The ability to sense and respond to changes in oxygen concentration is a fundamental requirement for the survival of all organisms. Throughout evolution, control of oxygen homeostasis has developed into a complex system, requiring both rapid adjustment to acute changes in oxygen and durable adaptation when the hypoxic stimulus is prolonged. There are numerous instances, in both physiologic and pathophysiologic conditions, during which the lung experiences localized or global hypoxia. It has become increasingly appreciated that adaptation to hypoxia requires the coordinated regulation of a large battery of genes, and that this collective response is controlled, to a large extent, at the level of transcription. In particular, the hypoxia-inducible factors (HIFs), have been identified as key mediators of adaptation to hypoxia. In this review, we will describe the HIF system and its role in a variety of developmental, physiologic, and pathogenic processes within the lung.
THE HIF SYSTEM
Originally identified as a protein that bound, under hypoxic conditions, to the hypoxia response element of the EPO gene (which encodes erythropoietin, the hormone controlling red blood cell production) (1), hypoxia-inducible factor 1 (HIF-1) is a highly conserved transcription factor that is now known to be present in almost all cell types, is tightly regulated by O2 availability, and regulates the expression of hundreds of genes. HIF-1 exists as a heterodimer, consisting of HIF-1α and HIF-1β subunits. HIF-1β is ubiquitously expressed, whereas HIF-1α is found at very low levels under normoxic conditions. In mouse lung, HIF-1α mRNA levels increase within 30 minutes of exposure to 7% O2 (2). Under normoxic conditions, HIF-1α protein is ubiquitinated and subjected to proteasomal degradation; however, acute exposure of pulmonary arterial smooth muscle cells (PASMCs) or endothelial cells (ECs) to hypoxia (1% O2) causes increased HIF-1α protein levels and HIF-1 DNA-binding activity (3). Thus, HIF-1α confers sensitivity and specificity for hypoxic induction of HIF-1 transcriptional activity.
The mechanism by which hypoxia is transduced into an increase in HIF-1 activity (and, consequently, induction of hypoxia-inducible gene expression) was unclear until the discovery that HIF-1α ubiquitination required hydroxylation at two proline residues, which are Pro-402 and Pro-564 in human HIF-1α (4–7). Under normoxic conditions, hydroxylation of HIF-1α is catalyzed by prolyl hydroxylase domain proteins (PHDs) using molecular O2 as a substrate (4, 5, 8). To date, four PHD isoforms have been identified, although only PHD1–3 appear to hydroxylate HIF-1, with evidence suggesting that PHD2 is the primary isoform responsible for HIF-1α hydroxylation in vivo (9–11).
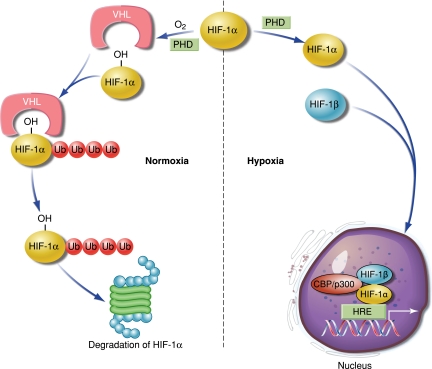
At reduced O2 concentrations, PHD activity decreases (Figure 1). Consequently, HIF-1α hydroxylation at the proline residues decreases, resulting in protein stabilization. HIF-1α then translocates into the nucleus, where it binds HIF-1β and recruits coactivator proteins to the HIF binding site within the hypoxia response element, activating the transcription of various target genes. Increased HIF-1α protein generally correlates with increased transcriptional activity, although HIF-1 transactivation is regulated by hydroxylation of HIF-1α at an asparagine residue within the C-terminal transactivation domain via factor inhibiting HIF-1 (FIH-1), which blocks the binding of the transcriptional co-activators CBP and p300 (12). Since PHD and FIH-1 activity require O2, hypoxia reduces the activity of both enzymes, leading to HIF-1α stabilization and transactivation of HIF-1 target genes.
Figure 1.
Regulation of HIF-1 by hypoxia. Under normal conditions, hydroxylation of HIF-1α by prolyl hydroxylase domain proteins (PHD), using molecular oxygen, leads to interaction with Von Hipple-Lindau (VHL) and addition of ubiquitin (Ub), targeting HIF-1α for proteosomal degradation. When oxygen levels fall, HIF-1α is not targeted for degradation, and translocates to the nucleus where it binds with HIF-1β and recruits co-activators at the hypoxia response element (HRE) to initiate gene transcription.
