Abstract
Adiponectin (Ad) is an abundant protein hormone regulatory of numerous metabolic processes. The 30 kDa protein originates from adipose tissue, with full-length and globular domain circulatory forms. A collagenous domain within Ad leads to spontaneous self-assemblage into various oligomeric isoforms, including trimers, hexamers, and high-molecular-weight multimers. Two membrane-spanning receptors for Ad have been identified, with differing concentration distribution in various body tissues. The major intracellular pathway activated by Ad includes phosphorylation of AMP-activated protein kinase, which is responsible for many of Ad's metabolic regulatory, anti-inflammatory, vascular protective, and anti-ischemic properties. Additionally, several AMP-activated protein kinase-independent mechanisms responsible for Ad's anti-inflammatory and anti-ischemic (resulting in cardioprotective) effects have also been discovered. Since its 1995 discovery, Ad has garnered considerable attention for its role in diabetic and cardiovascular pathology. Clinical observations have demonstrated the association of hypoadiponectinemia in patients with obesity, cardiovascular disease, and insulin resistance. In this review, we elaborate currently known information about Ad malfunction and deficiency pertaining to cardiovascular disease risk (including atherosclerosis, endothelial dysfunction, and cardiac injury), as well as review evidence supporting Ad resistance as a novel risk factor for cardiovascular injury, providing insight about the future of Ad research and the protein's potential therapeutic benefits. Antioxid. Redox Signal. 15, 1863–1873.
Introduction
Adiponectin (Ad) is a protein hormone responsible for the modulation of numerous metabolic processes. In this review, we shall elaborate currently known information about Ad malfunction or deficiency pertaining to cardiovascular disease risk, as well as review evidence supporting Ad resistance as a novel risk factor for cardiovascular injury, providing insight about the future of Ad research and the protein's potential therapeutic cardiovascular benefits.
Ad and Ad Receptors
First described in 1995, Ad had such similarity to complement factor C1q, the protein was termed Acrp30, as it was an adipocyte complement-related protein of 30 kDa (53). Three other groups isolated both mouse and human forms of Ad, and termed the adipocytokine AdipoQ, apM1, and GBP28, respectively (24, 36, 39). The human Ad gene exists on chromosome 3q26, a region associated with type-2 diabetes mellitus and metabolic syndrome susceptibility (52).
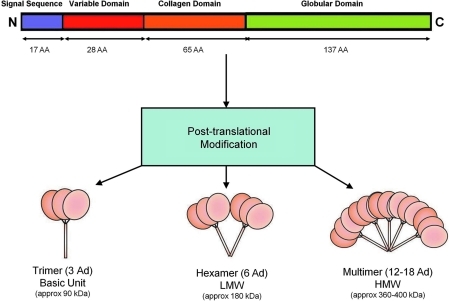
Modulating metabolic processes such as glucose regulation and fatty acid catabolism, Ad has a primary sequence 244 amino acids long, and contains a signal sequence (which targets the protein for extracellular section, and is cleaved in the mature peptide) and a nonconserved N-terminal domain, followed by 22 collagen repeats, and a C-terminal globular domain that has topologic similarities to tumor necrosis factor alpha (TNFα), despite dissimilar amino acid sequences (Fig. 1). Full-length Ad (fAd) requires post-translational modification for biologic activity (e.g., hydroxylation and glycosylation), and is secreted from adipocytes in three major size classes—trimers (∼90 kDa; the basic unit), low-molecular-weight (LMW) hexamers (∼180 kDa), and high-molecular-weight (HMW) isoforms consisting of 12-mers to 18-mers (which can exceed 400 kDa) (Fig. 1). Monomeric Ad not incorporated into trimers is predicted to be thermodynamically unstable and has not been observed under native conditions, but a proteolytic cleavage product of full-length Ad containing purely globular C-terminal domain has been postulated to exist in vivo. Recently, studies have shown that leukocyte elastase (secreted by activated monocytes and neutrophils) cleave fAd and generate globular Ad (gAd). Both in vivo studies and biochemical analysis of purified complexes suggest that the different Ad isoforms do not interconvert postcirculatory secretion. The contribution of varying isoforms of Ad to specific physiological processes is still incompletely understood. However, considerable evidence suggests that the HMW form is the biologically active form in the liver, and that the ratio of HMW form prevalence to total Ad isoforms quantity (the SA index) correlates to both insulin and Ad sensitivity (48). Mutations of the Ad gene (Arg112Cys and Ile164Thr) preventing trimer assemblage cause impaired secretion from the cell, and are clinically associated with hypoadiponectinemia (63). Gly84Arg and Gly90Ser mutants can assemble into trimers and hexamers, but are unable to form HMW multimers, and result in clinical diabetes (63), contributing more evidence to isoform specificity in insulin sensitization.
