Abstract
Schizophrenia (SZ) is a brain disorder that has been intensively studied for over a century; yet, its etiology and multifactorial pathophysiology remain a puzzle. However, significant advances have been made in identifying numerous abnormalities in key biochemical systems. One among these is the antioxidant defense system (AODS) and redox signaling. This review summarizes the findings to date in human studies. The evidence can be broadly clustered into three major themes: perturbations in AODS, relationships between AODS alterations and other systems (i.e., membrane structure, immune function, and neurotransmission), and clinical implications. These domains of AODS have been examined in samples from both the central nervous system and peripheral tissues. Findings in patients with SZ include decreased nonenzymatic antioxidants, increased lipid peroxides and nitric oxides, and homeostatic imbalance of purine catabolism. Reductions of plasma antioxidant capacity are seen in patients with chronic illness as well as early in the course of SZ. Notably, these data indicate that many AODS alterations are independent of treatment effects. Moreover, there is burgeoning evidence indicating a link among oxidative stress, membrane defects, immune dysfunction, and multineurotransmitter pathologies in SZ. Finally, the body of evidence reviewed herein provides a theoretical rationale for the development of novel treatment approaches. Antioxid. Redox Signal. 15, 2011–2035.
Introduction
Schizophrenia (SZ) is a major mental disorder that typically strikes in adolescence or early adulthood, and continues throughout the life span (256). Its prevalence is equal across all cultures, and it affects ∼1% of the general population worldwide (107, 124). SZ is characterized by a positive syndrome consisting of symptoms such as hallucinations, delusions, and paranoia, as well as a negative syndrome marked by alogia, anhedonia, avolition, and blunted affect (252, 262). SZ has been linked to widespread structural and functional brain alterations, the pathogenesis of which remains poorly understood.
The vast majority of research into the pathogenesis of SZ has thus far focused on the dopamine system. However, evidence for dopaminergic alterations in SZ is largely indirect, and is based on the fact that most antipsychotic medications act as antagonists at the dopamine receptor, particularly D2 (31, 119, 247). A host of alternative hypotheses regarding the pathophysiology of SZ have therefore been proposed over the years to conceptualize the pathophysiology of SZ. These include neuronal maldevelopment, impaired neurotransmission, role of viral infections, autoimmune dysfunction, and many others. Although there are a variety of apparently disparate biological findings reported in SZ (110, 141, 236), it is possible that there is etiologic heterogeneity, in which multiple etiological factors converge on a final common pathogenetic pathway (or very few pathways) may mediate the recognizable syndromes of SZ. The issue at hand, therefore, is to identify candidate pathological process(es) that account for the constellation of clinical and biological features in SZ patients.
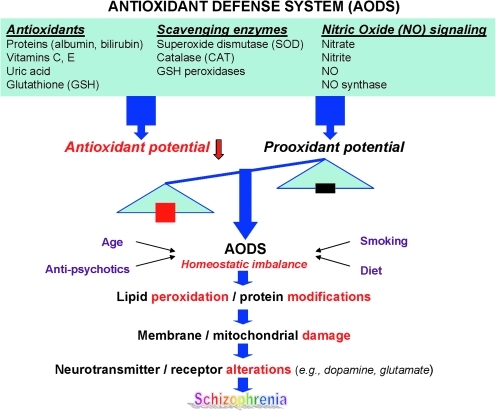
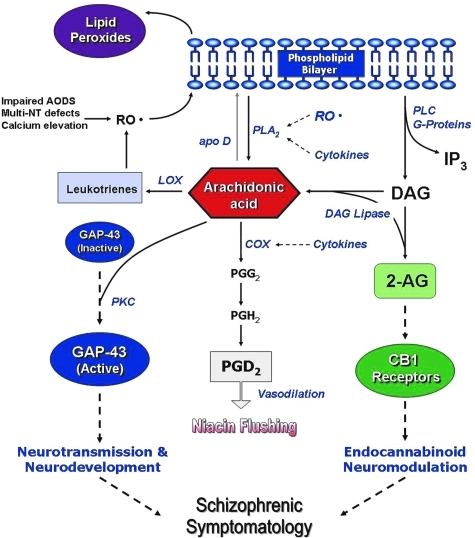
Free radicals are highly reactive chemical species generated during normal metabolic processes, which in excess can lead to membrane damage. Elaborate antioxidant defense systems exist to protect against oxidative stress. There is increasing evidence of antioxidant defense system (AODS) deficits in SZ (13, 27, 53, 66, 89, 147, 154, 157, 191, 204, 216, 293). Membrane dysfunction can be secondary to free radical-mediated pathology, and may contribute to specific aspects of SZ symptomatology and complications of its treatment. A diagram linking AODS imbalance to SZ pathology is illustrated in Figure 1. Specifically, free radical-mediated abnormalities may contribute to the development of a number of clinically significant consequences, including prominent negative symptoms, tardive dyskinesia, neurological signs, and Parkinsonian symptoms. The aim of this article is to provide a comprehensive review of recent research in antioxidant defense system and its possible role underlying the neuropathogenetic mechanism of SZ.
FIG. 1.
A schematic diagram linking homeostatic imbalance in antioxidant defense system to pathophysiology in schizophrenia (SZ). (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
Free radicals
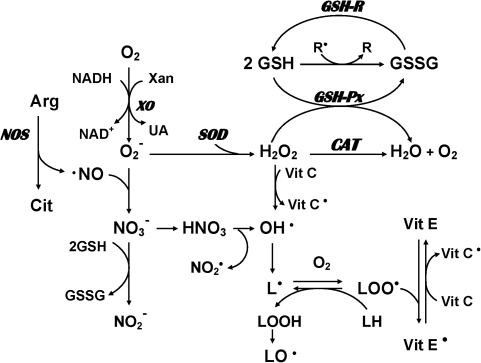
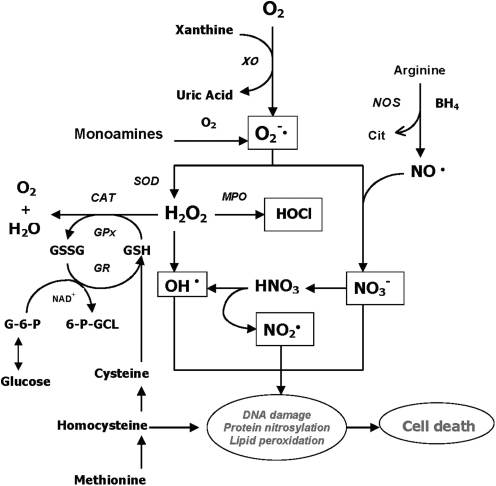
Free radicals are reactive chemical species generated during normal metabolic processes involving oxygen and nitric oxide (Fig. 2), including the mitochondrial electron transfer chain, nicotinamide adenine dinucleotide phosphate-dependent oxidase, and autooxidation of polyunsaturated fatty acids (PUFAs) and catecholamines (90, 221). These free radicals (primarily the reactive oxygen species [ROS], superoxide, and hydroxy radicals) play an important role in membrane lipid peroxidation.
FIG. 2.
Possible mechanisms involving production and removal of oxygen and nitrogen free radicals in mammalian cells. Molecular oxygen can be converted to superoxide radicals (O2−•) in the presence of xanthine oxidase (XO). Subsequently, superoxide dismutase (SOD) catalyzes the conversion of superoxide radicals to hydrogen peroxide (H2O2). Catalase (CAT) and glutathione peroxidase (GSH-Px) convert hydrogen peroxide to water. Glutathione (GSH) is utilized by GSH-Px to yield the oxidized form of glutathione (GSSG), which is converted back to GSH by glutathione reductase (GR). Hydrogen peroxide is susceptible to autoxidation to form hydroxyl radicals (OH•), particularly in the presence of metal catalysts such as iron. In addition, nitric oxide (NO), which is the product of a five-electron oxidation of the amino acid L-arginine, can also produce hydroxyl radicals as well as nitrogen dioxide radical. On the other hand, α-tocopherol (vitamin E) has the ability to inhibit lipid peroxidation as a chain-breaking antioxidant. Vitamin E radicals can be recycled back to their native form by ascorbic acid (vitamin C).
There are multiple pathways leading to excessive free radical generation and subsequent oxidative stress. In addition to the superoxide and hydroxyl radicals, another pathway is the formation of peroxynitrite by a reaction of nitric oxide radical and superoxide radical (Fig. 2). Nitric oxide (NO) can also produce hydroxyl radicals as well as nitrogen dioxide radicals. Nitric oxide is a free radical by its unpaired electron. Since NO radicals cannot produce initiation or propagation reactions, they do not generate free radical chain reactions. However, NO is a unique second messenger molecule that mediates a number of cellular functions, including neurotransmission, neurotoxicity and plasticity, vasodilation, regulation of blood flow, and inhibition of platelet aggregation (22, 105, 174). Elevated NO production has been linked to various neurodegenerative disorders, including Alzheimer's disease (186, 255), multiple sclerosis (94), and Parkinson's disease (19, 81, 103).
Antioxidant defense system
Biological systems have evolved complex protective strategies against free radical toxicity. Under physiological conditions the potential for free-radical-mediated damage is kept in check by the AODS, which is comprised of a series of enzymatic and nonenzymatic components. The critical antioxidant enzymes include superoxide dismutase (SOD; E.C.1.15.1.6), catalase (CAT; E.C.1.11.1.6), and glutathione peroxidase (GSH-Px; E.C.1.11.1.9). These enzymes act cooperatively at different sites in the metabolic pathway of free radicals (Fig. 2). Hydrogen peroxide produced by SOD is decomposed to water and oxygen by the heme protein, CAT, thereby preventing the formation of hydroxy radicals. Failure of this first-line antioxidant defense may lead to an initiation of lipid peroxidation. Selenium-dependent GSH-Px protects against lipid peroxidation by converting hydrogen peroxide to water, or more critically by converting toxic hydroperoxides to less toxic alcohols. Since SOD, CAT, and GSH-Px are critical to different stages of free radical metabolism, altered activity of one enzyme without compensatory changes in other enzymes may leave the membranes vulnerable to damage. Thus, the differential patterning of the antioxidant enzyme activities may provide important clues to the pathogenetic mechanisms of abnormal free radical metabolism (237).
In addition, the nonenzymatic antioxidant components, which may be equally important in the overall AODS (Fig. 2), consist of molecules that react with activated oxygen species and thereby prevent the propagation of free radical chain reactions. The most common nonenzymatic antioxidant molecules are albumin, uric acid, bilirubin, GSH, ascorbic acid (vitamin C), and α-tocopherol (vitamin E).
Altered AODS in SZ
Oxidative stress is a state when there is a dysequilibrium between pro-oxidant processes and the antioxidant defense system in favor of the former. There are potentially multiple pathological consequences of increased oxidative stress, due to increased free radical production and/or inefficient antioxidant systems (e.g., increased SOD and/or decreased CAT activity), which lead to lipid peroxidation. It has been suggested that increased scavenging activity of SOD in the absence of increased superoxide production can depress free radical-dependent reactions, such as those catalyzed by oxygenases (11), thus resulting in decreased catecholamine production. We review below the literature on AODS supporting a role of oxidative stress in SZ pathology (Table 1).
Table 1.
Alterations of Antioxidant Defense System in Patients with Schizophrenia
| AODS | SZ population | Source | Findings | References | Implications/comments |
|---|---|---|---|---|---|
| Antioxidants | |||||
| Proteins | |||||
| Albumin, Bilirubin | First-episode, neuroleptic-naive | Plasma | Decrease | 194, 220 | Increased bilirubin consumption may be secondary to oxidative stress. |
| Chronic | Plasma | Decrease | 194, 268, 275, 290, 292 | ||
| Biopyrrins | Chronic | Urine | Increase | 173 | Biopyrrins are oxidative metabolites of bilirubin. |
| Thioredoxin (TRX) | First-episode, neuroleptic-naive | Serum | Increase | 301 | Serum TRX levels were also positively correlated with positive symptoms of Positive and Negative Syndrome Scale. Following antipsychotic treatment, reduced TRX levels may provide us with biochemical index of therapeutic outcome. |
| UA | First-episode, neuroleptic-naive | Plasma | Decrease | 219, 284 | The potential for steady formation of antioxidant UA from purine catabolism is altered early in the course of SZ (284). UA and inosine (precursor of UA) may be beneficial in the treatment of oxidative stress related neurodegenerative diseases (60, 97, 144, 229, 241). |
| Chronic, treated | Plasma | Decrease | 289 | ||
| Vitamins | |||||
| Ascorbic acid | Chronic | Plasma | Decrease | 43, 249 | Some (44, 234), though not all (263), studies suggest that add-on supplementation of vitamin C may reduce oxidative stress and improve the outcome of SZ. |
| Urine | Decrease | 249 | |||
| Tocopherol | SZ with tardive dyskinesia | Plasma | Decrease | 23 | Vitamin E levels in plasma were corrected for total lipids. |
| Glutathione | Untreated | Plasma | Decrease | 212 | Adjunctive N-acetyl cysteine, a precursor for GSH synthesis, may provide us with a novel therapeutic intervention targeting GSH Dysregulation in SZ (15, 139). |
| Chronic | Plasma | Decrease | 51 | ||
| CSF | Decrease | 54 | |||
| Prefrontal cortex | Decrease | 54 | |||
| Postmortem caudate | Decrease | 286 | |||
| Free thiols | Chronic | Serum | Decrease | 101 | |
| Chronic | Platelets | Decrease | 49 | ||
| Scavenging enzymes | |||||
| Superoxide dismutase | Chronic | Serum | Increase | 43, 75, 137, 306 | |
| RBC | Increase | 1, 169, 217, 289, 305 | |||
| Platelets | Decrease | 50 | |||
| Postmortem brain | Increase | 168 | |||
| Chronic, untreated | RBC | Increase | 290 | ||
| Children, untreated | Platelets | Decrease | 84 | ||
| Early course, untreated | Blood | Increase | 129 | ||
| RBC | Decrease | 177, 212 | With progression of the illness, the superoxide dismutase levels may rise as a compensatory response to oxidative stress (177). | ||
| Treated | RBC | Decrease | 212 | ||
| CAT | Untreated | RBC | Decrease | 212 | |
| Treated | RBC | Decrease | 212 | ||
| GSH peroxidase | Chronic, treated | RBC | Decrease | 85, 169, 193, 212 | |
| Untreated | RBC | Decrease | 1 | ||
| Plasma | Increase | 307 | |||
| Neuroleptic-naive | Plasma | Increase | 307 | ||
| NO signaling | |||||
| NOS activity | Chronic | Postmortem cerebral cortex | Decrease | 277 | In light of inconsistent findings, it is surmised that NO and its metabolites may not be of diagnostic value to distinguish SZ from healthy controls or other brain disorders. |
| Platelets | Increase | 45 | |||
| NOS concentration | Chronic | Postmortem cerebellar vermis | Increase | 122 | |
| Neuronal NOS expression | Chronic | Postmortem prefrontal cortex | Increase | 9 | |
| Neuronal NOS expression | Chronic | Hypothalamus | Decrease | 16 | |
| NO | Chronic | Postmortem caudate | Increase | 287 | |
| Serum | Increase | 253 | |||
| Plasma | Decrease | 140, 184 | |||
| RBC | Increase | 96 | |||
| NO2−, NO3− | Chronic | CSF | Decrease | 213 | |
| NO2− | Chronic | Plasma | Increase | 308 | |
| Plasma | Decrease | 279 | |||
| NO3− | Chronic | Plasma | Decrease | 250 | |
| Lipid peroxidation | |||||
| Thiobarbituric acid reactive species (TBARS) | Chronic | Blood | Increase | 3, 65, 75, 85, 88, 137, 189, 193, 198, 207, 208, 302 | Findings to date suggest that oxidative stress occurs at very early in the course of illness, and independent of treatment. However, few other studies from Scottish SZ Research Group (230) and Skinner et al. (235) do not support the view that increased lipid peroxidation is associated with the SZ per se. |
| First-episode, neuroleptic-naive | Plasma | Increase | 153 | ||
| Chronic | Platelets | Increase | 50 | ||
| SZ with tardive dyskinesia | CSF7 | Increase | 146 | ||
| Pentane | Chronic | Breath | Increase | 134, 202 | |
| Ethane | Chronic | Breath | Increase | 211 | |
| Isoprostanes | Chronic | Urine | Increase | 52 | |
| Protein modifications | |||||
| 3-Nitrotyrosine | Chronic | Plasma | Increase | 51 | |
| Platelets | Increase | 49 | |||
| 4-hydroxynonenal | Chronic | Postmortem anterior cingulate | Increase | 269 | |
CSF, cerebrospinal fluid; GSH, glutathione; NOS, nitric oxide synthase; RBC, red blood cell; SZ, schizophrenia; UA, uric acid.
Decreased individual antioxidants in plasma
Albumin, uric acid, and ascorbic acid substantively contribute (>85%) to the total antioxidant capacity in human plasma (271) largely due to their high concentrations relative to those of other antioxidants in blood, for example, GSH, bilirubin, ascorbic acid (vitamin C), and α-tocopherol (vitamin E).
Proteins
Albumin and bilirubin are both metal-binding proteins with free radical scavenging properties. Albumin inhibits lipid peroxidation by binding copper ions and also serves as a scavenger of both oxygen- and carbon-centered free radicals (71, 238). Bilirubin, though less abundant, is a more efficient antioxidant than albumin. Plasma albumin and total bilirubin are reduced in early and chronic SZ (Table 1). Consequently, urinary excretion of biopyrrins (i.e., oxidative metabolites of bilirubin) is increased. In addition, serum thioredoxin, a redox-regulating protein with antioxidant activity, can be released from cells in response to oxidative stress (183). Increased levels of serum thioredoxin have been shown in first-episode, but not chronic SZ (301).
Earlier studies noted that SZ patients had a significantly higher frequency of hyperbilirubinemia than patients with other psychosis (172, 179). To eliminate the potential confounding effects of liver function, a recent case–control study by Vitek et al. (268) demonstrated that serum bilirubin levels were markedly lower in SZ patients with physiological range of liver function tests than those age- and gender-matched controls. These authors suggest that increased bilirubin consumption may be resulted from an increased oxidative stress accompanying SZ.
Uric acid
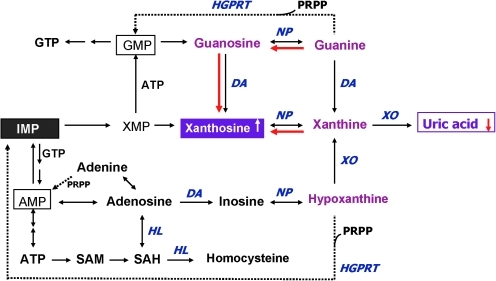
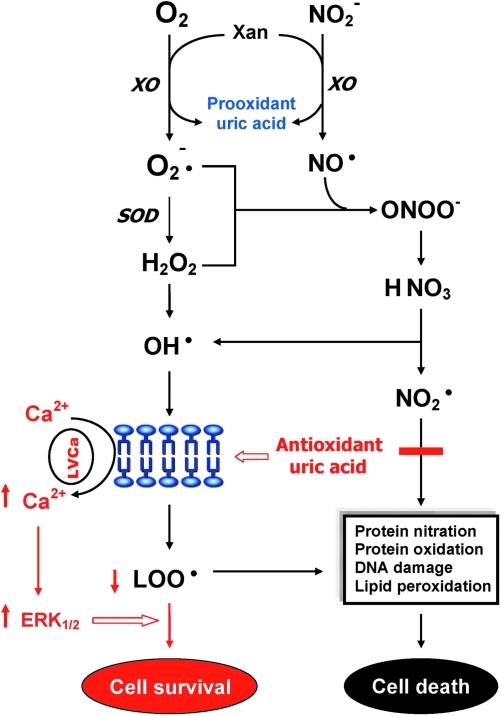
Uric acid (UA), an endproduct of purine catabolism in humans, is mainly synthesized from adenine- and guanine-based purines by the enzyme xanthine oxidase (Fig. 3). The blood levels of UA depend upon the dietary intake of purines, biosynthesis of UA, and the rate of UA excretion (138, 176). UA is a selective antioxidant that removes superoxide by preventing the degradation of SOD and subsequently inhibits its reaction with NO to form peroxynitrite (260). UA can also neutralize peroxynitrite (125) and hydroxyl radicals (12, 46) to inhibit protein nitration (192) and lipid peroxidation (181), respectively. UA, via astroglia, may protect dopaminergic neurons from glutamate toxicity (60). In addition, UA prevents the propagation of oxidative stress from the extracellular to the intracellular milieu by preserving the integrity of the plasma membrane at the lipid–aqueous interface (87). High K+-induced depolarization amplifies neuroprotection provided by UA through a mechanism involving Ca2+ elevation and extracellular signal-regulated kinases ½ activation (Fig. 4). Thus, decreased plasma UA may reflect decreased ability to prevent superoxide and peroxynitrite from damaging cellular components (138).