Several years after the discovery of HIF-1α, a closely related protein was identified based on its sequence similarity and subsequently named HIF-2α (13, 14). Like HIF-1α, HIF-2α is subjected to the same PHD-dependent degradation machinery, and dimerizes with HIF-1β; however, unlike HIF-1α, which is found in all nucleated cells, HIF-2α exhibits a much more restricted pattern of expression.
ROLE OF HIFS IN LUNG DEVELOPMENT
Given that development occurs in a hypoxic environment, it is not surprising that HIFs would be important contributors to embryogenesis. Indeed, HIF mRNA and protein levels are quite high in the fetal lung (15–17), with the entire HIF system in place as early as 8 weeks of gestation in the human (15). HIF-1α protein expression is induced by hypoxia in cultured cells derived from all cell types found in the adult lung (3), whereas HIF-2α protein expression is restricted to the vascular endothelium and type II pneumocytes (18). During fetal development, the lung of the normal embryo is hypoxic (19), and analysis of the spatial pattern of expression in human lungs during initial development of the pulmonary vasculature revealed high levels of HIF-1α protein localized predominantly in branching epithelium (15). HIF-2α protein was also present in epithelium, as well as in mesenchymal structures, which give rise to the vascular endothelium (15). Given the distinct expression pattern observed in the developing lung, HIF-1α and HIF-2α may serve different, and specific, functions in epithelial and vascular morphogenesis. This possibility is supported by results from studies examining HIF-1α and HIF-2α loss-of-function models. Genetic deletion of HIF-1α or HIF-1β results in fetal death at approximately Embryonic Day 10, with severe cardiovascular malformations (20–24). In contrast, loss of HIF-2α leads to fetal death in approximately 50% of embryos, with the remaining offspring exhibiting impaired lung development, reduced production of surfactant, postnatal respiratory distress, and neonatal lethality (23, 25). Although homozygous deletion of HIF-1α or HIF-2α is lethal, animals heterozygous for either factor develop normally and survive to adulthood, with the lung architecture and function appearing outwardly normal under normoxic conditions.
In the embryonic lung of sheep and primates, levels of both HIF-1α and HIF-2α protein levels are high in the third trimester, although HIF-1α protein levels quickly decrease upon delivery (16, 17). HIF-1α and HIF-2α protein levels are also markedly reduced in the lungs of mechanically ventilated preterm animals (16, 17), where the decline in HIF levels may have severe consequences for lung development, leading to vascular and alveolar hypoplasia, neonatal respiratory distress, and bronchopulmonary dysplasia. Consistent with this possibility, in vivo use of PHD inhibitors to increase HIF protein levels improved lung growth and function in a baboon model of prematurity (26, 27). Moreover, in vitro assays demonstrated that induction of HIF-1 protein in fetal lung buds was sufficient to induce lung development even under nonhypoxic conditions (28), whereas depletion of HIF-1α using antisense oligonucleotides reduced lung branching morphogenesis and vascularization (29). These results indicate that the HIF system plays a critical role in pulmonary development, and suggest the possibility that therapies aimed at increasing HIF levels might enhance lung angiogenesis and may be promising candidates for the treatment of bronchopulmonary dysplasia.
ROLE OF HIFS IN PULMONARY HYPERTENSION
Prolonged exposure to alveolar hypoxia, due to chronic lung disease or residence at high altitude, is a significant cause of pulmonary hypertension (World Health Organization Class 3). The role of HIF in the development of pulmonary hypertension associated with hypoxemia has been explored using murine models, where partial deficiency of either HIF-1α (Hif1a+/− mice) or HIF-2α (Hif2a+/− mice) markedly attenuated the increase in pulmonary arterial pressure and right ventricular hypertrophy that are induced by chronic hypoxic exposure of wild-type mice (25, 30).