FIG. 1.
Structure of Ad (human). Full-length Ad requires post-translational modifications (e.g., hydroxylation and glycosylation) for activity. Ad molecules are secreted from adipocytes as trimers (∼90 kDa; the basic unit), LMW hexamers (∼180 kDa), and HMW isoforms (12–18-mers; >400 kDa). AA, amino acid (length); Ad, adiponectin; C, carboxy-terminus; HMW, high molecular weight; LMW, low molecular weight; N, amino-terminus. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
At its high circulatory concentration (0.5–30 μg/ml), Ad comprises ∼0.01% of all plasma proteins (53), thereby exceeding other adipokines 100-fold, and most hormones 3-fold. Contrary to expectations given its major production source, Ad levels are inversely correlated with body fat percentage in adults (in stark contrast to leptin levels, which are proportional to adipose mass). fAd or gAd decrease free fatty acid release in the postprandial state. Systemic insulin sensitivity and plasma Ad levels are positively correlated, and Ad augments postprandial insulin's suppression of hepatic glucose output. Cnop and colleagues report Ad concentrations to be significantly higher in women than in men (7.4–2.9 vs. 5.4–2.3 μg/ml, p < 0.0001, 182 total subjects) (11), which has been attributed to the inhibitory effect of testosterone upon Ad secretion by epidemiological puberty data (7), and reaffirmed in hormonal replacement therapy in male to female transgender studies (5, 50). Sexual dimorphism exists regarding oligomeric distribution, as males harbor a predominance of LMW Ad, whereas females have a more balanced distribution of both LMW and HMW forms (48).
For many years, Ad was believed to be exclusively secreted by adipocytes. Although an overwhelming majority of the body's Ad is generated from white adipose tissue, it has been shown that the Ad gene is expressed in other cell types, including osteoblasts, skeletal muscle, and cardiomyocytes. Our recent study has shown that cardiomyocytes-derived Ad is biologically active and contributes to physiologic function (65).
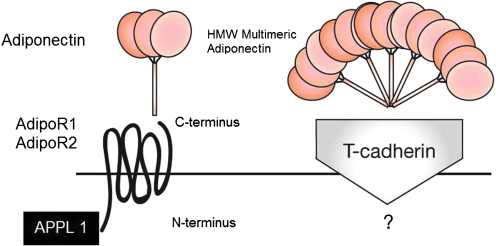
Ad regulates cellular function via binding and activation of its specific receptors, Ad receptor 1 (AdipoR1) and Ad receptor 2 (AdipoR2), each encoded by their respectively named genes. The Ad receptors belong to a new family of membrane receptors structurally predicted to contain seven transmembrane domains, but are topologically distinct from G-coupled receptor proteins. Ad binds to the C-terminal extracellular domain of AdipoR, whereas the N-terminal cytoplasmic domain interacts with an adaptor protein, APPL1 (Fig. 2). AdipoR1 is abundantly expressed in skeletal muscle and endothelial cells, and AdipoR2 is predominantly expressed in the liver (75). Both AdipoR1 and AdipoR2 are constitutively expressed in adult cardiomyocytes, but studies resolving each receptor's relative contribution to Ad cardioprotective signaling remain ongoing. Receptor expression levels in skeletal muscle and adipose tissue correlate with insulin levels, and are reduced in diabetic mouse models (6, 16). By Scatchard plot analysis, Yamuchi and colleagues have shown that AdipoR1 is a receptor with high affinity for globular Ad, whereas AdipoR2 is a receptor with intermediate affinity for both full-length and globular Ad (70, 75).
FIG. 2.
