Abstract
Objective:
This study determined whether CGG repeat length moderates the relationship between age and performance on selective measures of executive function in premutation carriers (PM) who are asymptomatic for a recently described late-onset neurodegenerative disorder, fragile X–associated tremor/ataxia syndrome (FXTAS).
Methods:
Forty PM men aged 18–69 years with a family history of fragile X syndrome underwent neuropsychological tests of inhibition and working memory. We examined only men who are asymptomatic for FXTAS. Multiple regression analyses were conducted to examine the moderating role of CGG repeat length on the relation between age and performance on inhibition and working memory tasks.
Results:
With increasing age and only in men with an FMR1 expansion in the upper premutation range (>100 CGG repeats) was there an association between age and poorer task performance on selective executive function measures involving inhibition (p < 0.05) and executive working memory (p < 0.01). Men in the lower premutation range (<100 CGG repeats) were relatively risk-free from any cognitive aging effects associated with CGG repeat expansions.
Conclusions:
We conclude that neural networks in the prefrontal cortex may be highly susceptible to age-related neurotoxic effects in the upper size range of the FMR1 premutation. Future longitudinal studies will be needed to determine whether specific executive markers may serve to distinguish those at greatest risk for severe cognitive decline or dementia associated with FXTAS.
Fragile X syndrome (FXS) is the leading cause of inherited intellectual disability worldwide and one of the few known single-gene causes of autism.1 The condition is caused by a large expansion of the trinucleotide CGG repeat region (>200 CGG repeats) within the 5′ untranslated region of the fragile X mental retardation 1 gene (FMR1), located at the long arm of the X chromosome. FMR1 is distinctive in that expansions can occur across successive generations, with individuals classified as demonstrating normal (7–44 CGG repeats), intermediate or gray-zone alleles (45–54), or moderate (55–200) repeat expansions. Individuals with moderate expansions of the FMR1 gene are referred to as premutation carriers (PM), and until a decade ago were assumed to be free of a deleterious phenotype; that is, without any discernible cognitive or brain impairment.
It is now well-documented that approximately 30%–40% of PM males and 8% of PM females will develop a recently described late-onset neurodegenerative disorder, fragile X–associated tremor/ataxia syndrome (FXTAS), which is associated with progressive dementia (>55 years and predominantly in men), intention tremor, ataxia, and parkinsonism alongside mood and executive function deficits.2–4
Although previous studies have identified a more generalized executive function impairment or “dysexecutive syndrome” in older men (>50 years) with FXTAS,5,6 other studies in asymptomatic PM carriers have found distinct executive function signatures of inhibitory and executive working memory decline that progressively deteriorate with increasing age, and correlate with increasing CGG repeat length.7,8 However, it remains unclear whether asymptomatic carriers have selective executive function impairments.9,10
Here we explore the possibility that recent findings may reflect a higher CGG repeat threshold within which a subgroup of asymptomatic carriers may be especially vulnerable to selective executive cognitive impairments.
METHODS
Participants.
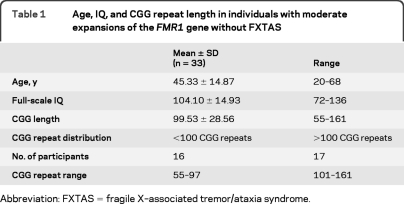
We recruited a total of 40 PM men with a family history of FXS ranging in age from 18 to 69 years through the UK Clinical Genetics Services and the UK Fragile X Society. Of these individuals, data from 7 participants were removed because of the confounding effects of FXTAS-related symptoms screened by a validated neurologic questionnaire.11 Thus, the final sample comprised 33 asymptomatic PM men aged between 20 and 68 years. Table 1 shows the distribution of CGG repeat length and descriptive statistics. Intellectual level was assessed using the Wechsler Abbreviated Scale of Intelligence,12 which comprises 4 subtests that tap both verbal and performance domains (see table 1).
Table 1.
Age, IQ, and CGG repeat length in individuals with moderate expansions of the FMR1 gene without FXTAS
Abbreviation: FXTAS = fragile X–associated tremor/ataxia syndrome.
Fragile X DNA testing.