The reduction in pulmonary hypertension was due, at least in part, to the reduced pulmonary vascular remodeling observed in these animals (25, 30). The components of hypoxia-induced pulmonary vascular remodeling include PASMC proliferation, migration, and hypertrophy. While the effects of HIF deficiency on pulmonary vascular smooth muscle migration and proliferation during hypoxic exposure have not been reported, electrophysiologic measurements revealed that PASMCs from wild-type (Hif1a+/+) mice exposed to 10% O2 for 3 weeks exhibited an increase in cell capacitance, which is a measure of cell size (31). In contrast, PASMCs isolated from normoxic and chronically hypoxic Hif1a+/− mice were not different, suggesting that HIF-1–dependent smooth muscle cell hypertrophy contributes to pulmonary vascular remodeling during hypoxia. In Hif1a+/+ mice, exposure to chronic hypoxia is associated with PASMC depolarization, reductions in K+ channel expression and activity, and elevated intracellular calcium concentration and pH, due to up-regulation of transient potential receptor proteins and Na+/H+ exchanger isoform 1, respectively (31–34). These alterations in PASMC ion homeostasis, which are associated with a more contractile, apoptosis-resistant, proliferative, and migratory phenotype, were significantly attenuated or absent in PASMCs from chronically hypoxic Hif1a+/− mice (31–34). Whether reduction in HIF-2α results in similar alterations in PASMC responses to hypoxia is presently unknown.
While it is easy to understand how HIF would be involved in the pathogenesis of hypoxic pulmonary hypertension, it is possible that HIFs also play a role in other forms of pulmonary hypertension that are not due to alveolar hypoxia. For example, HIF-1α levels are up-regulated in the Fawn-hooded rat, a genetic model of pulmonary arterial hypertension that exhibits several characteristics of the human disease, including reduced K+ channel expression, elevated intracellular calcium levels, and excessive PASMC proliferation even under normoxic conditions (35). Furthermore, immunohistochemical examination of tissues and cells from patients with non–hypoxia-associated pulmonary arterial hypertension also revealed elevated expression of HIF-1α (36). Although the mechanism by which HIF levels are elevated under these conditions is still incompletely understood, these studies would suggest that therapies aimed at reducing HIF-1 or HIF-2 levels and/or activity have clinical potential, even in cases of pulmonary hypertension not associated with hypoxemia.
ROLE OF HIFS IN LUNG INJURY
Hypoxia may be a consequence of acute lung injury, leading to aberrations in lung function and repair. Early events in acute lung injury include damage of the alveolar lining layer, apoptosis of alveolar epithelial cells, and lung edema, whereas at later phases, reactive hyperplasia of alveolar type II cells is predominant, leading to fibrosis. During acute lung injury, increased vascular permeability can result from hypoxia, ischemia, and/or inflammatory stimuli. The role of the HIF system in modulating pulmonary vascular leak is still unresolved, with only a handful of published studies. In a hypoxic ischemia/reperfusion model, up-regulation of HIF-1 protein levels was associated with increased vascular endothelial growth factor (VEGF) levels and augmented barrier disruption (37), whereas another study reported HIF-1–dependent down-regulation of adenosine kinase, the enzyme that converts adenosine to AMP, which attenuated hypoxia-induced pulmonary vascular leak (38). While neither study directly tested whether loss of HIF-1 altered the observed changes in vascular permeability, taken at face value these results imply that HIF-1 may play both barrier-protective and barrier-disruptive roles. With respect to epithelial cell damage and subsequent fibrotic lung disease, hypoxia has been reported to induce alveolar type II cell apoptosis via HIF-1α (39). Furthermore, up-regulation of HIF-1 by inflammatory levels of NO may lead to suppression of epithelial cell wound repair (40), suggesting that increased HIF-1 levels may render the injured, hypoxic lung less able to mount an appropriate healing response after epithelial injury. Recently, epithelial–mesenchymal transition (EMT) has been proposed to contribute to pulmonary fibrosis in patients with acute lung injury (41). Emerging evidence indicates that this process is under the control of hypoxia-induced increases in mitochondrial-derived reactive oxygen species (ROS) (42), which serve to stabilize HIF-1α in several cell types, including alveolar epithelial cells (43). While these studies provide circumstantial evidence for the involvement of HIF in the development of acute lung injury, direct testing of the role of HIF has only been performed in models of lung injury due to splanchnic ischemia–reperfusion and intestinal inflammation. In rodent models of traumatic hemorrhagic shock due to gut ischemia–reperfusion, there is a rapid and prolonged increase in nuclear HIF-1α protein levels (44), with mice partially deficient for HIF-1α exhibiting attenuated intestinal mucosal damage, bacterial translocation, and subsequent lung injury (45). Further experiments testing the effect of HIF-1α or HIF-2α loss-of-function in other models of lung injury will be required to delineate the exact role of HIF in acute lung injury and evaluate the suitability of therapies aimed at modulating HIF levels in this context.