Ad and the Ad receptor. Ad interacts with the extracellular C-terminus region of the Ad receptor, which spans the membrane in seven domains, with its N-terminus intracellular, interacting with APPL1. T-cadherin is postulated to be a receptor for multimeric HMW Ad isoforms, with yet unknown biologic function. AdipoR1/R2, Ad receptor 1/2; APPL1, adapter protein. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
In addition to the two Ad receptors, T-cadherin has been proposed to be a receptor for the hexameric and HMW Ad forms, and has been found to be capable of binding Ad in C2C12 myoblasts, but not in hepatocytes (25). However, as T-cadherin lacks an intracellular domain, the specific biologic function of any Ad/T-cadherin interaction remains unknown. Calreticulin (CRT), a multifunctional chaperone protein binding calcium ions, in concert with its adaptor protein CD91, has been identified by Walsh's group (40, 60) to be yet another receptor for Ad. Ad's interaction with CRT/CD91 has been shown to exert endothelial anti-inflammatory and vasculoprotective effects. As Walsh's group has acknowledged, CRT/CD91 does not constitute a classical receptor pathway, but, in addition to its overwhelming serum abundance, Ad is not a typical ligand with its unique property of higher-order oligimerization (40). The CRT/CD91 receptor pathway is discussed further in the section AMPK-independent signaling mechanisms.
Ad Intracellular Signaling
More than a decade of both clinical and experimental investigation has identified four central biological functions for Ad: (i) a metabolic regulatory function (via increasing insulin sensitivity, increasing glucose utilization, and increasing fatty acid oxidation); (ii) a vascular protective function (by enhancing nitric oxide [NO] production, and stimulating angiogenesis); (iii) an anti-inflammatory role (through decreasing both neutrophil adhesion and macrophage activation); and (iv) a cardioprotective/anti-ischemic function (19).
The specific mechanisms underlying these biologic functions of Ad can be separated by their dependency upon the AMP-activated protein kinase (AMPK) axis. AMPK is the first and most recognized molecule in Ad intracellular signaling. Ad regulates cellular metabolism through the AMPK/acetyl-CoA carboxylase signaling axis (71). Ad inhibits the inflammatory response and elicits vasodilatation/vasculoprotection largely through the AMPK/endothelial NO synthase (eNOS) axis (10, 55). However, Ad signaling involved with vasculoprotection, anti-inflammatory response, and cardioprotection all have been demonstrated to be mediated via AMPK-independent mechanisms.
AMPK-dependent signaling mechanisms
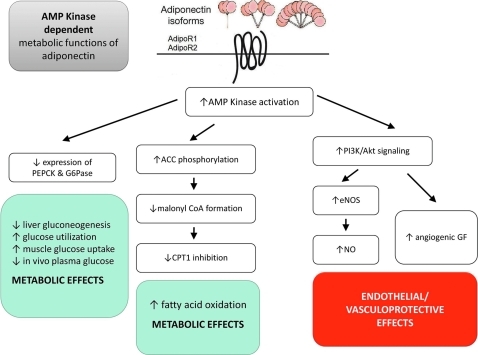
Figure 3 summarizes the AMPK-dependent metabolic functions of Ad. The major intracellular pathway activated by Ad is AMPK, a metabolic switch responsible for regulation of several intracellular systems, including glucose uptake, glucose transporter 4 generation, fatty acid oxidation, and mitochondrial genesis. The phosphorylative activation of AMPK by Ad causes resultant phosphorylative inactivation of acetyl co-A carboxylase, which in turn affects metabolic activity via various downstream cellular mechanisms. Inactive phosphorylated acetyl co-A carboxylase reduces malonyl-coA formation, which ceases inhibition of carnitine palmitoyltransferase 1, which increases fatty acid flux into the mitochondria for oxidation (2). AMPK activation additionally reduces the expression of molecules involved in gluconeogenesis, such as phosphoenolpyruvate carboxykinase and glucose-6-phosphatase in hepatocytes (34). Therefore, via the AMPK axis, Ad stimulates increased glucose utilization, increased fatty acid oxidation, increased muscle glucose uptake, increased myocyte lactate production, decreased liver gluconeogenesis, and decreased in vivo plasma glucose levels (71). The abrogration of each of Ad's metabolic effects by the blocking of AMPK via a dominant-negative mutation in an experimental model gives evidence to the integral involvement of AMPK for Ad's metabolic functions (8, 71, 72).
FIG. 3.
