Abstract
Objective:
The static ocular counterroll (OCR) reflex generates partially compensatory torsional eye movements during head roll. It is mediated by the utricle in the inner ear. Skew deviation is a vertical strabismus thought to be caused by imbalance in the utriculo-ocular pathway. We hypothesized that if skew deviation is indeed caused by damage to this reflex pathway, patients with skew deviation would show abnormal OCR.
Methods:
Eighteen patients with skew deviation caused by brainstem or cerebellar lesions and 18 normal participants viewed a target at 1 m. Ocular responses to static passive head roll-tilts of approximately 20° were recorded using search coils. Static OCR gain was calculated as the change in torsional eye position divided by the change in head position during sustained head roll. Perception of the subjective visual vertical (SVV) was also measured.
Results:
Group mean OCR gain was reduced by 45% in patients. At an individual level, OCR gains were asymmetric between eyes and between torsional directions in 90% of patients. In addition, the hypotropic eye incyclotorting gain was lower than the hypertropic eye excyclotorting gain during head roll toward the hypotropic eye in 94% of patients. No consistent pattern of gain asymmetry was found during head roll toward the hypertropic eye. The SVV was tilted toward the hypotropic eye.
Conclusion:
Static OCR gain is significantly reduced in skew deviation. Interocular and directional gain asymmetries are also prevalent. The asymmetries provide further evidence that disruption of the utriculo-ocular pathway is a mechanism for skew deviation.
Skew deviation is a vertical strabismus caused by supranuclear lesions.1–6 It is part of the ocular tilt reaction (OTR), a pathologic synkinetic triad of skew deviation, abnormal ocular torsion, and head tilt.7,8 Skew deviation has been attributed to an asymmetric disruption of the utriculo-ocular pathway causing an imbalance of vestibular tone in the roll plane.3,9–11 The utricle normally mediates the static ocular counterroll (OCR) reflex which generates partially compensatory torsional eye movements during static head roll.12–14 In a previous study,5 we found that the OCR response was asymmetrically reduced in patients with skew deviation caused by cerebellar lesions. However, a definitive pattern could not be drawn because of the study's small sample size (n = 3).5 It is also unclear whether the reduced OCR response was causally related to the underlying cerebellar process or to skew deviation. In the present study, we aim to investigate further the pattern of changes in OCR response in a larger number of patients with skew deviation caused by lesions in the brainstem or cerebellum.
METHODS
Participants.
Eighteen patients with skew deviation caused by brainstem or cerebellar lesions were recruited from the University Health Network. Skew deviation was diagnosed by the following criteria: 1) a vertical strabismus with a pattern that is inconsistent with that found in palsy of one or more cyclovertical muscles; 2) presence of associated neurologic symptoms and signs; and 3) presence of a lesion in the posterior fossa on MRI. Patients with a history of strabismus since childhood or prior surgery for strabismus were excluded.
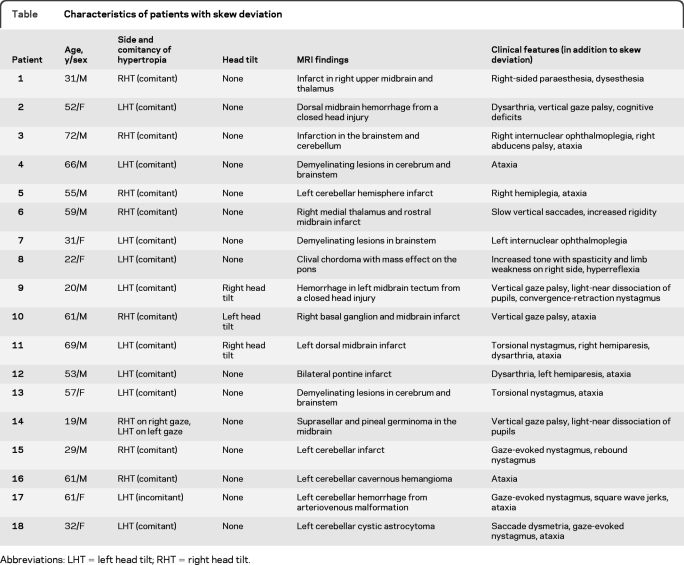
The clinical characteristics of the patients are shown in the table. The mean age (± SD) was 47 ± 18 years (range: 19–72 years; 6 women). Twelve (67%) of 18 patients had lesions in the brainstem, 5 (27%) in the cerebellum, and 1 (6%) in both the brainstem and cerebellum. Three of 18 patients (17%) had abnormal head tilt toward the hypotropic eye; the other 15 had no head tilt. Ten patients had purely unilateral lesions; 5 of these 10 patients had lesions in the midbrain-diencephalon with ipsilesional hypertropia, whereas the other 5 had lesions in the cerebellum with 3 having a contralesional hypertropia and 2 an ipsilesional hypertropia. The other 8 patients had diffuse/bilateral brainstem lesions, or lesions in the brainstem and cerebellum which preclude systematic correlation with the side of hypertropia. The vertical strabismus was comitant in 16 patients, incomitant in one, and alternating with right and left gaze in one. Two patients with internuclear ophthalmoplegia had ipsilateral hypertropia, and 2 patients had torsional nystagmus. The OCR findings of 3 patients with cerebellar skew deviation have been reported previously.5
Table.
