Abstract
Hypoxia-inducible transcription factor (HIF)-prolyl hydroxylases domain (PHD-1–3) are oxygen sensors that regulate the stability of the HIFs in an oxygen-dependent manner. Suppression of PHD enzymes leads to stabilization of HIFs and offers a potential treatment option for many ischemic disorders, such as peripheral artery occlusive disease, myocardial infarction, and stroke. Here, we show that homozygous disruption of PHD-1 (PHD-1−/−) could facilitate HIF-1α-mediated cardioprotection in ischemia/reperfused (I/R) myocardium. Wild-type (WT) and PHD-1−/− mice were randomized into WT time-matched control (TMC), PHD-1−/− TMC (PHD1TMC), WT I/R, and PHD-1−/− I/R (PHD1IR). Isolated hearts from each group were subjected to 30 min of global ischemia followed by 2 h of reperfusion. TMC hearts were perfused for 2 h 30 min without ischemia. Decreased infarct size (35% ± 0.6% vs. 49% ± 0.4%) and apoptotic cardiomyocytes (106 ± 13 vs. 233 ± 21 counts/100 high-power field) were observed in PHD1IR compared to wild-type ischemia/reperfusion (WTIR). Protein expression of HIF-1α was significantly increased in PHD1IR compared to WTIR. mRNA expression of β-catenin (1.9-fold), endothelial nitric oxide synthase (1.9-fold), p65 (1.9-fold), and Bcl-2 (2.7-fold) were upregulated in the PHD1IR compared with WTIR, which was studied by real-time quantitative polymerase chain reaction. Further, gel-shift analysis showed increased DNA binding activity of HIF-1α and nuclear factor-kappaB in PHD1IR compared to WTIR. In addition, nuclear translocation of β-catenin was increased in PHD1IR compared with WTIR. These findings indicated that silencing of PHD-1 attenuates myocardial I/R injury probably by enhancing HIF-1α/β-catenin/endothelial nitric oxide synthase/nuclear factor-kappaB and Bcl-2 signaling pathway. Antioxid. Redox Signal. 15, 1789–1797.
Introduction
The prolyl hydroxylase domain (PHD) enzymes PHD-1, PHD-2, and PHD-3 are oxygen sensors that regulate the stability of hypoxia-inducible transcription factor-1α (HIF-1α) in an oxygen-dependent manner, thus mediating cellular adaptive responses to changes in oxygen supply (12). Under normoxic condition, HIF-1α protein undergoes rapid ubiquitination followed by proteasomal degradation after interacting with von Hippel-Lindau protein (38). Interaction between von Hippel-Lindau protein and the oxygen-dependent degradation domain of the HIF-1α subunit is regulated through hydroxylation of 402/564 proline residues by PHDs (13). PHDs are consequently emerged as an attractive therapeutic target for ischemic diseases, since manipulation of responses to hypoxia is desirable in many disease states, including cardiovascular diseases. Recently, Bao et al. (2) have hypothesized that a novel, orally active PHD inhibitor GSK360A, could facilitate local and systemic HIF-1α signaling and protect the failing heart after myocardial infarction. Thus, research has been ongoing to inhibit the three PHD isoforms. PHD-2, among all three PHDs, is mostly studied because it is the most abundantly expressed isoform, whereas the information about PHD-1 isoform in cardiovascular pathology is scarce. However, recently, Schneider et al. (29) reported that mouse liver ischemia/reperfusion (I/R) injury was protected by silencing of the PHD-1. Further, Aragones et al. (1) showed the deficiency or inhibition of PHD-1 induces hypoxia tolerance of skeletal muscle by reprogramming basal metabolism.
The restoration of blood flow to a previously ischemic region is known to induce pathological events leading to myocardial tissue injury greater than original ischemic insult. Enhanced generation of highly reactive oxygen species by the heart during the acute reperfusion phase has been postulated to affect glycolysis, erythropoiesis, angiogenesis, and apoptosis by decreasing the stability of HIF-1α (13, 32). Under these conditions, the lack of PHD-1 expression leads to HIF-1α accumulation and its nuclear translocation. HIF-1α then dimerizes with its other subunits, and activates a multitude of angiogenic, glycolytic, erythropoiesis, and anti-apoptotic pathways, which ultimately leads to cardioprotection (36). Seeking to assess if inhibition of PHDs could offer the cardioprotection, Eckle et al. (4) have studied the effect of administration of dimethyloxallyl glycine (DMOG) to mice before subjection to I/R. They documented that the effect of DMOG is cardioprotective as seen in reduced infarct size and also they found this effect is equal to the benefit of ischemic preconditioning (4). Ockaili et al. (20) also confirmed the cardioprotective nature of DMOG via reduction in infarct size in DMOG-treated rabbit hearts. Both FibroGen (22) and GlaxoSmithKline (2) are in the process of developing prolyl-4-hydroxylase inhibitors as cardioprotective compounds.
HIF-1α mediates transcription of >2% of proteins in mammalian cells and modulates all angiogenic and apoptotic pathways, making control of HIF-1α vital for cardioprotection (7). The cardioprotective role of endothelial nitric oxide synthase (eNOS) has been previously well established (26). Jones et al. (10) have shown increased myocardial apoptosis and infarct size in eNOS knockout (KO) mice, demonstrating the role of eNOS in cardioprotection. Very recently, Ng et al. (19) reported that overexpression of HIF-1α in cultured embryonic stem cells showed increased differentiation into cardiomyocytes and this effect was abolished with the treatment of L-NG-Nitroarginine methyl ester, a well-known NOS inhibitor. These results demonstrate the importance of eNOS in HIF-1α-mediated cardioprotection (19). In addition, HIF-1α also known to propagate translation of nuclear factor-kappaB (NF-κB) (6), a well-known redox transcription factor (28).
From the understanding of PHD-1 regulation in HIF-1α-mediated cardioprotection, it has been hypothesized that PHD-1 inhibition could enhance cardiac recovery from myocardial I/R injury through expression of HIF-1α and its target proteins related to cardioprotection. Hence, the present study sought to verify whether PHD-1 inhibition could protect myocardium from I/R injury by upregulation of β-catenin/eNOS/NF-κB and Bcl-2-mediated cardioprotection through accumulation of HIF-1α.
