with control mice (Figure 1C). Furthermore, vascular endothelial growth factor (VEGF) concentrations in lung homogenates began to decline after 1 week of treatment, and were even further decreased after 2 weeks compared with control lungs (Figure 1D). These data suggest that intraperitoneal injections of monocrotaline lead to EC damage in the pulmonary arterioles, thereby initiating a process that ultimately leads to remodeling of the lung vasculature.
Perivascular Infiltration of Inflammatory Cells Follows EC Damage during Pulmonary Arteriolar Remodeling
Histologically, a perivascular inflammatory cell infiltration was evident in small and large vessels in mice treated with monocrotaline compared with control mice. To characterize these cells, staining was performed for T cells by the CD3 marker, for macrophages by F4/80 markers, for B cells by B220 markers, and for neutrophils by neutrophil-specific markers. This inflammatory cell infiltration was found to be T cell–predominant, beginning as early as 1 week after the first monocrotaline injection, peaking after 4 weeks of injections, and sustained until the withdrawal of monocrotaline (Figure 2A).
Figure 2.
Perivascular infiltration of inflammatory cells after endothelial cell injury is characterized by CD4+ T cells. (A, top) Percentage of perivascular cells positive for CD3 (squares). Control mice, 2.8% ± 2.9% total perivascular cells; 1 week, 27.6% ± 2.7% total perivascular cells (P < 0.001); 2 weeks, 40.6% ± 3.3% total perivascular cells (P < 0.001); 4 weeks, 63.2% ± 2.4% total perivascular cells (P < 0.001); 8 weeks, 62.1% ± 2.9% total perivascular cells (P < 0.001); recovery, 29.1% ± 2.8% total perivascular cells (P < 0.001). F4/80 (macrophage antigen) (light blue upside-down triangle), B cells (circles), or neutrophils (dark red upside-down triangle) staining. (A, bottom) Representative images of inflammatory cell staining in control mice and after 8 weeks of monocrotaline injections show CD3+ T cell–predominant inflammatory response. (B) IL-6. Control mice (n = 4), 9.9 ± 0.5 pg/ml; 1 week (n = 5), 11.5 ± 3.5 pg/ml; 2 weeks (n = 5), 16.7 ± 2.4 pg/ml; 4 weeks (n = 5), 27.7 ± 5.2 pg/ml (P = 0.01). H&E, hematoxylin and eosin. (C) Immunohistochemical staining for CD4 (row 1) and CD8 (row 2) in control mice and at 4-week and 8-week time points (scale bar, 50 μm) shows that inflammatory response consists predominantly of CD4+ T cells. Results are expressed as mean ± SD.
We found that the proinflammatory T-cell cytokine IL-6 was trending upward by the 2-week time point, and was significantly elevated after 4 weeks of exposure to monocrotaline (Figure 2B). To characterize further this T-cell response to injury, immunohistochemical staining for CD4 and CD8 was performed. This revealed the inflammatory infiltrate to be predominantly CD4+ T cells (Figure 2C). B cells, neutrophils, and macrophages constituted the minority of inflammatory cells recruited to the injured vasculature.
These results confirm that after EC damage, an inflammatory response occurs that appears to be mediated by CD4+ T cells. We speculate that this inflammatory response may be linked to the initiation of a process of pulmonary arteriolar remodeling.
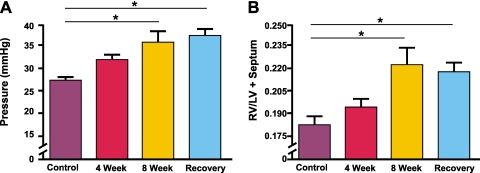
Elevations of RV Pressure and RV Hypertrophy Develop after EC Injury and the Ensuing Inflammatory Response
Pulmonary vascular changes, characterized by the muscularization of arterioles and inflammation, are associated with hemodynamic changes in animal models of pulmonary hypertension. Therefore, we explored the effects of our model on RV pressure and the Fulton index. We found that after 4 and 8 weeks of exposure to monocrotaline, an elevation in RV pressure was evident. Furthermore, after 4 weeks off treatment, RV pressures remained elevated (Figure 3A). We assessed the role that LV dysfunction might play in elevated right-sided pressures by measuring LV pressures directly. No difference was evident in LV systolic pressures between control mice and monocrotaline-treated mice (control mice, 8 weeks: 98.0 ± 1.3 mm Hg; monocrotaline-treated mice, 8 weeks: 98.5 ± 2.3 mm Hg; P > 0.05).
Figure 3.