Characteristics of patients with skew deviation
Abbreviations: LHT = left head tilt; RHT = right head tilt.
Eighteen normal participants (mean age: 44.6 ± 17 years; range: 20–77 years; 8 women) without any vestibular, neurologic, or eye diseases served as controls.
Visual stimuli and experimental protocol.
Participants fixated a central red laser spot 0.25° in diameter, rear projected onto a vertical flat screen. A photographic image of a cityscape that contained many vertical cues was mounted on the screen to serve as a background against which the red laser target was presented to elicit stronger OCR responses.14 The target was located 1 m from the participant's nasion at eye level and centered on the photographic image of the cityscape. With one eye occluded, static passive head roll of about 20° toward either the right or left shoulder were elicited. At each head roll position, the head was held steady for at least 6 seconds.13,15 Head movements were controlled by the experimenter, who tilted and held the participants' head by placing both hands over their parietal region. Five trials were performed for each head roll direction. The procedure was then repeated with the other eye fixating and the fellow eye occluded.
Recordings of eye movements and calibration.
A 3D magnetic search coil technique was used. The field system consisted of 6 ft (183 cm) diameter coils arranged in a cube (CNC Engineering, Seattle, WA), with 3 orthogonal magnetic fields. The patient wore a dual-lead scleral search coil on each eye (Skalar Instrumentation, Delft, Netherlands). Head position was recorded by an additional search coil taped to the participant's forehead. Each participant's head was kept within a 10-inch (25.4 cm) cube at the center of the magnetic field coils, where the magnetic field was uniform and was insensitive to translation.16
Horizontal, vertical, and torsional movements were calibrated by attaching each search coil to a rotating protractor in vitro before each experiment. To measure the offsets in the signal, each search coil was rotated through 360° to measure its maximum and minimum readings for each of the 3 source fields. If there was no offset for a particular field, the 2 corresponding readings would be equal and opposite. If they were not, the mean of the 2 readings was the offset, which was then subtracted from all recordings. After the offsets were determined, the gains (maximal signals) for each search coil from each of the 3 source fields were measured and set to a common standard fixed value.
At the onset of each trial, the participant fixed on the straight-ahead central target with the head upright. All eye positions were described by rotations from this reference position using quaternions.17,18 The torsional position of the eye in this reference position was arbitrarily defined as zero. All measurements of torsion during static head roll were relative to this arbitrarily defined zero. Analog position data from the eyes, head, and target were anti-alias filtered with a passband of 0 to 90 Hz using 8 pole low-pass Butterworth filters (Precision Filters, Ithaca, NY) and digitized at 500 Hz.
Data analyses.
Positive directions for horizontal, vertical, and torsional angles were defined as left, down, and clockwise, respectively, from the participant's viewpoint. The position of the eye in the head was the difference between head and gaze position signals. Responses containing blinks or rapid drifts were not analyzed. While eye movements were recorded continuously throughout the experiment, we analyzed a 1-s epoch (constituting 500 samples at a sampling frequency of 500 Hz) of this continuous record at the end of each 6-s head roll period. This was to ensure that the dynamic VOR semicircular canal signal had decayed13,15 and the static torsional eye response had stabilized. Mean static OCR gain was calculated as the change in torsional eye position divided by change in head position during sustained head roll. We assessed changes in torsional eye position relative to the arbitrarily defined zero torsion when the head was in the upright position. This was to ensure that any abnormal gain we observed was not due to the pathologic static ocular torsion (torsional offset) that typically accompanies skew deviation with the head in the upright position.2,3,7,19
Testing of the subjective visual vertical.