The repeat region in the 5′ end of the FMR1 gene was identified for each participant using direct PCR techniques and quantified through comparison to a female control of known repeat size using PAGE gel electrophoresis.13 A premutation is defined here as an allele ranging in size from 55 CGG repeats up to approximately 200 repeats without any evidence of abnormal methylation, which is indicative of gene silencing as occurs in full mutation FXS.
Neuropsychological testing.
In the cognitive domain of inhibition, we selected measures previously identified as most sensitive in the inhibition composite score7: the Hayling Sentence Completion and the Stroop Color-Word Tests. Several cognitive measures in the domain of working memory were chosen—in particular, letter-number sequencing and Paced Auditory Serial Addition Test (PASAT)—because they represent quite distinct neural circuits during performance.14,15
Statistical analysis.
Multiple regression analyses were conducted to test the moderating role of CGG repeat length on the relation between age and performance on inhibition or working memory tasks. The primary criterion measures were selected as the most sensitive inhibitory (Hayling category B errors, Stroop Color-Word interference score) and working memory (letter-number sequencing and PASAT accuracy scores) measures identified in our previous composite measures,7,8 and the predictor variables were age, CGG repeat, and their interaction. Preliminary analyses indicated no significant effects involving full-scale, verbal, or performance IQ as covariates, and thus we excluded IQ scores from subsequent analyses. CGG repeat length and age were centered prior to regression analyses and multiplied together to represent this interaction according to previously established guidelines.16
RESULTS
Response inhibition measures.
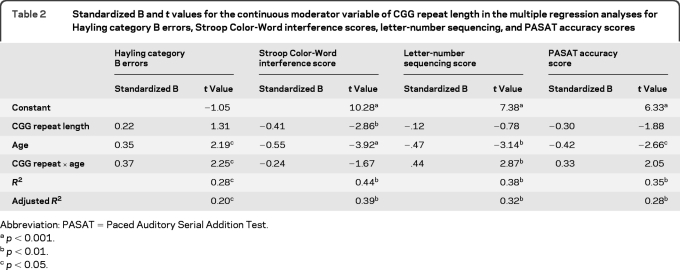
For Hayling category B errors, there was a significant association with age as well as an interaction between age and CGG repeat length (see table 2 for standardized B and t values). This analysis showed that CGG repeat length moderates the relationship between age and inhibitory control.
Table 2.
Standardized B and t values for the continuous moderator variable of CGG repeat length in the multiple regression analyses for Hayling category B errors, Stroop Color-Word interference scores, letter-number sequencing, and PASAT accuracy scores
Abbreviation: PASAT = Paced Auditory Serial Addition Test.
p < 0.001.
p < 0.01.
p < 0.05.
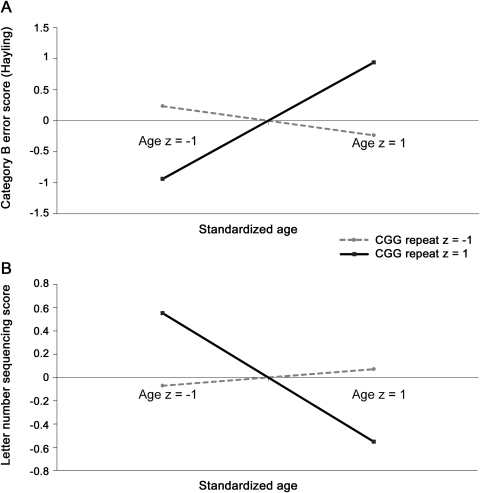
Following previously established guidelines,16 we graphed the interaction by calculating standardized values that represent the relationship between age and Hayling category B errors at high (+1 SD) and low (−1 SD) values of CGG repeat length using equations derived from the standardized B values (figure, A). The positive association between age and Hayling category B errors diminished as CGG repeats declined. Thus, when CGG repeats were relatively high (+1 SD), there was a deterioration in inhibitory control with increasing age. When CGG repeats were relatively low (−1 SD), this relationship diminished and there was no deterioration with increasing age. For Stroop Color-Word interference scores, there was a significant association with age but no interaction emerged between age and CGG repeat length (see table 2).
Figure. Relationship between age and inhibitory control or working memory at high and low values of CGG repeat length.