AMP Kinase-dependent metabolic functions of Ad. Ad interacts with the extracellular C-terminus region of the Ad receptor, which spans the membrane in seven domains, with its N-terminus intracellular, and causes activation of AMP kinase. Of the four identified central biological functions for Ad, its metabolic regulatory function and vascular protective function are dependent upon the AMP kinase signaling axis. ACC, acetyl co-A carboxylase; AMPK, AMP-activated protein kinase; CPT1, carnitine palmotyltransferase 1; G6Pase, glucose-6-phosphatase; GF, growth factors; PEPCK, phosphoenolpyruvate carboxykinase. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
The anti-inflammatory and vasculoprotective effects of Ad similarly share activation via the AMPK axis. Considerable evidence supports Ad as a pro-angiogenic regulator through AMPK signaling, eliciting angiogenic and antiapoptotic endothelial responses (27, 44). Ad-induced AMPK signaling promotes phosphatidylinositol 3-kinase-Akt signaling within muscle, leading to angiogenic growth factor synthesis (59). Ad also directly stimulates endothelial NO production via phosphatidylinositol 3-kinase-dependent pathways involving eNOS phosphorylation at Ser1179 by AMPK, contributing to its vasodilatory and antiatherogenic properties (10) (please refer to the section “Ad and endothelial dysfunction”). Either pharmacological inhibition of AMPK activity or genetic suppression of AMPK expression virtually abolishes Ad's anti-inflammatory (21), and vasculoprotective (44, 55) effects.
Two ex vivo studies demonstrate the importance of AMPK to fAd-mediated cardioprotection. Shinmura and colleagues revealed the value of short-term caloric restriction in elevating total serum Ad levels, with subsequent AMPK activation and resultant cardiac protection (58). However, as caloric restriction affects multiple physiologic processes, all positive benefits seen in the study could not be exclusively attributable to Ad alone. Later, Gonon and colleagues demonstrated fAd's protection against myocardial ischemia reperfusion injury via the AMPK/Akt/NO signaling axis (20). Despite demonstrating fAd indeed resulted in AMPK phosphorylation in an isolated heart perfusion model, no determination of resultant blockade of beneficial fAd effects in the setting of AMPK inhibition was made, and no cause–effect relationship was established. Although documented to be potent in magnitude, Ad's anti-ischemic/cardioprotective effects cannot be strictly attributed to AMPK mediation solely (15).
AMPK-independent signaling mechanisms
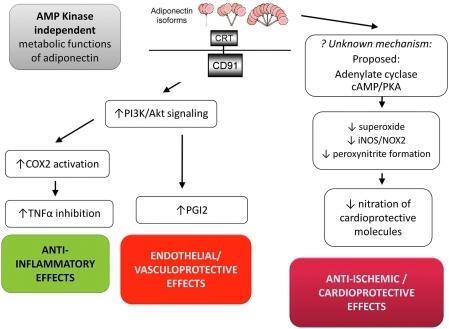
Figure 4 summarizes the AMPK-independent metabolic functions of Ad. Considerable evidence counters AMPK phosphorylation as an absolute prerequisite for Ad's anti-ischemic/cardioprotective effects. In wild-type as well as cardiomyocyte-specific AMPK-dominant negative transgenic mice, Ad significantly reduces ischemia/reperfusion-induced superoxide/NO overproduction, blocks inducible NOS (iNOS)/NOX-2 expression, and attenuates protein nitration (64). Although the precise intracellular molecules mediating Ad's AMPK-independent antioxidative/antinitrative action remain presently undefined, accumulating evidence suggests that the adenylate cyclase/cAMP/protein kinase-A signaling axis is involved (19). Moreover, Takemura and colleagues reported an AMPK-independent mechanism through which Ad reduces systemic inflammation by promoting early apoptotic cell clearance by macrophages via the CRT/CD91-dependent pathway (60). Walsh's group has reported that Ad exerts cardioprotection partially through AMPK-independent cyclo-oxygenase 2 (COX-2) activation and subsequent TNFα inhibition (56). Interestingly, this group recently demonstrated that Ad activation of COX-2 in endothelial cells is not mediated by the traditional receptors AdipoR1/2, but rather by the cell surface CRT/CD91 coreceptor (40). Ad was demonstrated to exert vasculoprotective effects via COX-2-dependent signaling, enhancing ischemic muscle tissue revascularization by generation of COX-2-derived prostaglandin I2. Via siRNA manipulation, Walsh's group identified that ablation of CRT or its adaptor protein CD91 resulted in reduced COX-2 induction by Ad, and blocked the PI3-kinase-Akt signaling pathway.