Participants sat in a natural upright posture in the dark during monocular viewing of a dimly illuminated straight line. The line was mounted on a linear rotating potentiometer, and was located 1 m away in the participant's midsagittal plane at eye level. Starting from a random nonvertical position, the examiner slowly rotated the line toward the earth-vertical and stopped when the participant perceived the line as vertical, as indicated verbally to the examiner. The signal from the potentiometer was amplified, and digitized at 100 Hz. The results of 5 trials per participant were averaged.
Standard protocol approvals, registrations, and patient consents.
The research protocol was approved by the University Health Network Ethics Committee. Informed consent was obtained from all participants.
Statistics.
Preliminary analysis showed that viewing eye had no significant effect on OCR gain. Therefore, data from both viewing conditions were combined. Group mean static OCR gain was analyzed using repeated measures mixed analysis of variance with one between-subjects factor: group (normal participants and patients), and 2 within-subjects factors: eye (2 levels: hypertropic vs hypotropic; for normal participants, right vs left) and torsional direction (2 levels: incyclotorsion vs excyclotorsion). Gain asymmetry was assessed by examining the normalized gain difference of 2 conditions (i.e., gain difference divided by the sum of gains). The normalized gain differences of each individual patient were compared to the 95% confidence interval around the mean gain difference for normal participants. All statistical analyses were performed using the SAS 9.2 statistical package. The significance level was set at p < 0.05. Any significant main effects and interactions were analyzed further using post hoc Tukey honestly significant difference tests.
Mean group subjective visual vertical (SVV) was compared between normal participants and patients using Student t test.
RESULTS
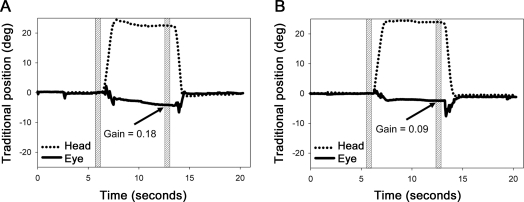
Representative tracings of clockwise head roll (i.e., toward the right shoulder) from 0° to approximately 20°, and the corresponding torsional eye movements, are shown in figure 1. In the normal participant, the eye reached a partially compensatory torsional position with a gain of 0.18 after the head maintained a static roll position for about 6 seconds (figure 1A). In contrast, in the patient with skew deviation, the OCR response was reduced, with a gain of 0.09 (figure 1B). Similar decrease in OCR gains was observed in each of the 18 patients.
Figure 1. Representative eye movement tracings during head roll.
Representative tracings of clockwise head roll (i.e., toward the right shoulder) from 0° to approximately 20°, and the corresponding partially compensatory torsional eye movements (ocular counterroll) of the right eye in a normal participant (A) and of the hypertropic eye in a representative patient (B). Positive y-axis values = clockwise; negative y-axis values = counterclockwise. The position measurements were made before and a minimum of 6 s after the head roll when the torsional values were stable as shown by the shaded region.
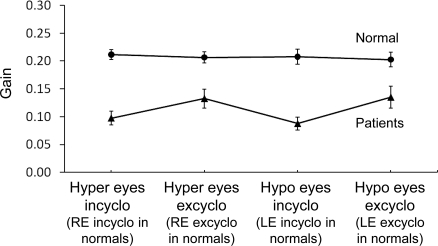
As a group, there was an overall main effect with patients exhibiting a significant reduction in OCR gain (mean and SD of both eyes and both torsional directions = 0.11 ± 0.07) compared to normal participants (0.21 ± 0.05; F1,33 = 36.43, p < 0.0001; figure 2). No other significant main effect was observed. There was no interaction between group and the 2 within-subjects factors, indicating that the difference in OCR gains between normal participants and patients as a group was not dependent on eye (hypertropic vs hypotropic) and torsional direction (incyclotorsion vs excyclotorsion).