ROLE OF HIFS IN LUNG CANCER
As solid tumors expand, lack of blood supply renders areas of the tumor hypoxic. Since hypoxia-induced angiogenesis is a requirement for tumor growth, hypoxia is both a consequence of, and contributor to, tumorigenesis. The degree of hypoxia within a tumor is associated with increased expression of angiogenic factors (46) and more aggressive tumors (47–49). Given the positive association between hypoxia and tumorigenesis, it is not surprising that HIF-1α protein expression is correlated with more aggressive and radiation-resistant tumor cells (50), while inhibition of HIF by treatment with digoxin or acriflavine prevents tumor xenograft growth and vascularization in mice (51, 52). With respect to the lung, both small cell and non–small cell lung cancers exhibit high levels of HIF-1α and HIF-2α, both of which are associated with poor prognosis (46, 53–56). Overexpressing HIF-2α leads to increased tumor size, invasion, and angiogenesis in the inducible LSL-KrasG12D murine model of non–small cell lung cancer (57), resulting in decreased survival. Surprisingly, deletion of HIF-2α in this model resulted in increased tumorigenesis (58), whereas in the A549 lung carcinoma cell line and in drug-resistant non–small cell lung cancer cells, silencing HIF-2α prevented tumor growth (59). A small molecule inhibitor of HIF-1α, PX-478, demonstrated effectiveness against tumor growth in an orthotopic mouse model of human lung cancer (54); however, in mice injected with A549 cells, silencing HIF-1α impaired tumor vascularization and increased the necrotic area, but did not reduce tumor cell proliferation and only slightly impacted tumor growth (59). A similar lack of effect of HIF-1α silencing on tumor growth was observed in LSL-KrasG12D mice (58). In contrast, reduction of HIF-1α levels markedly impaired metastasis in murine models of human lung and mammary cancer (54, 60). While these studies might imply a more prominent role for HIF-2α than HIF-1α in the growth of certain lung cancer tumors, the divergent effects of HIF-1α and HIF-2α silencing in the aforementioned studies suggest a complex regulation of tumor growth and invasion that is not controlled simply by the absence or presence of HIF-1 or HIF-2, but rather may be dependent on the absolute levels of HIF and/or the specific tumor cells involved. Nonetheless, addition of HIF inhibitors to current cancer therapies may prove beneficial in controlling tumor progression and/or metastasis.
CONCLUSIONS
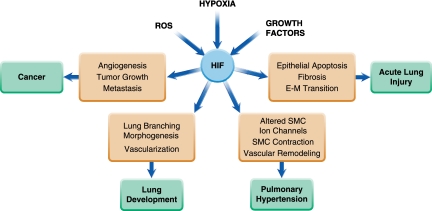
Since its discovery nearly 20 years ago, HIF has emerged as a central component of a myriad of O2-dependent physiologic and pathophysiologic processes and is now recognized as a master regulator of O2 homeostasis. Understanding the precise role of the HIF system in lung development and in lung diseases such as pulmonary hypertension, acute lung injury, and cancer (Figure 2), and the identification and/or creation of tools to manipulate HIF levels in vivo, hold promise for better therapeutic options to treat lung dysfunction in both the neonate and adult.
Figure 2.
Illustration of the role of HIF in various lung processes. E–M = endothelial–mesenchymal; SMC = smooth muscle cell; ROS = reactive oxygen species.
Acknowledgments
Work in the authors' laboratories is supported by Public Health Service grants from AHA, NCI, NHLBI, and NIGMS and by the Johns Hopkins Institute for Cell Engineering. G.L.S. is the C. Michael Armstrong Professor at The Johns Hopkins University School of Medicine.