Elevation of right ventricular (RV) pressure and RV hypertrophy in response to vascular remodeling. (A) RV pressures in control mice and at 4, 8, and 12 weeks (recovery). Control mice (n = 10), 27.0 ± 2.7 mm Hg; 4 weeks (n = 10), 31.6 ± 4.1 mm Hg (P < 0.01); 8 weeks (n = 10), 35.5 ± 7.6 mm Hg (P < 0.05); 4 weeks off treatment (n = 5), 37.0 ± 3.4 mm Hg (P < 0.01). (B) RV to left ventricle (LV) plus septum weight ratio (Fulton index) in control mice and at 4, 8, and 12 weeks (recovery). Control mice (n = 10), 0.18 ± 0.02; 4 weeks (n = 10), 0.2 ± 0.02 (P > 0.05); 8 weeks (n = 10), 0.22 ± 0.03 (P < 0.01); 4 weeks off treatment (n = 5), 0.22 ± 0.01 (P < 0.05). Results are expressed as mean ± SD.
Mice treated with monocrotaline showed evidence of RV hypertrophy, as assessed by the Fulton index. After 4 weeks of exposure, a trend toward an elevated Fulton index was evident, and this achieved statistical significance after 8 weeks. Furthermore, after 4 weeks off treatment, the Fulton index remained significantly elevated above control values (Figure 3B).
These data suggest that EC damage and the persistent CD4+ T-cell inflammatory response lead to a rise in pulmonary artery pressure, which in turn leads to RV hypertrophy. Furthermore, these changes appear fixed, and after the withdrawal of monocrotaline, elevations of pulmonary artery pressure and RV hypertrophy do not regress. We speculate that these physiologic changes in the heart are related to the pathologic remodeling of the pulmonary vasculature.
Smooth Muscle Cell Proliferation and Increased Medial Area Lead to Remodeling of the Pulmonary Vasculature in Response to EC Injury and Inflammation
The muscularization of small arteries is a characteristic histologic finding of pulmonary hypertension (20). The vascular area positive for smooth muscle actin staining was calculated for vessels less than 30 μm in diameter and for vessels 30–100 μm in diameter. In vessels less than 30 μm in diameter, the vessel wall area positive for smooth muscle actin staining was significantly increased in treated mice compared with control mice. This result was also found in vessels ranging from 30–100 μm in diameter (Figure 4A). Furthermore, we assessed changes in vessel wall thickness as an additional means of measuring vascular remodeling. The wall thickness/vessel lumen ratio in monocrotaline-treated mice was double that of control mice at both 4 and 8 weeks (Figure 4B).
Figure 4.
Pulmonary vascular remodeling is characterized by smooth muscle cell proliferation and increased arterial wall thickness in response to inflammation. (A) Area of smooth muscle actin (SMA) staining in controls and at 4-week and 8-week time points in vessels less than 30 μm in diameter. Control mice (n = 5), 157.7 ± 17.7 μm2; 4 weeks (n = 5), 270.5 ± 17.6 μm2 (P = 0.004); 8 weeks (n = 5), 280.1± 23.8 μm2 (P = 0.003). In vessels 30–100 μm in diameter, control mice (n = 5), 466.1 ± 45.6 μm2; 4 weeks (n = 5), 818.4 ± 129.1 μm2 (P < 0.05); 8 weeks (n = 5), 1,144 ± 353.5 μm2 (P < 0.05). (B) Evaluation of wall thickness as measured by wall thickness to vessel lumen ratio. Control mice (n = 5), 13.7 ± 2.4; 4 weeks (n = 5), 34.75 ± 4.5 (P < 0.05); 8 weeks (n = 5), 35.2 ± 8.9 (P < 0.05). (C) Bromodeoxyuridine (BRDU) (red) and α-actin (green) double staining of control mice and at 1-week time point shows smooth muscle cell proliferation in monocrotaline-treated mice (solid arrow). (D) Matrix metalloproteinase-2 (MMP2). Control mice (n = 5), 2,343 ± 710 pg/ml; 4 weeks (n = 5), 3,226 ± 473 pg/ml (P > 0.05); 8 weeks (n = 5), 4,588 ± 444 pg/ml (P < 0.001). Results are expressed as mean ± SD.
Bromodeoxyuridine/α actin double staining revealed proliferating smooth muscle cells, 1 week after the initiation of monocrotaline treatment. This suggests that the muscularization of the small pulmonary arteries is caused by smooth muscle cell proliferation (Figure 4C). Later time points (4 weeks and 8 weeks) did not reveal proliferating smooth muscle cells (data not shown).
Matrix metalloproteinases (MMPs) are involved in extracellular matrix turnover, contributing to endothelial and smooth muscle migration and proliferation. MMP-2 was shown to be associated with structural remodeling of the pulmonary vessel during experimental pulmonary hypertension (21). MMP-2 concentrations were determined from mouse lung homogenates. MMP-2 concentrations had not significantly changed after the 1-week and 2-week time points (data not shown). However, MMP-2 concentrations were elevated at both 4 and 8 weeks (Figure 4D).