Figure 2. Mean ocular counterroll gains for controls (n = 18) and patients (n = 18) by eye during incyclotorsion and excyclotorsion.
Error bars represent the SEM. Hyper and hypo eyes = hypertropic and hypotropic eyes in patients (right and left eyes in normal participants). Incyclo = incyclotorsion; excyclo = excyclotorsion.
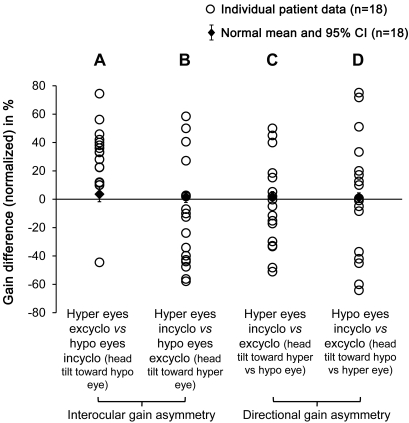
At an individual level, there was no significant gain difference between eyes or between torsional directions in each normal participant. In contrast, a significant interocular gain asymmetry (hypertropic vs hypotropic eyes) was found in 17 of 18 (94%) patients whose individual gain difference fell outside of the 95% confidence interval of the normal mean gain difference (figure 3, A and B). In addition, a consistent pattern was observed across patients during head roll toward the hypotropic eye (figure 3A)—the hypotropic eye incyclotorting gain was lower than the hypertropic eye excyclotorting gain in 17 of 18 (94%) patients (positive values in the figure; patient 3 was the only patient with a negative value). During head roll toward the hypertropic eye (figure 3B), however, there was no consistent pattern across patients, with 7 (39%) patients having a higher gain in the hypertropic incyclotorting eye (positive values), while the rest of the patients (61%) had a higher gain in the hypotropic excyclotorting eye (negative values).
Figure 3. Normalized ocular counterroll gain differences of individual patient.
Normalized gain differences of each individual patient were plotted against the mean and 95% confidence interval (CI) for 18 controls to show the interocular (A, B) and directional (C, D) gain asymmetries in patients. (A) Hypertropic eye excyclotorting gain vs hypotropic eye incyclotorting gain during head roll toward the hypotropic eye; positive values indicate that the former gain was higher than the latter, while negative values indicate the latter gain was higher than the former. (B) Hypertropic eye incyclotorting gain vs hypotropic eye excyclotorting gain during head roll toward the hypertropic eye. (C) Incyclotorting vs excyclotorting gains of the hypertropic eye. (D) Incyclotorting vs excyclotorting gains of the hypotropic eye. Hyper and hypo eyes = hypertropic and hypotropic eyes in patients (right and left eyes in normal participants). Incyclo = incyclotorsion; excyclo = excyclotorsion.
A significant directional gain asymmetry (incyclotorsion vs excyclotorsion) was also found in 16 of 18 (89%) patients whose individual gain difference fell outside of the 95% confidence interval of the normal mean gain difference (figure 3, C and D). However, no consistent pattern was observed across patients when we compared incyclotorsion vs excyclotorsion gain of the hypertropic eye (figure 3C), with 44% of patients having a higher incyclotorting gain and the other 56% having a higher excyclotorting gain. Similarly, when we compared incyclotorsion vs excyclotorsion gain of the hypotropic eye (figure 3D), 56% of patients had a higher incyclotorting gain and the other 44% had a higher excyclotorting gain. No correlation was found between the magnitude of the vertical separation of the eyes (skew) and the reduction in OCR gain. No consistent relationships were found between the eye with lower gain and the laterality or location of brain lesions.
Seventeen of 18 patients (94%) had tilt of their SVV toward the hypotropic eye with a mean net tilt of 3.13 ± 2.38°, compared with 0.72 ± 0.40° in normal participants (t34 = 4.24, p < 0.0010). One patient (patient 12) had no tilt of SVV. This patient was one of the 17 patients who exhibited a lower hypotropic eye incyclotorting gain than the hypertropic eye excyclotorting gain during head roll toward the hypotropic eye (i.e., positive value of 33% in figure 3A).