FIG. 3.
Altered purine catabolism in first-episode neuroleptic-naïve patients with SZ. Red arrows indicate shifts toward an increase of xanthosine and a decrease of uric acid productions in first-episode neuroleptic-naïve patients with SZ patients at baseline. Reactions shown with dotted lines represent the salvage pathways, which purine bases can be reutilized resulting in considerably energy saving for the cell. Reprinted with permission from Yao et al. (284). (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
FIG. 4.
Dual role of uric acid in the antioxidant defense system. Uric acid can neutralize peroxynitrite and hydroxyl radicals to inhibit protein nitration and lipid peroxidation, respectively. At increased levels, however, uric acid may be considered as a marker of oxidative stress due to accumulation of reactive oxygen species. Reprinted with permission from Yao et al. (284). (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
At increased levels, however, UA may also be considered as a marker of oxidative stress (12, 248) due to accumulation of ROS (104). Abnormally high plasma (or serum) UA has been related to cardiovascular disease, gout, hypertension, and renal disease (21, 36, 72, 112, 120), whereas low levels of plasma (or serum) UA have been linked to Alzheimer's disease, multiple sclerosis, optic neuritis, and Parkinson's disease (20, 38, 131, 133, 257). Although some studies have indicated that UA may play a role in the development or progression of such diseases (21, 112, 120, 226, 270), it remains unclear whether an increased UA contributes to the cause or is simply a consequence of these pathologic conditions (138). In short, UA can be served as both anti- and prooxidant in the AODS (Fig. 4). Plasma UA levels are reduced in chronic as well as first-episode neuroleptic-naïve patients with SZ (Table 1).
Vitamins
Ascorbic acid is a biological antioxidant that acts as a chain-breaking scavenger for peroxy radicals and acts synergistically with vitamin E (Fig. 2). Basal ganglia, which are rich in dopamine and glutamate, are also rich in ascorbic acid (188); the antioxidant function of ascorbic acid may thus protect against dopamine- or glutamate-induced neurodegenerative process (214). Both plasma and urinary vitamin C levels are decreased in SZ, relative to normal controls, even after controlling for diet. After vitamin C supplementation for 1 month, group differences were no longer significant (249). Some (44, 234), though not all (263), studies suggest that add-on supplementation of vitamin C may reduce oxidative stress and improve the outcome of SZ. Vitamin C requirements for schizophrenic patients may be higher than for normal subjects because they consumed substantially less antioxidant vitamins (C and E) and dietary fiber than a matched control group (159).
The fat-soluble vitamin E is involved in oxidative metabolism (Fig. 2) and is associated with diseases linked to oxidative stress. Brown et al. (23) reported decreased lipid-corrected vitamin E levels in SZ with tardive dyskinesia, relative to healthy controls, but not in patients without dyskinesia. However, there are no antioxidant monotherapy treatment trials for SZ treatment, and such a trial is unlikely to be ever conducted in the absence of demonstrable primary antipsychotic activity of an antioxidant molecule (215).
Reduced total antioxidant status in plasma
Although individual antioxidants play a specific role in the AODS, these antioxidants may act cooperatively in vivo to provide synergistic protection to the organs against oxidative damage. Therefore, it is more meaningful to evaluate AODS by measuring not only the individual levels but also the total antioxidant status (TAS) in plasma. Using a within-subject, on-off haloperidol treatment design, we have reported reduced plasma TAS in SZ compared to normal controls (291). Using a different method, similar reductions of plasma TAS were also shown in drug-free SZ (3, 259) and chronic SZ (267). On the other hand, plasma total oxidant status was not significantly different between SZ and healthy controls (267). It is likely that the alterations in antioxidant functions may be a consequence of changed symptom severity rather than direct effects of antipsychotic drug treatment. Thus, reduced plasma TAS may have pathophysiological significance in SZ, consistent with AODS alterations reported in association with tardive dyskinesia (35), negative symptoms, neurological signs, poor premorbid function, and computed tomography scan abnormalities (293). Taken together, the observed decreases in plasma TAS as well as individual antioxidants suggest an increased risk for oxidative damage in SZ.
GSH deficits
GSH plays an important role in metabolism, transport, redox signaling (Fig. 2), and cellular protection. The reduced form of GSH is the major nonprotein thiol present in virtually all cells. Its reducing and nucleophilic properties protect cells against destructive effects of ROS and free radicals (161). Using 1H-magnetic resonance spectroscopy (MRS) with double quantum coherence (DQC) filtering and water as an internal standard, Do et al. (54) first demonstrated GSH deficits in prefrontal cortex in vivo in SZ. Their findings were further supported by reduced GSH levels in cerebrospinal fluid (CSF) of drug-naïve SZ patients, and in plasma of early course or untreated SZ patients (Table 1). Free thiols are also reduced in serum and platelets as well as postmortem caudate in SZ patients (Table 1). Such GSH deficits have been linked to a genetic association between SZ and a GAG trinucleotide repeat polymorphism in the catalytic subunit of the glutamate cysteine ligase, the key enzyme for GSH synthesis (53).
Using 1H-MRS with Point RESolved Spectroscopy sequences and LCModel, Wood et al. (276) demonstrated 22% higher levels of GSH in SZ patients than in control subjects. A higher GSH level was more evident in nonresponders to niacin treatment. These authors suggest that GSH is increased in a compensatory manner. However, the mean percent standard deviation of the fit of their GSH was higher than the recommended 20% (or less), making the spectroscopic measurement of GSH less reliable (209). Berger et al. (14) failed to detect GSH level using the standard Point RESolved Spectroscopy sequence with TE = 30, suggesting that the GSH measurement by Wood et al. (276) might be an artifact. Recent advances in the MRS study of the AODS were reviewed in details by Matsuzawa and Hashimoto in this Forum (158).
Altered levels of scavenging antioxidant enzymes
Among three key scavenging antioxidant enzymes (Fig. 2), SOD has been the most frequently studied. Increased SOD activities have been reported in serum, red blood cells (RBCs), and in frontal cortex and substantia innominata regions of postmortem brains of SZ patients (Table 1) but not by others (233). Neuroleptic-naïve first-episode schizophreniform and schizophrenic patients show both increased SOD activity (129) and decreased SOD activity (84, 177, 212). It is possible that with progression of the illness, the SOD levels rise as a compensatory response to oxidative stress (177). On the other hand, GSH-Px activity was found to be lower, relative to normal controls, in neuroleptic-treated chronic SZ, and in drug-free female schizophrenic patients (Table 1). In plasma, Zhang et al. (307) have reported increased GSH-Px activities in long-term neuroleptic free as well as neuroleptic-naïve SZ patients, whereas Yao et al. (288) did not find any significant difference between chronic SZ and normal subjects, but is correlated with psychosis severity. Further, no differences were found in GSH-Px levels from skin fibroblasts of SZ patients and normal controls (307), suggesting that plasma GSH-Px elevation in patients may be state-dependent changes, and not a consequence of course of illness, treatment effects, or neuroleptics.
Examining indvidual enzymes may have limited value for elucidating the role of abnormal AODS in disease processes (217). Since SOD, CAT, and GSH-Px are critical to different stages of free radical metabolism, altered activity of one enzyme without compensatory changes in other enzymes may leave membranes vulnerable to damage. Thus, the differential patterning of the antioxidant enzyme activities may provide important clues to the pathogenetic mechanisms of abnormal free radical metabolism (217). Using a within-subject, repeated measures, on-off haloperidol treatment design, we have further evaluated three critical enzymes of the AODS (SOD, CAT, and GSH-Px) in SZ (288, 290). Among the three major AODS enzymes in erythrocytes and plasma, only RBC-SOD was found significantly higher in drug-free SZ patients than in age- and sex-matched normal volunteers (290). Our findings are consonant with most previous reports of increased SOD activity that have been reported in erythrocytes of drug-free and treated SZ patients.
Nitric oxide signaling
NO is the product of a five-electron oxidation of the amino acid L-arginine by nitric oxide synthase (NOS) (195). There are three isoforms of the NOS family: neuronal (nNOS), inducible (iNOS), and endothelial (eNOS) (70). In human brain, nNOS is primarily responsible for the synthesis of NO (18). Under physiologic conditions, NO and its metabolites react with various thiols (e.g., GSH) to form stable S-nitrosothiols (130). These thiol compounds play an important role in the suppression of NO functions in cellular membranes. Previously, Gegg et al. (80) have demonstrated an increase of GSH in astrocytes exposed to NO. Thus, to suppress the NO radical function, the level of cytosolic thiol compounds may be elevated in response to an increased NO production (287). The reaction of NO with free thiols may compete with a substrate such as GSH for decomposition of hydrogen peroxide by GSH peroxidase (Fig. 2).
Changes in cellular expression of brain-associated NOS/nicotinamide adenine dinucleotide phosphate diaphorase have been variable in SZ (2). Increased levels of nNOS were found in cerebellar vermis of SZ (Table 1), but no differences in the expression of NOS in cerebellar granule cells (55). Later, Xing et al. (277) demonstrated decreased constitutive NOS activity but normal nNOS protein in the cerebral cortex of SZ patients. In addition, increased levels of nNOS mRNA (9) and reduced hypothalamic nNOS expression (16) were also reported in SZ. Utilizing a sensitive fluorometric assay, Yao et al. (287) showed an increased NO production independent of age, brain weight, postmortem interval, sample storage time, alcohol use, or cigarette smoking in postmortem caudate nucleus in SZ. On the other hand, NO metabolites, nitrate and nitrite, were reduced in the CSF samples of SZ patients (213). Peripheral findings have also been inconsistent (Table 1), suggesting that NO and its metabolites may not be of diagnostic values.
Homeostatic imbalance of AODS
GSH redox coupling
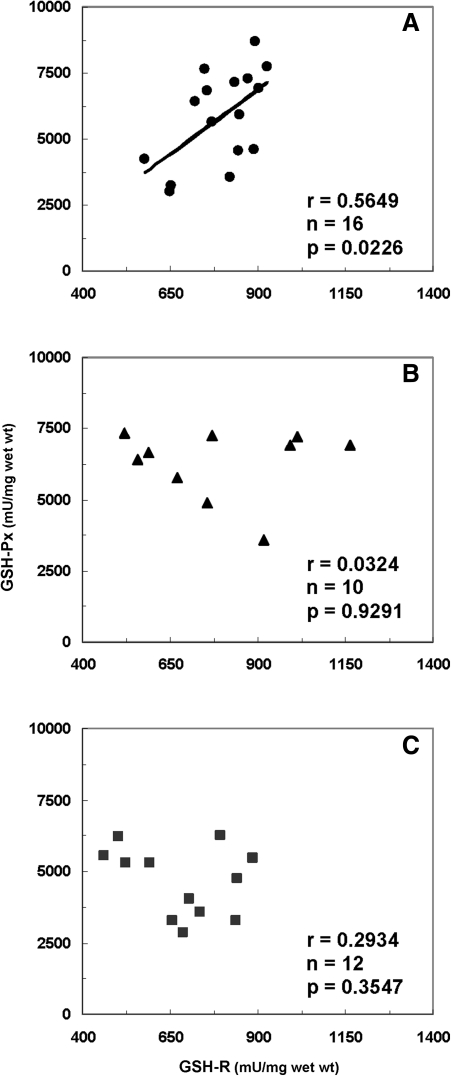
The GSH deficiency induced in the animal model has been related to mitochondrial damage (161, 231) and increased free radical insult (5, 185). Moreover, several pathological conditions, including Alzheimer's, Huntington's, and Parkinson's diseases, have been associated with a deficiency of GSH and imbalanced ratio of GSH to oxidized GSH (GSSG) (111, 123). In postmortem caudate region, we (286) have also demonstrated positive correlations between GSH levels and GSH-Px or glutathione reductase (GSH-R) activities in postmortem caudate from control subjects without any psychiatric disorders. Concomitantly, GSH-Px was significantly correlated with GSH-R activities in control subjects (Fig. 5). These positive correlations suggest that a dynamic state regulates the redox coupling under normal conditions. By contrast, lack of such correlations in SZ point to a disturbance of redox coupling mechanisms in the AODS, possibly resulting from a decreased level of GSH as well as age-related decreases of GSSG and GSH-R activities (Fig. 6; for details see subsection Age under section Factors influencing antioxidant status).
FIG. 5.
Correlations of GSH-Px to glutathione reductase (GSH-R) activities in postmortem caudate region. (A) Control subjects without mental disorders, (B) control subjects with bipolar and/or depression, and (C) patients with SZ. Reprinted with permission from Yao et al. (286).
FIG. 6.
Correlations of GSSG or GSH-R activities to age in postmortem caudate region. (A) Control subjects without mental disorders, and (B) patients with SZ. Reprinted with permission from Yao et al. (286).
A recent integrative review by Do et al. (53) posited that redox dysregulation resulting from the convergence between genetic impairments of GSH synthesis and oxidative stress generating environmental insults would lead to hypoactive N-methyl-d-aspartate (NMDA) receptors, impairment of fast-spiking parvalbumin GABA interneurons, and a deficit in myelination. Such a defect in GSH redox signaling not only attenuates deaminase (DA) modulation of calcium responses to NMDA (245) but also affects the structural and functional connectivity in the brain, which may contribute to the development of SZ (244). Following an add-on clinical trial with the GSH precursor and antioxidant N-acetyl-cysteine, both the negative symptoms (15) and the auditory evoked potential (139) were significantly improved in clinically stable patients with chronic SZ. These findings suggest a novel therapeutic intervention targeting GSH dysregulation in SZ.
Purine catabolism
Mitochondria process most of the cellular oxygen to provide energy that drives almost all metabolic processes, and also are the site of significant free radical production. About 3% of all oxygen consumed is converted to superoxide, and subsequently to hydrogen peroxide (69). Thus, there is an enormous and continuous free-radical burden. Antioxidant systems keep this in check. When the equilibrium between pro-oxidant and antioxidant systems are disturbed in favor of the former, mitochondrial damage can occur. Mitochondrial membranes, similar to neuronal membranes, are vulnerable to lipid peroxidation. Any impairment in mitochondrial oxidative phosphorylation can lead to a broad range of cellular disturbances, including decreased neurotransmission, decreased DNA repair, and, finally, cell death. Cytochrome-c oxidase is a key enzyme in the mitochondrial electron transport chain. Decreased activity of this enzyme has been reported in the frontal cortex and caudate nucleus of schizophrenic patients. Several lines of evidence suggest decreased oxidative metabolism in some brain areas in SZ (24, 286, 287), and may be explained in part by mitochondrial dysfunction. We propose that this is secondary to oxidative stress due to decreased antioxidant capacity and/or increased free radical burden.
An early study by Kristal et al. (136) indicated that purine catabolism may contribute to mitochondrial antioxidant defense by producing UA. Failure to maintain elevated xanthine and UA occurred contemporaneously with progressive mitochondrial dysfunction. Thus, purine catabolism appears to be a homeostatic response of mitochondria to oxidant stress and may protect against progressive mitochondrial dysfunction in certain disease states (136). In response to oxidative stress, decreased energy charge, or nucleic acid damage, purine metabolism shifts to favor breakdown to xanthine and uric acid.
During the de novo synthesis of purine nucleotides, many reactions require a great deal of energy utilizing the hydrolysis of adenosine triphosphate. To provide energy saving for the cell, the purine bases can be reutilized via salvage pathways (41) by converting adenine, guanine, or hypoxanthine (Hx) to AMP, GMP, or IMP, respectively (shown dotted arrow in Fig. 3). The unsalvaged Hx is then converted to xanthine (Xan), which is further converted to UA by xanthine oxidase. In humans, UA is the final product of purine catabolism (142), which has been implicated as a risk factor and cause of numerous pathological conditions (see below).
Recently, we have evaluated the purine pathway by quantitative determinations of six major purine metabolites consisting of xanthosine (Xant), Xan, Hx, guanine (G), guanosine (Gr), and UA (284). During the purine degradations, both conversions from Gr to G and from Xant to Xan are readily reversible (Fig. 3). Altered ratios of product to precursor, that is, significantly decreased ratios of G/Gr, UA/Gr, and UA/Xant, and the increased ratio of Xant/G, suggested a shift favorable to the Xant formation (approximately twofold increase) in the first-episode neuroleptic-naïve patients with schizophrenia (FENNS). Consequently, the potential for steady formation of UA from purines was reduced at first testing of the patients, which is shown in the red arrow of Figure 3. In addition, there are tightly correlated precursor and product relationships within purine pathways; although some of these correlations persist across disease or medication status, others appear to be lost among FENNS. Taken together, these results suggest that the potential for steady formation of antioxidant UA from purine catabolism is altered early in the course of SZ and may be independent of treatment effects.
Increased lipid peroxidation and protein modifications
Lipid peroxidation
Changes in antioxidant defense system such as decreased plasma TAS do not necessarily reflect an increased oxidative stress and subsequent membrane lipid damage. While evidence of peroxidative damage is limited to SZ patients, the findings have been consistent. Increased blood levels of malondialdehyde (MDA) were found in SZ patients (Table 1). A similar increase of lipid peroxides was also demonstrated in CSF samples (146) and platelets (50) of SZ patients. In addition, elevated plasma lipid peroxides were also shown at the onset of psychosis in never-medicated, first-episode SZ patients (153), suggesting the presence of oxidative stress at very early in the course of illness, and independent of treatment. Increased concentrations of pentane and ethane, which are other markers of lipid peroxidation, have also been reported in SZ relative to normal controls (Table 1). By contrast, findings from Scottish SZ Research Group (230) and Skinner et al. (235) do not support the view that increased lipid peroxidation is associated with the SZ per se.
Although the classical determination of MDA by complexion with thiobarbituric acid reactive substances is widely used for assaying lipid peroxides, clinical application is somewhat limited because of concerns relating to analytical specificity, sensitivity, reproducibility, recovery, sample instability, and drug interference (109). One alternative approach is to determine isoprostanes, which are prostaglandin isomers resulting from free radical insult of arachidonic acid (AA) in situ on membrane phospholipids (175). Isoprostanes are chemically stable end-products of lipid peroxidation and are excreted in urine (8). The increased plasma MDA levels were supported by a significantly increased excretion of isoprostanes in urine of the same SZ patient individuals as compared to control subjects (52).
Protein modifications
There are high levels of free radicals that are formed by the mitochondrial respiratory chain, with subsequent damage to mitochondrial proteins, DNA, and lipids (182). Protein oxidation is identified as carbonyl formation resulting from ROS, and 3-nitrotyrosine as a marker of reactive nitrogen species. Increased oxidative/nitrative modifications have been demonstrated in both plasma and platelet proteins of SZ patients (Table 1). By application of immunohistochemistry determining 4-hydroxynonenal protein adducts, Wang et al. (269) have recently shown that levels of 4-hydroxynonenal (a major product of lipid proxidation) were also significantly increased in the postmortem anterior cingulate brain from SZ patients. Recent advances in proteomic studies have provided considerable evidence further supporting a role of oxidative stress in SZ (156).
Factors influencing antioxidant status
Age
Previous studies have linked aging process to oxidative injury (58, 73). The balance between free radical production and antioxidant defense system is an important mediator of the mechanism of aging (92). In general, the rate of oxidative damage by free radicals to membranes provides us an index to determine life span (91, 171, 232). Previously, we have reported that reduction of plasma antioxidant proteins appears to be age related in patients with chronic SZ (292). Contrary to normal control subjects, there was an inverse correlation between plasma albumin and age in SZ. On the other hand, plasma total bilirubin levels were positively correlated with age in normal control subjects, but not in SZ patients. Such age-related correlations were not found in the FENNS and age-matched normal controls (219), perhaps resulting from a narrow range of age (mean age, 28 years; range, 18–40 years) in the FENNS group as compared to the previously reported patients with chronic SZ (mean age, 35 years; range, 18–55 years).
Recently, in the caudate region of postmortem brains, we (286) have further shown that both GSSG and GSH-R levels were significantly and inversely correlated to age of SZ patients, but not control subjects (Fig. 6). However, the plasma AODS enzyme activities were not significantly correlated with subject age, age of onset of illness, or the duration of illness (288, 290). It is likely that decreased levels of GSH result from a decrease of GSH-R in the brain with aging, suggesting an increased susceptibility to oxidative damage with accelerated aging. In addition, several lines of evidence indicate accelerated aging processes in SZ (132). Certain biomarkers of aging, including telomere functioning (68, 121, 205), deficient insulin-like growth factor-1 levels (264), and increased interleukin (IL)-6 levels (206, 282), were also abnormal in SZ patients. Taken together, age appears to be an important factor modifying AODS in SZ.