FIG. 4.
AMP Kinase-independent metabolic and antioxidant functions of Ad. Recent experimental results from many investigators, including our own, demonstrated that several AMPK-independent pathways exist that mediate Ad's anti-inflammatory, vascular protective, and cardioprotective/anti-ischemic functions. The interaction of Ad with calreticulin/CD91 complex has been demonstrated to lead to both anti-inflammatory and vascular protective effects in an AMPK-independent manor. A still un-identified mechanism is responsible for Ad's antioxidative/cardioprotective effects (likely via adenylate 29 cyclase/cAMP/PKA). cAMP, cyclic AMP; COX-2, cyclooxygenase 2; CRT, calreticulin; iNOS, inducible nitric oxide synthase; NOX2, NADPH oxidase 2/gp91phox; PKA, protein kinase A; TNFα, tumor necrosis factor alpha. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
Shibata and colleagues' work established varying degrees of AMPK importance in fAd's cardioprotective effect in different models (56). While fAd blocks hypoxia/reoxygenation-induced neonatal cardiomyocyte apoptosis largely through AMPK activation, Ad's inhibitory effect on LPS-induced TNFα production is completely AMPK independent. In vivo administration of NS398, a cyclooxgenase-2 (COX-2) inhibitor, attenuated the infarct sparing effects of exogenous fAd, suggesting that COX-2 is also a significant player in fAd's cardioprotection s/p myocardial ischemia/reperfusion (MI/R). Ouedraogo and colleges reported that gAd administration suppressed glucose-induced superoxide overproduction in culture endothelial cells, a phenomenon that could not be abrogated with AMPK inhibitors, suggesting that gAd reduced high glucose-induced superoxide overproduction in AMPK-independent fashion (47). In an in vivo animal study, we demonstrated that AMPK deficiency enhanced myocardial I/R injury but had minimal effect on Ad's antioxidant/antinitrative protection (64). These findings were confirmed in our later study utilizing an in vitro model demonstrating that although Ad's metabolic and eNOS activation effect is largely mediated by AMPK, its suppression of superoxide is not, and this AMPK-independent antioxidant property of Ad increased nitric oxide bioavailability and exerted significant antiapoptotic effect (66). It also provided new evidence demonstrating that iNOS/NADPH oxidase overexpression is sufficient to increase nitrative/oxidative stress such that gAd exerts its AMPK-independent cardioprotection partially through iNOS/NADPH oxidase suppression.
There is understandable reasoning for the mediation of Ad's cardioprotective effects through AMPK-independent mechanisms. The energy-sensing capability of AMPK originates from its ability to detect and react to AMP:ATP ratio fluctuations occurring during muscle stimulation in rest and exercise. The major substrate of AMPK is AMP, which is grossly abundant during times of ischemic duress. If Ad is to have any significant cardioprotective properties, such effects must be executed through AMPK-independent pathways, as the dependent pathway would already be AMP-saturated. Ad's biological functions are largely determined by the intracellular environment, particularly AMP concentration. Under pathological conditions in which intracellular AMP concentrations are not elevated (i.e., muscular insulin resistance and vascular inflammation), AMPK might be the most important molecule in Ad intracellular signaling. In contrast, under pathological conditions in which intracellular AMP concentrations are elevated, and AMPK has already been significantly activated (such as myocardial ischemia), Ad may summarily exert its biological actions largely through signaling molecules other than AMPK.
Ad Deficiency as a Risk Factor of Cardiovascular Disease
Ad and atherosclerosis
It has been increasingly recognized that the diabetic and obesity state is synonymous with chronic inflammation of the microcirculation. The resultant deleterious effects of ambient TNF and other cytokines, as well as high-glucose levels, further trigger inflammatory signaling cascades, enhancing leukocyte–endothelial interactions, leading to elementary atherosclerotic processes. Ouedraogo's study investigated the relationship between decreased Ad levels in obese and type-2 diabetic patients and observed exacerbated microvascular inflammation; in Ad knockout mice, globular Ad replacement therapy mitigated the inflamed microvascular endothelium phenotype (46).