DISCUSSION
We investigated the pattern of changes in OCR responses in skew deviation. We found that each patient exhibited a marked decrease in static OCR gains with a mean reduction of 45% across both eyes and both directions (see figure 2). In addition, at an individual level, the OCR gains were asymmetric between eyes (interocular gain asymmetry) and between torsional directions (directional gain asymmetry) in about 90% of patients (see figure 3). Despite the heterogeneity of the type and location of lesions, a consistent pattern of interocular gain asymmetry was observed, with the hypotropic eye incyclotorting gain being lower than the hypertropic eye excyclotorting gain in 94% of patients during head roll toward the hypotropic eye.
Skew deviation has been attributed to asymmetric disruption of projections from otolith receptors in the utricles to the oculomotor and trochlear nuclei,2,4,7 largely based on stimulation and lesion studies in animals. For example, experimental stimulation of specific regions of the utricular macula in guinea pigs evokes vertical or horizontal eye movements.20 Stimulating the utricular nerve in the cat produces eye movements similar to those seen in OTR: the ipsilateral eye elevates and incyclotorts, whereas the contralateral eye depresses and excyclotorts.21 A comparable phenomenon also occurs with stimulation in the region of the interstitial nucleus of Cajal in monkeys9 and the midbrain in humans.22 However, in this case, the contralateral eye elevates and incyclotorts, whereas the ipsilateral eye depresses and excyclotorts, with head tilts toward the side of stimulation. Conversely, muscimol inactivation of undetermined fibers in the supraoculomotor area caudal to the interstitial nucleus of Cajal in monkeys causes the ipsilesional eye to elevate and the contralesional eye to depress, with head tilt toward the contralesional side.23
Relatively few studies have documented abnormal utricular function quantitatively in patients with skew deviation.5,24–26 The otolith receptors act as gravito-inertial force sensors and contribute to 3 major functions: 1) perception of spatial orientation (earth verticality); 2) generation of the translational VOR during lateral (heave), vertical (bob), and fore-aft (surge) motion of the head; and 3) generation of OCR during static head roll.
The SVV test is a psychophysical measure of the angle between perceptual vertical and true (gravitational) earth-vertical. It quantifies erroneous tilt perception of the true earth-vertical that might occur after a unilateral lesion to the utricular nerve or its projections in the brainstem.10,26–29 The dentate nucleus30 and nodulus31,32 of the cerebellum are also critical in the processing of gravito-inertial signals for the perception of verticality. Our finding that 17 patients had abnormal SVV tilt toward the hypotropic eye supports the notion that SVV is a sensitive test for detecting abnormal utricular function in skew deviation. However, because abnormal SVV can also be found in patients with posterior fossa lesions without skew deviation,31–33 SVV is not a specific correlate of skew deviation.
A second function of the utricle is to mediate the translational VOR during lateral and fore-aft head movements. We have shown previously that patients with skew deviation have an asymmetric binocular reduction in translational VOR responses during sudden and brief head heaves,26 providing evidence that imbalance in the utriculo-ocular pathway is a mechanism of skew deviation. Additionally, sustained off-vertical axis rotation of the body at a constant speed induces continuous translational head acceleration against gravity, such that the dynamic otolithic-ocular reflex persists, but the angular VOR, which is generated by the semicircular canals, fades away. Patients with skew deviation have directionally asymmetric responses to off-vertical axis rotation, indicating that asymmetric dynamic otolith signal of the translational VOR is associated with skew deviation.25
A third function of the utricle is to mediate the OCR during static head roll. In a previous study, we have shown that the static OCR gains were asymmetrically reduced in 3 patients with cerebellar skew deviation.5 One patient had decreased OCR gains in one eye in both directions, a second had decreased gains in both eyes in one direction, and a third had asymmetric gain in one direction in one eye alone. In the present study, we found that patients with skew deviation from brainstem or cerebellar lesions had a significant reduction in OCR gains, and that at an individual level, the OCR gains were asymmetric between eyes and between torsional directions. In addition, we found a consistent pattern of interocular asymmetry during head roll toward the hypotropic eye, despite the heterogenous nature of our patients' pathology.