These data suggest that after EC injury and T-cell infiltration, the pulmonary vasculature begins a process of remodeling, as evidenced by smooth muscle cell proliferation, medial thickening of the artery, and the elevation of MMP-2 (Figure E1).
Monocrotaline-Induced Perivascular Inflammation and Vascular Remodeling Predominantly Involves the Monocyte Chemoattractant Protein–1–Associated Recruitment of CD4+ T Cells
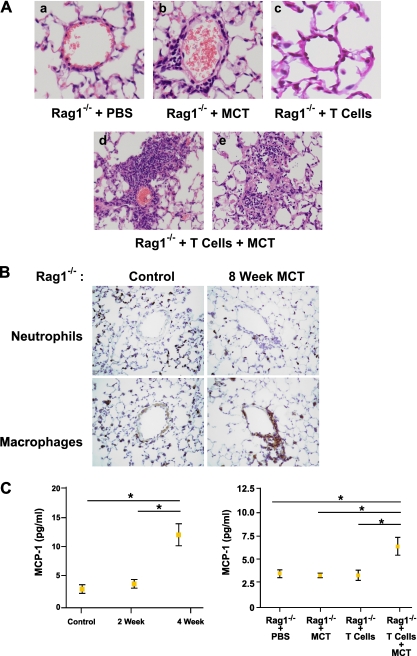
To confirm the role of CD4+ T cells in the pathology of monocrotaline-induced vascular injury and remodeling, we used the Rag1−/− strain of mice. Mice homozygous for the Rag1−/− mutation produce no mature T or B cells, and have no CD3+ cells. We exposed Rag1−/− mice to serial injections with monocrotaline and reintroduced CD4+ T cells via an adoptive T-cell transfer, in an attempt to reproduce the inflammatory phenotype.
Rag1−/− mice receiving T cells and treatment with monocrotaline produced a marked perivascular inflammatory response (Figure 5A). In contrast, Rag1−/− mice without reintroduced T cells and treated with monocrotaline showed evidence of only minimal perivascular inflammation, involving predominantly neutrophils and macrophages (Figure 5B). We used CD4+ T cells from both untreated wild-type mice and wild-type mice treated with monocrotaline for 8 weeks. We found no difference in the inflammatory response to monocrotaline when reintroduced CD4+ T cells were from monocrotaline-treated or untreated mice. This suggests that monocrotaline does not prime T cells to induce pulmonary vascular EC injury (data not shown).
Figure 5.
(A) Hematoxylin and eosin staining of pulmonary vasculature in Rag1−/− mice receiving (a) serial injections with PBS, (b) serial injections with MCT, (c) adoptive T-cell transfer and serial injections with PBS, and (d and e) adoptive T-cell transfer and serial injections with MCT. (B) Neutrophil and macrophage staining of Rag1−/− mice receiving serial injections with monocrotaline. (C) Monocyte chemoattractant protein–1 (MCP-1) concentrations in wild-type mice receiving monocrotaline injection. Control mice (n = 5), 3.1 ± 0.5 pg/ml/mg; 2 weeks (n = 5), 4.1 ± 0.5 pg/ml/mg (P > 0.05); 4 weeks (n = 5), 12.4 ± 3.3 pg/ml/mg (P < 0.01). MCP-1 concentrations in Rag1−/− receiving either monocrotaline and/or T cells: Rag1−/−, 3.5 ± 0.9 pg/ml; Rag1−/− + MCT, 3.3 ± 0.3 pg/ml; Rag1−/− + CD4 cells, 3.3 ± 1.1 pg/ml; Rag1−/− + CD4 cells + MCT, 6.4 ± 1.8 pg/ml (P < 0.05). Results are expressed as mean ± SD.
Monocyte chemoattractant protein–1 (MCP-1), part of the C-C chemokine family, exerts its affects predominantly on the trafficking of monocytes and T lymphocytes. However, it was also shown to exert immunomodulatory effects on CD4+ T lymphocytes (22, 23). In C57Bl6 mice, MCP-1 was significantly elevated after 4 weeks of exposure to monocrotaline (Figure 5C). In Rag1−/− mice, a twofold rise in MCP-1 concentrations was found to be T cell–dependent (Figure 5C). To highlight the role of T cells further, we measured IL-6 concentrations. In alignment with minimal perivascular inflammation, the IL-6 concentrations of Rag1−/− mice treated with monocrotaline were unchanged compared with control mice. When T cells were reintroduced and mice were exposed to monocrotaline, a significant rise in IL-6 concentrations occurred (Figure 6A).
Figure 6.