Materials and Methods
Experimental animals
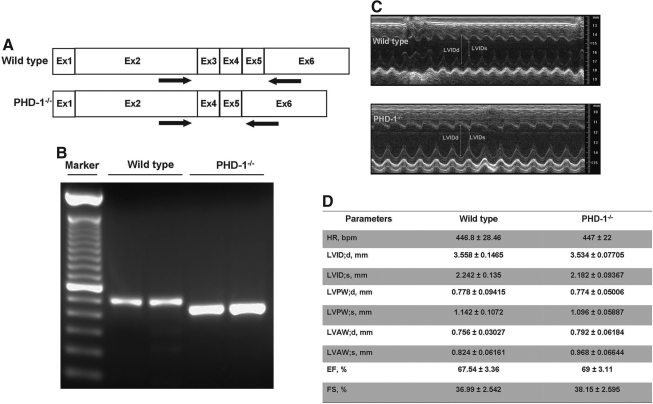
All animals received care in compliance with the principles of laboratory animal care formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health. PHD-1 KO mice (referred to as PHD-1−/−) used in this study were generated as described previously (31) and were a generous gift from Dr. Fong GH, Vascular Biology, UCONN Health Centre, CT. Briefly, mice carrying floxed PHD-1 exon 3 (PHD-1f/+) were initially created by homologous recombination method, and further crossed with transgenic mice expressing Cre under the control of adenoviral EIIa promoter (The Jackson Laboratory, Maine) to obtain PHD-1+/− mice. PHD-1−/− mice were obtained by PHD-1+/− intercrossing. All procedures used in handling mice were approved by the Animal Care Committee at the University of Connecticut Health Center in compliance with Animal Welfare Assurance. The genotyping of PHD-1−/− animals were conformed by polymerase chain reaction (PCR) from tail purified DNA as well as real-time (RT) PCR from myocardial RNA and the representative genotyping data from myocardial RNA is given in Figure 1A and B.
FIG. 1.
PHD-1 knockout does not appear to affect cardiac morphology and functions (echocardiographic evaluation) at baseline. (A) The validation of PHD-1−/− mice. The position of real-time polymerase chain reaction primers for PHD-1 mRNA is shown. Note that the exon 3-encoded region is deleted in PHD-1−/− mRNA. (B) Real-time polymerase chain reaction was performed using RNA isolated from myocardial tissue in WT and PHD-1−/− mice. The bands in PHD-1−/− mice were 120 bp shorter than WT bands. (C) Representative echocardiograph pictures of parasternal short-axis M mode images at baseline from WT and PHD-1−/− mice. (D) The quantitative data of systolic and diastolic LVID, and LVAW and LVPW thickness, and ejection fraction, fractional shortening, and heart rate from five different animals in each group. These results demonstrate that there was no difference in dynamic cardiac morphology and cardiac performance in the PHD-1−/− mice compared with WT mice. PHD-1−/− indicates PHD-1 knockout mice; HR, heart rate; bpm, beats per minute; LVIDs, left ventricular internal diameter in systole; LVIDd, LVID in diastole; LVPWs, left ventricular posterior wall thickness in systole; LVPWd, LVPW in diastole; LVAWd, left ventricular anterior wall thickness in diastole; LVAWs, LVAW in systole; EF, ejection fraction; FS, fractional shortening; WT, wild type.
Experimental protocol
PHD-1−/− (KO) mice and corresponding wild-type (WT) mice were randomized into four groups, WT time-matched control (TMC), PHD-1−/− TMC (PHD1TMC), wild-type ischemia/reperfusion (WTIR), and PHD-1−/− I/R (PHD1IR). To create I/R injury, after a 10 min of washout period and stabilization, isolated hearts were exposed to zero-flow normothermic global ischemia for 30 min followed by 120 min of reperfusion, and, for TMC, after stabilization, hearts were perfused totally for 2 h and 30 min without ischemia.
Langendorff's perfusion
The mice were anesthetized with sodium pentobarbital (150–200 mg/kg bw, ip injection; Abbott Laboratories) and anticoagulated with heparin (500 U/kg bw, ip injection; Elkins–Sinn Inc.). The hearts were excised and immediately immersed in ice-cold perfusion buffer, consisted of a modified Krebs–Henseleit bicarbonate buffer [composed of (in mM) 118 NaCl, 4.7 KCl, 1.2 MgSO4, 25 NaHCO3, 10 glucose, and 1.7 CaCl2; gassed with 95% O2:5% CO2; and filtered through a 5-μm filter to remove any particulate contaminants, pH 7.4]. Same buffer was maintained at a constant temperature of 37°C and was gassed continuously in Langendorff's apparatus for the entire duration of the experiment (33, 35). The aorta was cannulated and did retrograde perfusion in the Langendorff's mode through the aortic cannula at a perfusion pressure of 60 mm Hg.
Measurement of infarct size
At the end of reperfusion, the hearts were perfused with 1% (w/v) solution of triphenyl tetrazolium chloride in phosphate buffer through the aortic cannula (35). Then, the hearts were excised and stored at −70°C for later analysis. Sections of frozen hearts were fixed in 10% formalin by placing between two coverslips, and digitally imaged with the use of an Epson scanner. To quantitate the areas of interest in pixels, Scion Image (Scion Corp) analyzing software was used.
Determination of cardiomyocyte apoptosis
After reperfusion, formaldehyde-fixed left ventricle was embedded in paraffin, cut into transverse sections (4 μm thick), and deparaffinized with a graded series of histoclear and ethanol solutions. Immunohistochemical detection of apoptotic cells was carried out by terminal dUTP nick end-labeling (TUNEL) reaction using an In Situ Cell Death Detection Kit (Roche Diagnostics) and fluorescein as per the kit protocol. In brief, the TUNEL reaction preferentially labels DNA strand breaks generated during apoptosis, which can be identified by labeling free 3′-OH termini with modified nucleotides in an enzymatic reaction catalyzed by terminal deoxynucleotidyltransferase. Fluorescein labels incorporated in nucleotide polymers are detected by fluorescence microscopy. The sections were washed three times in phosphate-buffered saline (PBS), blocked with 10% normal goat serum in PBS, and incubated with mouse monoclonal sarcomeric actin (Sigma) followed by staining with Alexa Fluor 555 donkey anti-mouse IgG (1:200 dilution; Invitrogen). After incubation, the sections were rinsed 3 times in PBS and mounted with Vectashield mounting medium (Vector Laboratories). The sections were observed, and images were captured using a confocal laser Zeiss LSM 510 Meta microscope. For quantitative purposes, the number of TUNEL-positive cardiomyocytes was counted on 100 high-power fields (25).