One might expect a consistent directional asymmetry across patients with lower gain after head roll toward the hypotropic eye in skew deviation. However, a prior investigation of 4 patients with skew deviation from brainstem lesions found no consistent directional OCR gain asymmetries to rightward vs leftward head roll.24 Similarly, we did not detect any directional asymmetry in our patients. It is difficult to explain the lack of directional asymmetry and the varied gain asymmetries we (see figure 3) and others24 have observed for several reasons. First, unlike the semicircular canals, the morphologic arrangement of maculae in the utricles is complex—the axes of polarity of the hair cells are multidirectional and they reverse direction across a curvilinear midline landmark called the striola.34,35 Thus, signals of opposing directions of stimulation can be elicited from either side of the striola (lateral or medial) in each utricle and from either labyrinth (right or left). Second, the exact anatomy of the utriculo-ocular pathway is not well understood. Although it has traditionally been thought to be disynaptic,11,36,37 there is evidence that the utricles also project to the ocular motoneurons indirectly via polysynaptic pathways through the cerebellum and brainstem tegmentum.38–40 In addition, the utriculo-ocular pathway decussates in the brainstem, possibly at the level between the vestibular and abducens nuclei.11 Third, our patients had diverse etiology and location of damage, making it difficult to correlate the OCR responses systematically with their clinical and neuroimaging findings. Although the exact mechanism for the varied gain asymmetries remains to be elucidated, the asymmetric OCR responses we found provide additional support to the growing evidence that skew deviation is caused by disruption of the utriculo-ocular pathway which results in imbalanced static vestibular tone in the roll plane.
GLOSSARY
- OCR
ocular counterroll
- OTR
ocular tilt reaction
- SVV
subjective visual vertical
AUTHOR CONTRIBUTIONS
Manokaraananthan Chandrakumar: drafting/revising the manuscript for content, including medical writing for content, analysis or interpretation of data, acquisition of data, statistical analysis. Alan Blakeman: drafting/revising the manuscript for content, including medical writing for content, analysis or interpretation of data, acquisition of data. Herbert C. Goltz: drafting/revising the manuscript for content, including medical writing for content, analysis or interpretation of data, study supervision or coordination. James A. Sharpe: drafting/revising the manuscript for content, including medical writing for content, study concept or design, analysis or interpretation of data, contribution of vital reagents/tools/patents. Agnes M.F. Wong: drafting/revising the manuscript for content, including medical writing for content, study concept or design, analysis or interpretation of data, contribution of vital reagents/tools/patents, study supervision or coordination, statistical analysis, obtaining funding. Principal Investigator: I have access to all the data and take responsibility for the data, accuracy of the data analysis, and the conduct of the research. I have the right to publish any and all data, separate and apart from the guidance of any sponsor of the research.
DISCLOSURE
M. Chandrakumar and A. Blakeman report no disclosures. Dr. Goltz receives research support from CIHR and the Canadian National Institute for the Blind. Dr. Sharpe serves on the editorial board of Neuro-ophthalmology and as an Associate Editor for The Scientific World Journal, Neurology; and receives/has received research support from the CIHR and Fight for Sight. Dr. Wong serves as Associate Editor for the Journal of Neuro-Ophthalmology, Section Editor for the Canadian Journal of Ophthalmology, and on the editorial board of Strabismus; receives publishing royalties for Eye Movement Disorders (Oxford University Press, 2008); and receives research support from CIHR, the NIH, the Canada Foundation for Innovation, and the Canadian National Institute for the Blind.