CD4+ T cells are required for pulmonary artery inflammatory response and vessel remodeling. (A) IL-6 concentrations in lung homogenates in control mice and in adaptive T-cell transfer. Rag1−/−, 9.9 ± 3.1 pg/ml; Rag1−/− + MCT, 6.5 ± 1.0 pg/ml; Rag1−/− + CD4 cells, 9.0 ± 5.1 pg/ml; Rag1−/− + CD4 cells + MCT, 16.6 ± 6.4 pg/ml (P < 0.05). (B) RV pressure measurements in adaptive T-cell transfer experiment. Control mice (n = 4), 30.5 ± 0.65 mm Hg; 4 weeks (n = 5), 30.6 ± 1.3 mm Hg; 8 weeks (n = 4), 31.8 ± 1.3 mm Hg; Adaptive T-cell transfer (ATCT), 8 weeks (n = 4), 38.0 ± 1.7 mm Hg (P < 0.05). (C) RV hypertrophy measurements in adaptive T-cell transfer experiment. Rag1−/−, 0.18 ± 0.008; Rag1−/− + MCT, 0.19 ± 0.017; Rag1−/− + CD4 cells, 0.2 ± 0.008; Rag1−/− + CD4 cells + MCT, 0.23 ± 0.009 (P < 0.05). (D) MMP2 concentrations in lung homogenates in control and in adaptive T-cell transfer. Rag1−/−, 3,186 ± 598 pg/ml; Rag1−/− + MCT, 7,756 ± 3,329 pg/ml; Rag1−/− + CD4 cells, 7,278 ± 2,722 pg/ml; Rag1−/− + CD4 cells + MCT, 14,846 ± 2,813 pg/ml (P < 0.001). Results are expressed as mean ± SD.
Furthermore, in Rag1−/− mice treated with 4 or 8 weeks of monocrotaline, no difference in RV pressure was evident, compared with control mice. However, in Rag1−/− mice that underwent adoptive T-cell transfer and were treated with 8 weeks of monocrotaline injections, a significant rise in RV pressure and an elevation of the Fulton index were evident, compared with control mice (Figures 6B and 6C). These changes correlated with evidence of vascular remodeling. In Rag1−/− mice treated with 4 and 8 weeks of monocrotaline injections, concentrations of MMP-2 were not significantly elevated. When CD4+ T cells were reintroduced and mice were treated with monocrotaline, concentrations of MMP-2 were increased fivefold above control concentrations (Figure 6D). This suggests that the concentrations of MMP-2 involved in vessel remodeling after vascular injury and inflammation are dependent on CD4+ T cells.
These data suggest that CD4+ T cells play a pivotal role in the pathology of monocrotaline-induced pulmonary vascular injury and remodeling. The absence of CD 4+ T cells leads to an attenuated inflammatory response after exposure to monocrotaline, and in turn protects against a rise in RV pressure and RV hypertrophy. The reintroduction of CD4+ T cells leads to marked perivascular inflammation, vessel remodeling, the elevation of RV pressures, and RV hypertrophy after exposure to monocrotaline.
DISCUSSION
PAH exists in both an idiopathic form and in association with a wide variety of diseases such as HIV, connective tissue diseases such as scleroderma, liver disease, and exposure to certain drugs and toxins (24). Despite the wide variety of underlying causes, patients with PAH tend to develop common histologic findings of the lung vasculature. These findings include a proliferation of smooth muscle cells with medial hypertrophy and the muscularization of arterioles, in situ thrombosis, the proliferation of ECs with the formation of neointima and plexogenic lesions, and the infiltration of vascular lesions with inflammatory cells (macrophages and lymphocytes) (6–8). Vascular injury with a secondary inflammatory response appears to be a common finding that may help explain the shared histologic endpoint for the diverse variety of diseases causing pulmonary hypertension.
Inflammation plays a key role in vascular biology. The role of inflammatory cells (and particularly T cells) in peripheral vascular disease and atherosclerosis is well known. Markers of chronic inflammation, such as C-reactive protein and macrophage colony–stimulating factor, were shown to be predictors of cardiovascular disease, including PAH in humans (2, 25). T and B cells were shown to be key players in the development of atherosclerotic lesions, with immunodeficient mice showing a 40–80% decrease in the formation of lesions (26, 27). When apoprotein E–deficient mice, which are hypercholesterolemic and prone to the formation of atherosclerosis, are crossed with severe combined immunodeficiency mice, which lack T and B cells, the formation of atherosclerotic lesions drops by 70%. Interestingly, when CD4+ T cells are transferred into these mice, the development of atherosclerosis is similar to that in immunocompetent control mice (28). Similarly, in a wire injury model of vascular disease, the importance of T cells was demonstrated by the observation that Rag1−/− mice were protected from vascular proliferation after injury, as were mice that had undergone a thymectomy (17). This shows that an inflammatory response (including CD4+ T cells) to a vascular injury seems to play a key role in the remodeling process of the vessel wall.