Quantitative RT-PCR
RNA was isolated from mouse left heart ventricular tissue by Qiagen extraction kit. According to the manufacturer's instructions, RNA was converted to cDNA with iScript cDNA Synthesis Kit (Bio-Rad). Quantitative real-time (qRT)-PCR was carried out by Bio-Rad IQ5 cycler. All samples were analyzed in duplicates using IQ SYBR Green Super mix (Bio-Rad) and β-actin as an internal control. The RT-PCR cycling conditions include initial denaturation at 95°C for 3 min followed by 35 cycles of denaturation at 95°C for 10 s, annealing at 60°C for 15 s, and extension at 72°C for 15 s. The data were analyzed with the help of BIO-RAD IQ5 software (35). The primer sequences used for qRT-PCR were as follows: Mu Bcl-2 forward: 5′-GGG ATG CCT TTG TGG AAC TA-3′, reverse: 5′-CTC ACT TGT GGC CCA GGT AT-3′; Mu p65 forward: 5′-TCT GCT TCC AGG TGA CAG TG-3′, reverse: 5′-ATC TTG AGC TCG GCA GTG TT-3′; Mu β-catenin forward: 5′-ACA GGG TGC TAT TCC ACG AC-3′, reverse: 5′-TTA CCC CAC CCC ATT TAC AA-3′; and Mu eNOS forward: 5′-TCT ACC GGG ACG AGG TAC TG-3′, reverse: 5′-CTG TCC TCA GGA GGT CTT GC-3′ (34, 35).
Immunohistochemistry for HIF-1α expression and β-catenin nuclear translocation
The immunohistochemical expression of HIF-1α and nuclear translocation of β-catenin was performed after 30 min ischemia followed by 2 h reperfusion in the paraffin-embedded heart sections (34). Myocardial sections were analyzed for the reactivity with 1:100 dilution of polyclonal anti-rabbit HIF-1α (Novus Biologicals) or 1:100 diluted monoclonal anti-rabbit β-catenin (Santa Cruz Biotechnology). Bound antibody was detected with the Vectastain ABC kit (Vector Laboratories) and observed with DAB, and the nucleus was counter stained with hematoxylin (for β-catenin only) (Sigma) and observed under light microscope.
Electrophoretic mobility shift assay for HIF-1α and NF-κB
Electrophoretic mobility shift assay (EMSA)/gel shift was performed by using 10 μg of the nuclear extracts, and the extract was incubated for 20 min at room temperature with 32P end-labeled oligonucleotides containing the putative HIF-1α (5′-TCTGTACGTGACCACACTCACCTC-3′) (Santa Cruz Biotechnology) or NF-κB (5′-AGTTGAGGGGACTTTCCCA GGC-3′) (Promega) binding site. Reaction products were resolved on 5% nondenaturing polyacrylamide gel. The specificity of the DNA–protein interaction was established by competition experiments using 10× cold HIF-1α oligonucleotide as the competitor or adding p65 antibody for supershift for NF-κB. After electrophoresis, gels were dried and observed by autoradiography (24).
Echocardiographic evaluation of cardiac morphology and functions
The 8–12-week-old WT or PHD-1−/− mice were sedated using isoflurane (2%, vol/vol). When adequately sedated, the mice were secured with tape in the supine position on a custom-built mold designed to maintain the mouse's natural body contours. The chest hair was removed using a commercially available hair remover from Nair and cotton swab. Ultrasound gel was spread over the precordium and ultrasound biomicroscopy (Vevo 770; Visual-Sonics Inc.) with a 25-MHz transducer was employed to observe the left ventricle. The left ventricle was imaged in apical, parasternal long axis, and parasternal short axis views for ventricular systolic function, LV cavity diameter, wall thickness, diastolic function, and end-systolic and end diastolic volume determination. Two-dimensional M-mode images of the LV short axis were taken just below the level of the papillary muscles to analyze ventricular wall thickness and chamber diameter. Ejection fraction and fractional shortening were assessed for LV systolic function. All measurements represent the mean of at least three consecutive cardiac cycles. Throughout the procedure, the electrocardiogram, respiratory rate, and heart rate were monitored as described previously (25).
Statistical analysis
Results were expressed as mean ± SEM. Two-tailed unpaired t-test (Graph Pad Prism software) was carried out to determine differences between the mean values of all groups. The results were considered significant at p ≤ 0.05.
Results
Cardiac morphology and ventricular function of PHD-1−/− mice compared to WT at baseline (echocardiographic evaluation)
The validation of PHD-1−/− mice is provided in Figure 1A and B. The position of RT-PCR primers for PHD-1 mRNA is shown (Fig. 1A). Note that the exon 3-encoded region is deleted in PHD-1−/− mRNA. RT-PCR was performed using RNA isolated from myocardial tissue in WT and PHD-1−/− mice (Fig. 1B). The bands in PHD-1−/− mice were 120 bp shorter than WT bands. Left ventricular functional parameters in WT and PHD-1−/− mice were studied by echocardiography (n = 5) at baseline. Left ventricular function was estimated by quantitating ejection fraction and fractional shortening on the short axis. M mode images (Fig. 1C) of WT and PHD-1−/− and functional parameters (Fig. 1D) demonstrate no difference in the PHD-1−/− mice when compared to the WT mice at baseline. Further, there were differences neither between systolic and diastolic left ventricular internal diameter nor between anterior and posterior wall thickness, whether in systole or in diastole between WT and PHD-1−/− mice. Therefore, PHD-1 KO does not appear to affect dynamic cardiac morphology or cardiac performance at baseline.