Antipsychotic drugs
Applying the controlled discontinuation of haloperidol in patients with chronic SZ, we have previously examined the antipsychotic effects on plasma antioxidant status. Neither individual antioxidant levels nor total antioxidant status were significantly affected by antipsychotic treatment (194, 267, 289, 291, 292). More importantly, the altered AODS were observed independent of treatment since patients were antipsychotic drug-naïve at entry into the study (194, 212, 219, 267). Although decrease of plasma uric acid levels were observed in patients after haloperidol withdrawal (289), it is unlikely that the decreased uric acid levels in patients with chronic SZ are due to the antipsychotic effects since the haloperidol treatment was associated with increased uric acid levels within SZ patients. Similarly, neither increased serum nitric oxide levels (253) nor increased lipid peroxidation (75, 193, 302) in SZ patients were affected by antipsychotic treatment. A recent study by Lee and Kim (140) further indicated that plasma NO levels in SZ patients were significantly lower than in normal controls both before and after 6-week treatment with risperidone.
On the other hand, several other studies have shown that blood levels of TAS (3), SOD (43, 306), MDA (3), IL-2 (306), and thioredoxin (301) may be regulated by antipsychotic treatment in SZ patients. Miljevic et al. suggested that atypical antipsychotic drugs (e.g., clozapine)-induced metabolic syndrome may be mediated through the oxidative stress mechanisms (169).
In postmortem studies, it is possible that alterations in the GSH redox state are attributable to the long-term treatment with antipsychotic drugs that SZ patients likely received. In our previous study (286), all patients were on antipsychotic medications at the time of death. No significant differences, however, were shown in the caudate mercaptans between control subjects with or without nonschizophrenic psychiatric disorders. Grima et al. (86) have proposed that GSH deficit in SZ may be associated with dopamine-induced oxidative stress. Antipsychotic drugs are known to block DA receptors and, consequently, may result in increased levels of GSH. Therefore, the GSH deficit is unlikely due to antipsychotic effects, because GSH levels may be lower in SZ patients in the absence of antipsychotic treatment.
Data on the effect of antipsychotic drugs on molecular markers are complicated by the lack of complete database on the dose and duration of different antipsychotic drugs taken by the patients. Data from animal model studies have indicated that antipsychotic drugs may have differential effects (time and dose dependent) on molecules in redox signaling (203).
Diet
Diet, caloric intake, and alcohol can affect both the antioxidant system and the production of free radicals (196). Malnutrition can cause low plasma albumin levels (170). In our previous studies examining antioxidant capacity in plasma (289–292, 297), SZ patients were all hospitalized and maintained on a controlled and balanced diet without alcohol consumption. Subsequent studies in FENNS (219), all participants including controls were recruited from the general population, without a controlled diet. In general, patients were not undernourished as indicated by the normal range of body mass indices. Systematically examining the associations between diet, sociodemographic, and physical characteristics in community-dwelling SZ patients, Strassnig et al. (246) concluded that SZ patients do not make dietary choices different from those of people in the general population. Thus, dietary factors may not have predominant confounding effects on plasma antioxidant capacity.
Although diet has profound effect on the developing brain, the mature brain is more resistant to undernutrition (82). It is unlikely that diet alone accounts for the findings of AODS defects in SZ patient groups from different countries and different continents.
Cigarette smoke
Cigarette smoke contains many pro-oxidants in both the gas and tar phases, and may contribute directly to oxidative stress and decreases in antioxidants (37, 210). One of the major compounds in the gas phase of tobacco smoke is nitric oxide. It has been suggested that nitric oxide reacts with smoke olefins to form carbon-centered radicals (210). The tar phase consists of a semiquinone radical that promotes hydrogen peroxide formation. Moreover, tobacco smoke may increase free radical formation by activating neutrophils.
On the other hand, several in vivo studies have shown antioxidant properties of nicotine (67, 143), which might explain the protective effects of smoking against the development of Parkinson's disease (239). Cigarette smoking by SZ patients exceeds the rates in the general population by 2–3-folds (102, 145, 189). Some investigators have suggested that cigarette smoking in schizophrenic patients may be a method of self-treatment (189) or smoking may serve as a behavioral filler (102), or there may be an underlying neurobiological cause (243). Further, smoking may help to offset the sedative or other adverse effects of psychotropic medications. It has been shown that smoking decreases the blood levels of many psychoactive agents by activating hepatic microsomal enzyme systems, thereby increasing their metabolism (102), and may help overcome the dopaminergic blockade and associated medication side effects, including akathisia, drowsiness, dystonia, and Parkinsonism (145). Some, or all, of these factors may underlie the high rates of smoking in schizophrenic patients (218).
Previously, we have shown that cigarette smoking can significantly reduce the levels of plasma bilirubin (292) but not of albumin (219, 292) and uric acid (219, 289). Although bilirubin is a more efficient antioxidant than albumin, the overall contribution of plasma TAS from bilirubin is only <5% as compared to that of >70% from albumin and uric acid (169, 271). Moreover, plasma TAS was not correlated with levels of plasma cotinine (291), which is the major metabolite of nicotine. Recent findings from Ustundag et al. (259) also showed significantly lower levels of plasma TAS in medication-free SZ patients independent of smoking habits. Similarly, levels of plasma MDA were significantly higher in chronic SZ patients than in age-, gender-, and smoking-matched healthy control subjects (302). Approximately 68%–70% of subjects were smokers in both patient and control groups. In addition, serum thioredoxin levels were not significantly different between smokers and nonsmokers (301). Taken together, cigarette smoking alone does not appear to be a confounding factor in altering AODS in SZ patients, although increased oxidative stress may be associated more with the smokers than the nonsmokers (230, 259, 304).
Oxidative Stress, Membrane Dynamics, Immune Response, and Neurotransmission
Much of the research focus in SZ has been on neurotransmitter systems. Although the role of dopamine in the pathophysiology of SZ remains preeminent, recent findings suggest instead that multiple neurotransmitter systems may be involved. Whether these are primary or secondary to other pathological processes, such as oxidative stress and membrane dysfunction, will need to be determined in future studies. It is important to recognize, however, that alterations in the metabolism of several neurotransmitter systems can both contribute to, and be modified by oxidative stress (or membrane dysfunction).
Altered phospholipids and PUFAs
Membrane PUFAs highly susceptible to free radical insult
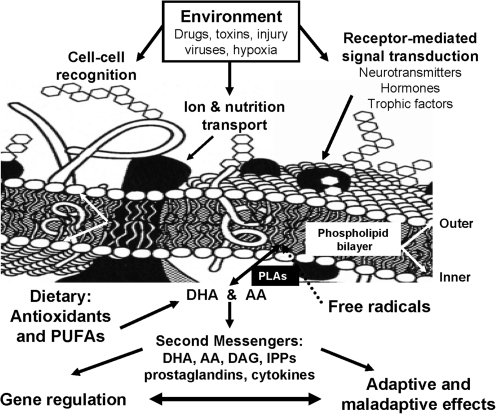
Lipids account for ∼50% of the brain's dry weight. Phospholipids in particular constitute 50%–70% of the makeup of brain lipids (251), which contain high levels of PUFAs, most notably AA (n-6) and docosahexaenoic acid (DHA; n-3). PUFAs are highly susceptible to free radical insult and autooxidation to form peroxyradicals and lipid peroxide intermediates, the existence of which within cell membranes (Fig. 7) results in unstable membrane structure, altered membrane fluidity and permeability, and impaired signal transduction (62). Hydroperoxides can further decompose to other toxic species (aldehydes, including malonyldialdehyde), which can damage adjacent cells, membrane-bound enzymes, and receptors, cause cross-linking between various types of molecules, and result in membrane breakdown, cytotoxicity, mutagenicity, and enzyme modification (33, 34, 62). Aldehydes can react with lipids and proteins forming lipofuscin, which accumulates in neuronal cells, particularly in regions of active free radical metabolism.
FIG. 7.
Schematic membrane structure illustrating the key components and their functional role in cell–cell interaction, interaction with environmental factors, and receptor-mediated signal transduction. Membrane model shows the phospholipid bilayer with embedded in it the receptors for neurotransmitters, physiological mediators and growth factors, transporters for ions and nutritional ingredients, and signal transduction machinery. Phospholipid bilayer is made up of four major phospholipids, phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylinositol (PI), and phosphatidylserine (PS), which are asymmetrically localized, that is, PC predominantly on outer lipid layer and PE, PI, and PS on inner lipid layer. PE, PI, and PS are highly enriched in arachidonic acid (AA) and docosahexaenoic acid (DHA), which are released by receptor-mediated phospholipases (PLAs). AA and DHA, and their metabolic products, diacylglycerol (DAG), inositol polyphosphates (IPPs), and prostaglandins work as second messengers and physiological mediators, including gene modulation, and thus lead to adaptive and maladaptive cellular changes. Reprinted with permission from Mahadik and Yao (151).
Thus, the unchecked effects of free radicals can result in cellular dysfunction, loss of membrane integrity, and even cell death. The brain, which is rich in PUFAs, is particularly vulnerable to free-radical-mediated damage.
Abnormal phospholipid metabolism
There are a variety of findings indicative of defects in phospholipid metabolism and cell signaling in patients with chronic SZ as well as in first-episode neuroleptic naïve patients with SZ (59, 151, 236). These findings include (i) decreased PUFAs and altered phospholipids in plasma (98, 115), RBC (7, 83, 118, 128, 187, 199, 220, 296), platelets (77), skin fibroblasts (150, 152), and postmortem brain tissues (98, 160, 227, 285); (ii) increased levels of DHA in CSF (117); (iii) increased turnover of inositol phospholipids (48, 64, 298) and production of second messengers (116, 295); (iv) decreased incorporation of AA in platelets (48, 295); and (v) increased lipid peroxidation in plasma (128, 153), CSF (146, 193) and platelets (50).
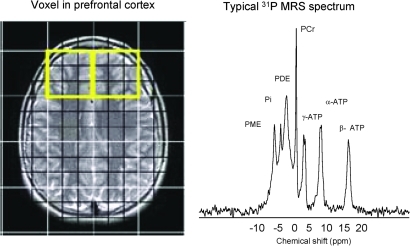
Support for central membrane dysregulation in SZ is also derived by in vivo 31P MRS techniques, which assay the relative concentrations of phospholipid precursors and breakdown products, phosphmonoesters, and phosphodiesters, respectively (Fig. 8). Using this methodology, Pettegrew et al. (200, 201) demonstrated a significant reduction of phosphmonoester and significantly increased levels of phosphodiester in frontal cortices of FENNS as compared to controls. In addition, an increased adenosine triphosphate and decreased inorganic orthophosphate levels were also found in the frontal cortex. Pettegrew and colleagues (201) suggested that changes in membrane phospholipids might be related to molecular changes that precede the onset of clinical symptoms and brain structural changes in SZ, whereas changes in high-energy phosphate metabolism may be state dependent. Other groups (47, 74, 242, 273) also reported similar findings in membrane phospholipid perturbations in both acutely and chronically ill patients. Also, based on 31P MRS findings, Keshavan et al. (126, 127) suggested a possible familial basis for membrane phospholipid changes in SZ. Yao et al. (294) have shown that 31P MRS and peripheral indices of phospholipid metabolism may be correlated. Given the obvious need for central measures of brain structure and function, these techniques may prove highly valuable in the further explication of the specific phospholipid and fatty acid abnormalities evident in SZ (158).
FIG. 8.
A typical in vivo 31P magnetic resonance spectrum from the prefrontal region of a healthy subject. The left panel shows the voxel placement for the spectral acquisition, and the right panel shows the spectrum. The x axis represents the frequency in parts per million (ppm). ATP, adenosine triphosphate; PCr, phosphocreatine; PDE, phosphodiester; Pi, inorganic phosphate; PME, phosphomonoesters. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
Taken collectively, there is ample evidence for the existence of membrane phospholipid and fatty acid defects in early course and chronic SZ. It is thus reasonable to hypothesize that increased oxidative stress may be one of the mechanisms responsible for reduction of membrane PUFAs.
PUFAs, immune dysfunction, and oxidative stress
PUFAs and immune response
Both n-6 and n-3 PUFA are involved in the regulation of inflammatory response system. The n-6 PUFAs, particularly AA, have the pro-inflammatory features, since AA is the precursor of pro-inflammatory eicosanoids, prostaglandin E2, and leukotriene B4 and increase production of IL-1, tumor necrosis factor, and IL-6 (93, 240, 254). On the other hand, the n-3 PUFAs eicosapentaenoic acid (EPA) and DHA suppress the production of AA-derived eicosanoids, thus having anti-inflammatory and immunosuppressive effects (28, 166). Previous studies on n-3 PUFA-enriched diets (e.g., fish oil) have shown partial replacement of AA by EPA in inflammatory cell membranes and significantly reduce the ex vivo production of pro-inflammatory cytokines (28, 61, 63, 108, 166, 240). Therefore, an imbalance of n-6/n-3 PUFAs may result in an increased production of pro-inflammatory cytokines.
Inflammatory response and ROS production
The inflammatory response is intimately connected with oxidative stress via free radical generation (57). During inflammatory processes, infiltrating cells can produce large amounts of ROS. In addition being cytotoxic, these ROS also act as important mediators regulating various cellular and immunological processes. Under physiologically relevant concentration, hydrogen peroxide was shown to either increase the production of T-cell growth factor (223) or induce the gene expression of IL-2 (148) and IL-6 (113). The enhancement of IL-2 production was associated with a decrease in the intracellular GSH level (148) and was reversed by the addition of exogenous GSH (222). Hehner et al. (95) have further demonstrated enhancement of T cell receptor signaling by a shift in the intracellular GSH redox state. Above findings thus suggest that the intact immune system requires a delicate balance between antioxidant and prooxidant status (56).
In SZ, the unspecific, innate immune system appears to be overactivated as evident by an increase of monocytes (272) and gamma/delta cells (180), resulting in an increased release of IL-6 (76, 261). By contrast, there is a decreased in vitro production of IL-2 (17, 32, 265) as well as decreased production of interferon-γ (6, 224, 272), which suggests that the Th-1 system is underactivated in SZ. Since the Th2 system can produce IL-6, Müller et al. (178) suggested an imbalance of adaptive immune system with a shift to the Th2-like immune reactivity in a subgroup of patients with SZ. This subgroup is further characterized by more pronounced negative symptoms and poor outcome (228).
However, data published so far have been inconsistent, which may be the result of differences in assay methodology, sample size, sample handling, diagnostic criteria, and comparison groups (282). In addition, several confounding factors, including age, gender, ethnic background, smoking, alcohol, substance abuse, and antipsychotic treatment may also explain these discrepancies (10). In a recent meta-analysis of 62 cross-sectional studies involving 2298 SZ patients and 1858 healthy volunteers, Potvin et al. (206) concluded that SZ patients have increased levels of in vivo IL-1 receptor antagonist, soluble IL-2 receptor, and IL-6, and decreased levels of in vitro IL-2, establishing an inflammatory syndrome in SZ.
Oxidative stress, immune dysfunction, and membrane defects
A number of putative mechanisms have been identified to explain the decreased PUFA levels in SZ (299), notably increased breakdown of phospholipids and decreased incorporation of AA. Both increased oxidative stress and altered immune function may play a role in an induction of phospholipase activities, for example, phospholipase A2 (PLA2) responsible for breakdown of membrane phospholipids (Fig. 9). This association is particularly relevant in relation to phospholipids/PUFA, because AA can be converted to a variety of biologically active eicosanoids that serve as potent messengers in regulating the inflammatory response. Indirect evidence for a dysregulated inflammatory response in SZ stems from the observation of a negative association between SZ and rheumatoid arthritis (163, 258, 266) and decreased prostaglandin-dependent niacin skin-flushing in SZ (99, 164, 165).
FIG. 9.
A putative model integrating lipid peroxidation, phospholipids turnover, AA signaling, and SZ symptomatology. As shown, several possible mechanisms can lead to increased phospholipid breakdown and AA release, including decreased AA incorporation and increased phospholipase activities (PLA2 and PLC), possibly resulting from increased oxidative stress and cytokine release. The resulting changes in AA level could then affect more downstream processes, including neurodevelopment via growth-associated protein (GAP)-43, neurotransmitter homeostasis, phosphatidylinositol signaling, and neuromodulatory actions of endocannabinoids. It is proposed that the specific behavioral symptomatology of SZ is related mostly to the effect of AA changes on the neurochemistry of deaminase, glutamate release, and circulating levels of the endocannabinoids anandamide and 2-arachidonoylglycerol (2-AG). In addition, alterations in AA may also affect the inflammatory response, which can then affect PLA2 release via cytokines, further exacerbating phospholipid turnover and AA release. Hence, in the current conceptualization, AA is at a nexus point in the cascade leading to the syndrome of SZ, and represents a common biochemical pathway leading to the highly heterogeneous symptomatology of psychosis. Reprinted with permission from Skosnik and Yao (236). (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
Direct evidence of immune changes in SZ have also come to light, particularly in the activities of several cytokines (e.g., IL-2 and IL-6) known to be abnormal in autoimmune dysfunction (78, 282, 306). Given the diverse physiological function of AA, the specific behavioral symptomatology of SZ is related mostly to the effect of AA changes that regulate neurodevelopment, neurotransmitter homeostasis, second messenger signaling, and neuromodulatory activity in SZ. Hence, in the current conceptualization, AA may be at a nexus point in the cascade leading to the syndrome of SZ (Fig. 9), and represents a common biochemical pathway leading to the highly heterogeneous symptomatology and course of SZ (236).
Neurotransmitters as contributors to free radical burden
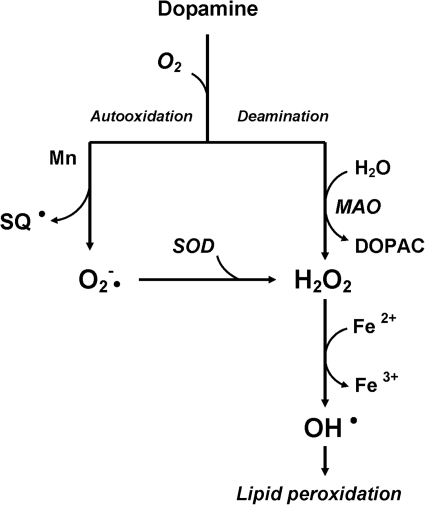
Previous studies have shown that dopamine metabolism yields free radicals under normal physiological conditions (40). A number of DA metabolic pathways exist that lead to the generation of hydroxyl radicals (Fig. 10). DA is susceptible to autoxidation when the antioxidant defense system is weak (300). Moreover, the progressive loss of DA triggers an increase in DA turnover in the remaining neurons and facilitates the accumulation of toxic byproducts of DA metabolism. Interestingly, DA-induced toxicity is also mediated through DA actions on the NMDA glutamate receptors (14, 26, 167). There is accumulating evidence that NMDA-mediated excitotoxicity involves free radicals such as superoxide and nitric oxide (42, 197). In fact, antioxidants (e.g., ascorbate and α-tocopherol) protect neurons against glutamate neurotoxicity (39, 149).
FIG. 10.
Autooxidation and oxidative deamination of dopamine. In the presence of Mn, the auto-oxidation of dopamine produces semiquinones (SQ) and superoxide radicals (O2−•), as well as H2O2, which is readily converted to OH• in the presence of Fe2+. On the other hand, the enzymatic oxidation of dopamine by monoamine oxidase (MAO) can also produce H2O2 and, subsequently, generate the toxic OH•.
Other neurotransmitters, particularly glutamate, can induce other metabolic processes that increase free radical production. Activation of NMDA by glutamate stimulates PLA2 activity to release AA to act as a second messenger, which in turn can lead to the formation of free radicals (106). Decreased availability of AA, due either to increased PLA2 activity or lipid peroxidation, can lead to impaired glutamatergic neurotransmission, which has been proposed as a pathogenetic mechanism in SZ (29, 190). A dopamine-glutamate imbalance has also been implicated in SZ (30). Neuroleptic drugs that block dopamine receptors may also enhance the glutamatergic neurotransmission.
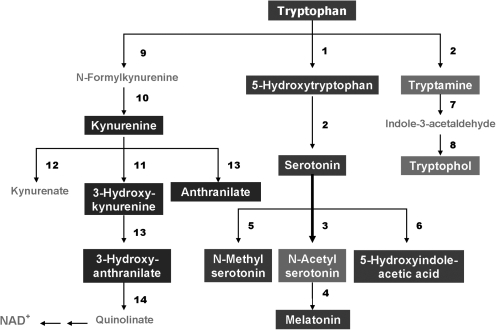
Recently, using a targeted electrochemistry-based metabolomics platform, we have compared metabolic signatures consisting of 13 plasma tryptophan-metabolites simultaneously (Fig. 11) between FENNS patients and healthy controls, and between FENNS at baseline (BL) and 4 weeks after antipsychotic treatment (283). It was noted that conversion of serotonin to N-acetylserotonin by serotonin N-acetyltransferase may be upregulated in FENNS patients, possibly related to the observed alteration in tryptophan and 5-hydroxyindoleacetic acid correlation. Considering N-acetylserotonin as a potent antioxidant (79, 274), such increases in N-acetylserotonin might be a compensatory response to increased oxidative stress, implicated in the pathogenesis of SZ.