The role of inflammatory cells in pulmonary vascular disease is less clear. Although they were described as present in lesions of PAH, whether they play a role in the pathogenesis and progression of the disease or are incidental findings is unclear. Evidence suggests that the immune system may play an active role in disease progression. PAH, a rare disease with an incidence of about six cases per million in the idiopathic form, has an increased prevalence in patients with autoimmune disorders such as systemic sclerosis and lupus (4, 5, 29). Patients with PAH were found to have elevated concentrations of autoantibodies (30, 31). Patients with idiopathic PAH have increased concentrations of FoxP3+CD4+ T cells or T-regulatory cells in peripheral blood compared with control subjects (32). These findings suggest that the immune system, and particularly T cells, may play a role in the pathogenesis of pulmonary hypertension.
Here we describe a murine model of monocrotaline-induced pulmonary vascular injury and remodeling. Monocrotaline-induced pulmonary hypertension in rats is a well-established animal model of the disease (33). It is characterized by monocrotaline-induced EC injury, followed by an inflammatory response and vascular remodeling (34). Few publications have described this model in mice (35, 36). Interspecies differences in the amount of liver cytochrome P450 3A enzyme required to convert monocrotaline to its toxic pyrrole metabolite may explain the difficulty in establishing a murine model of monocrotaline-induced pulmonary hypertension. Mice require a much higher exposure to this toxin than do rats to induce pulmonary vascular injury (35, 37, 38).
Within 1 week of injection with monocrotaline, we began to see histologic evidence of EC injury. Serum ACE concentrations, previously shown to correlate with lung injury, were significantly upregulated (39). Furthermore, VEGF concentrations were decreased, which was shown to occur in the rat monocrotaline model as well as in a rat model of pulmonary fibrosis with pulmonary hypertension. This decrease in VEGF concentrations is thought to be related to EC injury and apoptosis (40, 41).
We found that serial injections with monocrotaline led to a perivascular CD4+ T-cell inflammatory response. The inflammation was characterized by elevations in the T-cell inflammatory cytokine IL-6. This response began as early as 1 week after the initial injection with monocrotaline, and lasted as long as the exposure to monocrotaline. The chemokine MCP-1 appeared to play a role in the trafficking of CD4+ T cells. MCP-1 was observed to be trending upward by 2 weeks of injections with monocrotaline, and was significantly elevated by 4 weeks, which correlated with the infiltration of CD3+CD4+ T cells. This chemokine was previously shown to play a role in experimental animal models of pulmonary hypertension and in human disease (42, 43). Specifically, blocking the MCP-1 signaling pathway was shown to attenuate the development of pulmonary hypertension in the rat monocrotaline model of pulmonary hypertension (43). MCP-1 concentrations were shown to be significantly elevated in the serum of patients with idiopathic PAH, compared with control subjects (42).
The inflammatory reaction induced by monocrotaline led to a remodeling of the pulmonary vasculature. This was characterized by medial thickening and smooth muscle proliferation, with increased muscularization (as assessed by smooth muscle actin staining) of the small (<30 μm) pulmonary arterioles. MMP-2 concentrations were elevated and likely play a role in remodeling of the vessel wall. MMP-2 participates in the degradation of basement membrane, facilitates smooth muscle proliferation, and was previously shown to play a role in rat models of pulmonary hypertension (21). These vascular changes coincide with an increase in RV pressures and RV hypertrophy. The remodeling of vessels persisted even after 4 weeks off monocrotaline therapy, despite a drop in the number of perivascular inflammatory cells. RV pressure and the Fulton index remained significantly elevated above control levels. This suggests that inflammatory remodeling appears to be fixed, and that it may lead to a progressive rise in pressures and right heart failure, even after the removal of the inflammatory insult.
We found that CD4+ T cells are necessary for the induction of pulmonary hypertension by monocrotaline in mice. When Rag1−/− mice are exposed to monocrotaline, they have an attenuated inflammatory response, and markers of vascular remodeling such as MMP-2 are not significantly elevated. Furthermore, they do not manifest significant elevations in RV pressures or increased RV hypertrophy. When CD 4+ cells are transplanted from normal control mice into Rag1−/− mice and are then exposed to serial injections of monocrotaline, they experience exuberant perivascular inflammation, a threefold rise in MMP-2 concentrations, and a significant rise in RV pressures and the Fulton index.