Author Disclosure: L.A.S. has received sponsored grants from the National Institutes of Health (over $100,000), and AHA ($50,001–$100,000). G.L.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol 1992;12:5447–5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiener CM, Booth G, Semenza GL. In vivo expression of mRNAs encoding hypoxia-inducible factor 1. Biochem Biophys Res Commun 1996;225:485–488. [DOI] [PubMed] [Google Scholar]
- 3.Yu AY, Frid MG, Shimoda LA, Wiener CM, Stenmark K, Semenza GL. Temporal, spatial, and oxygen-regulated expression of hypoxia-inducible factor-1 in the lung. Am J Physiol 1998;275:L818–L826. [DOI] [PubMed] [Google Scholar]
- 4.Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG Jr. HIFα targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 2001;292:464–468. [DOI] [PubMed] [Google Scholar]
- 5.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, et al. Targeting of HIFα to the von hippel-lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 2001;292:468–472. [DOI] [PubMed] [Google Scholar]
- 6.Yu F, White SB, Zhao Q, Lee FS. HIF-1α binding to VHL is regulated by stimulus-sensitive proline hydroxylation. Proc Natl Acad Sci USA 2001;98:9630–9635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masson N, Willam C, Maxwell PH, Pugh CW, Ratcliffe PJ. Independent function of two destruction domains in hypoxia-inducible factor-α chains activated by prolyl hydroxylation. EMBO J 2001;20:5197–5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, et al. C. elegans egl-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 2001;107:43–54. [DOI] [PubMed] [Google Scholar]
- 9.Appelhoff RJ, Tian YM, Raval RR, Turley H, Harris AL, Pugh CW, Ratcliffe PJ, Gleadle JM. Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor. J Biol Chem 2004;279:38458–38465. [DOI] [PubMed] [Google Scholar]
- 10.Berra E, Benizri E, Ginouves A, Volmat V, Roux D, Pouyssegur J. HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1α in normoxia. EMBO J 2003;22:4082–4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Metzen E, Berchner-Pfannschmidt U, Stengel P, Marxsen JH, Stolze I, Klinger M, Huang WQ, Wotzlaw C, Hellwig-Burgel T, Jelkmann W, et al. Intracellular localisation of human HIF-1α hydroxylases: implications for oxygen sensing. J Cell Sci 2003;116:1319–1326. [DOI] [PubMed] [Google Scholar]
- 12.Mahon PC, Hirota K, Semenza GL. FIH-1: a novel protein that interacts with HIF-1α and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev 2001;15:2675–2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ema M, Taya S, Yokotani N, Sogawa K, Matsuda Y, Fujii-Kuriyama Y. A novel bHLH-PAS factor with close sequence similarity to hypoxia-inducible factor 1α regulates the VEGF expression and is potentially involved in lung and vascular development. Proc Natl Acad Sci USA 1997;94:4273–4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tian H, McKnight SL, Russell DW. Endothelial pas domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev 1997;11:72–82. [DOI] [PubMed] [Google Scholar]
- 15.Groenman F, Rutter M, Caniggia I, Tibboel D, Post M. Hypoxia-inducible factors in the first trimester human lung. J Histochem Cytochem 2007;55:355–363. [DOI] [PubMed] [Google Scholar]
- 16.Grover TR, Asikainen TM, Kinsella JP, Abman SH, White CW. Hypoxia-inducible factors HIF-1α and HIF-2α are decreased in an experimental model of severe respiratory distress syndrome in preterm lambs. Am J Physiol Lung Cell Mol Physiol 2007;292:L1345–L1351. [DOI] [PubMed] [Google Scholar]
- 17.Asikainen TM, Ahmad A, Schneider BK, White CW. Effect of preterm birth on hypoxia- inducible factors and vascular endothelial growth factor in primate lungs. Pediatr Pulmonol 2005;40:538–546. [DOI] [PubMed] [Google Scholar]