FIG. 11.
Tryptophan metabolic pathways. The bolded arrow indicates that pathway may be upregulated in first-episode neuroleptic-naïve patients with SZ. 1, tryptophan hydroxylase; 2, aromatic L-amino acid decarboxylase; 3, serotonin N-acetyltransferase; 4, 5-hydroxyindole-O-methyltransferase; 5, serotonin N-methyltransferase; 6, monoamine oxidase and aldehyde dehydrogenase; 7, monoamine oxidase; 8, alcohol dehydrogenase; 9, tryptophan 2,3-dioxygenase; 10, formamidase; 11, kynurenine 3-monooxygenase; 12, kynurenine transaminase; 13, kynureninase; 14, 3-hydroxyanthranilate oxigenase. Reprinted with permission from Yao et al. (283).
Clinical Implications
Since the pathophysiology in SZ still remains unclear, understanding the clinical implications of oxidative stress and membrane deficits is also limited. Many of the previously unexplained clinical characteristics of SZ, including high pain tolerance, differences in severity and prognosis across different countries (due to dietary variations), the inverse relationship between SZ and some inflammatory disorders, and the remission of symptoms that occur during high fever, may be explained by membrane phospholipid abnormalities (100, 236). However, to directly link the observed AODS defects with SZ pathogenesis, it is necessary to establish a relationship between the traditional positive/negative symptoms of the disease and the noted aberrations in AODS. While preliminary evidence in this regard has been admittedly correlative, such associations between altered AODS indices and clinical symptoms may provide the building blocks for determining causal relationships and developing novel treatment options.
To date, several laboratories have demonstrated associations between AODS enzymes and symptom severity. In platelets, Buckman et al. (25) found that GSH-Px activity was inversely correlated with computed tomographic scan measures of brain atrophy in patients with chronic SZ, specifically nonparanoid SZ with a predominance of negative symptoms. Positive symptoms, however, have been correlated with SOD activity (129). Decreased SOD activity is associated with impaired premorbid school functioning (177) and tardive dyskinesia (278).
Systematic evaluation of relations between three key scavenging antioxidant enzymes and various psychosis rating scales in drug-free schizophrenic patient groups, both RBC and plasma GSH-Px, was found to be significantly and positively correlated to the Bunney-Hamburg psychosis ratings (BHPR) (288, 293). Such a correlation was present in patients both on and off haloperidol treatment. On the other hand, RBC-SOD activities were inversely and significantly correlated with the BHPR or the Scale for the Assessment of Negative Symptoms (SANS) scores. Later, Zhang et al. (305) also demonstrated that blood SOD levels were positively correlated with the Brief Psychiatric Rating Scale (BPRS) and Scale for the Assessment of Positive Symptoms (SAPS) total scores in SZ patients.
In addition, significant inverse correlations between plasma TAS and the SANS were demonstrated in drug-free schizophrenic patients (259, 291). A similar inverse correlation between plasma uric acid and BHPR scale was also demonstrated in schizophrenic patients both on and off haloperidol treatment (289). Moreover, plasma bilirubin levels were significantly lower in the negative subgroup of the SZ patient group (194), whereas SZ patients with higher levels of plasma bilirubin showed higher scores on the positive subscales of the Positive and Negative Syndrome Scale (172).
Recently, Zhang et al. (303) have demonstrated that plasma MDA levels were positively correlated with Abnormal Involuntary Movement Scale total score and the SANS in SZ patients with tardive dyskinesia. In addition, serum thioredoxin was significantly correlated with SAPS (301), and plasma NO levels were negatively correlated with SANS in SZ patients (184). Taken together, above findings suggest that alterations in AODS enzymes and antioxidant status may be a related to changed symptom severity rather than being direct effects of antipsychotic drug treatment, and further suggest the possibility of therapeutic approaches using currently available treatments (215).
Summary and Future Directions
SZ is a major mental disorder without a clearly identified pathophysiology. A number of putative mechanisms have been proposed to explain the etiopathogenesis and illness presentation of SZ, including abnormal neuronal development, impaired neurotransmission, viral infections in utero, autoimmune dysfunction, and many others. Extensive, though fragmentary, findings from neurochemical and neuroendocrine studies of SZ have not provided conclusive evidence for any specific etiologic theory of SZ, perhaps due to etiopathogenetic heterogeneity. However, there exists a point of convergence for many of these theoretical models, one that occurs at the level of the neuronal membrane, which is the site of neurotransmitter receptors, ion channels, signal transduction, and drug effects. Membrane deficits, specifically free radical-mediated, can significantly alter a broad range of membrane functions. There is abundant evidence that alterations in key neurotransmitters can both be modified by and contribute to oxidative stress and membrane dysfunction, suggesting a link among oxidative stress, membrane dysfunction, and multineurotransmitter pathologies in SZ (59, 236, 282).
Research into the pathophysiology of SZ is replete with findings across a large variety of discrete biological systems that in many instances are replicable. It must be assumed that these findings in multiple systems in the same disorder are likely linked to each other in meaningful ways, and explicating such relationships will permit a more accurate understanding of the dynamics of the underlying pathology. In this review, defects in the antioxidant system, membrane composition, immune response, and neurotransmission were reported in SZ patients. A recent study by Kale et al. (118) reported reduced levels of folic acid and vitamin B12, and increased levels of homocysteine and cortical in never-medicated SZ patients, further indicating an altered one-carbon metabolism in SZ. Conceptually, these abnormalities can be linked (Fig. 12) but until now there has been no practical way of simultaneously examining these discrete, but interrelated, large systems. Therefore, future studies should focus to identify candidate pathological process(es) that account for the complex constellation of clinical and biological features in SZ.
FIG. 12.
Antioxidant defense system involving multiple biochemical pathways. It is surmised that some, or all, of these alterations in purine catabolism, neurotransmission, glutathione redox coupling, glucose phosphorylation, one-carbon metabolism, and nitric oxide synthase activation may contribute to oxidative stress and membrane dysfunction in SZ. Reprinted with permission from Yao and Reddy (281).
Novel, powerful, and rapid multidimensional separation and characterization methods, for example, high-pressure liquid chromatography coupled with a 16-channel coulometric multielectrode array system (Fig. 13), can lead to revolutionary changes in our understanding at the molecular level (114, 135, 225, 280, 281). The resolving power of these methods is superior to one-dimensional approaches, enabling the comprehensive metabolic analyses particularly in the targeted biochemical pathways. A metabolomic analytic approach has the potential to yield valuable insights into the likely complex pathophysiological mechanisms that affect multiple metabolic pathways (283, 284), and thereby offer multiple windows of therapeutic opportunities. Examples of such interventions include dietary supplementation with a combination of n-3 PUFAs and antioxidants for treatment of SZ patients, particularly in the early stages of illness (59, 155), and in reducing the risk of progression to psychotic disorder in patients with prodromal symptoms (4), and the use of N-acetyl cysteine, a GSH precursor and antioxidant, in treatment of negative symptoms of SZ. Clearly, the redox signaling hypothesis of SZ offers unique ways of thinking out of the box to develop novel, hypothesis-driven interventions to treat this highly disabling disease.
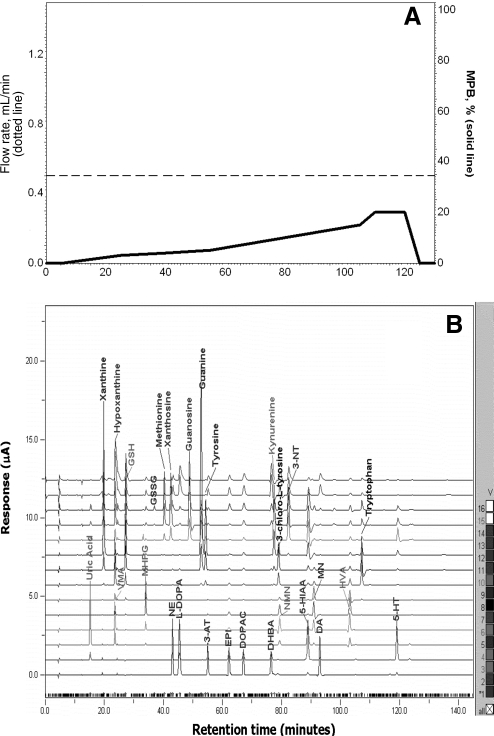
FIG. 13.
Separation of low-molecular-weight, redox-active compounds by high-pressure liquid chromatography coupled with a Coulometric Multielectrode Array System. (A) Flow rate and mobile phase gradient profile; (B) 16-channel chromatograms obtained by separation of standard mixture in a single column (ESA Meta-250, 5 μm ODS, 250 × 4.6 mm ID) under a 150-min gradient elution that ranged from 0% to 20% MPB with a fixed flow rate of 0.5 ml/min (A). The temperature of both cells and column was maintained at 25°C. The Coulometric Multielectrode Array System was set to have increments from 0 to 900 mV in 60 mV steps. Reprinted with permission from Yao and Reddy (281).
Abbreviations Used
- 2-AG
2-arachidonoyl glycerol
- AA
arachidonic acid
- AODS
antioxidant defense system
- ATP
adenosine triphosphate
- BHPR
Bunney-Hamburg psychosis ratings
- CAT
catalase
- CSF
cerebrospinal fluid
- DA
deaminase
- DAG
diacylglycerol
- DHA
docosahexaenoic acid
- eNOS
endothelial nitric oxide synthase
- FENNS
first-episode neuroleptic-naïve patient with schizophrenia
- GAP
growth-associated protein
- GSH
glutathione
- GSH-Px
glutathione peroxidase
- GSH-R
glutathione reductase
- GSSG
oxidized glutathione
- H2O2
hydrogen peroxide
- Hx
hypoxanthine
- IL
interleukin
- iNOS
inducible nitric oxide synthase
- IPPs
inositol polyphosphates
- MAO
monoamine oxidase
- MDA
malondialdehyde
- MRS
magnetic resonance spectroscopy
- NMDA
N-methyl-d-aspartate
- nNOS
neuronal nitric oxide synthase
- NO
nitric oxide
- NOS
nitric oxide synthase
- OH•
hydroxyl radical
- PC
phosphatidylcholine
- PCr
phosphocreatine
- PDEs
phosphodiesters
- PE
phosphatidylethanolamine
- Pi
inorganic phosphate
- PI
phosphatidylinositol
- PLA2
phospholipase A2
- PLC
phosphorlipase C
- PMEs
phosphmonoesters
- PS
phosphatidylserine
- PUFAs
polyunsaturated fatty acids
- RBC
red blood cell
- ROS
reactive oxygen species
- SANS
Scale for the Assessment of Negative Symptoms
- SOD
superoxide dismutase
- SZ
schizophrenia
- TAS
total antioxidant status
- UA
uric acid
- Xan
xanthine
- Xant
xanthosine
- XO
xanthine oxidase
Acknowledgments
This material is based upon work supported in part by the grants from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory R&D (Merit Reviews and Senior Research Career Scientist Award), VA Pittsburgh Healthcare System, and National Institute of Health [MH58141 (J.K.Y.), and MH45203 (M.S.K.)]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the article. The contents of this article do not represent the views of the Department of Veterans Affairs or the U.S. Government.
References
- 1.Abdalla DS. Manteiro HP. Olivera JA. Bechara EJ. Activities of superoxide dismutase and glutathione peroxidase in schizophrenic and manic depressive patients. Clin Chem. 1986;32:805–807. [PubMed] [Google Scholar]
- 2.Akyol O. Zoroglu S. Armutcu F. Sahin S. Gurel A. Nitric oxide as a physiopathological factor in neuropsychiatric disorders. In Vivo. 2004;18:377–390. [PubMed] [Google Scholar]
- 3.Al-Chalabi BM. Thanoon IA. Ahmed FA. Potential effect of olanzapine on total antioxidant status and lipid peroxidation in schizophrenic patients. Neuropsychobiology. 2009;59:8–11. doi: 10.1159/000202823. [DOI] [PubMed] [Google Scholar]
- 4.Amminger GP. Schäfer MR. Papageorgiou K. Klier CM. Cotton SM. Harrigan SM. Mackinnon A. McGorry PD. Berger GE. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Arch Gen Psychiatry. 2010;67:146–154. doi: 10.1001/archgenpsychiatry.2009.192. [DOI] [PubMed] [Google Scholar]
- 5.Anderson ME. Glutathione: an overview of biosynthesis and modulation. Chem Biol Interact. 1998;111–112:1–14. doi: 10.1016/s0009-2797(97)00146-4. [DOI] [PubMed] [Google Scholar]
- 6.Arolt A. Rothermund M. Wandinger KP. Kirchner H. Decreased in vitro production of interferon-gamma and interleukin-2 in whole blood of patients with schizophrenia during treatment. Mol Psychiatry. 2000;5:150–158. doi: 10.1038/sj.mp.4000650. [DOI] [PubMed] [Google Scholar]
- 7.Arvindakshan M. Sitasawad S. Debsikdar V. Ghate M. Evans D. Horrobin DF. Bennett C. Ranjekar PK. Mahadik SP. Essential polyunsaturated fatty acid and lipid peroxide levels in never-medicated and medicated schizophrenia patients. Biol Psychiatry. 2003;53:56–64. doi: 10.1016/s0006-3223(02)01443-9. [DOI] [PubMed] [Google Scholar]
- 8.Awad JA. Morrow JD. Takahashi K. Roberts II LJ. Identification of non-cyclooxygenase-derived prostanoid (F2- isoprostane) metabolites in human urine and plasma. J Biol Chem. 1993;268:4161–4169. [PubMed] [Google Scholar]
- 9.Baba H. Suzuki T. Arai H. Emson PC. Expression of nNOS and soluble guanylate cyclase in schizophrenic brain. Neuroreport. 2004;15:677–680. doi: 10.1097/00001756-200403220-00020. [DOI] [PubMed] [Google Scholar]
- 10.Banks RE. Measurement of cytokines in clinical samples using immunoassays: problems and pitfalls. Crit Rev Clin Lab Sci. 2000;37:131–182. doi: 10.1080/10408360091174187. [DOI] [PubMed] [Google Scholar]
- 11.Bartosz G. Commentary on: free radicals and the developmental pathology of schizophrenic burnout. Integr Psychiatry. 1987;5:43–44. [Google Scholar]
- 12.Becker BF. Towards the physiological function of uric acid. Free Radical Biol Med. 1993;14:615–631. doi: 10.1016/0891-5849(93)90143-i. [DOI] [PubMed] [Google Scholar]
- 13.Ben-Shachar D. Laifenfeld D. Mitochondria, synaptic plasticity, and schizophrenia. Int Rev Neurobiol. 2004;59:273–296. doi: 10.1016/S0074-7742(04)59011-6. [DOI] [PubMed] [Google Scholar]
- 14.Berger GE. Wood SJ. Wellard RM. Proffitt TM. McConchie M. Amminger GP. Jackson GD. Velakoulis D. Pantelis C. McGorry PD. Ethyl-eicosapentaenoic acid in first-episode psychosis. A 1H-MRS study. Neuropsychopharmacology. 2008;33:2467–2473. doi: 10.1038/sj.npp.1301628. [DOI] [PubMed] [Google Scholar]
- 15.Berk M. Copolov D. Dean O. Lu K. Jeavons S. Schapkaitz I. Anderson-Hunt M. Judd F. Katz F. Katz P. Ording-Jespersen S. Little J. Conus P. Cuenod M. Do KQ. Bush AI. N-acetyl cysteine as a glutathione precursor for schizophrenia—a double-blind, randomized, placebo-controlled trial. Biol Psychiatry. 2008;64:361–368. doi: 10.1016/j.biopsych.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Bernstein HG. Heinemann A. Krell D. Dobrowolny H. Bielau H. Keilhoff G. Bogerts B. Hypothalamic nitric oxide synthase in affective disorder: focus on the suprachiasmatic nucleus. Cell Mol Biol (Noisy-le-grand) 2005;51:279–284. [PubMed] [Google Scholar]
- 17.Bessler H. Levental Z. Karp L. Modai I. Djaldetti M. Weizman A. Cytokine production in drug-free and neuroleptic-treated schizophrenic patients. Biol Psychiatry. 1995;38:297–302. doi: 10.1016/0006-3223(94)00299-I. [DOI] [PubMed] [Google Scholar]
- 18.Blum-Degen D. Heinemann T. Lan J. Pedersen V. Leblhuber F. Paulus W. Riederer P. Gerlach M. Characterization and regional distribution of nitric oxide synthase in the human brain during normal ageing. Brain Res. 1999;834:128–135. doi: 10.1016/s0006-8993(99)01444-4. [DOI] [PubMed] [Google Scholar]
- 19.Bockelmann R. Wolf G. Ransmayr P. Riederer P. NADPH-diaphorase/nitric oxide synthase containing neurons in normal and Parkinson's disease putamen. J Neural Transm. 1994;7:115–121. doi: 10.1007/BF02260966. [DOI] [PubMed] [Google Scholar]
- 20.Bogdanov M. Matson WR. Wang L. Matson T. Saunders-Pullman R. Bressman SS. Flint Beal M. Metabolomic profiling to develop blood biomarkers for Parkinson's disease. Brain. 2008;131:389–396. doi: 10.1093/brain/awm304. [DOI] [PubMed] [Google Scholar]
- 21.Bos MJ. Koudstaal PJ. Hofman A. Witteman JC. Breteler MM. Uric acid is a risk factor for myocardial infarction and stroke: the Rotterdam study. Stroke. 2006;37:1503–1507. doi: 10.1161/01.STR.0000221716.55088.d4. [DOI] [PubMed] [Google Scholar]
- 22.Bredt DS. Snyder SH. Nitric oxide, a novel neuronal messenger. Neuron. 1992;8:3–11. doi: 10.1016/0896-6273(92)90104-l. [DOI] [PubMed] [Google Scholar]
- 23.Brown K. Reid A. White T. Henderson T. Hukin S. Johnstone C. Glen A. Vitamin E, lipids, lipid peroxidation products and tardive dyskinesia. Biol Psychiatry. 1998;43:863–867. doi: 10.1016/s0006-3223(97)00197-2. [DOI] [PubMed] [Google Scholar]
- 24.Buchsbaum MS. Neuchterlein KH. Haier RJ. Wu J. Sicotte N. Hazlett E. Asarnow R. Potkin S. Guich S. Glucose metabolic rate in normals and schizophrenics during the continuous performance test assessed by Positron Emission Tomography. Br J Psychiatry. 1990;156:217–227. doi: 10.1192/bjp.156.2.216. [DOI] [PubMed] [Google Scholar]
- 25.Buckman TD. Kling A. Sutphin MS. Steinberg A. Eiduson S. Platelet glutathione peroxidase and monoamine oxidase activity in schizophrenics with CT scan abnormalities: relation to psychosocial variables. Psychiatry Res. 1990;31:1–14. doi: 10.1016/0165-1781(90)90103-c. [DOI] [PubMed] [Google Scholar]
- 26.Cadet JL. Kahler LA. Free radical mechanisms in schizophrenia and tardive dyskinesia. Neurosc Biobehav Rev. 1994;18:457–467. doi: 10.1016/0149-7634(94)90001-9. [DOI] [PubMed] [Google Scholar]
- 27.Cadet JL. Lohr JB. Free radicals and the developmental pathology of schizophrenic burnout. Integr Psychiatry. 1987;5:40–48. [Google Scholar]
- 28.Calder PC. Immunoregulatory and anti-inflammatory effects of n-3 polyunsaturated fatty acids. Braz J Med Biol Res. 1998;31:467–490. doi: 10.1590/s0100-879x1998000400002. [DOI] [PubMed] [Google Scholar]