REFERENCES
- 1. Smith JL, David NJ, Klintworth G. Skew deviation. Neurology 1964;14:96–105 [DOI] [PubMed] [Google Scholar]
- 2. Keane JR. Ocular skew deviation: analysis of 100 cases. Arch Neurol 1975;32:185–190 [DOI] [PubMed] [Google Scholar]
- 3. Brandt T, Dieterich M. Skew deviation with ocular torsion: a vestibular brainstem sign of topographic diagnostic value. Ann Neurol 1993;33:528–534 [DOI] [PubMed] [Google Scholar]
- 4. Brodsky MC, Donahue SP, Vaphiades M, Brandt T. Skew deviation revisited. Surv Ophthalmol 2006;51:105–128 [DOI] [PubMed] [Google Scholar]
- 5. Wong AM, Sharpe JA. Cerebellar skew deviation and the torsional vestibulo-ocular reflex. Neurology 2005;65:412–419 [DOI] [PubMed] [Google Scholar]
- 6. Fesharaki M, Karagiannis P, Tweed D, Sharpe JA, Wong AM. Adaptive neural mechanism for Listing's law revealed in patients with skew deviation caused by brainstem or cerebellar lesion. Invest Ophthalmol Vis Sci 2008;49:204–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rabinovitch HE, Sharpe JA, Sylvester TO. The ocular tilt reaction: a paroxysmal dyskinesia associated with elliptical nystagmus. Arch Ophthalmol 1977;95:1395–1398 [DOI] [PubMed] [Google Scholar]
- 8. Zackon DH, Sharpe JA. The ocular tilt reaction and skew deviation. In: Sharpe JA, Barber HO. eds. Vestibulo-Ocular Reflex and Vertigo. New York: Raven Press; 1993:129–140 [Google Scholar]
- 9. Westheimer G, Blair SM. The ocular tilt reaction–a brainstem oculomotor routine. Invest Ophthalmol 1975;14:833–839 [PubMed] [Google Scholar]
- 10. Dieterich M, Brandt T. Ocular torsion and tilt of subjective visual vertical are sensitive brainstem signs. Ann Neurol 1993;33:292–299 [DOI] [PubMed] [Google Scholar]
- 11. Zwergal A, Cnyrim C, Arbusow V, et al. Unilateral INO is associated with ocular tilt reaction in pontomesencephalic lesions: INO plus. Neurology 2008;71:590–593 [DOI] [PubMed] [Google Scholar]
- 12. Collewijn H, Van der Steen J, Ferman L, Jansen TC. Human ocular counterroll: assessment of static and dynamic properties from electromagnetic scleral coil recordings. Exp Brain Res 1985;59:185–196 [DOI] [PubMed] [Google Scholar]
- 13. Hamasaki I, Hasebe S, Ohtsuki H. Static ocular counterroll: video-based analysis after minimizing the false-torsion factors. Jpn J Ophthalmol 2005;49:497–504 [DOI] [PubMed] [Google Scholar]
- 14. Goltz HC, Mirabella G, Leung JC, et al. Effects of age, viewing distance and target complexity on static ocular counterroll. Vision Res 2009;49:1848–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moore ST, Clement G, Raphan T, Cohen B. Ocular counterrolling induced by centrifugation during orbital space flight. Exp Brain Res 2001;137:323–335 [DOI] [PubMed] [Google Scholar]
- 16. Robinson DA. The measurement of eye movement using magnetic induction in a contact lens coil. Biomed Sci Instrum 1964;2:97–106 [PubMed] [Google Scholar]
- 17. Tweed D, Vilis T. Implications of rotational kinematics for the oculomotor system in three dimensions. J Neurophysiol 1987;58:832–849 [DOI] [PubMed] [Google Scholar]
- 18. Wong AM, Sharpe JA, Tweed D. Adaptive neural mechanism for Listing's law revealed in patients with fourth nerve palsy. Invest Ophthalmol Vis Sci 2002;43:1796–1803 [PubMed] [Google Scholar]
- 19. Parulekar MV, Dai S, Buncic JR, Wong AM. Head position-dependent changes in ocular torsion and vertical misalignment in skew deviation. Arch Ophthalmol 2008;126:899–905 [DOI] [PubMed] [Google Scholar]
- 20. Curthoys IS. Eye movements produced by utricular and saccular stimulation. Aviat Space Environ Med 1987;58(suppl 9):A192–A197 [PubMed] [Google Scholar]
- 21. Suzuki J-I, Tokumasu K, Goto K. Eye movements from single utricular nerve stimulation in the cat. Acta Otolaryngol 1969;68:350–362 [DOI] [PubMed] [Google Scholar]