- 18.Wiesener MS, Jurgensen JS, Rosenberger C, Scholze CK, Horstrup JH, Warnecke C, Mandriota S, Bechmann I, Frei UA, Pugh CW, et al. Widespread hypoxia-inducible expression of HIF-2α in distinct cell populations of different organs. FASEB J 2003;17:271–273. [DOI] [PubMed] [Google Scholar]
- 19.Lee YM, Jeong CH, Koo SY, Son MJ, Song HS, Bae SK, Raleigh JA, Chung HY, Yoo MA, Kim KW. Determination of hypoxic region by hypoxia marker in developing mouse embryos in vivo: a possible signal for vessel development. Dev Dyn 2001;220:175–186. [DOI] [PubMed] [Google Scholar]
- 20.Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1α. Genes Dev 1998;12:149–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maltepe E, Schmidt JV, Baunoch D, Bradfield CA, Simon MC. Abnormal angiogenesis and responses to glucose and oxygen deprivation in mice lacking the protein ARNT. Nature 1997;386:403–407. [DOI] [PubMed] [Google Scholar]
- 22.Kotch LE, Iyer NV, Laughner E, Semenza GL. Defective vascularization of HIF-1α-null embryos is not associated with VEGF deficiency but with mesenchymal cell death. Dev Biol 1999;209:254–267. [DOI] [PubMed] [Google Scholar]
- 23.Compernolle V, Brusselmans K, Franco D, Moorman A, Dewerchin M, Collen D, Carmeliet P. Cardia bifida, defective heart development and abnormal neural crest migration in embryos lacking hypoxia-inducible factor-1α. Cardiovasc Res 2003;60:569–579. [DOI] [PubMed] [Google Scholar]
- 24.Ryan HE, Lo J, Johnson RS. HIF-1α is required for solid tumor formation and embryonic vascularization. EMBO J 1998;17:3005–3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brusselmans K, Compernolle V, Tjwa M, Wiesener MS, Maxwell PH, Collen D, Carmeliet P. Heterozygous deficiency of hypoxia-inducible factor-2α protects mice against pulmonary hypertension and right ventricular dysfunction during prolonged hypoxia. J Clin Invest 2003;111:1519–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asikainen TM, Chang LY, Coalson JJ, Schneider BK, Waleh NS, Ikegami M, Shannon JM, Winter VT, Grubb P, Clyman RI, et al. Improved lung growth and function through hypoxia-inducible factor in primate chronic lung disease of prematurity. FASEB J 2006;20:16981700. [DOI] [PubMed] [Google Scholar]
- 27.Asikainen TM, Waleh NS, Schneider BK, Clyman RI, White CW. Enhancement of angiogenic effectors through hypoxia-inducible factor in preterm primate lung in vivo. Am J Physiol Lung Cell Mol Physiol 2006;291:L588–L595. [DOI] [PubMed] [Google Scholar]
- 28.Groenman FA, Rutter M, Wang J, Caniggia I, Tibboel D, Post M. Effect of chemical stabilizers of hypoxia-inducible factors on early lung development. Am J Physiol Lung Cell Mol Physiol 2007;293:L557–L567. [DOI] [PubMed] [Google Scholar]
- 29.van Tuyl M, Liu J, Wang J, Kuliszewski M, Tibboel D, Post M. Role of oxygen and vascular development in epithelial branching morphogenesis of the developing mouse lung. Am J Physiol Lung Cell Mol Physiol 2005;288:L167–L178. [DOI] [PubMed] [Google Scholar]
- 30.Yu AY, Shimoda LA, Iyer NV, Huso DL, Sun X, McWilliams R, Beaty T, Sham JS, Wiener CM, Sylvester JT, et al. Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1α. J Clin Invest 1999;103:691–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shimoda LA, Manalo DJ, Sham JS, Semenza GL, Sylvester JT. Partial HIF-1α deficiency impairs pulmonary arterial myocyte electrophysiological responses to hypoxia. Am J Physiol Lung Cell Mol Physiol 2001;281:L202–L208. [DOI] [PubMed] [Google Scholar]
- 32.Shimoda LA, Fallon M, Pisarcik S, Wang J, Semenza GL. HIF-1 regulates hypoxic induction of NHE1 expression and alkalinization of intracellular pH in pulmonary arterial myocytes. Am J Physiol Lung Cell Mol Physiol 2006;291:L941–L949. [DOI] [PubMed] [Google Scholar]