(A) Relationship between Hayling category B errors and age at high (z = 1) and low (z = −1) levels of CGG repeat length, and (B) relationship between letter-number sequencing score and age at high (z = 1) and low (z = −1) levels of CGG repeat length.
Working memory measures.
For letter-number sequencing correct responses, there was a significant association with age as well an interaction between CGG repeat size and age (see table 2). The relationship between age and letter-number sequencing score becomes stronger with increasing CGG repeat length (figure, B). When CGG repeats were relatively high (+1 SD), letter-number sequencing was inversely associated with age. When CGG repeats were relatively low (−1 SD), the relationship between age and performance on letter-number sequencing was diminished and performance did not deteriorate with increasing age. For PASAT accuracy scores, there was a significant association with age, but no interaction emerged between CGG repeat and age (see table 2).
DISCUSSION
Our findings are the first to demonstrate that individuals in the upper size range of the FMR1 premutation may be at risk from an age-related decline in selective inhibitory and working memory measures (Hayling task and letter-number sequencing, respectively). Furthermore, these findings suggest that specific executive markers may incur variable degrees of risk for developing a recently identified late-onset neurodegenerative disorder, FXTAS, which is associated with global executive function deficits, intention tremor, and gait ataxia.
The identification of specific executive markers in an at-risk subgroup of the FMR1 premutation suggests that executive function tasks that place demands on focal processing may be more vulnerable to the effects FMR1 mRNA toxicity. This interpretation is in line with the extant studies that show larger CGG repeat sizes result in up to a 10-fold increase in FMR1 mRNA levels, which appear to be toxic to neurons.17,18 We conjecture that the toxic effects of high levels of FMR1 mRNA and moderately lowered FMRP levels in patients with larger CGG repeat sizes17–19 initially affect vulnerable prefrontal circuits resulting in the subtle yet specific impairments described here. This is consistent with numerous studies that imply toxicity increases with increasing CGG repeat length in patients with FXTAS, with significant correlations between CGG repeat size and age at onset of motor signs,20 the presence of intranuclear inclusions throughout the brain,21 MRI changes in carriers with and without FXTAS,22 and the onset of marked cognitive impairments.23
The current findings of an age-related cognitive decline using selective measures of inhibition and working memory indicate the greater sensitivity in the FMR1 premutation to deficits in specific neural networks. Functional imaging has demonstrated that performance on the Hayling task activates the left dorsolateral prefrontal cortex (DLPFC) underlying executive processes,24 whereas the Stroop task has been shown to activate a broadly distributed network of integrated cortical regions that extend beyond the prefrontal cortex.25–27 In contrast, functional imaging studies indicate that performance on letter-number sequencing activates a distinct pattern of neural activation in the orbital frontal lobe, DLPFC, and posterior parietal cortex underlying the executive working memory network.14 Cortical activation during performance on the PASAT involves more extensive networks underlying verbal working memory and semantic memory retrieval networks.15 Together, these findings indicate that the sensitivity in detecting an age-related decline may only become discernible in asymptomatic male carriers on measures that tap central executive functions—that is, when performing complex mental operations such as planning, manipulation, and organization. We therefore posit that only central executive functions that require increasing demands to inhibitory and working memory control may confer at-risk profiles in asymptomatic carriers.
The cross-sectional design of this study is a limitation in predicting whether cognitive decline manifested in the preclinical state may eventually develop into FXTAS. Therefore, whether specific executive markers may serve as cognitive precursors to the later onset of FXTAS should remain tentative in light of developmental cognitive problems proposed to be associated with the FMR1 premutation including autism and attention-deficit/hyperactivity disorder.28,29 Declarative learning and memory recall problems have been described in asymptomatic carriers of premutation alleles6,30; however, it remains unclear whether these impairments are an indication of developmental changes as they manifest across the lifespan or presymptomatic vulnerability to later neurodegenerative decline associated with FXTAS.
Our results provide the first evidence that the age-related decline in inhibitory control and working memory in the FMR1 premutation may only become discernible in an at-risk premutation repeat range. Future longitudinal studies will be needed to determine whether selective executive markers may be precursors to more severe forms of dementia reported in patients with FXTAS.