Our findings highlight the important role of inflammation, and specifically of CD4+ T cells, in the propagation of vascular remodeling in a monocrotaline model of vascular injury. These findings seem to be in conflict with the findings of Taraseviciene-Stewart and colleagues, who showed a protective role for T cells in the development of a proliferative vasculopathy in athymic nude rats exposed to vascular endothelial growth factor receptor 2 inhibitor (44). Several key differences between our studies may help explain these seemingly disparate findings. First, the athymic nude rat is known to be deficient in T cells, but with normal B-cell function and increased natural killer and macrophage cell populations. In contrast, wild-type mice obviously have an intact immune system, and Rag1−/− mice are characterized by an absence of mature T or B cells. These three different animal lines have very different immunologic backgrounds, and thus likely different immunologic responses to vascular injury. Furthermore, the vascular injury caused by the VEGF inhibitor is likely different from that caused by serial monocrotaline injections. The end result of the injury in each model is also quite different. The model of Taraseviciene-Stewart and colleagues (44) attempted to model PAH by inducing an EC proliferative response, whereas the monocrotaline model involves inflammatory injury that is not primarily characterized by EC proliferation. The presence of T cells appears to play an important role in the response of athymic nude rats to exposure to SU5416. The absence of T cells likely alters the immune response and allows for EC proliferation under normoxic conditions. The introduction of T cells into the athymic rat presumably normalizes the immune response and confers protection from SU5416 during normoxia. These two studies are linked insofar as they both highlight the importance of inflammation and immune response to vascular injury and vascular remodeling. However, we feel that the specific role of T cells differs because of the differences in animal models used and the mode of injury in the separate experiments.
We found that monocrotaline induces EC damage in the pulmonary vasculature of mice. Vascular remodeling in response to this EC injury was characterized by CD4+ T cell–dominated inflammation and smooth muscle cell proliferation. This CD4+ T cell–dependent remodeling of the pulmonary vasculature leads to a rise in RV pressure and RV hypertrophy. This model of monocrotaline-induced pulmonary artery injury and remodeling allows for insights into the role of inflammation in the remodeling of the pulmonary vasculature, and further facilitates the use of genetic murine models in pulmonary vascular research. This, in turn, may help us understand the role of inflammation in vascular lesions in human pulmonary hypertension.
Supplementary Material
Acknowledgments
The authors thank Robin Schwartzbeck and Hong San for their assistance with murine microsurgical techniques and specimen processing.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2009-0365OC on September 2, 2010
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Archer S, Rich S. Primary pulmonary hypertension: a vascular biology and translational research “work in progress”. Circulation 2000;102:2781–2791. [DOI] [PubMed] [Google Scholar]
- 2.Quarck R, Nawrot T, Meyns B, Delcroix M. C-reactive protein: a new predictor of adverse outcome in pulmonary arterial hypertension. J Am Coll Cardiol 2009;53:1211–1218. [DOI] [PubMed] [Google Scholar]
- 3.Nicolls MR, Taraseviciene-Stewart L, Rai PR, Badesch DB, Voelkel NF. Autoimmunity and pulmonary hypertension: a perspective. Eur Respir J 2005;26:1110–1118. [DOI] [PubMed] [Google Scholar]
- 4.Mukerjee D, St George D, Coleiro B, Knight C, Denton CP, Davar J, Black CM, Coghlan JG. Prevalence and outcome in systemic sclerosis associated pulmonary arterial hypertension: application of a registry approach. Ann Rheum Dis 2003;62:1088–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fagan KA, Badesch DB. Pulmonary hypertension associated with connective tissue disease. Prog Cardiovasc Dis 2002;45:225–234. [DOI] [PubMed] [Google Scholar]
- 6.Voelkel NF, Tuder RM. Cellular and molecular mechanisms in the pathogenesis of severe pulmonary hypertension. Eur Respir J 1995;8:2129–2138. [DOI] [PubMed] [Google Scholar]