- 29.Carlsson M. Carlsson A. Interactions between glutamatergic and monoaminergic systems within the basal ganglia—implications for schizophrenia and Parkinson's disease. Trends Neurosci. 1990;13:272–276. doi: 10.1016/0166-2236(90)90108-m. [DOI] [PubMed] [Google Scholar]
- 30.Carlsson M. Carlsson A. Schizophrenia a subcortical neurotransmitter imbalance syndrome? Schizophr Bull. 1990;16:425–432. doi: 10.1093/schbul/16.3.425. [DOI] [PubMed] [Google Scholar]
- 31.Carpenter WT., Jr. Buchanan RW. Schizophrenia. N Engl J Med. 1994;330:681–690. doi: 10.1056/NEJM199403103301006. [DOI] [PubMed] [Google Scholar]
- 32.Cazzullo CL. Scarone S. Grassi B. Vismara C. Trabattoni D. Clerici M. Clerici M. Cytokines production in chronic schizophrenia patients with or without paranoid behavior. Prog Neuro-Psychopharmacol Biol Psychiatry. 1998;22:947–957. doi: 10.1016/s0278-5846(98)00059-1. [DOI] [PubMed] [Google Scholar]
- 33.Chan PH. Fishman RA. Transient formation of superoxide radicals in polyunsaturated fatty acid-induced brain swelling. J Neurochem. 1980;35:1004–1007. doi: 10.1111/j.1471-4159.1980.tb07100.x. [DOI] [PubMed] [Google Scholar]
- 34.Chan PH. Fishman RA. Longar S. Chen S. Yu A. Cellular and molecular effects of polyunsaturated fatty acids in brain ischemia and injury. Prog Brain Res. 1985;63:227–235. doi: 10.1016/S0079-6123(08)61986-X. [DOI] [PubMed] [Google Scholar]
- 35.Chen DC. Xiu MH. Liu H. Zhang BS. Wang Y. Kosten TR. Zhang XY. Reduced status of plasma total antioxidant capacity in schizophrenia with tardive dyskinesia. J Psychiat Res. 2010;44:1111–1112. doi: 10.1016/j.jpsychires.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 36.Choi HK. Mount DB. Reginato AM. Pathogenesis of gout. Ann Intern Med. 2005;143:499–516. doi: 10.7326/0003-4819-143-7-200510040-00009. [DOI] [PubMed] [Google Scholar]
- 37.Chow KC. Vitamin E and cigarette smoking-induced oxidative damage. In: Packer L, editor; Fuchs J, editor. Vitamin E in Health and Disease. New York: Marcel Dekker, Inc.; 1992. pp. 683–697. [Google Scholar]
- 38.Church WH. Ward VL. Uric acid is reduced in the substantia nigra in Parkinson's disease: effect on dopamine oxidation. Brain Res Bull. 1994;33:419–425. doi: 10.1016/0361-9230(94)90285-2. [DOI] [PubMed] [Google Scholar]
- 39.Ciani E. Groneng L. Voltattorni M. Rolseth V. Contestabile A. Paulsen RE. Inhibition of free radical production or free radical scavenging protects from excitatoxic cell death mediated by gutamate in cultures of cerebellar granule cells. Brain Res. 1996;728:1–6. [PubMed] [Google Scholar]
- 40.Cohen G. Enzymatic/nonenzymatic sources of oxyradicals and regulation of antiodant defences. Ann N Y Acad Sci. 1994;738:8–14. doi: 10.1111/j.1749-6632.1994.tb21784.x. [DOI] [PubMed] [Google Scholar]
- 41.Cory JG. Purine and pyrimidine nucleotide metabolism. In: Devlin TM, editor. Text Book of Biochemistry with Clinical Correlations. New York: John Wiley & Sons; 1982. pp. 489–528. [Google Scholar]
- 42.Coyle JT. Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262:689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- 43.Dakhale G. Khanzode S. Khanzode S. Saoji A. Khobragade L. Turankar A. Oxidative damage and schizophrenia: the potential benefit by atypical antipsychotics. Neuropsychobiology. 2004;49:205–209. doi: 10.1159/000077368. [DOI] [PubMed] [Google Scholar]
- 44.Dakhale GN. Khanzode SD. Khanzode SS. Saoji A. Supplementation of vitamin C with atypical antipsychotics reduces oxidative stress and improves the outcome of schizophrenia. Psychopharmacology (Berl) 2005;182:494–498. doi: 10.1007/s00213-005-0117-1. [DOI] [PubMed] [Google Scholar]
- 45.Das I. Khan NS. Puri BK. Sooranna SR. de Belleroche J. Hirsch SR. Elevated platelet calcium mobilization and nitric oxide synthase activity may reflect abnormalities in schizophrenic brain. Biochem Biophys Res Commun. 1995;212:375–380. doi: 10.1006/bbrc.1995.1980. [DOI] [PubMed] [Google Scholar]
- 46.Davies KJ. Sevanian A. Muakkassah-Kelly SF. Hochstein P. Uric acid-iron ion complexes. A new aspect of the antioxidant functions of uric acid. Biochem J. 1986;235:747–754. doi: 10.1042/bj2350747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deicken RF. Calabrese G. Merrin EL. Meyerhoff DJ. Dillon WP. Weine MW. Fein G. 31Phosphorus magnetic resonance spectroscopy of the frontal and parietal lobes in chronic schizophrenia. Biol Psychiatry. 1994;34:503–510. doi: 10.1016/0006-3223(94)90613-0. [DOI] [PubMed] [Google Scholar]
- 48.Demisch L. Heinz K. Gerbaldo H. Kirsten R. Increased concentrations of phosphatidylinositol and decreased esterification of arachidonic acid into phospholipids in platelets from patients with schizoaffective disorders or atypic phasic psychoses. Prostaglandins Leukot. Essent Fatty Acids. 1992;46:47–52. doi: 10.1016/0952-3278(92)90058-q. [DOI] [PubMed] [Google Scholar]
- 49.Dietrich-Muszalska A. Olas B. Modifications of blood platelet proteins of patients with schizophrenia. Platelets. 2009;20:90–96. doi: 10.1080/09537100802641499. [DOI] [PubMed] [Google Scholar]
- 50.Dietrich-Muszalska A. Olas B. Rabe-Jablonska J. Oxidative stress in blood platelets from schizophrenic patients. Platelets. 2005;16:386–391. doi: 10.1080/09537100500128872. [DOI] [PubMed] [Google Scholar]
- 51.Dietrich-Muszalska A. Olas B. Glowacki R. Bald E. Oxidative/nitrative modifications of plasma proteins and thiols from patients with schizophrenia. Neuropsychobiology. 2009;59:1–7. doi: 10.1159/000202822. [DOI] [PubMed] [Google Scholar]
- 52.Dietrich-Muszalska A. Olas B. Isoprostenes as indicators of oxidative stress in schizophrenia. World J Biol Psychiatry. 2009;10:27–33. doi: 10.1080/15622970701361263. [DOI] [PubMed] [Google Scholar]
- 53.Do KQ. Cabungcal JH. Frank A. Steullet P. Cuenod M. Redox dysregulation, neurodevelopment, and schizophrenia. Curr Opin Neurobiol. 2009;19:220–230. doi: 10.1016/j.conb.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 54.Do KQ. Trabesinger AH. Kirsten-Krüger M. Lauer CJ. Dydak U. Hell D. Holsboer F. Boesiger P. Cuénod M. Schizophrenia: glutathione deficit in cerebrospinal fluid and prefrontal cortex in vivo. Eur J Neurosci. 2000;12:3721–3728. doi: 10.1046/j.1460-9568.2000.00229.x. [DOI] [PubMed] [Google Scholar]
- 55.Doyle CA. Slater P. Application of [3H]L-N(G)-nitro-arginine labelling to measure cerebellar nitric oxide synthase in patients with schizophrenia. Neurosci Lett. 1995;202:49–52. doi: 10.1016/0304-3940(95)12196-x. [DOI] [PubMed] [Google Scholar]
- 56.Droge W. Mihm S. Bockstette M. Roth S. Effect of reactive oxygen intermediates and antioxidants on proliferation and function of T lymphocytes. Methods Enzymol. 1994;234:135–151. doi: 10.1016/0076-6879(94)34084-6. [DOI] [PubMed] [Google Scholar]
- 57.Droge W. Free radicals in the physiological control of cell function. Pysiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 58.Droge W. The plasma redox state and ageing. Ageing Res Rev. 2002;1:257–278. doi: 10.1016/s1568-1637(01)00008-3. [DOI] [PubMed] [Google Scholar]
- 59.du Bois TM. Deng C. Huang XF. Membrane phospholipid composition, alterations in neurotransmitter systems and schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:878–888. doi: 10.1016/j.pnpbp.2005.04.034. [DOI] [PubMed] [Google Scholar]
- 60.Du Y. Chen CP. Tseng CY. Eisenberg Y. Firestein BL. Astroglia-mediated effects of uric acid to protect spinal cord neurons from glutamate toxicity. Glia. 2007;55:463–472. doi: 10.1002/glia.20472. [DOI] [PubMed] [Google Scholar]
- 61.Endres S. Meydani SN. Ghrobani E. Schindler R. Dinarello CA. Dietary supplementation with n-3 fatty acids suppresses interleukin-2 production and mononuclear cell proliferation. J Leukoc Biol. 1993;54:599–603. [PubMed] [Google Scholar]
- 62.Ernster L. Lipid peroxidation in biological membranes mechanisms and implications. In: Yagi K, editor. Active Oxygens, Lipid Peroxides, and Antioxidants. New York, NY: CRC Press; 1993. pp. 1–38. [Google Scholar]
- 63.Espersen GT. Grunnet N. Lervang HN. Nielsen GL. Thomsen BS. Faarvang KL, et al. Decreased interleukin-1 beta levels in plasma from rheumatoid arthritis patients after dietary supplementation with n-3 polyunsaturated fatty acids. Clin Rheumatol. 1992;11:393–395. doi: 10.1007/BF02207200. [DOI] [PubMed] [Google Scholar]
- 64.Essali MA. Das I. Belleroche J. Hirsch SR. The platelet phosphoinositide system in schizophrenia: the effects of neuroleptic treatment. Biol Psychiatry. 1990;28:475–487. doi: 10.1016/0006-3223(90)90481-g. [DOI] [PubMed] [Google Scholar]
- 65.Evans DR. Puczkovski PY. Brandsma MJ. Mahadik JS. Mahadik SP. Elevated plasma lipid peroxides in schizophrenic patients without dementia. Biol Psychiatry. 1996;39:588. [Google Scholar]
- 66.Fendri C. Mechri A. Khiari G. Othman A. Kerkeni A. Gaha L. Oxidative stress involvement in schizophrenia pathophysiology: a review. Encephale. 2006;32:244–252. doi: 10.1016/s0013-7006(06)76151-6. [DOI] [PubMed] [Google Scholar]
- 67.Ferger B. Spratt C. Earl CD. Teismann P. Oertel WH. Kuschinsky K. Effects of nicotine on hydroxyl free radical formation in vitro and on MPTP-induced neurotoxicity in vivo. Naunyn Schmiedebergs Arch Pharmacol. 1998;358:351–359. doi: 10.1007/pl00005264. [DOI] [PubMed] [Google Scholar]
- 68.Fernandez-Egea E. Bernardo M. Heaphy CM. Griffith JK. Parellada E. Esmatjes E. Conget I. Nguyen L. George V. Stöppler H. Kirkpatrick B. Telomere length and pulse pressure in newly diagnosed, antipsychotic-naive patients with nonaffective psychosis. Schizophr Bull. 2009;35:437–442. doi: 10.1093/schbul/sbn169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Floyd RA. Mitochondrial damage in neurodegenerative disease. In: Packer L, editor; Hiramatsu M, editor; Yoshikawa T, editor. Free Radicals in Brain Physiology and Disorders. San Diego, CA: Academic Press; 1996. pp. 313–329. [Google Scholar]
- 70.Forstermann U. Schmidt HHHW. Pollock J. Sheng H. Mitchell JA. Warner TD. Nakane M. Murad F. Isoforms of nitric oxide synthase: characterization and purification from different cell types. Biochem Pharmacology. 1991;42:1849–1857. doi: 10.1016/0006-2952(91)90581-o. [DOI] [PubMed] [Google Scholar]
- 71.Frankel EN. Lipid oxidation. Dundee, Scotland: The Oily Press Ltd.; 1998. [Google Scholar]
- 72.Freedman DS. Williamson DF. Gunter EW. Byers T. Relation of serum uric acid to mortality and ischemic heart disease: the NHANES I Epidemiologic Follow-up Study. Am J Epidemiol. 1995;141:637–644. doi: 10.1093/oxfordjournals.aje.a117479. [DOI] [PubMed] [Google Scholar]
- 73.Fukagawa NK. Aging: is oxidative stress a marker or is it causal? Proc Soc Exp Biol Med. 1999;222:293–298. doi: 10.1046/j.1525-1373.1999.d01-146.x. [DOI] [PubMed] [Google Scholar]
- 74.Fukuzako H. Fukuzako T. Takeuchi K. Ohbo Y. Ueyama K. Takigawa M. Fujimoto T. Phosphorus magnetic resonance spectroscopy in schizophrenia: correlation between membrane phospholipid metabolism in temporal lobe and positive symptoms. Prog Neuro-Psychopharmacol Biol Psychiatry. 1996;20:629–640. doi: 10.1016/0278-5846(96)00036-x. [DOI] [PubMed] [Google Scholar]
- 75.Gama CS. Salvador M. Andreazza AC. Kapczinski F. Belmonte-de-Abreu PS. Elevated serum superoxide dismutase and thiobarbituric acid reactive substances in schizophrenia: a study of patients treated with haloperidol or clozapine. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:512–515. doi: 10.1016/j.pnpbp.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 76.Ganguili R. Yang Z. Shurin G. Chengappa R. Brar JS. Gubbi AV. Rabin BS. Serum interleukin-6 concentration in schizophrenia: elevation associated with duration of illness. Psychiatry Res. 1994;51:1–10. doi: 10.1016/0165-1781(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 77.Gattaz WF. Schmitt A. Maras A. Increased platelet phospholipase A2 activity in schizophrenia. Schizophr Res. 1995;16:1–6. doi: 10.1016/0920-9964(94)00060-l. [DOI] [PubMed] [Google Scholar]
- 78.Gaughran F. Immunity and schizophrenia: autoimmunity, cytokines, and immune responses. Int Rev Neurobiol. 2002;52:275–302. doi: 10.1016/s0074-7742(02)52013-4. [DOI] [PubMed] [Google Scholar]
- 79.Gavazza MB. Català A. Protective effect of N-acetyl-serotonin on the nonenzymatic lipid peroxidation in rat testicular microsomes and mitochondria. J Pineal Res. 2004;37:153–160. doi: 10.1111/j.1600-079X.2004.00150.x. [DOI] [PubMed] [Google Scholar]
- 80.Gegg ME. Beltran B. Salas-Pino S. Bolanos JP. Clark JB. Moncada S. Heales SJR. Differential effect of nitric oxide on glutathione metabolism and mitochondrial function in astrocytes and neurones: implications for neuroprotection/neurodegeneration? J Neurochem. 2003;86:228–237. doi: 10.1046/j.1471-4159.2003.01821.x. [DOI] [PubMed] [Google Scholar]
- 81.Gerlach M. Blum-Degen D. Lan J. Riederer P. Nitric oxide in the pathogenesis of Parkinson's disease. Adv Neurol. 1999;80:239–245. [PubMed] [Google Scholar]
- 82.Gibson RA. McMurchie EJ. Charnok JS. Kneebone GM. Homeostatic control of membrane fatty acid composition in the rat after dietary lipid treatment. Lipids. 1984;19:942–951. doi: 10.1007/BF02534730. [DOI] [PubMed] [Google Scholar]
- 83.Glen AIM. Glen EMT. Horrobin DF. Vaddadi KS. Spellman M. Morse-Fisher N. Ellis K. Skinner FS. A red cell membrane abnormality in a sub-group of schizophrenic patients: evidence for two diseases. Schizophr Res. 1994;12:53–61. doi: 10.1016/0920-9964(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 84.Golse B. Debray-Ritzen P. Puget K. Michelson AM. Analysis of platelet superoxide dismutase 1 in the development of childhood psychoses. Nouv Presse Med. 1977;6:2449. [PubMed] [Google Scholar]
- 85.Gora D. Sandhya M. Shiv G. Praveen S. Evaluation of antioxidant deficit in schizophrenia. Indian J Psychiatry. 2008;50:16–20. doi: 10.4103/0019-5545.39753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Grima G. Benz B. Parpura V. Cuenod M. Do KQ. Dopamine-induced oxidative stress in neurons with glutathione deficit: implication for schizophrenia. Schizophr Res. 2003;62:213–224. doi: 10.1016/s0920-9964(02)00405-x. [DOI] [PubMed] [Google Scholar]
- 87.Guerreiro S. Ponceau A. Toulorge D. Martin E. Alvarez-Fischer D. Hirsch EC. Michel PP. Protection of midbrain dopaminergic neurons by the end-product of purine metabolism uric acid: potentiation by low-level depolarization. J Neurochem. 2009;109:1118–1128. doi: 10.1111/j.1471-4159.2009.06040.x. [DOI] [PubMed] [Google Scholar]
- 88.Guliaeva NV. Levshina IP. Obidin AM. Indices of lipid free-radical oxidation and the antiradical protection of the brain-the neurochemical correlates of the development of the general adaptation syndrome. Zh Vyssh Nerv Deiat. 1988;38:731–737. [PubMed] [Google Scholar]
- 89.Gysin R. Kraftsik R. Sandell J. Bovet P. Chappuis C. Conus P. Deppen P. Preisig M. Ruiz V. Steullet P. Tosic M. Werge T. Cuenod M. Do KQ. Impaired glutathione synthesis in schizophrenia: convergent genetic and functional evidence. Proc Natl Acad Sci U S A. 2007;104:16621–16626. doi: 10.1073/pnas.0706778104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Halliwell B. Gutteridge JMC. Oxygen radicals and the central nervous system. TINS. 1984;8:22–26. [Google Scholar]
- 91.Harman D. Aging and disease: extending functional life span. Ann N Y Acad Sci. 1996;786:321–336. doi: 10.1111/j.1749-6632.1996.tb39074.x. [DOI] [PubMed] [Google Scholar]
- 92.Harman D. Aging prospects for future increases in the functional life span. Age. 1996;17:119–146. [Google Scholar]
- 93.Hayashi N. Tashiro T. Yammamori H. Takagi K. Morishima Y. Otsubo Y. Sugiura T. Furukawa K. Nitta H. Nakajama N. Suzuki N. Ito I. Effects of intravenous omega-3 and omega-6 fat emulsion on cytokine production and delayed type hypersensitivity in burned rats receiving total parenteral nutrition. J Parenter Enteral Nutr. 1998;22:363–367. doi: 10.1177/0148607198022006363. [DOI] [PubMed] [Google Scholar]
- 94.Heales SJR. Bolanos JP. Stewart VC. Brookes PS. Land JM. Clark JB. Nitric oxide, mitochondria and neurological disease. Biochim Biophys Acta. 1999;1410:215–228. doi: 10.1016/s0005-2728(98)00168-6. [DOI] [PubMed] [Google Scholar]
- 95.Hehner SP. Breitkreutz R. Shubinsky G. Unsoeld H. Schulze-Osthoff K. Schmitz ML. Droge W. Enhancement of t cell receptor signaling by a mild oxidative shift in the intracellular thiol pool. J Immunology. 2000;165:4319–4328. doi: 10.4049/jimmunol.165.8.4319. [DOI] [PubMed] [Google Scholar]
- 96.Herken H. Uz E. Ozyurt H. Akyol O. Red blood cell nitric oxide levels in patients with schizophrenia. Schizophr Res. 2001;52:289–290. doi: 10.1016/s0920-9964(00)00169-9. [DOI] [PubMed] [Google Scholar]
- 97.Hooper DC. Scott GS. Zborek A. Mikheeva T. Kean RB. Koprowski H. Spitsin SV. Uric acid, a peroxynitrite scavenger, inhibits CNS inflammation, blood-CNS barrier permeability changes, and tissue damage in a mouse model of multiple sclerosis. FASEB J. 2000;14:691–698. doi: 10.1096/fasebj.14.5.691. [DOI] [PubMed] [Google Scholar]
- 98.Horrobin DF. Manku MS. Hillman H. Lain A. Glen M. Fatty acid levels in the brains of schizophrenics and normal controls. Biol Psychiatry. 1991;30:795–805. doi: 10.1016/0006-3223(91)90235-e. [DOI] [PubMed] [Google Scholar]
- 99.Horrobin DF. Niacin flushing, prostaglandin E and evening primrose oil: a possible objective test for monitoring therapy in schizophrenia. J Orthomo Psychiatr. 1980;9:33–34. [Google Scholar]
- 100.Horrobin DF. The membrane phospholipid hypothesis as a biochemical basis for the neurodevelopmental concept of schizophrenia. Schizophr Res. 1998;30:193–208. doi: 10.1016/s0920-9964(97)00151-5. [DOI] [PubMed] [Google Scholar]