PHD-1−/− significantly reduced infarct size and apoptotic cardiomyocytes after I/R injury
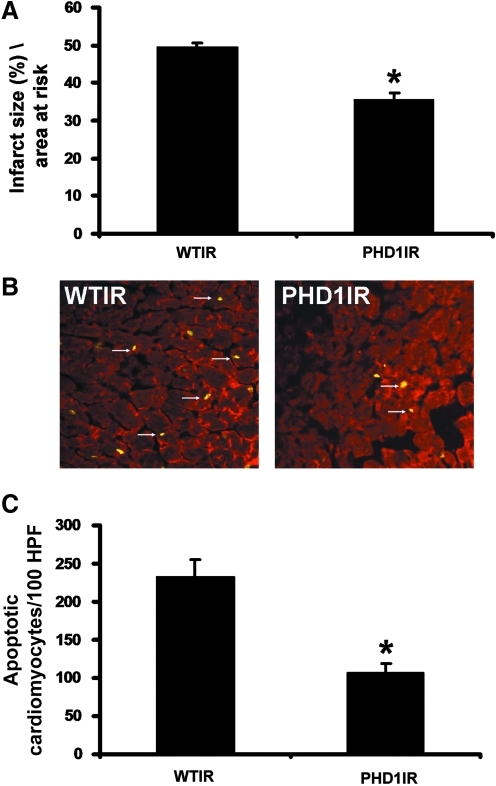
To understand whether PHD-1 KO is cardioprotective by reduction in infarct size and cardiomyocyte apoptosis, we performed TTC staining for infarct size (n = 6), and a TUNEL assay followed by antibody staining with anti-α-sarcomeric actin to measure cardiomyocyte apoptosis (n = 4 per group) after 30 min of ischemia followed by 2 h of reperfusion. The results showed significant decrease in infarct size in PHD1IR group when compared to WTIR group (35%±Ԅ 0.6% vs. 49% ± 0.4%) (Fig. 2A). We also observed a significant decrease in apoptotic cardiomyocytes in PHD1IR group compared with the WTIR group (106± 13 vs. 233± 21 counts/100 high-power field) (Fig. 2B and C). These results demonstrated that inhibition of PHD-1 reduces apoptotic signal during I/R.
FIG. 2.
PHD-1−/− mice showed decreased infarct size and cardiomyocyte apoptosis after I/R. (A) The quantitative analysis from 6 different animals (n = 6) showed decreased infarct size in PHD1IR when compared to WTIR. (B) Representative digital micrographs showing cardiomyocyte apoptosis of PHD1IR and WTIR groups. (C) Quantitative analysis of cardiomyocyte apoptosis after I/R injury from 4 different animals in each group, in counts/100 high-power field (HPF). The apoptotic cardiomyocytes were significantly reduced in PHD1IR compared to WTIR. WTIR indicates WT animals subjected to I/R injury; PHD1IR, PHD-1−/− animals subjected to I/R injury. *p ≤ 0.05, versus WTIR. I/R, ischemia/reperfusion. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
Molecular basis for the cardioprotective effect of PHD-1 KO during myocardial I/R injury
KO of PHD-1 increased the protein expression and also DNA binding activity of HIF-1 α
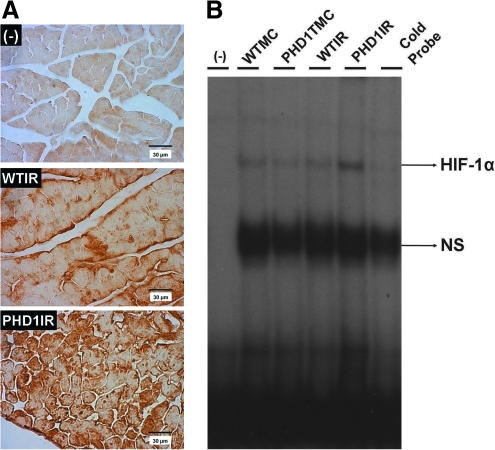
PHD1IR group showed significantly increased HIF-1α (mol. wt. 115 kDa) protein expression (immunohistochemical analysis) compared to WTIR (Fig. 3A). The EMSA results also showed increased DNA binding activity of HIF-1α in PHD1IR when compared to WTIR (Fig. 3B). These results suggested that cardioprotective property of PHD-1 inhibition is probably through HIF-1α expression followed by upregulation of its target genes.
FIG. 3.
Effect of PHD-1 knockout on protein expression and DNA binding activity of HIF-1α after I/R. (A) Representative micrographs shows the protein expression of HIF-1α (mol. wt. ∼115 kDa) after I/R from different groups. The experiments were performed from three to four animals of each group. (B) EMSA analysis of DNA binding activity of HIF-1α (n = 4 from each group). These results showed increased expression, nuclear translocation, and DNA binding activity of HIF-1α in PHD1IR compared with WTIR. In EMSA, the position of the HIF-1α band was confirmed by the disappearance of the corresponding band in the last lane from right side when we used unlabelled probe (cold probe). WTMC indicates WT animals' time-matched control; PHD1TMC, PHD-1−/− animals time-matched control. HIF-1α, hypoxia-inducible transcription factor-1α; EMSA, electrophoretic mobility shift assay. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
PHD-1 KO significantly increased the mRNA expression of eNOS and Bcl-2
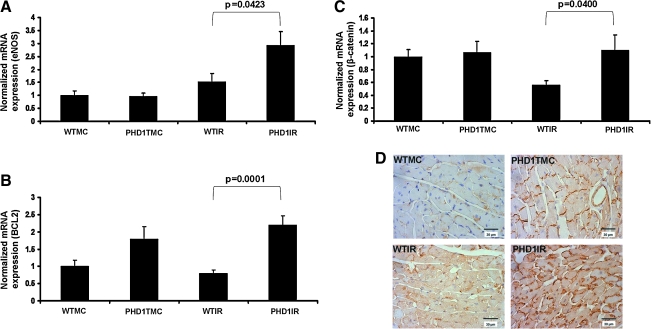
To examine whether PHD-1 inhibition modulates the expression of eNOS and Bcl-2 after I/R, we performed qRT-PCR. The extent of mRNA expression related to eNOS (Fig. 4A) and Bcl-2 (Fig. 4B) after 30 min of ischemia followed by 2 h of reperfusion documented increased eNOS (1.9-fold) and Bcl-2 (2.7-fold) in PHD1IR as compared to WTIR. These results suggested that the cardioprotective response of PHD-1 inhibition against I/R injury may be through upregulation of eNOS and Bcl-2.