- 7.Tuder RM, Groves B, Badesch DB, Voelkel NF. Exuberant endothelial cell growth and elements of inflammation are present in plexiform lesions of pulmonary hypertension. Am J Pathol 1994;144:275–285. [PMC free article] [PubMed] [Google Scholar]
- 8.Tuder RM, Voelkel NF. Angiogenesis and pulmonary hypertension: a unique process in a unique disease. Antioxid Redox Signal 2002;4:833–843. [DOI] [PubMed] [Google Scholar]
- 9.Guillevin L. Vasculopathy and pulmonary arterial hypertension. Rheumatology (Oxford) 2009;48:iii54–iii57. [DOI] [PubMed] [Google Scholar]
- 10.Ramos-Casals M, Nardi N, Lagrutta M, Brito-Zeron P, Bove A, Delgado G, Cervera R, Ingelmo M, Font J. Vasculitis in systemic lupus erythematosus: prevalence and clinical characteristics in 670 patients. Medicine (Baltimore) 2006;85:95–104. [DOI] [PubMed] [Google Scholar]
- 11.Guillevin L. Vasculitides in the context of HIV infection. AIDS 2008;22:S27–S33. [DOI] [PubMed] [Google Scholar]
- 12.Fleming JN, Schwartz SM. The pathology of scleroderma vascular disease. Rheum Dis Clin North Am 2008;34:41–55. (vi.). [DOI] [PubMed] [Google Scholar]
- 13.Fernandes SR, Singsen BH, Hoffman GS. Sarcoidosis and systemic vasculitis. Semin Arthritis Rheum 2000;30:33–46. [DOI] [PubMed] [Google Scholar]
- 14.Turner JH, Lalich JJ. Experimental cor pulmonale in the rat. Arch Pathol 1965;79:409–418. [PubMed] [Google Scholar]
- 15.Kane GC, Keogh KA. Involvement of the heart by small and medium vessel vasculitis. Curr Opin Rheumatol 2009;21:29–34. [DOI] [PubMed] [Google Scholar]
- 16.Sanchez O, Sitbon O, Jais X, Simonneau G, Humbert M. Immunosuppressive therapy in connective tissue diseases–associated pulmonary arterial hypertension. Chest 2006;130:182–189. [DOI] [PubMed] [Google Scholar]
- 17.Boehm M, Olive M, True AL, Crook MF, San H, Qu X, Nabel EG. Bone marrow–derived immune cells regulate vascular disease through a p27(Kip1)-dependent mechanism. J Clin Invest 2004;114:419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tuder RM, Cool CD, Yeager M, Taraseviciene-Stewart L, Bull TM, Voelkel NF. The pathobiology of pulmonary hypertension: endothelium. Clin Chest Med 2001;22:405–418. [DOI] [PubMed] [Google Scholar]
- 19.Voelkel NF, Cool C, Lee SD, Wright L, Geraci MW, Tuder RM. Primary pulmonary hypertension between inflammation and cancer. Chest 1998;114:225S–230S. [DOI] [PubMed] [Google Scholar]
- 20.Galie N, Torbicki A, Barst R, Dartevelle P, Haworth S, Higenbottam T, Olschewski H, Peacock A, Pietra G, Rubin LJ, et al. Guidelines on diagnosis and treatment of pulmonary arterial hypertension: the Task Force on Diagnosis and Treatment of Pulmonary Arterial Hypertension of the European Society of Cardiology. Eur Heart J 2004;25:2243–2278. [DOI] [PubMed] [Google Scholar]
- 21.Frisdal E, Gest V, Vieillard-Baron A, Levame M, Lepetit H, Eddahibi S, Lafuma C, Harf A, Adnot S, Dortho MP. Gelatinase expression in pulmonary arteries during experimental pulmonary hypertension. Eur Respir J 2001;18:838–845. [DOI] [PubMed] [Google Scholar]
- 22.Carr MW, Roth SJ, Luther E, Rose SS, Springer TA. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc Natl Acad Sci USA 1994;91:3652–3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hogaboam CM, Lukacs NW, Chensue SW, Strieter RM, Kunkel SL. Monocyte chemoattractant protein–1 synthesis by murine lung fibroblasts modulates CD4+ T cell activation. J Immunol 1998;160:4606–4614. [PubMed] [Google Scholar]
- 24.Simonneau G, Galie N, Rubin LJ, Langleben D, Seeger W, Domenighetti G, Gibbs S, Lebrec D, Speich R, Beghetti M, et al. Clinical classification of pulmonary hypertension. J Am Coll Cardiol 2004;43:5S–12S. [DOI] [PubMed] [Google Scholar]
- 25.Ikonomidis I, Lekakis J, Revela I, Andreotti F, Nihoyannopoulos P. Increased circulating C-reactive protein and macrophage-colony stimulating factor are complementary predictors of long-term outcome in patients with chronic coronary artery disease. Eur Heart J 2005;26:1618–1624. [DOI] [PubMed] [Google Scholar]
- 26.Reardon CA, Blachowicz L, White T, Cabana V, Wang Y, Lukens J, Bluestone J, Getz GS. Effect of immune deficiency on lipoproteins and atherosclerosis in male apolipoprotein E–deficient mice. Arterioscler Thromb Vasc Biol 2001;21:1011–1016. [DOI] [PubMed] [Google Scholar]