- 101.Huang TL. Liou CW. Lin TK. Serum thiobarbituric acid-reactive substances and free thiol levels in schizophrenia patients: effects of antipsychotic drugs. Psychiatry Res. 2010;177:18–21. doi: 10.1016/j.psychres.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 102.Hughes JR. Hatsukami DK. Mitchell JE. Dahlgren LA. Prevalence of smoking among psychiatric outpatients. Am J Psychiatry. 1986;143:993–997. doi: 10.1176/ajp.143.8.993. [DOI] [PubMed] [Google Scholar]
- 103.Hunot S. Boissiere F. Faucheux B. Brugg B. Mouatt-Prigent A. Agid Y. Hirsch EC. Nitric oxide synthase and neuronal vulnerability in Parkinson's disease. Neuroscience. 1996;72:355–363. doi: 10.1016/0306-4522(95)00578-1. [DOI] [PubMed] [Google Scholar]
- 104.Hyden MR. Tyagi SC. Uric acid: a new look at an old risk marker for cardiovascular disease, metabolic syndrome, and type 2 diabetes mellitus: the urate redox shuttle. Nutr Metab (Lond) 2004;1:10. doi: 10.1186/1743-7075-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ignarro LJ. Cirino G. Casini A. Napoli C. Nitric oxide as a signaling molecule in the vascular system: an overview. J Cardiovascular Pharmacology. 1999;34:879–886. doi: 10.1097/00005344-199912000-00016. [DOI] [PubMed] [Google Scholar]
- 106.Iuliano L. Pedersen JZ. Pratico D. Rotilio G. Violi F. Role of hydroxyl radicals in the activation of human platelets. Eur J Biochem. 1994;221:695–704. doi: 10.1111/j.1432-1033.1994.tb18782.x. [DOI] [PubMed] [Google Scholar]
- 107.Jablensky A. Sartorius N. Ernberg G. Anker M. Korten A. Cooper JE. Day R. Bertelsen A. Schizophrenia: manifestations, incidence and course in different cultures. A World Health Organization ten-country study. Psychol Med Monogr Suppl. 1992;20:1–97. doi: 10.1017/s0264180100000904. [DOI] [PubMed] [Google Scholar]
- 108.James MJ. Gibson RA. Cleland LG. Dietary polyunsaturated fatty acids and inflammatory mediator production. Am J Clin Nutr. 2000;71:343S–348S. doi: 10.1093/ajcn/71.1.343s. [DOI] [PubMed] [Google Scholar]
- 109.Janero DR. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radical Biol Med. 1990;9:515–540. doi: 10.1016/0891-5849(90)90131-2. [DOI] [PubMed] [Google Scholar]
- 110.Javitt DC. Laruelle M. Neurochemical theories. In: Lieberman JA, editor; Stroup TS, editor; Perkins DO, editor. Text Book of Schizophrenia. Washington, DC: The American Psychiatric Publishing; 2006. pp. 85–116. [Google Scholar]
- 111.Jha N. Jurma O. Lalli G. Liu Y. Pettus EH. Greenamyre JT. Liu R-M. Forman HJ. Andersen JK. Glutathione depletion in PC12 results in selective inhibition of mitochondrial complex I activity, implications for Parkinson's disease. J Biol Chem. 2000;275:26096–26101. doi: 10.1074/jbc.M000120200. [DOI] [PubMed] [Google Scholar]
- 112.Jossa F. Farinaro E. Panico S. Krogh V. Celentano E. Galasso R. Mancini M. Trevisan M. Serum uric acid and hypertension: the Olivetti heart study. J Hum Hypertens. 1994;8:677–681. [PubMed] [Google Scholar]
- 113.Junn E. Lee NL. Ju HR. Han SH. Im JY. Kang HS. Lee TH. Bae YS. Ha KS. Rhee SG. Choi I. Requirement of hydrogen peroxide generation in TGF-β1 signal transduction in human lung fibroblast cells: involvement of hydrogen peroxide in Ca2 + in TGF-β1-induced IL-6 expression. J Immunology. 2000;165:2190–2197. doi: 10.4049/jimmunol.165.4.2190. [DOI] [PubMed] [Google Scholar]
- 114.Kaddurah-Daouk R. Kristal BS. Weinshilboum RM. Metabolomics: a global biochemical approach to drug response and disease. Ann Rev Pharmacol Toxicol. 2008;48:653–683. doi: 10.1146/annurev.pharmtox.48.113006.094715. [DOI] [PubMed] [Google Scholar]
- 115.Kaiya H. Horrobin DF. Manku MS. Fisher NM. Essential and other fatty acids in plasma in schizophrenics and normal individuals from Japan. Biol Psychiatry. 1991;30:357–362. doi: 10.1016/0006-3223(91)90291-s. [DOI] [PubMed] [Google Scholar]
- 116.Kaiya H. Nishida A. Imai A. Nakashima S. Nozawa Y. Accumulation of diacylglycerol in platelet phosphoinositide turnover in schizophrenia: a biological marker of good prognosis? Biol Psychiatry. 1989;26:669–676. doi: 10.1016/0006-3223(89)90101-7. [DOI] [PubMed] [Google Scholar]
- 117.Kale A. Joshi S. Naphade N. Sapkale S. Raju MS. Pillai A. Nasrallah H. Mahadik SP. Opposite changes in predominantly docosahexaenoic acid (DHA) in cerebrospinal fluid and red blood cells from never-medicated first-episode psychotic patients. Schizophr Res. 2008;98:295–301. doi: 10.1016/j.schres.2007.09.036. [DOI] [PubMed] [Google Scholar]
- 118.Kale A. Naphade N. Sapkale S. Kamaraju M. Pillai A. Joshi S. Mahadik S. Reduced folic acid, vitamin B12 and docosahexaenoic acid and increased homocysteine and cortisol in never-medicated schizophrenia patients: implications for altered one-carbon metabolism. Psychiatry Res. 2010;175:47–53. doi: 10.1016/j.psychres.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 119.Kane JM. Marder SR. Psychopharmacologic treatment of schizophrenia. Schizophr Bull. 1993;19:287–302. doi: 10.1093/schbul/19.2.287. [DOI] [PubMed] [Google Scholar]
- 120.Kang DH. Nakagawa T. Feng L. Watanabe S. Han L. Mazzali M. Truong L. Harris R. Johnson RJ. A role for uric acid in the progression of renal disease. J Am Soc Nephrol. 2002;13:2888–2897. doi: 10.1097/01.asn.0000034910.58454.fd. [DOI] [PubMed] [Google Scholar]
- 121.Kao H-T. Cawthon RM. Delisi LE. Bertisch HC. Ji F. Gordon D. Li P. Benedict MM. Greenberg WM. Porton B. Rapid telomere erosion in schizophrenia. Mol Psychiatry. 2008;13:118–119. doi: 10.1038/sj.mp.4002105. [DOI] [PubMed] [Google Scholar]
- 122.Karson CN. Griffin WS. Mrak RE. Husain M. Dawson TM. Snyder SH. Moore NC. Sturner WQ. Nitric oxide synthase (NOS) in schizophrenia: increases in cerebellar vermis. Mol Chem Neuropathol. 1996;27:275–284. doi: 10.1007/BF02815109. [DOI] [PubMed] [Google Scholar]
- 123.Keelan J. Allen NJ. Antcliffe D. Pal S. Duchen MR. Quantitative imaging of glutathione in hippocampal neurons and glia in culture using monochlorobimane. J Neurosci Res. 2001;66:873–884. doi: 10.1002/jnr.10085. [DOI] [PubMed] [Google Scholar]
- 124.Keith SJ. Regier DA. Rae DS. Schizophrenic disorders. In: Robins LN, editor; Regier D, editor. Psychiatric Disorders in America, the Epidemiologic Catchment Area Study. New York: Free Press; 1991. pp. 33–52. [Google Scholar]
- 125.Keller JN. Kindy MS. Holtsberg FW. St Clair DK. Yen HC. Germeyer A. Steiner SM. Bruce-Keller AJ. Hutchins JB. Mattson MP. Mitochondrial manganese superoxide dismutase prevents neural apoptosis and reduces ischemic brain injury: suppression of peroxynitrite production, lipid peroxidation, and mitochondrial dysfunction. J Neurosci. 1998;18:687–697. doi: 10.1523/JNEUROSCI.18-02-00687.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Keshavan MS. Stanley JA. Montrose DM. Minshew NJ. Pettegrew JW. Prefrontal membrane phospholipid metabolism of child and adolescent offspring at risk for schizophrenia or schizoaffective disorder: an in vivo 31P MRS study. Mol Psychiatry. 2003;8:316–323. doi: 10.1038/sj.mp.4001325. [DOI] [PubMed] [Google Scholar]
- 127.Keshaven MS. Pettegrew JW. Ward R. Are membrane phospholipid changes in schizophrenia familial? Biol Psychiatry. 1993;33:45A. [Google Scholar]
- 128.Khan MM. Evans DR. Gunna V. Scheffer RE. Parikh VV. Mahadik SP. Reduced erythrocyte membrane essential fatty acids and increased lipid peroxides in schizophrenia at the never-medicated first-episode of psychosis and after years of treatment with antipsychotics. Schizophr Res. 2002;58:1–10. doi: 10.1016/s0920-9964(01)00334-6. [DOI] [PubMed] [Google Scholar]
- 129.Khan NS. Das I. Oxidative stress and superoxide dismutase in schizophrenia. Biochem Soc Transact. 1997;25:418S. doi: 10.1042/bst025418s. [DOI] [PubMed] [Google Scholar]
- 130.Kharitonov SA. Robbins RA. Yates D. Keatings V. Barnes PJ. Acute and chronic effects of cigarette smoking on exhaled nitric oxide. Am J Respiratory Critical Care Med. 1995;152:609–612. doi: 10.1164/ajrccm.152.2.7543345. [DOI] [PubMed] [Google Scholar]
- 131.Kim TS. Pae CU. Yoon SJ. Jang WY. Lee NJ. Kim JJ. Lee SJ. Lee C. Paik IH. Lee CU. Decreased plasma antioxidants in patients with Alzheimer's disease. Int J Geriatr Psychiatry. 2006;21:344–348. doi: 10.1002/gps.1469. [DOI] [PubMed] [Google Scholar]
- 132.Kirkpatrick B. Messias E. Harvey PD. Fernandez-Egea E. Bowie CR. Is schizophrenia a syndrome of accelerated aging? Schizophr Bull. 2008;34:1024–1032. doi: 10.1093/schbul/sbm140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Knapp CM. Constantinescu CS. Tan JH. McLean R. Cherryman GR. Gottlob I. Serum uric acid levels in optic neuritis. Mult Scler. 2004;10:278–280. doi: 10.1191/1352458504ms1042oa. [DOI] [PubMed] [Google Scholar]
- 134.Kovaleva ES. Orlov ON. Tsutsul'kovskia MIA. Vladimirova TV. Beliaev BS. Lipid peroxidation processes in patients with schizophrenia. Zh Nevropatol Psikiatr. 1989;89:108–110. [PubMed] [Google Scholar]
- 135.Kristal BS. Shurubor YI. Kaddurah-Daouk R. Matson WR. High-performance liquid chromatography separations coupled with coulometric electrode array detectors: a unique approach to metabolomics. Methods Mol Biol. 2007;358:159–174. doi: 10.1007/978-1-59745-244-1_10. [DOI] [PubMed] [Google Scholar]
- 136.Kristal BS. Vigneau-Callahan KE. Moskowitz AJ. Matson WR. Purine catabolism: links to mitochondrial respiration and antioxidant defenses? Arch Biochem Biophys. 1999;370:22–33. doi: 10.1006/abbi.1999.1387. [DOI] [PubMed] [Google Scholar]
- 137.Kunz M. Gama CS. Andreazza AC. Salvador M. Cereser KM. Gomes FA. Belmonte-de-Abreu PS. Berk M. Kapczinski F. Elevated serum superoxide dismutase and thiobarbituric acid reactive substances in different phases of bipolar disorder and in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1677–1681. doi: 10.1016/j.pnpbp.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 138.Kutzing MK. Firestein BL. Altered uric acid levels and disease states. J Pharm Exp Therapeutics. 2007;324:1–7. doi: 10.1124/jpet.107.129031. [DOI] [PubMed] [Google Scholar]
- 139.Lavoie S. Murray MM. Deppen P. Knyazeva MG. Berk M. Boulat O. Bovet P. Bush AI. Conus P. Copolov D. Fornari E. Meuli R. Solida A. Vianin P. Cuenod M. Buclin T. Do KQ. Glutathione precursor, N-acetyl-cysteine, improves mismatch negativity in schizophrenia patients. Neuropsychopharmacology. 2008;33:2187–2199. doi: 10.1038/sj.npp.1301624. [DOI] [PubMed] [Google Scholar]
- 140.Lee BH. Kim YK. Reduced plasma nitric oxide metabolites before and after antipsychotic treatment in patients with schizophrenia compared to controls. Schizophr Res. 2008;104:36–43. doi: 10.1016/j.schres.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 141.Lieberman JA. Koreen AR. Neurochemistry and neuroendocrinology of schizophrenia, a selective review. Schizophr Bull. 1993;19:371–429. doi: 10.1093/schbul/19.2.371. [DOI] [PubMed] [Google Scholar]
- 142.Linden J. Rosin DL. Purinergic system. In: Siegel GJ, editor; Albers RW, editor; Brady ST, editor; Price DL, editor. Basic Neurochemistry, Molecular, Cellular and Medical Aspects. 7th. Boston: Elsevier; 2006. pp. 303–316. [Google Scholar]
- 143.Linert W. Bridge MH. Huber M. Bjugstad KB. Grossman S. Arendash GW. In vitro and in vivo studies investigating possible antioxidant actions of nicotine: relevance to Parkinson's and Alzheimer's diseases. Biochim Biophys Acta. 1999;1454:143–152. doi: 10.1016/s0925-4439(99)00029-0. [DOI] [PubMed] [Google Scholar]
- 144.Liu F. You SW. Yao LP. Liu HL. Jiao XY. Shi M. Zhao QB. Ju G. Secondary degeneration reduced by inosine after spinal cord injury in rats. Spinal Cord. 2006;44:421–426. doi: 10.1038/sj.sc.3101878. [DOI] [PubMed] [Google Scholar]
- 145.Lohr JB. Flynn K. Smoking and schizophrenia. Schizophr Res. 1992;8:93–102. doi: 10.1016/0920-9964(92)90024-y. [DOI] [PubMed] [Google Scholar]
- 146.Lohr JB. Kuczenski R. Bracha HS. Moir M. Jeste DV. Increased indices of free radical activity in the cerebrospinal fluid of patients with tardive dyskinesia. Biol Psychiatry. 1990;28:535–539. doi: 10.1016/0006-3223(90)90490-s. [DOI] [PubMed] [Google Scholar]
- 147.Lohr JB. Oxygen radicals and neuropsychiatric illness: some speculations. Arch Gen Psychiatry. 1991;48:1097–1106. doi: 10.1001/archpsyc.1991.01810360061009. [DOI] [PubMed] [Google Scholar]
- 148.Los M. Droge W. Stricker K. Baeuerle PA. Schulze-Osthoff K. Hydrogen peroxide as a potent activator of T lymphocyte functions. Eur J Immunol. 1995;25:159–165. doi: 10.1002/eji.1830250127. [DOI] [PubMed] [Google Scholar]
- 149.MacGregor DG. Higgins MJ. Jones PA. Maxwell WL. Watson MW. Graham DI. Stone TW. Ascorbate attenuates the systemic kainate-induced neurotoxicity in the rat hippocampus. Brain Res. 1996;727:133–144. doi: 10.1016/0006-8993(96)00362-9. [DOI] [PubMed] [Google Scholar]
- 150.Mahadik SP. Mukherjee S. Correnti E. Kelkar HS. Wakade CG. Costa RM. Scheffer R. Distribution of plasma membrane phospholipids and cholesterol in skin fibroblasts from drug-naïve patients at the onset of psychosis. Schizophr Res. 1994;13:239–247. doi: 10.1016/0920-9964(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 151.Mahadik SP. Yao JK. Phospholipids in schizophrenia. In: Lieberman JA, editor; Stroup TS, editor; Perkins DO, editor. Textbook of Schizophrenia. Washington, DC: The American Psychiatric Publishing, Inc.; 2006. pp. 117–135. [Google Scholar]
- 152.Mahadik SP. Mukherjee S. Horrobin DF. Jenkins K. Correnti EE. Scheffer RE. Plasma membrane phospholipid fatty acid composition of cultured skin fibroblasts from schizophrenic patients: comparison with bipolar patients and normal subjects. Psychiatry Res. 1996;63:133–142. doi: 10.1016/0165-1781(96)02899-5. [DOI] [PubMed] [Google Scholar]
- 153.Mahadik SP. Mukherjee S. Scheffer R. Correnti EE. Mahadik JS. Elevated plasma lipid peroxides at the onset of nonaffective psychosis. Biol Psychiatry. 1998;43:674–679. doi: 10.1016/s0006-3223(97)00282-5. [DOI] [PubMed] [Google Scholar]
- 154.Mahadik SP. Parikh V. Khan MM. The role of oxidative stress in modulating membrane and phospholipid function in schizophrenia. In: Peet M, editor; Glen I, editor; Horrobin D, editor. Phospholipid Spectrum Disorders in Psychiatry and Neurology. Carnforth, UK: Marius Press; 2003. pp. 277–288. [Google Scholar]
- 155.Mahadik SP. Pillai A. Joshi S. Foster A. Prevention of oxidative stress-mediated neuropathology and improved clinical outcome by adjunctive use of a combination of antioxidants and omega-3 fatty acids in schizophrenia. Int Rev Psychiatry. 2006;18:119–131. doi: 10.1080/09540260600581993. [DOI] [PubMed] [Google Scholar]
- 156.Martins-de-Souza D. Harris LW. Guest PC. Bahn S. The role of energy metabolism dysfunction and oxidative stress in schizophrenia revealed by proteomics. Antioxid Redox Signal. 2011;15:2067–2079. doi: 10.1089/ars.2010.3459. [DOI] [PubMed] [Google Scholar]
- 157.Matsuzawa D. Hashimoto K. Hashimoto T. Shimizu E. Watanabe H. Fujita Y. Lyo M. Association study between the genetic polymorphisms of glutathione-related enzymes and schizophrenia in a Japanese population. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:86–94. doi: 10.1002/ajmg.b.30776. [DOI] [PubMed] [Google Scholar]
- 158.Matsuzawa D. Hashimoto K. MRS study of the antioxidant defense system in schizophrenia. Antioxid Redox Signal. 2011;15:2057–2065. doi: 10.1089/ars.2010.3453. [DOI] [PubMed] [Google Scholar]
- 159.McCreadie R. Macdonald E. Blacklock C. Tilak-Singh D. Wiles D. Halliday J. Paterson J. Dietary intake of schizophrenic patients in Nithsdale, Scotland: case-control study. Brit Med J. 1998;317:784–785. doi: 10.1136/bmj.317.7161.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.McNamara RK. Jandacek R. Rider T. Tso P. Hahn CG. Richtand NM. Stanford KE. Abnormalities in the fatty acid composition of the postmortem orbitofrontal cortex of schizophrenic patients: gender differences and partial normalization with antipsychotic medication. Schizophr Res. 2007;91:37–50. doi: 10.1016/j.schres.2006.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Meister A. Mitochondrial changes associated with glutathione deficiency. Biochim Biophys Acta. 1995;1271:35–42. doi: 10.1016/0925-4439(95)00007-q. [DOI] [PubMed] [Google Scholar]
- 162.Meister A. Selective modification of glutathione metabolism. Science. 1983;220:472–477. doi: 10.1126/science.6836290. [DOI] [PubMed] [Google Scholar]
- 163.Mellsop GW. Schizophrenia and rheumatoid arthritis. Aust N Z J Psychiatry. 1972;6:214. doi: 10.3109/00048677209159713. [DOI] [PubMed] [Google Scholar]
- 164.Messamore E. Hoffman WF. Yao JK. Niacin sensitivity and the arachidonic acid pathway in schizophrenia. Schizophr Res. 2010;122:248–256. doi: 10.1016/j.schres.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Messamore E. Relationship between the niacin skin flush response and essential fatty acids in schizophrenia. Prostaglandins Leukot Essent Fatty Acids. 2003;69:413–419. doi: 10.1016/j.plefa.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 166.Meydani SN. Endres S. Woods MM. Goldin BR. Soo C. Morill-Labrode A. Dinarello CA. Gorbach SL. Oral (n-3) fatty acid supplementation suppresses cytokine production and lymphocyte proliferation: comparison between young and older women. J Nutr. 1991;121:547–555. doi: 10.1093/jn/121.4.547. [DOI] [PubMed] [Google Scholar]
- 167.Michel PP. Hefti F. Toxicity of 6-hydroxydopamine and dopamine for dopaminergic neurons in culture. J Neurosci Res. 1990;76:428–435. doi: 10.1002/jnr.490260405. [DOI] [PubMed] [Google Scholar]
- 168.Michel TM. Thome J. Martin D. Nara K. Zwerina S. Tatschner T. Weijers HG. Koutsilieri E. Cu, Zn-, and Mn-superoxide dismutase levels in brains of patients with schizophrenic psychosis. J Neural Transm. 2004;111:1191–1201. doi: 10.1007/s00702-004-0160-9. [DOI] [PubMed] [Google Scholar]