FIG. 4.
Effect of PHD-1 knockout on mRNA expression of β-catenin, eNOS and Bcl-2, and nuclear translocation of β-catenin after I/R. Bar graphs show the mRNA expression of eNOS (A), Bcl-2 (B), β-catenin (C), and after I/R from different groups. The values are mean ± SEM of 4–5 animals from each group. The results showed increased mRNA expression of eNOS, β-catenin, and Bcl-2 in PHD1IR compared to WTIR. (D) Representative digital micrographs (immunohistochemical analysis by DAB staining and counter nuclear staining by hematoxylin) showing β-catenin (mol. wt. ∼92 kDa) nuclear translocation after I/R in different experimental groups; n = 4–5 per group. There was a significant increase in the expression of β-catenin as well as translocation of β-catenin into the nucleus in PHD1IR compared to WTIR. The value on top of the bars represents the two-tailed p-value (statistical difference) between WTIR and PHD1IR. eNOS, endothelial nitric oxide synthase. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
PHD-1 KO significantly increased the mRNA expression and also nuclear translocation of β-catenin
We found significantly increased β-catenin (mol. wt. 92 kDa) expression at mRNA level in PHD1IR group when compared to WTIR (1.9-fold) (Fig. 4C), indicating the possible role of β-catenin in PHD-1 inhibition-mediated cardioprotection during I/R. Similarly, immunohistochemical analysis showed significant increase in nuclear translocation of β-catenin in PHD1IR when compared to WTIR (n = 4 to 5 per group) (Fig. 4D).
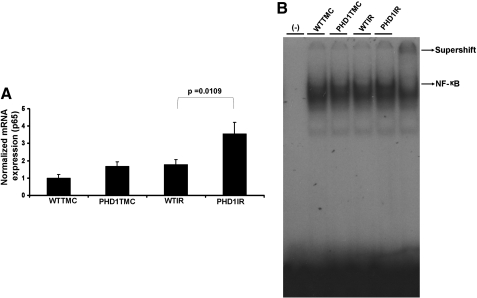
PHD-1 KO increased the mRNA expression of p65 and also DNA binding activity of NF-κB
qRT-PCR analysis showed significant increase in the mRNA expression of p65 subunit of NF-κB in PHD1IR when compared to WTIR (1.9-fold) (Fig. 5A). Further, significant increase in the DNA binding activity of NF-κB in PHD1IR as compared to WTIR (Fig. 5B) was revealed by super shift analysis.
FIG. 5.
Effect of PHD-1 knockout on expression of p65 (subunit of NF-κB) and DNA binding activity of NF-κB after I/R. (A) Bar graph shows the mRNA expression of p65 in different groups after I/R injury. The values are mean ± SEM of 3–4 animals from each group. (B) EMSA analysis of DNA binding activity of NF-κB (n = 4 from each group). These results showed increased expression, nuclear translocation, and DNA binding activity of NF-κB in PHD1IR compared to WTIR. The position of the NF-κB band was confirmed by the super shift band with NF-κB antibody. The super shift band is given in separate box, which was longer exposure of the same blot of NF-κB. The value on top of the bars represents the two-tailed p-value (statistical difference) between WTIR and PHD1IR. NF-κB, nuclear factor-kappaB.
Discussion
The present study documents that homozygous KO of PHD-1 protects the myocardium from I/R injury through HIF-1α followed by the increased expression of β-catenin (also nuclear translocation), eNOS, and P65 (NF-κB) at mRNA and protein level. In addition, we observed increased expression of anti-apoptotic protein Bcl-2 and reduced cardiomyocyte apoptosis in PHD-1−/− mice. In association with these molecular events, we also observed reduced infarct size. The present basic mechanistic study results indicate that increased expression of HIF-1α by inhibiting PHD-1 has beneficial effects in the treatment of I/R-mediated cardiac injury. Hence, our present study is of significant clinical importance to seek development of pharmacological inhibitors of PHD-1 to treat human cardiovascular complications.
Increased cardiomyocyte and endothelial cell apoptosis and reduction of antioxidative and angiogenic factors have been previously reported in the I/R myocardium (5, 15). Oxidative stress due to excessive reactive oxygen species generation in the reperfused myocardium plays a key role in apoptotic cardiomyocyte death (15). In the present study, we observed that the PHD-1 inhibition significantly reduces cardiomyocyte apoptosis after I/R, as evidenced by the reduction in TUNEL-positive cardiomyocytes. This reduction of apoptosis in PHD-1 KO mice might be due to decreased oxidative stress via increased expression of HIF-1α (1, 32) followed by anti-apoptotic protein Bcl-2 expression (37, 39). Malhotra et al. (14) have shown that HIF-1α is a critical mediator of hypoxia-induced apoptosis in cardiac H9c2 and kidney epithelial HK-2 cells. Further, hearts of PHD-2 hypomorphic mice showed protection against acute I/R injury by attenuating apoptosis via induction of Bcl-2 family proteins (8).
We also observed increased expression of eNOS levels in PHD-1−/− mice during I/R when compared to WT animals. The increased eNOS levels by PHD-1 inhibition might be due to increased HIF-1α expression, which was evident in the present study by immunohistochemistry as well as DNA binding activity by EMSA. Jiang et al. (9) showed that ischemic limbs were protected by transplantation of HIF-1α-modified endothelial progenitor cells and this was through increased expression of eNOS, indicating the possible role of eNOS in HIF-1α-mediated protection. Therefore, HIF-1α stabilization might represent an alternative strategy, and indeed, several preclinical studies reported that pharmacological HIF-1α stabilization induced angiogenesis and arteriogenesis in different animal models of cardiac ischemia (18) or cerebral ischemia (3, 30). Further, nitric oxide (NO) produced by eNOS is a signaling molecule with multiple protective functions in the heart especially during I/R injury (11). It has been previously reported that NO inhibits lipid peroxidation and thus regulate apoptosis in heart (17). Thus, the increased eNOS expression and/or NO activation in PHD1IR may be involved in the decreased apoptosis and ultimately cardioprotection. However, further in vivo studies are needed to confirm the exact role of eNOS in PHD-1 inhibition-mediated cardioprotection, which are ongoing in our laboratory.