Carotid intima-media thickness, an early marker for atherosclerosis, is inversely related to Ad levels in men (53). Experimental studies support Ad as an antiatherogenic molecule relevant for the prevention of atherosclerotic plaques. In vitro studies have demonstrated that Ad is inhibitory upon monocyte–endothelial adhesion, myeloid differentiation, and macrophage cytokine production—processes intimately related with atherosclerotic plaque genesis (41). Ad is directly inhibitive of atherogenic molecules such as intracellular adhesion molecule-1, vascular cellular adhesion molecule-1, and E-selectin (molecules associated with heightened leukocyte trafficking) (41).
Ad attenuates expression of scavenger receptor class A-1 macrophages and consequent low-density lipoprotein uptake, diminishing foam cell formation (42). Ad inhibits various growth factors in cultured smooth muscle cells, including platelet-derived growth factor, basic fibroblast growth factor, heparin-binding epidermal growth factor (EGF)-like growth factor, and EGF, as well as the cell proliferation and migration induced by these elements (1). Additionally, Ad exerts anti-inflammatory effects on macrophages (73). Most importantly, increased neointimal formation is observed in Ad knock-out mice in response to external vascular cuff injury (29). Globular Ad overexpression results in decreased atherosclerotic lesions in even mice with proatherogenic apoE−/− background (1).
Ad and endothelial dysfunction
Endothelial dysfunction, the earliest alteration in vascular pathology, plays a critical role in atherosclerosis development. Clinical observations have demonstrated that hypoadiponectinemia is closely correlated to endothelial dysfunction in peripheral arteries (30, 35, 45, 57, 61). Our in vitro studies demonstrated that aortic vascular rings from Ad knockout mice manifest endothelial dysfunction in terms of increased superoxide production, decreased eNOS phosphorylation, and aberrant vasodilatory response to acetylcholine (9). Exogenous globular Ad supplementation to the Ad−/− mice in vivo attenuated the endothelial dysfunction measured by these parameters. Morever, we demonstrated that acute Ad treatment significantly attenuated hyperlipidemia-induced endothelial dysfunction in rats via reduction of oxidative and nitrative stress, by differential regulation of eNOS and iNOS activity (32), giving further evidence that reduced Ad in metabolic disorders disrupts endothelial function, a pivotal event in the genesis and progression of macroangiopathy (14), a significant cause of mortality in patients with metabolic syndrome and type-2 diabetes (12).
Many of Ad's anti-inflammatory effects have been associated with eNOS activation, increasing endothelial bioavailable nitric oxide (NO) (10, 22), via Ad-invoked binding of regulatory heat shock protein 90 to eNOS (68) (Fig. 5). Pharmacologic blockade of eNOS with NΩ-nitro-l-arginine methyl ester attenuates Ad-inhibition of in vivo TNF-inflamed microvasculature leukocyte adhesion (46).
FIG. 5.
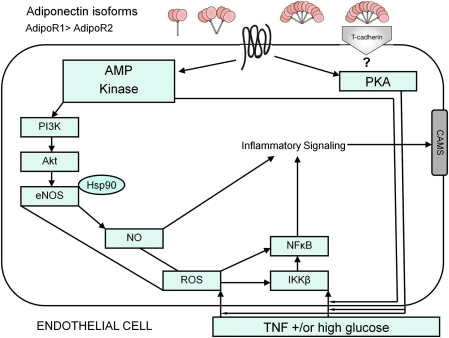
Ad signaling in endothelial cells. Ad signaling transduction pathways suppress endothelial cell activation elicited by high glucose levels and agonists such as TNF, and suppresses inflammatory responses (IKKβ and NFκβ activation). Ad enhances NO generation via AMP kinase cascade activation, and the cAMP-PKA pathway has been shown to mediate Ad's antioxidative and anti-inflammatory protective effects. CAMs, cell adhesion molecules; eNOS, endothelial nitric oxide synthase; Hsp90, heat shock protein 90; IKKβ, Iκβ kinase; NFκβ, nuclear factor κβ; NO, nitric oxide; ROS, reactive oxygen species. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
AMPK mediates Ad's vascular effects in endothelial cells involving eNOS activation, as AMPK activation by Ad increases endothelial NO availability, as well as signaling through phosphatidylinositol 3′-kinase (10). The influence of Ad upon angiogenesis is dependent upon Ad-induced phosphorylation of both AMPK and Akt (protein kinase B), with AMPK upstream of Akt, as disruption of AMPK activation inhibits Ad-induced Akt phosphorylation (44).