- 169.Miljevic C. Nikolic M. Nikolic-Kokic A. Jones DR. Niketic V. Lecic-Tosevski D. Spasic MB. Lipid status, anti-oxidant enzyme defence and haemoglobin content in the blood of long-term clozapine-treated schizophrenic patients. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:303–307. doi: 10.1016/j.pnpbp.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 170.Millward DJ. Protein deficiency, starvation and protein metabolism. Proc Nutr Soc. 1979;38:77–88. doi: 10.1079/pns19790011. [DOI] [PubMed] [Google Scholar]
- 171.Miquel J. Economos AC. Fleming J. Johnson JE., Jr. Mitochondrial role in cell aging. Exp Gerontol. 1998;15:575–591. doi: 10.1016/0531-5565(80)90010-8. [DOI] [PubMed] [Google Scholar]
- 172.Miyaoka T. Seno H. Itoga M. Iigima M. Inagaki T. Horiguchi J. Schizophrenia-associated idiopathic unconjuated hyperbilirubinemia (Gilbert's syndrome) J Clin Psychiatry. 2000;61:868–871. doi: 10.4088/jcp.v61n1110. [DOI] [PubMed] [Google Scholar]
- 173.Miyaoka T. Yasukawa R. Yasuda H. Shimizu M. Mizuno S. Sukegawa T. Inagaki T. Horiguchi J. Urinary excretion of biopyrrins, oxidative metabolites of bilirubin, increases in patients with psychiatric disorders. Eur Neuropsychopharmacol. 2005;15:249–252. doi: 10.1016/j.euroneuro.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 174.Moncada S. Palmer RMJ. Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacology Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 175.Morrow JD. Awad JA. Boss HJ. Blair IA. LJ Roberts II. Non-cyclooxygenase-derived prostanoids (F2-isoprostanes) are formed in situ on phospholipids. Proc Natl Acad Sci U S A. 1992;89:10721–10725. doi: 10.1073/pnas.89.22.10721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mount DB. Kwon CY. Zandi-Nejad K. Renal urate transport. Rheum Dis Clin North Am. 2006;32:313–331. doi: 10.1016/j.rdc.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 177.Mukherjee S. Mahadik SP. Scheffer R. Correnti EE. Kelkar H. Impaired antioxidant defense at the onset of psychosis. Schizophr Res. 1996;19:19–26. doi: 10.1016/0920-9964(95)00048-8. [DOI] [PubMed] [Google Scholar]
- 178.Müller N. Riedel M. Ackenheil M. Schwarz MJ. Cellular and humoral immune system in schizophrenia: a conceptual re-evaluation. World J Biol Psychiatry. 2000;1:173–179. doi: 10.3109/15622970009150588. [DOI] [PubMed] [Google Scholar]
- 179.Muller N. Schiller P. Ackenheil M. Coincidence of schizophrenia and hyperbillrubinemia. Pharmacopsychiatry. 1991;24:225–228. doi: 10.1055/s-2007-1014472. [DOI] [PubMed] [Google Scholar]
- 180.Müller N. Schlesinger BC. Hadjamu M. Riedel M. Schwarz M. Primbs J. Ackenheil M. Wank R. Gruber R. Cytotoxic gamma/delta cells (γ/δCD8+) are elevated in unmedicated schizophrenic patients and related to the Blood-Brain Barrier and the HLA allele DPA 02011. Schizophr Res. 1998;12:69–71. doi: 10.1016/s0920-9964(98)00036-x. [DOI] [PubMed] [Google Scholar]
- 181.Muraoka S. Miura T. Inhibition by uric acid of free radicals that damage biological molecules. Pharmacol Toxicol. 2003;93:284–289. doi: 10.1111/j.1600-0773.2003.pto930606.x. [DOI] [PubMed] [Google Scholar]
- 182.Murray J. Oquendo CE. Willis JH. Marusich MF. Capaldi RA. Monitoring oxidative and nitrative modification of cellular proteins; a paradigm for identifying key disease related markers of oxidative stress. Adv Drug Deliv Rev. 2008;60:1497–1503. doi: 10.1016/j.addr.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 183.Nakamura H. Thioredoxin and its related molecules: update 2005. Antioxid Redox Signal. 2005;7:823–828. doi: 10.1089/ars.2005.7.823. [DOI] [PubMed] [Google Scholar]
- 184.Nakano Y. Yoshimura R. Nakano H. Ikenouchi-Sugita A. Hori H. Umene-Nakano W. Ueda N. Nakamura J. Association between plasma nitric oxide metabolites levels and negative symptoms of schizophrenia: a pilot study. Hum Psychopharmacol. 2010;25:139–144. doi: 10.1002/hup.1102. [DOI] [PubMed] [Google Scholar]
- 185.Navarro J. Obrador E. Pellicer JA. Aseni M. Vina J. Estrela JM. Blood glutathione as an index of radiation-induced oxidative stress in mice and humans. Free Radic Biol Med. 1997;22:1203–1209. doi: 10.1016/s0891-5849(96)00554-0. [DOI] [PubMed] [Google Scholar]
- 186.Norris PJ. Faull RL. Emson PC. Neuronal nitric oxide synthase (nNOS) mRNA expression and NADPH-diaphorase staining in the frontal cortex, visual cortex and hippocampus of control and Alzheimer's disease brains. Mol Brain Res. 1996;41:36–49. doi: 10.1016/0169-328x(96)00064-2. [DOI] [PubMed] [Google Scholar]
- 187.Nuss P. Tessier C. Ferreri F. De Hert M. Peuskens J. Trugnan G. J Masliah J. Wolf C. Abnormal transbilayer distribution of phospholipids in red blood cell membranes in schizophrenia. Psychiatry Res. 2009;169:91–96. doi: 10.1016/j.psychres.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 188.Oke AF. May L. Adams RN. Ascorbic acid distribution patterns in human brain. Ann New York Acad Sci. 1987;498:1–12. doi: 10.1111/j.1749-6632.1987.tb23747.x. [DOI] [PubMed] [Google Scholar]
- 189.Olincy A. Young DA. Freedman R. Increased levels of the nicotine metabolite cotinine in schizophrenic smokers compared to other smokers. Biol Psychiatry. 1997;42:1–5. doi: 10.1016/S0006-3223(96)00302-2. [DOI] [PubMed] [Google Scholar]
- 190.Olney JW. Farber NB. Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry. 1995;52:998–1007. doi: 10.1001/archpsyc.1995.03950240016004. [DOI] [PubMed] [Google Scholar]
- 191.Othmen LB. Mechri A. Fendri C. Bost M. Chazot G. Gaha L. Kerkeni A. Altered antioxidant defense system in clinically stable patients with schizophrenia and their unaffected siblings. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:155–159. doi: 10.1016/j.pnpbp.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 192.Pacher P. Beckman JS. Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Padurariu M. Ciobica A. Dobrin I. Stefanescu C. Evaluation of antioxidant enzymes activities and lipid peroxidation in schizophrenic patients treated with typical and atypical antipsychotics. Neurosci Lett. 2010;479:317–320. doi: 10.1016/j.neulet.2010.05.088. [DOI] [PubMed] [Google Scholar]
- 194.Pae CU. Paik IH. Lee C. Lee SJ. Kim JJ. Lee CU. Decreased plasma antioxidants in schizophrenia. Neuropsychobiology. 2004;50:54–56. doi: 10.1159/000077942. [DOI] [PubMed] [Google Scholar]
- 195.Palmer RMJ. Ashton DS. Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333:664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- 196.Papas AM. Determinants of antioxidant status in humans. Lipids. 1996;31:S77–S82. doi: 10.1007/BF02637055. [DOI] [PubMed] [Google Scholar]
- 197.Patel M. Day BJ. Crapo JD. Fridovich I. McNamara JO. Requirement for superoxide in excitotoxic cell death. Neuron. 1996;16:345–355. doi: 10.1016/s0896-6273(00)80052-5. [DOI] [PubMed] [Google Scholar]
- 198.Peet M. Laugharne J. Rangarajan N. Reynolds GP. Tardive dyskinesia, lipid peroxidation, and sustained amelioration with vitamin E treatment. Intl Clin Psychopharmacology. 1993;8:151–153. doi: 10.1097/00004850-199300830-00003. [DOI] [PubMed] [Google Scholar]
- 199.Peet M. Laugharne JDE. Mellor J. Ramchand CN. Essential fatty acid deficiency in erythrocyte membranes from chronic schizophrenic patients, and the clinical effects of dietary supplementation. Prostaglandins Leukot Essent Fatty Acids. 1996;55:71–75. doi: 10.1016/s0952-3278(96)90148-9. [DOI] [PubMed] [Google Scholar]
- 200.Pettegrew JW. Keshavan MS. Panchalingam K. Strychor S. Kaplan DB. Tretta MG. Allen M. Alterations in brain high energy phosphate and membrane phospholipid metabolism in first episode drug naive schizophrenics. Arch Gen Psychiatry. 1991;48:563–568. doi: 10.1001/archpsyc.1991.01810300075011. [DOI] [PubMed] [Google Scholar]
- 201.Pettegrew JW. Keshaven MS. Minshew NJ. 31P nuclear magnetic resonance spectroscopy: neuro-development and schizophrenia. Schizophr Bull. 1993;19:335–353. doi: 10.1093/schbul/19.1.35. [DOI] [PubMed] [Google Scholar]
- 202.Phillips M. Sabas M. Greenberg J. Increased pentane and carbon disulfide in the breath of patients with schizophrenia. J Clin Pathol. 1993;46:861–864. doi: 10.1136/jcp.46.9.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Pillai A. Parikh V. Terry AV., Jr Mahadik SP. Long-term antipsychotic treatments and crossover studies in rats: differential effects of typical and atypical agents on the expression of antioxidant enzymes and membrane lipid peroxidation in rat brain. J Psychiatr Res. 2007;41:372–386. doi: 10.1016/j.jpsychires.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 204.Pillai A. Reddy R. Yao JK. Oxidative stress in schizophrenia. In: Reddy R, editor; Yao JK, editor. Fatty Acids and Oxidative Stress in Neuropsychiatric Disorders. New York: Nova Biochemical Books; 2007. pp. 29–42. [Google Scholar]
- 205.Porton B. Delisi LE. Bertisch HC. Ji F. Gordon D. Li P. Benedict MM. Greenberg WM. Kao H-T. Telomerase levels in schizophrenia: a preliminary study. Schizophr Res. 2008;106:242–247. doi: 10.1016/j.schres.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Potvin S. Stip E. Sepehry AA. Gendron A. Bah R. Kouassi E. Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biol Psychiatry. 2008;63:801–808. doi: 10.1016/j.biopsych.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 207.Prilipko LL. Lideman RR. Lipid peroxidation processes as one of the factors modifying neuronal membrane-bound proteins in schizophrenia. Vestn Akad Med Nauk SSSR. 1982;1:33–37. [PubMed] [Google Scholar]
- 208.Prilipko LL. The possible role of lipid peroxidation in the pathophysiology of mental disorders. In: Packer L, editor; Prilipko L, editor; Christen Y, editor. Free Radicals in the Brain. Berlin: Spinger-Verlag; 1992. pp. 146–152. [Google Scholar]
- 209.Provencher SW. LCModel and LCMgui User's Manual. http://s-provenchercom/pages/lcm-manualshtml. 2001. http://s-provenchercom/pages/lcm-manualshtml
- 210.Pryor WA. Stone K. Oxidants in cigarette smoke. Ann N Y Acad Sci. 1992;686:29. doi: 10.1111/j.1749-6632.1993.tb39148.x. [DOI] [PubMed] [Google Scholar]
- 211.Puri BK. Counsell SJ. Ross BM. Hamilton G. Bustos MG. Treasaden IH. Evidence from in vivo 31-phosphorus magnetic resonance spectroscopy phosphodiesters that exhaled ethane is a biomarker of cerebral n-3 polyunsaturated fatty acid peroxidation in humans. BMC Psychiatry. 2008;8:S2. doi: 10.1186/1471-244X-8-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Raffa M. Mechri A. Othman LB. Fendri C. Gaha L. Kerkeni A. Decreased glutathione levels and antioxidant enzyme activities in untreated and treated schizophrenic patients. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1178–1183. doi: 10.1016/j.pnpbp.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 213.Ramirez J. Garnica R. Boll MC. Montes S. Rios C. Low concentration of nitrite and nitrate in the cerebrospinal fluid from schizophrenic patients: a pilot study. Schizophr Res. 2004;68:357–361. doi: 10.1016/S0920-9964(03)00070-7. [DOI] [PubMed] [Google Scholar]
- 214.Rebec GV. Ascorbate: an antioxidant neuroprotectant and extracellular neuromodulator. In: Connor JR, editor. Metals and Oxidative Damage in Neurological Disorders. New York: Plenum Press; 1997. pp. 149–173. [Google Scholar]
- 215.Reddy R. Reddy R. Antioxidant therapeutics in schizophrenia. Antioxid Redox Signal. 2011;15:2047–2055. doi: 10.1089/ars.2010.3571. [DOI] [PubMed] [Google Scholar]
- 216.Reddy R. Yao JK. Free radical pathology in schizophrenia: a review. Prostaglandins Leukot Essent Fatty Acids. 1996;55:33–43. doi: 10.1016/s0952-3278(96)90143-x. [DOI] [PubMed] [Google Scholar]
- 217.Reddy R. Sahebarao MP. Mukherjee S. Murthy JN. Enzymes of the antioxidant system in chronic schizophrenic patients. Biol Psychiatry. 1991;30:409–412. doi: 10.1016/0006-3223(91)90298-z. [DOI] [PubMed] [Google Scholar]
- 218.Reddy RD. Yao JK. Environmental factors and membrane polyunsaturated fatty acids in schizophrenia. Prostaglands Leukot Essent Fatty Acids. 2003;69:385–391. doi: 10.1016/j.plefa.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 219.Reddy RD. Keshavan MS. Yao JK. Reduced plasma antioxidants in first-episode patients with schizophrenia. Schizophr Res. 2003;62:205–212. doi: 10.1016/s0920-9964(02)00407-3. [DOI] [PubMed] [Google Scholar]
- 220.Reddy RD. Keshavan MS. Yao JK. Reduced red blood cell membrane essential polyunsaturated fatty acids in first-episode schizophrenia at neuroleptic-naïve baseline. Schizophr Bull. 2004;30:901–911. doi: 10.1093/oxfordjournals.schbul.a007140. [DOI] [PubMed] [Google Scholar]
- 221.Rice-Evans CA. Formation of free radicals and mechanisms of action in normal biochemical processes and pathological states. In: Rice-Evans CA, editor; Burdon RH, editor. Free Radical Damage and Its Control. Amsterdam: Elsevier; 1994. pp. 131–153. [Google Scholar]
- 222.Roth S. Droge W. Regulation of interlukin-2 production, interleukin 2 mRNA expression and intracellular glutathioine levels in ex vivo derived T lymphocytes by lactate. Eur J Immunol. 1991;21:1933–1937. doi: 10.1002/eji.1830210823. [DOI] [PubMed] [Google Scholar]
- 223.Roth S. Droge W. Regulation of T-cell activation and T-cell growth factor (TCGF) production by hydrogen peroxide. Cell Immunol. 1987;108:417–424. doi: 10.1016/0008-8749(87)90224-3. [DOI] [PubMed] [Google Scholar]
- 224.Rothermundt M. Arolt V. Weitzsch C. Eckhoff D. Kirchner H. Immunological dysfunction in schizophrenia: a systematic approach. Neuropsychobiology. 1998;37:186–193. doi: 10.1159/000026501. [DOI] [PubMed] [Google Scholar]
- 225.Rozen S. Cudkowicz ME. Bogdanov M. Matson WR. Kristal BS. Beecher C. Harrison S. Vouros P. Flarakos J. Vigneau-Callahan K. Matson TD. Newhall KM. Beal MF. Brown RH., Jr. Kaddurah-Daouk R. Metabolomic analysis and signatures in motor neuron disease. Metabolomics. 2005;1:101–108. doi: 10.1007/s11306-005-4810-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Saito I. Saruta T. Kondo K. Nakamura R. Oguro T. Yamagami K. Ozawa Y. Kato E. Serum uric acid and the renin-angiotensin system in hypertension. J Am Geriatr Soc. 1978;26:241–247. doi: 10.1111/j.1532-5415.1978.tb02396.x. [DOI] [PubMed] [Google Scholar]
- 227.Schmitt A. Wilczek K. Blennow K. Maras A. Jatzko A. Petroianu G. Braus DF. Gattaz WF. Altered thalamic membrane phospholipids in schizophrenia: a postmortem study. Biol Psychiatry. 2004;56:41–45. doi: 10.1016/j.biopsych.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 228.Schwarz MJ. Muller N. Riedel M. Ackenheil M. The Th2-hypothesis of schizophrenia: a strategy to identify a subgroup of schizophrenia caused by immune mechanisms. Med Hypotheses. 2001;56:483–486. doi: 10.1054/mehy.2000.1203. [DOI] [PubMed] [Google Scholar]
- 229.Scott GS. Spitsin SV. Kean RB. Mikheeva T. Koprowski H. Hooper DC. Therapeutic intervention in experimental allergic encephalomyelitis by administration of uric acid precursors. Proc Natl Acad Sci U S A. 2002;99:16303–16308. doi: 10.1073/pnas.212645999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Scottish Schizophrenia Research Group. Smoking habits and plasma lipid peroxide and vitamin E levels in never-treated first-episode patients with schizophrenia. Brit J Psychiatry. 2000;176:290–293. doi: 10.1192/bjp.176.3.290. [DOI] [PubMed] [Google Scholar]
- 231.Seaton TA. Jenner P. Marsden CD. Mitochondrial respiratory enzyme function, superoxide dismutase activity following brain glutathione depletion in the rat. Biochem Pharmacol. 1996;52:1657–1663. doi: 10.1016/s0006-2952(96)00452-2. [DOI] [PubMed] [Google Scholar]
- 232.Shigenaga MK. Hagen T. Ames BN. Oxidative damage and mitochondrial decay in aging. Proc Natl Acad Sci U S A. 1994;91:10771–10778. doi: 10.1073/pnas.91.23.10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Sinet PM. Debray Q. Carmagnol F. Pelicier Y. Nicole A. Jerome H. Normal erythrocyte SOD values in two human diseases: schizophrenia and cystic fibrosis. In: Greenwald RA, editor; Cohen G, editor. Oxy Radicals and Their Scavenger Systems, Cellular and Medical Aspects. II. New York: Elsevier; 1983. pp. 302–304. [Google Scholar]
- 234.Sivrioglu EY. Kirli S. Sipahioglu D. Gursoy B. Sarandol E. The impact of omega-3 fatty acids, vitamins E and C supplementation on treatment outcome and side effects in schizophrenia patients treated with haloperidol: an open-label pilot study. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1493–1499. doi: 10.1016/j.pnpbp.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 235.Skinner AO. Mahadik SP. Garver DL. Thiobarbituric acid reactive substances in the cerebrospinal fluid in schizophrenia. Schizophr Res. 2005;76:83–87. doi: 10.1016/j.schres.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 236.Skosnik PD. Yao JK. From phospholipid and fatty acid defects to altered neurotransmission: is arachidonic acid a nexus in the pathophysiology of schizophrenia? Prostaglands Leukot Essent Fatty Acids. 2003;69:367–384. doi: 10.1016/j.plefa.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 237.Smith CV. Free radical mechanisms of tissue injury. In: Moslen MT, editor; Smith CV, editor. Free Radical Mechanisms of Tissue Injury. Boca Raton, FL: CRC Press; 1992. pp. 1–22. [Google Scholar]
- 238.Soriani M. Pietraforte D. Minetti M. Antioxidant potential of anaerobic human plasma: role of serum albumin and thiols as scavengers of carbon radicals. Arch Biochem Biophys. 1994;312:180–188. doi: 10.1006/abbi.1994.1297. [DOI] [PubMed] [Google Scholar]
- 239.Soto-Otero R. Mendez-Alvarez E. Hermida-Ameijeiras A. Lopez-Real AM. Labandeira-Garcia JL. Effects of (-)-nicotine and (-)-cotinine on 6-hydroxydopamine-induced oxidative stress and neurotoxicity: relevance for Parkinson's disease. Biochem Pharmacol. 2002;64:125–135. doi: 10.1016/s0006-2952(02)01070-5. [DOI] [PubMed] [Google Scholar]