We further documented the increased expression of β-catenin as well as its nuclear translocation in PHD-1−/− mice subjected to I/R injury when compared to WT mice. β-catenin, the downstream target of glycogen synthase kinase-β plays a vital role during development and also apoptosis (21, 34). Our group has already documented the importance of β-catenin in ischemic preconditioning-mediated cardioprotection and angiogenesis by using Ad-sh-β-catenin (34). In the same study, we further reported that inhibition of β-catenin significantly attenuated the ischemic preconditioning-mediated reduction in apoptosis and increased expression of Bcl-2 (34). This could explain the reduction of apoptotic cardiomyocytes in PHD1IR might be, at least partially, due to increased expression of β-catenin. Therefore, the reduced apoptosis and increased survival factors in PHD1IR might be due to, partially, increased expression and translocation of β-catenin in the (I/R) myocardium.
The importance of NF-κB activation and its role in hypoxic preconditioning-mediated myocardial angiogenesis has been previously documented from our group (27, 28). HIF-1α has been previously shown to increase the expression of NF-κB during inflammation and ischemia (6). Further, both vascular endothelial growth factor and Ang-1 have been shown to increase the DNA binding activity of NF-κB, thereby modulating endothelial cell proliferation, survival, and migration (16). Studies have documented that NF-κB activates several pro-survival genes, cytokines, and inhibitors of apoptosis necessary for endothelial cell survival (23). Our group has recently shown that combination gene therapy using vascular endothelial growth factor and Ang-1 significantly increased myocardial angiogenesis and reduced ventricular remodeling through the induction of NF-κB activation in ischemic hearts of diabetic rats (24). The observed reduction in apoptosis and the increase in the expression of survival proteins by HIF-1α expression in PHD-1 KO might have led to a reduction in infarct size in PHD1IR group as compared to WTIR.
In conclusion, the present findings indicate that PHD-1 inhibition protects myocardium from (I/R) injury by reducing apoptosis and infarct size. The molecular mechanism behind the PHD-1-mediated cardioprotection is probably HIF-1α expression followed by regulation of its target genes, including β-catenin, eNOS, NF-κB, and Bcl-2. This basic mechanistic study also warrants researchers to develop pharmacological inhibitors of PHD-1 to treat human cardiovascular complications. However, further investigations using in vivo model of myocardial infarction and I/R are necessary to confirm the cardioprotective role and the underlying molecular mechanism of PHD-1 inhibition.
Abbreviations Used
- DMOG
dimethyloxallyl glycine
- EMSA
electrophoretic mobility shift assay
- eNOS
endothelial nitric oxide synthase
- HIF-1α
hypoxia inducible transcription factor-1α
- HPF
high-power field
- I/R
ischemia/reperfusion
- KO
knockout
- LVAWd
left ventricular anterior wall thickness in diastole
- LVAWs
left ventricular anterior wall thickness in systole
- LVIDd
left ventricular internal diameter in diastole
- LVIDs
left ventricular internal diameter in systole
- NF-κB
nuclear factor-kappaB
- PBS
phosphate-buffered saline
- PCR
polymerase chain reaction
- PHD-1E/E
homozygous knockout prolyl hydroxylase domain enzymes mice
- PHD1IR
PHD-1E/E ischemia/reperfusion
- PHD1TMC
PHD-1E/E time-matched control
- PHDs
prolyl hydroxylase domain enzymes
- qRT-PCR
quantitative real-time PCR
- TMC
time-matched control
- TUNEL
terminal dUTP nick end-labeling
- WT
wild type
- WTIR
wild-type ischemia/reperfusion
- WTMC
wild-type time-matched control
Acknowledgments
This study was supported by the grant HL-85804 (N. Maulik) and also 5R01EY019721 (G.-H. Fong).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Aragones J. Schneider M. Van Geyte K. Fraisl P. Dresselaers T. Mazzone M. Dirkx R. Zacchigna S. Lemieux H. Jeoung NH. Lambrechts D. Bishop T. Lafuste P. Diez-Juan A. Harten SK. Van Noten P. De Bock K. Willam C. Tjwa M. Grosfeld A. Navet R. Moons L. Vandendriessche T. Deroose C. Wijeyekoon B. Nuyts J. Jordan B. Silasi-Mansat R. Lupu F. Dewerchin M. Pugh C. Salmon P. Mortelmans L. Gallez B. Gorus F. Buyse J. Sluse F. Harris RA. Gnaiger E. Hespel P. Van Hecke P. Schuit F. Van Veldhoven P. Ratcliffe P. Baes M. Maxwell P. Carmeliet P. Deficiency or inhibition of oxygen sensor Phd1 induces hypoxia tolerance by reprogramming basal metabolism. Nat Genet. 2008;40:170–180. doi: 10.1038/ng.2007.62. [DOI] [PubMed] [Google Scholar]
- 2.Bao W. Qin P. Needle S. Erickson-Miller CL. Duffy KJ. Ariazi JL. Zhao S. Olzinski AR. Behm DJ. Pipes GC. Jucker BM. Hu E. Lepore JJ. Willette RN. Chronic inhibition of hypoxia-inducible factor prolyl 4-hydroxylase improves ventricular performance, remodeling, and vascularity after myocardial infarction in the rat. J Cardiovasc Pharmacol. 2010;56:147–155. doi: 10.1097/FJC.0b013e3181e2bfef. [DOI] [PubMed] [Google Scholar]