- 22. Lueck CJ, Hamlyn P, Crawford TJ, et al. A case of ocular tilt reaction and torsional nystagmus due to direct stimulation of the midbrain in man. Brain 1991;114:2069–2079 [DOI] [PubMed] [Google Scholar]
- 23. Das VE, Leigh RJ, Swann M, Thurtell MJ. Muscimol inactivation caudal to the interstitial nucleus of Cajal induces hemi-seesaw nystagmus. Exp Brain Res 2010;205:405–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Averbuch-Heller L, Rottach KG, Zivotofsky AZ, et al. Torsional eye movements in patients with skew deviation and spasmodic torticollis: Responses to static and dynamic head roll. Neurology 1997;48:506–514 [DOI] [PubMed] [Google Scholar]
- 25. Tilikete C, Ventre-Dominey J, Denise P, Nighoghossian N, Vighetto A. Otolith dysfunction in skew deviation after brain stem lesions: abnormalities of eye movements induced by off-vertical-axis rotation (OVAR). J Vestib Res 2000;10:179–192 [PubMed] [Google Scholar]
- 26. Schlenker M, Mirabella G, Goltz HC, Kessler P, Blakeman AW, Wong AM. The linear vestibulo-ocular reflex in patients with skew deviation. Invest Ophthalmol Vis Sci 2009;50:168–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dieterich M, Brandt T. Wallenberg's syndrome: lateropulsion, cyclorotation, and subjective visual vertical in thirty-six patients. Ann Neurol 1992;31:399–408 [DOI] [PubMed] [Google Scholar]
- 28. Brandt T, Dieterich M. Vestibular syndromes in the roll plane: topographic diagnosis from brain stem to cortex. Ann Neurol 1994;36:337–347 [DOI] [PubMed] [Google Scholar]
- 29. Halmagyi M. New clinical tests of unilateral vestibular dysfunction. J Laryngol Otol 2004;118:589–600 [DOI] [PubMed] [Google Scholar]
- 30. Baier B, Bense S, Dieterich M. Are signs of ocular tilt reaction in patients with cerebellar lesions mediated by the dentate nucleus? Brain 2008;131:1445–1454 [DOI] [PubMed] [Google Scholar]
- 31. Kim HA, Lee H, Yi HA, Lee SR, Lee SY, Baloh RW. Pattern of otolith dysfunction in posterior inferior cerebellar artery territory cerebellar infarction. J Neurol Sci 2009;280:65–70 [DOI] [PubMed] [Google Scholar]
- 32. Moon IS, Kim JS, Choi KD, et al. Isolated nodular infarction. Stroke 2009;40:487–491 [DOI] [PubMed] [Google Scholar]
- 33. Cnyrim CD, Newman-Toker D, Karch C, Brandt T, Strupp M. Bedside differentiation of vestibular neuritis from central “vestibular pseudoneuritis.” J Neurol Neurosurg Psychiatry 2008;79:458–460 [DOI] [PubMed] [Google Scholar]
- 34. Lindeman HH. Studies on the morphology of the sensory regions of the vestibular apparatus. Ergeb Anat Entwicklungs Gesch 1969;42:1–113 [PubMed] [Google Scholar]
- 35. Fernandez C, Goldberg JM. Physiology of peripheral neurons innervating otolith organs of the squirrel monkey: II: directional selectivity and force-response relations. J Neurophysiol 1976;39:985–995 [DOI] [PubMed] [Google Scholar]
- 36. Uchino Y, Ikarashi K, Sasaki H, Endo K, Imagawa M, Isu N. Monosynaptic and disynaptic connections in the utriculo-ocular reflex arc of the cat. J Neurophysiol 1994;71:950–958 [DOI] [PubMed] [Google Scholar]
- 37. Uchino Y, Sasaki M, Sato H, Imagawa M, Suwa H, Isu N. Utriculoocular reflex arc of the cat. J Neurophysiol 1996;76:1896–1903 [DOI] [PubMed] [Google Scholar]
- 38. Wiest G, Tian JR, Baloh RW, Crane BT, Demer JL. Otolith function in cerebellar ataxia due to mutations in the calcium channel gene CACNA1A. Brain 2001;124:2407–2416 [DOI] [PubMed] [Google Scholar]
- 39. Newlands SD, Vrabec JT, Purcell IM, Stewart CM, Zimmerman BE, Perachio AA. Central projections of the saccular and utricular nerves in macaques. J Comp Neurol 2003;466:31–47 [DOI] [PubMed] [Google Scholar]
- 40. Angelaki DE. Eyes on target: what neurons must do for the vestibuloocular reflex during linear motion. J Neurophysiol 2004;92:20–35 [DOI] [PubMed] [Google Scholar]