- 33.Whitman EM, Pisarcik S, Luke T, Fallon M, Wang J, Sylvester JT, Semenza GL, Shimoda LA. Endothelin-1 mediates hypoxia-induced inhibition of voltage-gated K+ channel expression in pulmonary arterial myocytes. Am J Physiol Lung Cell Mol Physiol 2008;294:L309–L318. [DOI] [PubMed] [Google Scholar]
- 34.Wang J, Weigand L, Lu W, Sylvester JT, Semenza GL, Shimoda LA. Hypoxia inducible factor 1 mediates hypoxia-induced TRPC expression and elevated intracellular Ca2+ in pulmonary arterial smooth muscle cells. Circ Res 2006;98:1528–1537. [DOI] [PubMed] [Google Scholar]
- 35.Bonnet S, Michelakis ED, Porter CJ, Andrade-Navarro MA, Thebaud B, Bonnet S, Haromy A, Harry G, Moudgil R, McMurtry MS, et al. An abnormal mitochondrial-hypoxia inducible factor-1α-KV channel pathway disrupts oxygen sensing and triggers pulmonary arterial hypertension in fawn hooded rats: Similarities to human pulmonary arterial hypertension. Circulation 2006;113:2630–2641. [DOI] [PubMed] [Google Scholar]
- 36.Tuder RM, Chacon M, Alger L, Wang J, Taraseviciene-Stewart L, Kasahara Y, Cool CD, Bishop AE, Geraci M, Semenza GL, et al. Expression of angiogenesis-related molecules in plexiform lesions in severe pulmonary hypertension: evidence for a process of disordered angiogenesis. J Pathol 2001;195:367–374. [DOI] [PubMed] [Google Scholar]
- 37.Becker PM, Alcasabas A, Yu AY, Semenza GL, Bunton TE. Oxygen-independent upregulation of vascular endothelial growth factor and vascular barrier dysfunction during ventilated pulmonary ischemia in isolated ferret lungs. Am J Respir Cell Mol Biol 2000;22:272–279. [DOI] [PubMed] [Google Scholar]
- 38.Morote-Garcia JC, Rosenberger P, Kuhlicke J, Eltzschig HK. HIF-1-dependent repression of adenosine kinase attenuates hypoxia-induced vascular leak. Blood 2008;111:5571–5580. [DOI] [PubMed] [Google Scholar]
- 39.Krick S, Eul BG, Hanze J, Savai R, Grimminger F, Seeger W, Rose F. Role of hypoxia-inducible factor-1α in hypoxia-induced apoptosis of primary alveolar epithelial type II cells. Am J Respir Cell Mol Biol 2005;32:395–403. [DOI] [PubMed] [Google Scholar]
- 40.Bove PF, Hristova M, Wesley UV, Olson N, Lounsbury KM, van der Vliet A. Inflammatory levels of nitric oxide inhibit airway epithelial cell migration by inhibition of the kinase ERK1/2 and activation of hypoxia-inducible factor-1α. J Biol Chem 2008;283:17919–17928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, Sheppard D, Chapman HA. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci USA 2006;103:13180–13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou G, Dada LA, Wu M, Kelly A, Trejo H, Zhou Q, Varga J, Sznajder JI. Hypoxia-induced alveolar epithelial-mesenchymal transition requires mitochondrial ROS and hypoxia-inducible factor 1. Am J Physiol Lung Cell Mol Physiol 2009;297:L1120–L1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schroedl C, McClintock DS, Budinger GR, Chandel NS. Hypoxic but not anoxic stabilization of HIF-1α requires mitochondrial reactive oxygen species. Am J Physiol Lung Cell Mol Physiol 2002;283:L922–L931. [DOI] [PubMed] [Google Scholar]
- 44.Koury J, Deitch EA, Homma H, Abungu B, Gangurde P, Condon MR, Lu Q, Xu DZ, Feinman R. Persistent HIF-1α activation in gut ischemia/reperfusion injury: potential role of bacteria and lipopolysaccharide. Shock 2004;22:270–277. [DOI] [PubMed] [Google Scholar]
- 45.Feinman R, Deitch EA, Watkins AC, Abungu B, Colorado I, Kannan KB, Sheth S, Caputo FJ, Lu Q, Ramanathan M, et al. HIF-1 mediates pathogenic inflammatory responses to intestinal ischemia reperfusion injury. Am J Physiol Gastrointest Liver Physiol 2010;299:G833–G843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giatromanolaki A, Koukourakis MI, Sivridis E, Turley H, Talks K, Pezzella F, Gatter KC, Harris AL. Relation of hypoxia inducible factor 1α and 2α in operable non-small cell lung cancer to angiogenic/molecular profile of tumours and survival. Br J Cancer 2001;85:881–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brizel DM, Scully SP, Harrelson JM, Layfield LJ, Bean JM, Prosnitz LR, Dewhirst MW. Tumor oxygenation predicts for the likelihood of distant metastases in human soft tissue sarcoma. Cancer Res 1996;56:941–943. [PubMed] [Google Scholar]