- 3.Baranova O. Miranda LF. Pichiule P. Dragatsis I. Johnson RS. Chavez JC. Neuron-specific inactivation of the hypoxia inducible factor 1 alpha increases brain injury in a mouse model of transient focal cerebral ischemia. J Neurosci. 2007;27:6320–6332. doi: 10.1523/JNEUROSCI.0449-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eckle T. Kohler D. Lehmann R. El Kasmi K. Eltzschig HK. Hypoxia-inducible factor-1 is central to cardioprotection: a new paradigm for ischemic preconditioning. Circulation. 2008;118:166–175. doi: 10.1161/CIRCULATIONAHA.107.758516. [DOI] [PubMed] [Google Scholar]
- 5.Foadoddini M. Esmailidehaj M. Mehrani H. Sadraei SH. Golmanesh L. Wahhabaghai H. Valen G. Khoshbaten A. Pretreatment with hyperoxia reduces in vivo infarct size and cell death by apoptosis with an early and delayed phase of protection. Eur J Cardiothorac Surg. 2011;39:233–240. doi: 10.1016/j.ejcts.2010.05.036. [DOI] [PubMed] [Google Scholar]
- 6.Fraisl P. Aragones J. Carmeliet P. Inhibition of oxygen sensors as a therapeutic strategy for ischaemic and inflammatory disease. Nat Rev Drug Discov. 2009;8:139–152. doi: 10.1038/nrd2761. [DOI] [PubMed] [Google Scholar]
- 7.Hirota K. Semenza GL. Regulation of angiogenesis by hypoxia-inducible factor 1. Crit Rev Oncol Hematol. 2006;59:15–26. doi: 10.1016/j.critrevonc.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Hyvarinen J. Hassinen IE. Sormunen R. Maki JM. Kivirikko KI. Koivunen P. Myllyharju J. Hearts of hypoxia-inducible factor prolyl 4-hydroxylase-2 hypomorphic mice show protection against acute ischemia-reperfusion injury. J Biol Chem. 2010;285:13646–13657. doi: 10.1074/jbc.M109.084855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang M. Wang B. Wang C. He B. Fan H. Guo TB. Shao Q. Gao L. Liu Y. Angiogenesis by transplantation of HIF-1 alpha modified EPCs into ischemic limbs. J Cell Biochem. 2008;103:321–334. doi: 10.1002/jcb.21416. [DOI] [PubMed] [Google Scholar]
- 10.Jones SP. Girod WG. Palazzo AJ. Granger DN. Grisham MB. Jourd'Heuil D. Huang PL. Lefer DJ. Myocardial ischemia-reperfusion injury is exacerbated in absence of endothelial cell nitric oxide synthase. Am J Physiol. 1999;276:H1567–H1573. doi: 10.1152/ajpheart.1999.276.5.H1567. [DOI] [PubMed] [Google Scholar]
- 11.Jones SP. Greer JJ. Kakkar AK. Ware PD. Turnage RH. Hicks M. van Haperen R. de Crom R. Kawashima S. Yokoyama M. Lefer DJ. Endothelial nitric oxide synthase overexpression attenuates myocardial reperfusion injury. Am J Physiol Heart Circ Physiol. 2004;286:H276–H282. doi: 10.1152/ajpheart.00129.2003. [DOI] [PubMed] [Google Scholar]
- 12.Katschinski DM. In vivo functions of the prolyl-4-hydroxylase domain oxygen sensors: direct route to the treatment of anaemia and the protection of ischaemic tissues. Acta Physiol (Oxf) 2009;195:407–414. doi: 10.1111/j.1748-1716.2008.01952.x. [DOI] [PubMed] [Google Scholar]
- 13.Ke Q. Costa M. Hypoxia-inducible factor-1 (HIF-1) Mol Pharmacol. 2006;70:1469–1480. doi: 10.1124/mol.106.027029. [DOI] [PubMed] [Google Scholar]
- 14.Malhotra R. Tyson DW. Rosevear HM. Brosius FC., 3rd Hypoxia-inducible factor-1alpha is a critical mediator of hypoxia induced apoptosis in cardiac H9c2 and kidney epithelial HK-2 cells. BMC Cardiovasc Disord. 2008;8:9. doi: 10.1186/1471-2261-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moens AL. Claeys MJ. Timmermans JP. Vrints CJ. Myocardial ischemia/reperfusion-injury, a clinical view on a complex pathophysiological process. Int J Cardiol. 2005;100:179–190. doi: 10.1016/j.ijcard.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 16.Munoz-Chapuli R. Quesada AR. Angel Medina M. Angiogenesis and signal transduction in endothelial cells. Cell Mol Life Sci. 2004;61:2224–2243. doi: 10.1007/s00018-004-4070-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Napoli C. Ignarro LJ. Nitric oxide and pathogenic mechanisms involved in the development of vascular diseases. Arch Pharm Res. 2009;32:1103–1108. doi: 10.1007/s12272-009-1801-1. [DOI] [PubMed] [Google Scholar]
- 18.Natarajan R. Salloum FN. Fisher BJ. Ownby ED. Kukreja RC. Fowler AA., 3rd Activation of hypoxia-inducible factor-1 via prolyl-4 hydoxylase-2 gene silencing attenuates acute inflammatory responses in postischemic myocardium. Am J Physiol Heart Circ Physiol. 2007;293:H1571–H1580. doi: 10.1152/ajpheart.00291.2007. [DOI] [PubMed] [Google Scholar]
- 19.Ng KM. Lee YK. Chan YC. Lai WH. Fung ML. Li RA. Siu CW. Tse HF. Exogenous expression of HIF-1 alpha promotes cardiac differentiation of embryonic stem cells. J Mol Cell Cardiol. 2010;48:1129–1137. doi: 10.1016/j.yjmcc.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 20.Ockaili R. Natarajan R. Salloum F. Fisher BJ. Jones D. Fowler AA., 3rd Kukreja RC. HIF-1 activation attenuates postischemic myocardial injury: role for heme oxygenase-1 in modulating microvascular chemokine generation. Am J Physiol Heart Circ Physiol. 2005;289:H542–H548. doi: 10.1152/ajpheart.00089.2005. [DOI] [PubMed] [Google Scholar]
- 21.Park S. Choi J. Inhibition of beta-catenin/Tcf signaling by flavonoids. J Cell Biochem. 2010;110:1376–1385. doi: 10.1002/jcb.22654. [DOI] [PubMed] [Google Scholar]