ACKNOWLEDGMENT
The authors thank the regional genetic centers that took part in the study, the Fragile X Society UK for their support in recruitment, and the families who participated.
GLOSSARY
- DLPFC
dorsolateral prefrontal cortex
- FXS
fragile X syndrome
- FXTAS
fragile X–associated tremor/ataxia syndrome
- PASAT
Paced Auditory Serial Addition Test
- PM
premutation carrier
Footnotes
Editorial, page 612
Disclosure: The authors report no disclosures.
AUTHOR CONTRIBUTIONS
Dr. Cornish conceptualized and designed the study, wrote the first draft of the manuscript, and provided intellectual input into the interpretation of the data. Dr. Hocking analyzed the data, provided intellectual input into the interpretation of the data, and co-wrote the first draft of the manuscript. Dr. Moss provided statistical analysis and input on the interpretation of the study findings. Dr. Kogan provided intellectual input into the interpretation of the data and co-wrote the first draft of the manuscript.
REFERENCES
- 1. Cornish KM, Turk J, Hagerman R. The fragile X continuum: new advances and perspectives. J Intellect Disabil Res 2008;52:469–482 [DOI] [PubMed] [Google Scholar]
- 2. Hagerman PJ, Hagerman RJ. Fragile X-associated tremor/ataxia syndrome: an older face of the fragile X gene. Nat Clin Pract Neurol 2007;3:107–112 [DOI] [PubMed] [Google Scholar]
- 3. Hagerman RJ, Leehey M, Heinrichs W, et al. Intention tremor, parkinsonism, and generalized brain atrophy in male carriers of fragile X. Neurology 2001;57:127–130 [DOI] [PubMed] [Google Scholar]
- 4. Jacquemont S, Hagerman RJ, Leehey M, et al. Fragile X premutation tremor/ataxia syndrome: molecular, clinical, and neuroimaging correlates. Am J Hum Genet 2003;72:869–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brega AG, Goodrich G, Bennett RE, et al. The primary cognitive deficit among males with fragile X-associated tremor ataxia syndrome is a dysexecutive syndrome. J Clin Exp Neuropsychol 2008;30:853–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grigsby J, Brega AG, Engle K, et al. Cognitive profile of fragile X premutation carriers with and without fragile X-associated tremor ataxia syndrome. Neuropsychology 2008;22:48–60 [DOI] [PubMed] [Google Scholar]
- 7. Cornish KM, Li L, Kogan CS, et al. Age-dependent cognitive changes in carriers of the fragile X syndrome. Cortex 2008;44:628–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cornish KM, Kogan CS, Li L, Turk J, Jacquemont S, Hagerman RJ. Lifespan changes in working memory in fragile X premutation males. Brain Cogn 2009;69:551–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hunter JE, Allen EG, Abramowitz A, et al. No evidence for a difference in neuropsychological profile among carriers and noncarriers of the FMR1 premutation in adults under the age of 50. Am J Hum Genet 2008;83:692–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hunter JE, Abramowitz A, Rusin M, Sherman SL. Is there evidence for neuropsychological and neurobehavioral phenotypes among adults without FXTAS who carry the FMR1 premutation? A review of current literature. Genet Med 2009;11:79–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jacquemont S, Hagerman RJ, Leehey MA, et al. Penetrance of the fragile X-associated tremor/ataxia syndrome in a premutation carrier population. JAMA 2004;291:460–469 [DOI] [PubMed] [Google Scholar]
- 12. Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: The Psychological Corporation; 1999 [Google Scholar]
- 13. Kogan CS, Turk J, Hagerman RJ, Cornish KM. Impact of the fragile X mental retardation 1 (FMR1) gene premutation on neuropsychiatric functioning in adult males without fragile X-associated tremor/ataxia syndrome: a controlled study. Am J Med Genet B Neuropsychiatr Genet 2008;147B:859–872 [DOI] [PubMed] [Google Scholar]