- 240.Soyland E. Lea T. Sandstand B. Drevon A. Dietary supplementation with very long-chain n-3 fatty acids in man decreases expression of the interleukin-2 receptor (CD25) on mitogen-stimulated lymphocytes from patients with infammatory skin disease. Eur J Clin Invest. 1994;24:236–242. doi: 10.1111/j.1365-2362.1994.tb01080.x. [DOI] [PubMed] [Google Scholar]
- 241.Spitsin S. Hooper DC. Leist T. Streletz LJ. Mikheeva T. Koprowskil H. Inactivation of peroxynitrite in multiple sclerosis patients after oral administration of inosine may suggest possible approaches to therapy of the disease. Mult Scler. 2001;7:313–319. doi: 10.1177/135245850100700507. [DOI] [PubMed] [Google Scholar]
- 242.Stanley JA. Williamson PC. Drost DJ. Carr T. Rylett J. Morrison-Stewart S. Thompson RT. Membrane phospholipid metabolism and schizophrenia: an in vivo 31P-MR spectroscopy study. Schizophr Res. 1994;13:209–215. doi: 10.1016/0920-9964(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 243.Stassen HH. Bridler R. Hagele S. Hergersberg M. Mehmann B. Schinzel A. Weisbrod M. Scharfetter C. Schizophrenia and smoking: evidence for a common neurobiological basis? Am J Med Genet. 2000;96:173–177. [PubMed] [Google Scholar]
- 244.Steullet P. Cabungcal JH. Kulak A. Kraftsik R. Chen Y. Dalton TP. Cuenod M. Do KQ. Redox dysregulation affects the ventral but not dorsal hippocampus: impairment of parvalbumin neurons, gamma oscillations, and related behaviors. J Neurosci. 2010;30:2547–2558. doi: 10.1523/JNEUROSCI.3857-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Steullet P. Lavoie S. Kraftsik R. Guidi R. Gysin R. Cuenod M. Do KQ. A glutathione deficit alters dopamine modulation of L-type calcium channels via D2 and ryanodine receptors in neurons. Free Radic Biol Med. 2008;44:1042–1054. doi: 10.1016/j.freeradbiomed.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 246.Strassnig M. Brar JS. Ganguli R. Nutritional assessment of patients with schizophrenia: a preliminary study. Schizophr Bull. 2003;29:393–397. doi: 10.1093/oxfordjournals.schbul.a007013. [DOI] [PubMed] [Google Scholar]
- 247.Strauss JS. Carpenter WT., Jr Prediction of outcome in schizophrenia. III. Five-year outcome and its predictors. Arch Gen Psychiatry. 1977;34:159–163. doi: 10.1001/archpsyc.1977.01770140049005. [DOI] [PubMed] [Google Scholar]
- 248.Strazzullo P. Puig JG. Uric acid and oxidative stress: relative impact on cardiovascular risk? Nutr Metab Cardiovasc Dis. 2007;17:409–414. doi: 10.1016/j.numecd.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 249.Suboticanec K. Folnegovic-Smalc V. Korbar M. Mestrovic B. Buzina R. Vitamin C status in chronic schizophrenia. Biol Psychiatry. 1990;28:959–966. doi: 10.1016/0006-3223(90)90061-6. [DOI] [PubMed] [Google Scholar]
- 250.Suzuki E. Nakaki T. Nakamura M. Miyaoka H. Plasma nitrate levels in deficit versus non-deficit forms of schizophrenia. J Psychiatry Neurosci. 2003;28:288–292. [PMC free article] [PubMed] [Google Scholar]
- 251.Suzuki K. Chemistry and metabolism of brain lipids. In: Siegel GJ, editor; Albers RW, editor; Agranoff BW, editor; Katzman R, editor. Basic Neurochemistry. 3rd. Boston, MA: Little, Brown & Co.; 1981. pp. 355–370. [Google Scholar]
- 252.Tandon R. Nasrallah HA. Keshavan MS. Schizophrenia, “just the facts” 4. Clinical features and conceptualization. Schizophr Res. 2009;110:1–23. doi: 10.1016/j.schres.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 253.Taneli F. Pirildar S. Akdeniz F. Uyanik BS. Ari Z. Serum nitric oxide metabolite levels and the effect of antipsychotic therapy in schizophrenia. Arch Med Res. 2004;35:401–405. doi: 10.1016/j.arcmed.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 254.Tashiro T. Yamamori H. Takagi K. Hayashi N. Furukawa K. Nakajima N. N-3 versus n-6 polyunsaturated fatty acids in critical illness. Nutrition. 1998;14:551–553. doi: 10.1016/s0899-9007(98)00048-3. [DOI] [PubMed] [Google Scholar]
- 255.Thorns V. Hansen L. Masliah E. nNOS expressing neurons in the entorhinal cortex and hippocampus are affected in patients with Alzheimer's disease. Exp Neurology. 1998;150:14–20. doi: 10.1006/exnr.1997.6751. [DOI] [PubMed] [Google Scholar]
- 256.Tien AY. Eaton WW. Psychopathologic precursors and sociodemographic risk factors for the schizophrenia syndrome. Arch Gen Psychiatry. 1992;49:37–46. doi: 10.1001/archpsyc.1992.01820010037005. [DOI] [PubMed] [Google Scholar]
- 257.Toncev G. Milicic B. Toncev S. Samardzic G. Serum uric acid levels in multiple sclerosis patients correlate with activity of disease and blood-brain barrier dysfunction. Eur J Neurol. 2002;9:221–226. doi: 10.1046/j.1468-1331.2002.00384.x. [DOI] [PubMed] [Google Scholar]
- 258.Torrey EF. Yolken RH. The schizophrenia-rheumatoid arthritis connection, infectious, immune, or both? Brain Behav Immun. 2001;15:401–410. doi: 10.1006/brbi.2001.0649. [DOI] [PubMed] [Google Scholar]
- 259.Ustundag B. Atmaca M. Kirtas O. Selek S. Metin K. Tezcan E. Total antioxidant response in patients with schizophrenia. Psychiatry Clin Neurosci. 2006;60:458–464. doi: 10.1111/j.1440-1819.2006.01532.x. [DOI] [PubMed] [Google Scholar]
- 260.van der Veen RC. Hinton DR. Incardonna F. Hofman FM. Extensive peroxynitrite activity during progressive stages of central nervous system inflammation. J Neuroimmunol. 1997;77:1–7. doi: 10.1016/s0165-5728(97)00013-1. [DOI] [PubMed] [Google Scholar]
- 261.van Kammen DP. McAllister-Sistilli CG. Kelley ME. Gurklis JA. Yao JK. Elevated interleukin-6 in schizophrenia. Psychiatry Res. 1999;87:129–136. doi: 10.1016/s0165-1781(99)00053-0. [DOI] [PubMed] [Google Scholar]
- 262.van Os J. Kapur S. Schizophrenia. Lancet. 2009;374:635–645. doi: 10.1016/S0140-6736(09)60995-8. [DOI] [PubMed] [Google Scholar]
- 263.Vaughan K. McConaghy N. Megavitamin and dietary treatment in schizophrenia: a randomised, controlled trial. Aust N Z J Psychiatry. 1999;33:84–88. doi: 10.1046/j.1440-1614.1999.00527.x. [DOI] [PubMed] [Google Scholar]
- 264.Venkatasubramanian G. Chittiprol S. Neelakantachar N. Naveen MN. Thirthall J. Gangadhar BN. Shetty K. Insulin and insulin-like growth factor-1 abnormalities in antipsychotic-naive schizophrenia. Am J Psychiatry. 2007;164:1557–1560. doi: 10.1176/appi.ajp.2007.07020233. [DOI] [PubMed] [Google Scholar]
- 265.Villemain F. Chate-noud L. Galinowski A. Homo-Delar-che F. Genestet D. Loo H. Zarifarain E. Bach JF. Aberrant T-cell-mediated immunity in untreated schizophrenic patients: deficient Interleukin-2 production. Am J Psychiatry. 1989;146:609–616. doi: 10.1176/ajp.146.5.609. [DOI] [PubMed] [Google Scholar]
- 266.Vinogradov S. Gottesman II. Moises HW. Nicol S. Negative association between schizophrenia and rheumatoid arthritis. Schizophr Bull. 1991;17:669–678. doi: 10.1093/schbul/17.4.669. [DOI] [PubMed] [Google Scholar]
- 267.Virit O. Altindag A. Yumru M. Dalkilic A. Savas HA. Selek S. Erel O. Herken H. A defect in the antioxidant defense system in Schizophrenia. Neuropsychobiology. 2009;60:87–93. doi: 10.1159/000239684. [DOI] [PubMed] [Google Scholar]
- 268.Vitek L. Lenicek M. Jirsa M. Novotna M. Novotny L. Eberova J. Petrasek J. Jirsa M. Serum bilirubin levels and UGT1A1 promoter variations in patients with schizophrenia. Psychiatry Res. 2010;178:449–450. doi: 10.1016/j.psychres.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 269.Wang JF. Shao L. Sun X. Young LT. Increased oxidative stress in the anterior cingulate cortex of subjects with bipolar disorder and schizophrenia. Bipolar Disord. 2009;11:523–529. doi: 10.1111/j.1399-5618.2009.00717.x. [DOI] [PubMed] [Google Scholar]
- 270.Waring WS. Webb DJ. Maxwell SR. Uric acid as a risk factor for cardiovascular disease. Quart J Med. 2000;93:707–713. doi: 10.1093/qjmed/93.11.707. [DOI] [PubMed] [Google Scholar]
- 271.Wayner DDM. Burton GW. Ingold KU. Barclay LRC. Locke SJ. The relative contribution of vitamine E, urate, ascorbate and proteins to the total peroxyl radical-trapping antioxidant activity of human blood plasma. Biochim Biophys Acta. 1987;924:408–419. doi: 10.1016/0304-4165(87)90155-3. [DOI] [PubMed] [Google Scholar]
- 272.Wilke I. Arolt V. Rothermundt M. Weitzsch CH. Hornberg M. Kirchner H. Investigations of cytokine production in whole blood cultures of paranoid and residual schizophrenic patients. Eur Arch Psychiatry Clin Neurosci. 1996;246:279–284. doi: 10.1007/BF02190280. [DOI] [PubMed] [Google Scholar]
- 273.Williamson P. Drost D. Stanley J. Carr T. Morrison S. Merskey H. Localized phosphorus 31 magnetic resonance spectroscopy in chronic schizophrenic patients and normal controls. Arch Gen Psychiatry. 1991;48:578. doi: 10.1001/archpsyc.1991.01810300090013. [DOI] [PubMed] [Google Scholar]
- 274.Wolfler A. Abuja PM. Schauenstein K. Liebmann PM. N-acetylserotonin is a better extra- and intracellular antioxidant than melatonin. FEBS Lett. 1999;449:206–210. doi: 10.1016/s0014-5793(99)00435-4. [DOI] [PubMed] [Google Scholar]
- 275.Wong C-T. Tsoi W-F. Saha N. Acute phase proteins in male Chinese schizophrenic patients in Singapore. Schizophr Res. 1996;22:165–171. doi: 10.1016/s0920-9964(96)00037-0. [DOI] [PubMed] [Google Scholar]
- 276.Wood SJ. Berger GE. Wellard RM. Proffitt TM. McConchie M. Berk M. McGorry PD. Pantelis C. Medial temporal lobe glutathione concentration in first episode psychosis: a 1H-MRS investigation. Neurobiol Dis. 2009;33:354–357. doi: 10.1016/j.nbd.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 277.Xing G. Chavko M. Zhang LX. Yang S. Post RM. Decreased calcium-dependent constitutive nitric oxide synthase (cNOS) activity in prefrontal cortex in schizophrenia and depression. Schizophr Res. 2002;58:21–30. doi: 10.1016/s0920-9964(01)00388-7. [DOI] [PubMed] [Google Scholar]
- 278.Yamada K. Kanba S. Anamizu S. Ohnishi K. Ashikari I. Yagi G. Asai M. Low superoxide dismutase activity in schizophrenic patients with tardive dyskinesia. Psychol Med. 1997;27:1223–1225. doi: 10.1017/s0033291797005114. [DOI] [PubMed] [Google Scholar]
- 279.Yanik M. Vural H. Kocyigit A. Tutkun H. Zoroglu SS. Herken H. Savas HA. Koylu A. Akyol O. Is the arginine-nitric oxide pathway involved in the pathogenesis of schizophrenia? Neuropsychobiology. 2003;47:61–65. doi: 10.1159/000070010. [DOI] [PubMed] [Google Scholar]
- 280.Yao JK. Cheng P. Determination of multiple redox-active compounds by high-performance liquid chromatography with coulometric multi-electrode array system. J Chromatogr B. 2004;810:93–100. doi: 10.1016/j.jchromb.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 281.Yao JK. Reddy RD. Metabolic investigation in psychiatric disorders. Mol Neurobiology. 2005;31:193–203. doi: 10.1385/MN:31:1-3:193. [DOI] [PubMed] [Google Scholar]
- 282.Yao JK. van Kammen DP. Membrane phospholipids and cytokine interaction in schizophrenia. Int Rev Neurobiol. 2004;59:297–326. doi: 10.1016/S0074-7742(04)59012-8. [DOI] [PubMed] [Google Scholar]
- 283.Yao JK. Dougherty GG. Reddy RD. Keshavan MS. Montrose DM. Matson WR. Rozen S. Krishnan RR. McEvoy J. Kaddurah-Daouk R. Altered interactions of tryptophan metabolites in first-episode neuroleptic-naïve patients with schizophrenia. Mol Psychiatry. 2010;15:938–953. doi: 10.1038/mp.2009.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Yao JK. Dougherty GG. Reddy RD. Keshavan MS. Montrose DM. Matson WR. McEvoy J. Kaddurah-Daouk R. Homeostatic imbalance of purine catabolism in first-episode neuroleptic-naïve patients with schizophrenia. PLoS One. 2010;5:e9508. doi: 10.1371/journal.pone.0009508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Yao JK. Leonard S. Reddy R. Membrane phospholipid abnormalities in postmortem brains from schizophrenic patients. Schizophr Res. 2000;42:7–17. doi: 10.1016/s0920-9964(99)00095-x. [DOI] [PubMed] [Google Scholar]
- 286.Yao JK. Leonard S. Reddy RD. Altered glutathione redox state in schizophrenia. Disease Markers. 2006;22:83–93. doi: 10.1155/2006/248387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Yao JK. Leonard S. Reddy RD. Increased nitric oxide radicals in postmortem brains from schizophrenic patients. Schizophr Bull. 2004;30:923–934. doi: 10.1093/oxfordjournals.schbul.a007142. [DOI] [PubMed] [Google Scholar]
- 288.Yao JK. Reddy R. van Kammen DP. Human plasma glutathione peroxidase and symptom severity in schizophrenia. Biol Psychiatry. 1999;45:1512–1515. doi: 10.1016/s0006-3223(98)00184-x. [DOI] [PubMed] [Google Scholar]
- 289.Yao JK. Reddy R. van Kammen DP. Reduced level of plasma antioxidant uric acid in schizophrenia. Psychiatry Res. 1998;80:29–39. doi: 10.1016/s0165-1781(98)00051-1. [DOI] [PubMed] [Google Scholar]
- 290.Yao JK. Reddy R. McElhinny LG. van Kammen DP. Effects of haloperidol on antioxidant defense system enzymes in schizophrenia. J Psychiatry Res. 1998;32:385–391. doi: 10.1016/s0022-3956(98)00028-4. [DOI] [PubMed] [Google Scholar]
- 291.Yao JK. Reddy R. McElhinny LG. van Kammen DP. Reduced status of plasma total antioxidant capacity in schizophrenia. Schizophr Res. 1998;32:1–8. doi: 10.1016/s0920-9964(98)00030-9. [DOI] [PubMed] [Google Scholar]
- 292.Yao JK. Reddy RD. van Kammen DP. Abnormal age-related changes of plasma antioxidant proteins in schizophrenia. Psychiatry Res. 2000;97:137–151. doi: 10.1016/s0165-1781(00)00230-4. [DOI] [PubMed] [Google Scholar]
- 293.Yao JK. Reddy RD. van Kammen DP. Oxidative damage and schizophrenia: an overview of the evidence and its therapeutic implications. CNS Drugs. 2001;15:287–310. doi: 10.2165/00023210-200115040-00004. [DOI] [PubMed] [Google Scholar]
- 294.Yao JK. Stanley JA. Reddy RD. Keshavan MS. Pettegrew JW. Correlations between peripheral polyunsaturated fatty acid content and in vivo membrane phospholipid metabolites. Biol Psychiatry. 2002;52:823–830. doi: 10.1016/s0006-3223(02)01397-5. [DOI] [PubMed] [Google Scholar]
- 295.Yao JK. van Kammen DP. Gurklis J. Abnormal incorporation of arachidonic acid into platelets of drug-free patients with schizophrenia. Psychiatry Res. 1996;60:11–21. doi: 10.1016/0165-1781(95)02832-3. [DOI] [PubMed] [Google Scholar]
- 296.Yao JK. van Kammen DP. Welker JA. Red blood cell membrane dynamics in schizophrenia. II. Fatty acid composition. Schizophr Res. 1994;13:217–226. doi: 10.1016/0920-9964(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 297.Yao JK. van Kammen DP. Reddy RD. Kelley ME. Superoxide dismutase and negative symptoms in schizophrenia. Biol Psychiatry. 1998;43:123S–124S. [Google Scholar]
- 298.Yao JK. Yasaei P. van Kammen DP. Increased turnover of platelet phosphatidylinositol in schizophrenia. Prostaglandins Leukot Essent Fatty Acids. 1992;46:39–46. doi: 10.1016/0952-3278(92)90057-p. [DOI] [PubMed] [Google Scholar]
- 299.Yao JK. Abnormalities of fatty acid metabolism in red cells, platelets and brain in schizophrenia. In: Peet M, editor; Glen I, editor; Horrobin DF, editor. Phospholipid Spectrum Disorders in Psychiatry and Neurology. 2nd. Lancashire, UK: Marius Press; 2003. pp. 193–212. [Google Scholar]
- 300.Zhang F. Dryhurst G. Effects of l-cysteine on the oxidative chemistry of dopamine: new reaction pathways of potential relevance to ideopathic Parkinson's disease. J Med Chem. 1994;37:1084–1098. doi: 10.1021/jm00034a006. [DOI] [PubMed] [Google Scholar]
- 301.Zhang XY. Chen DC. Xiu MH. Wang F. Qi LY. Sun HQ. Chen S. He SC. Wu GY. Haile CN. Kosten TA. Lu L. Kosten TR. The novel oxidative stress marker thioredoxin is increased in first-episode schizophrenic patients. Schizophr Res. 2009;113:151–157. doi: 10.1016/j.schres.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 302.Zhang XY. Tan YL. Cao LY. Wu GY. Xu Q. Shen Y. Zhou DF. Antioxidant enzymes and lipid peroxidation in different forms of schizophrenia treated with typical and atypical antipsychotics. Schizophr Res. 2006;81:291–300. doi: 10.1016/j.schres.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 303.Zhang XY. Tan YL. Zhou DF. Cao LY. Wu GY. Haile CN. Kosten TA. Kosten TR. Disrupted antioxidant enzyme activity and elevated lipid peroxidation products in schizophrenic patients with tardive dyskinesia. J Clin Psychiatry. 2007;68:754–760. doi: 10.4088/jcp.v68n0513. [DOI] [PubMed] [Google Scholar]
- 304.Zhang XY. Tan YL. Zhou DF. Haile CN. Wu GY. Cao LY. Kosten TA. Kosten TR. Nicotine dependence, symptoms and oxidative stress in male patients with schizophrenia. Neuropsychopharmacology. 2007;32:2020–2024. doi: 10.1038/sj.npp.1301317. [DOI] [PubMed] [Google Scholar]
- 305.Zhang XY. Zhou DF. Cao LY. Zhang PY. Wu GY. Elevated blood superoxide dismutase in neuroleptic-free schizophrenia: association with positive symptoms. Psychiatry Res. 2003;117:85–88. doi: 10.1016/s0165-1781(02)00303-7. [DOI] [PubMed] [Google Scholar]
- 306.Zhang XY. Zhou DF. Qi LY. Chen S. Cao LY. Chen DC. Xiu MH. Wang F. Wu GY. Lu L. Kosten TA. Kosten TR. Superoxide dismutase and cytokines in chronic patients with schizophrenia: association with psychopathology and response to antipsychotics. Psychopharmacology (Berl) 2009;204:177–184. doi: 10.1007/s00213-008-1447-6. [DOI] [PubMed] [Google Scholar]
- 307.Zhang ZJ. Ramchand CN. Ramchand R. Milner E. Telang SD. Peet M. Glutathione peroxidase (GSHPx) activity in plasma and fibroblasts from schizophrenics and control. Biol Psychiatry. 1998;29:103–104. [Google Scholar]
- 308.Zoroglu SS. Herken H. Yurekli M. Uz E. Tutkun H. Savas HA. Bagci C. Ozen ME. Cengiz B. Cakmak EA. Dogru MI. Akyol O. The possible pathophysiological role of plasma nitric oxide and adrenomedullin in schizophrenia. J Psychiatry Res. 2002;36:309–315. doi: 10.1016/s0022-3956(02)00014-6. [DOI] [PubMed] [Google Scholar]