- 22.Philipp S. Jurgensen JS. Fielitz J. Bernhardt WM. Weidemann A. Schiche A. Pilz B. Dietz R. Regitz-Zagrosek V. Eckardt KU. Willenbrock R. Stabilization of hypoxia inducible factor rather than modulation of collagen metabolism improves cardiac function after acute myocardial infarction in rats. Eur J Heart Fail. 2006;8:347–354. doi: 10.1016/j.ejheart.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 23.Romashkova JA. Makarov SS. NF-kappaB is a target of AKT in anti-apoptotic PDGF signalling. Nature. 1999;401:86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- 24.Samuel SM. Akita Y. Paul D. Thirunavukkarasu M. Zhan L. Sudhakaran PR. Li C. Maulik N. Coadministration of adenoviral vascular endothelial growth factor and angiopoietin-1 enhances vascularization and reduces ventricular remodeling in the infarcted myocardium of type 1 diabetic rats. Diabetes. 2010;59:51–60. doi: 10.2337/db09-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samuel SM. Thirunavukkarasu M. Penumathsa SV. Koneru S. Zhan L. Maulik G. Sudhakaran PR. Maulik N. Thioredoxin-1 gene therapy enhances angiogenic signaling and reduces ventricular remodeling in infarcted myocardium of diabetic rats. Circulation. 2010;121:1244–1255. doi: 10.1161/CIRCULATIONAHA.109.872481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samuel SM. Thirunavukkarasu M. Penumathsa SV. Paul D. Maulik N. Akt/FOXO3a/SIRT1-mediated cardioprotection by n-tyrosol against ischemic stress in rat in vivo model of myocardial infarction: switching gears toward survival and longevity. J Agric Food Chem. 2008;56:9692–9698. doi: 10.1021/jf802050h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sasaki H. Ray PS. Zhu L. Otani H. Asahara T. Maulik N. Hypoxia/reoxygenation promotes myocardial angiogenesis via an NF kappa B-dependent mechanism in a rat model of chronic myocardial infarction. J Mol Cell Cardiol. 2001;33:283–294. doi: 10.1006/jmcc.2000.1299. [DOI] [PubMed] [Google Scholar]
- 28.Sasaki H. Zhu L. Fukuda S. Maulik N. Inhibition of NF kappa B activation by pyrrolidine dithiocarbamate prevents in vivo hypoxia/reoxygenation-mediated myocardial angiogenesis. Int J Tissue React. 2000;22:93–100. [PubMed] [Google Scholar]
- 29.Schneider M. Van Geyte K. Fraisl P. Kiss J. Aragones J. Mazzone M. Mairbaurl H. De Bock K. Jeoung NH. Mollenhauer M. Georgiadou M. Bishop T. Roncal C. Sutherland A. Jordan B. Gallez B. Weitz J. Harris RA. Maxwell P. Baes M. Ratcliffe P. Carmeliet P. Loss or silencing of the PHD1 prolyl hydroxylase protects livers of mice against ischemia/reperfusion injury. Gastroenterology. 2010;138:1143–1154.e1-2. doi: 10.1053/j.gastro.2009.09.057. [DOI] [PubMed] [Google Scholar]
- 30.Siddiq A. Ayoub IA. Chavez JC. Aminova L. Shah S. LaManna JC. Patton SM. Connor JR. Cherny RA. Volitakis I. Bush AI. Langsetmo I. Seeley T. Gunzler V. Ratan RR. Hypoxia-inducible factor prolyl 4-hydroxylase inhibition. A target for neuroprotection in the central nervous system. J Biol Chem. 2005;280:41732–41743. doi: 10.1074/jbc.M504963200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takeda K. Ho VC. Takeda H. Duan LJ. Nagy A. Fong GH. Placental but not heart defects are associated with elevated hypoxia-inducible factor alpha levels in mice lacking prolyl hydroxylase domain protein 2. Mol Cell Biol. 2006;26:8336–8346. doi: 10.1128/MCB.00425-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tekin D. Dursun AD. Xi L. Hypoxia inducible factor 1 (HIF-1) and cardioprotection. Acta Pharmacol Sin. 2010;31:1085–1094. doi: 10.1038/aps.2010.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thirunavukkarasu M. Addya S. Juhasz B. Pant R. Zhan L. Surrey S. Maulik G. Menon VP. Maulik N. Heterozygous disruption of Flk-1 receptor leads to myocardial ischaemia reperfusion injury in mice: application of affymetrix gene chip analysis. J Cell Mol Med. 2008;12:1284–1302. doi: 10.1111/j.1582-4934.2008.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thirunavukkarasu M. Han Z. Zhan L. Penumathsa SV. Menon VP. Maulik N. Adeno-sh-beta-catenin abolishes ischemic preconditioning-mediated cardioprotection by downregulation of its target genes VEGF, Bcl-2, and survivin in ischemic rat myocardium. Antioxid Redox Signal. 2008;10:1475–1484. doi: 10.1089/ars.2008.2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thirunavukkarasu M. Juhasz B. Zhan L. Menon VP. Tosaki A. Otani H. Maulik N. VEGFR1 (Flt-1 + /-) gene knockout leads to the disruption of VEGF-mediated signaling through the nitric oxide/heme oxygenase pathway in ischemic preconditioned myocardium. Free Radic Biol Med. 2007;42:1487–1495. doi: 10.1016/j.freeradbiomed.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vincent KA. Feron O. Kelly RA. Harnessing the response to tissue hypoxia: HIF-1 alpha and therapeutic angiogenesis. Trends Cardiovasc Med. 2002;12:362–367. doi: 10.1016/s1050-1738(02)00186-x. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y. Sun A. Xue J. Feng C. Li J. Wu J. Bone marrow derived stromal cells modified by adenovirus-mediated HIF-1alpha double mutant protect cardiac myocytes against CoCl2-induced apoptosis. Toxicol In Vitro. 2009;23:1069–1075. doi: 10.1016/j.tiv.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 38.Yuan Y. Hilliard G. Ferguson T. Millhorn DE. Cobalt inhibits the interaction between hypoxia-inducible factor-alpha and von Hippel-Lindau protein by direct binding to hypoxia-inducible factor-alpha. J Biol Chem. 2003;278:15911–15916. doi: 10.1074/jbc.M300463200. [DOI] [PubMed] [Google Scholar]
- 39.Zhou YF. Zheng XW. Zhang GH. Zong ZH. Qi GX. The effect of hypoxia-inducible factor 1-alpha on hypoxia-induced apoptosis in primary neonatal rat ventricular myocytes. Cardiovasc J Afr. 2010;21:37–41. [PMC free article] [PubMed] [Google Scholar]