- 14. Haut MW, Kuwabara H, Leach S, Arias RG. Neural activation during performance of number-letter sequencing. Appl Neuropsychol 2000;7:237–242 [DOI] [PubMed] [Google Scholar]
- 15. Audoin B, Ibarrola D, Au Duong MV, et al. Functional MRI study of PASAT in normal subjects. MAGMA 2005;18:96–102 [DOI] [PubMed] [Google Scholar]
- 16. Aiken LS, West SG. Multiple Regression: Testing and interpreting interactions. Newbury Park, CA: Sage; 1991 [Google Scholar]
- 17. Tassone F, Hagerman RJ, Taylor AK, Gane LW, Godfrey TE, Hagerman PJ. Elevated levels of FMR1 mRNA in carrier males: a new mechanism of involvement in the fragile-X syndrome. Am J Hum Genet 2000;66:6–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tassone F, Hagerman RJ, Loesch DZ, Lachiewicz A, Taylor AK, Hagerman PJ. Fragile X males with unmethylated, full mutation trinucleotide repeat expansions have elevated levels of FMR1 messenger RNA. Am J Med Genet 2000;94:232–236 [DOI] [PubMed] [Google Scholar]
- 19. Kenneson A, Zhang F, Hagedorn CH, Warren ST. Reduced FMRP and increased FMR1 transcription is proportionally associated with CGG repeat number in intermediate-length and premutation carriers. Hum Mol Genet 2001;10:1449–1454 [DOI] [PubMed] [Google Scholar]
- 20. Tassone F, Adams J, Berry-Kravis EM, et al. CGG repeat length correlates with age of onset of motor signs of the fragile X-associated tremor/ataxia syndrome (FXTAS). Am J Med Genet 2007;144B:566–569 [DOI] [PubMed] [Google Scholar]
- 21. Greco CM, Berman RF, Martin RM, et al. Neuropathology of fragile X-associated tremor/ataxia syndrome (FXTAS). Brain 2006;129:243–255 [DOI] [PubMed] [Google Scholar]
- 22. Cohen S, Masyn K, Adams J, et al. Molecular and imaging correlates of the fragile X-associated tremor/ataxia syndrome. Neurology 2006;67:1426–1431 [DOI] [PubMed] [Google Scholar]
- 23. Sevin M, Kutalik Z, Bergman S, et al. Penetrance of marked cognitive impairment in older male carriers of the FMR1 gene premutation. J Med Genet 2009;46:818–824 [DOI] [PubMed] [Google Scholar]
- 24. Nathaniel-James DA, Frith CD. The role of the dorsolateral prefrontal cortex: evidence from the effects of contextual constraint in a sentence completion task. Neuroimage 2002;16:1094–1102 [DOI] [PubMed] [Google Scholar]
- 25. Leung HC, Skudlarski P, Gatenby JC, Peterson BS, Gore JC. An event-related functional MRI study of the Stroop Color Word interference task. Cereb Cortex 2000;10:552–560 [DOI] [PubMed] [Google Scholar]
- 26. Peterson BS, Skudlarski P, Gatenby JC, Zhang H, Anderson AW, Gore JC. An fMRI study of Stroop word-color interference: evidence for cingulate subregions subserving multiple distributed attentional systems. Biol Psychiatry 1999;45:1237–1258 [DOI] [PubMed] [Google Scholar]
- 27. Peterson BS, Kane MJ, Alexander GM, et al. An event-related functional MRI study comparing interference effects in the Simon and Stroop tasks. Brain Res Cogn Brain Res 2000;13:427–440 [DOI] [PubMed] [Google Scholar]
- 28. Farzin F, Perry H, Hessl D, et al. Autism spectrum disorders and attention-deficit/hyperactivity disorder in boys with the fragile X premutation. J Dev Behav Pediatr 2006;27:S137–S144 [DOI] [PubMed] [Google Scholar]
- 29. Aziz M, Stathopulu E, Callias M, et al. Clinical features of boys with fragile X premutations and intermediate alleles. Am J Med Genet 2003;121B:119–127 [DOI] [PubMed] [Google Scholar]
- 30. Koldewyn K, Hessl D, Adams J, et al. Reduced hippocampal activation during recall is associated with elevated FMR1 mRNA and psychiatric symptoms in men with fragile x premutation. Brain Imaging Behav 2008;2:105–116 [DOI] [PMC free article] [PubMed] [Google Scholar]