- 48.Hockel M, Schlenger K, Aral B, Mitze M, Schaffer U, Vaupel P. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res 1996;56:4509–4515. [PubMed] [Google Scholar]
- 49.Le QT, Chen E, Salim A, Cao H, Kong CS, Whyte R, Donington J, Cannon W, Wakelee H, Tibshirani R, et al. An evaluation of tumor oxygenation and gene expression in patients with early stage non-small cell lung cancers. Clin Cancer Res 2006;12:1507–1514. [DOI] [PubMed] [Google Scholar]
- 50.Kim WY, Oh SH, Woo JK, Hong WK, Lee HY. Targeting heat shock protein 90 overrides the resistance of lung cancer cells by blocking radiation-induced stabilization of hypoxia-inducible factor-1α. Cancer Res 2009;69:1624–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee K, Zhang H, Qian DZ, Rey S, Liu JO, Semenza GL. Acriflavine inhibits HIF-1 dimerization, tumor growth, and vascularization. Proc Natl Acad Sci USA 2009;106:17910–17915. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 52.Zhang H, Qian DZ, Tan YS, Lee K, Gao P, Ren YR, Rey S, Hammers H, Chang D, Pili R, et al. Digoxin and other cardiac glycosides inhibit HIF-1α synthesis and block tumor growth. Proc Natl Acad Sci USA 2008;105:19579–19586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ioannou M, Papamichali R, Kouvaras E, Mylonis I, Vageli D, Kerenidou T, Barbanis S, Daponte A, Simos G, Gourgoulianis K, et al. Hypoxia inducible factor-1α and vascular endothelial growth factor in biopsies of small cell lung carcinoma. Lung 2009;187:321–329. [DOI] [PubMed] [Google Scholar]
- 54.Jacoby JJ, Erez B, Korshunova MV, Williams RR, Furutani K, Takahashi O, Kirkpatrick L, Lippman SM, Powis G, O'Reilly MS, et al. Treatment with HIF-1α antagonist PX-478 inhibits progression and spread of orthotopic human small cell lung cancer and lung adenocarcinoma in mice. J Thorac Oncol 2010;5:940–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Swinson DE, Jones JL, Richardson D, Wykoff C, Turley H, Pastorek J, Taub N, Harris AL, O'Byrne KJ. Carbonic anhydrase IX expression, a novel surrogate marker of tumor hypoxia, is associated with a poor prognosis in non-small-cell lung cancer. J Clin Oncol 2003;21:473482. [DOI] [PubMed] [Google Scholar]
- 56.Yohena T, Yoshino I, Takenaka T, Kameyama T, Ohba T, Kuniyoshi Y, Maehara Y. Upregulation of hypoxia-inducible factor-1α mRNA and its clinical significance in non-small cell lung cancer. J Thorac Oncol 2009;4:284–290. [DOI] [PubMed] [Google Scholar]
- 57.Kim WY, Perera S, Zhou B, Carretero J, Yeh JJ, Heathcote SA, Jackson AL, Nikolinakos P, Ospina B, Naumov G, et al. HIF2α cooperates with RAS to promote lung tumorigenesis in mice. J Clin Invest 2009;119:2160–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mazumdar J, Hickey MM, Pant DK, Durham AC, Sweet-Cordero A, Vachani A, Jacks T, Chodosh LA, Kissil JL, Simon MC, et al. HIF-2α deletion promotes Kras-driven lung tumor development. Proc Natl Acad Sci USA 2010;107:14182–14187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Franovic A, Holterman CE, Payette J, Lee S. Human cancers converge at the HIF-2α oncogenic axis. Proc Natl Acad Sci USA 2009;106:21306–21311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liao D, Corle C, Seagroves TN, Johnson RS. Hypoxia-inducible factor-1α is a key regulator of metastasis in a transgenic model of cancer initiation and progression. Cancer Res 2007;67:563–572. [DOI] [PubMed] [Google Scholar]