- 27.Song L, Leung C, Schindler C. Lymphocytes are important in early atherosclerosis. J Clin Invest 2001;108:251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou X, Nicoletti A, Elhage R, Hansson GK. Transfer of CD4(+) T cells aggravates atherosclerosis in immunodeficient apolipoprotein E knockout mice. Circulation 2000;102:2919–2922. [DOI] [PubMed] [Google Scholar]
- 29.Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaici A, Weitzenblum E, Cordier JF, Chabot F, et al. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med 2006;173:1023–1030. [DOI] [PubMed] [Google Scholar]
- 30.Tamby MC, Chanseaud Y, Humbert M, Fermanian J, Guilpain P, Garcia-de-la-Pena-Lefebvre P, Brunet S, Servettaz A, Weill B, Simonneau G, et al. Anti-endothelial cell antibodies in idiopathic and systemic sclerosis associated pulmonary arterial hypertension. Thorax 2005;60:765–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tamby MC, Humbert M, Guilpain P, Servettaz A, Dupin N, Christner JJ, Simonneau G, Fermanian J, Weill B, Guillevin L, et al. Antibodies to fibroblasts in idiopathic and scleroderma-associated pulmonary hypertension. Eur Respir J 2006;28:799–807. [DOI] [PubMed] [Google Scholar]
- 32.Ulrich S, Nicolls MR, Taraseviciene L, Speich R, Voelkel N. Increased regulatory and decreased CD8+ cytotoxic T cells in the blood of patients with idiopathic pulmonary arterial hypertension. Respiration 2008;75:272–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lalich JJ, Merkow L. Pulmonary arteritis produced in rat by feeding Crotalaria spectabilis. Lab Invest 1961;10:744–750. [PubMed] [Google Scholar]
- 34.Wilson DW, Segall HJ, Pan LC, Dunston SK. Progressive inflammatory and structural changes in the pulmonary vasculature of monocrotaline-treated rats. Microvasc Res 1989;38:57–80. [DOI] [PubMed] [Google Scholar]
- 35.Goto J, Ishikawa K, Kawamura K, Watanabe Y, Matumoto H, Sugawara D, Maruyama Y. Heme oxygenase–1 reduces murine monocrotaline-induced pulmonary inflammatory responses and resultant right ventricular overload. Antioxid Redox Signal 2002;4:563–568. [DOI] [PubMed] [Google Scholar]
- 36.Molteni A, Ward WF, Ts'ao CH, Solliday NH. Monocrotaline pneumotoxicity in mice. Virchows Arch B Cell Pathol Incl Mol Pathol 1989;57:149–155. [DOI] [PubMed] [Google Scholar]
- 37.Yasuhara K, Mitsumori K, Shimo T, Onodera H, Takahashi M, Hayashi Y. Mice with focal pulmonary fibrosis caused by monocrotaline are insensitive to urethane induction of lung tumorigenesis. Toxicol Pathol 1997;25:574–581. [DOI] [PubMed] [Google Scholar]
- 38.Hayashi S, Mitsumori K, Imaida K, Imazawa T, Yasuhara K, Uneyama C, Hayashi Y. Establishment of an animal model for pulmonary fibrosis in mice using monocrotaline. Toxicol Pathol 1995;23:63–71. [DOI] [PubMed] [Google Scholar]
- 39.Atochina EN, Muzykantov VR, Al-Mehdi AB, Danilov SM, Fisher AB. Normoxic lung ischemia/reperfusion accelerates shedding of angiotensin converting enzyme from the pulmonary endothelium. Am J Respir Crit Care Med 1997;156:1114–1119. [DOI] [PubMed] [Google Scholar]
- 40.Farkas L, Farkas D, Ask K, Moller A, Gauldie J, Margetts P, Inman M, Kolb M. VEGF ameliorates pulmonary hypertension through inhibition of endothelial apoptosis in experimental lung fibrosis in rats. J Clin Invest 2009;119:1298–1311. [DOI] [PMC free article] [PubMed]
- 41.Partovian C, Adnot S, Eddahibi S, Teiger E, Levame M, Dreyfus P, Raffestin B, Frelin C. Heart and lung VEGF mRNA expression in rats with monocrotaline- or hypoxia-induced pulmonary hypertension. Am J Physiol 1998;275:H1948–H1956. [DOI] [PubMed] [Google Scholar]
- 42.Itoh T, Nagaya N, Ishibashi-Ueda H, Kyotani S, Oya H, Sakamaki F, Kimura H, Nakanishi N. Increased plasma monocyte chemoattractant protein–1 level in idiopathic pulmonary arterial hypertension. Respirology 2006;11:158–163. [DOI] [PubMed] [Google Scholar]
- 43.Ikeda Y, Yonemitsu Y, Kataoka C, Kitamoto S, Yamaoka T, Nishida K, Takeshita A, Egashira K, Sueishi K. Anti-monocyte chemoattractant protein–1 gene therapy attenuates pulmonary hypertension in rats. Am J Physiol Heart Circ Physiol 2002;283:H2021–H2028. [DOI] [PubMed] [Google Scholar]
- 44.Taraseviciene-Stewart L, Nicolls MR, Kraskauskas D, Scerbavicius R, Burns N, Cool C, Wood K, Parr JE, Boackle SA, Voelkel NF, et al. Absence of T cells confers increased pulmonary arterial hypertension and vascular remodeling. Am J Respir Crit Care Med 2007;175:1280–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.