Ad also affects endothelium via pathways involving protein kinase A (PKA, or cyclic AMP [cAMP]-dependent protein kinase) signaling (43, 69). Ouchi and colleagues demonstrated the accumulation of cAMP within endothelial cells, whereas gAd suppressed TNF-induced NFκB activation and phosphorylated IκBα (an NFκB inhibitor); such action was blocked by inhibitors of adenylate cyclase or PKA (43). Oligomers of recombinant gAd and fAd have been shown to suppress high-glucose-induced reactive oxygen species production, as well as increase cellular cAMP content, and blocking PKA inhibition in endothelial cells (47).
In addition to suppressing TNF-induced or high-glucose-induced NFκB activation, gAd also potently blocks upstream enzyme Iκβ kinase action (67). Whereas forskolin (an adenylate cyclase activator) mimics the action of gAd, dideoxyadenosine (an adenylate cyclase inhibitor) as well as Rp-cAMP (a PKA inhibitor) block gAd inhibition of TNF-induced Iκβ kinase activity (67). Altogether, these findings suggest the great complexity of multiple pathways involved in Ad-mediated suppression of endothelial inflammatory signaling (summarized in Fig. 5).
Ad and cardiac injury
Numerous epidemiological studies have revealed the correlation of reduced Ad levels with increased cardiovascular disease risk (18, 23, 30, 74), and the relationship persists even after adjustment for diabetes, dyslipidemia, hypertension, smoking, and BMI (28, 30). High plasma Ad concentrations are associated with a lower risk of MI in men (17, 30, 49). Ethnic disparities in Ad levels might explain predilection in African Americans and those of South Asian descent for increased type-2 diabetes and coronary artery disease (13, 26, 51). Persistently, low plasma Ad concentration after acute myocardial infarction is predictive of future adverse cardiac events (3). In addition, recent clinical observations have demonstrated that post-MI plasma Ad levels correlated positively with myocardial salvage index and ejection fraction recovery (54). As such, reduced Ad production has been recognized as a risk factor of cardiovascular disease.
Experimental studies have generated more direct evidence supporting the link between hypoadiponectinemia and cardiomyocyte injury. In vitro studies have demonstrated that Ad promotes cell survival and inhibits cell death, suggesting that Ad may have direct cardioprotective effects atop its vasculo-protective properties, which indirectly shield cardiomyocytes from ischemia/reperfusion injury. Indeed, we and others have recently demonstrated that, in addition to microvascular defects, Ad knock-out (AdKO) mice manifest worse MI/R injury compared to control mice, in measures such as increased myocardial apoptosis and infarct size, and decrease cardiac function (56, 62). Compared to controls, there is increased MI/R injury in heterozygous Ad knock-out mice (+/−), in which circulating Ad levels are reduced by approximately half, but the severity was significantly less than that seen in homozygous knock-out animals (62). This finding suggests that Ad levels not only inversely correlate with the risk of development of ischemic heart disease (as indicated by the human epidemiological data), but also are inversely related to the severity of an MI/R insult. Provision of fAd or gAd in dose-dependent manner ameliorates exacerbated MI/R injury in AdKO mice (56, 62). These exciting results suggest that Ad might eventually be utilized as a novel therapeutic agent in the treatment of ischemic cardiac injury.
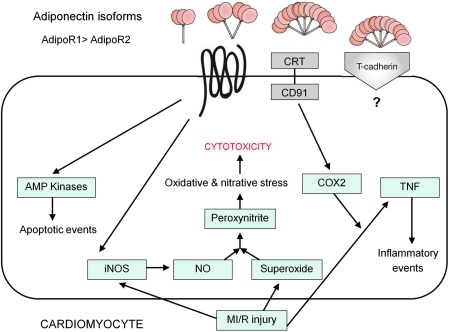
As stated earlier, Ad is known to stimulate NO production via AMPK-mediated eNOS phosphorylation. Lack of Ad, therefore, might conceivably reduce NO production, thus rendering cardiomyocytes more susceptible to MI/R injury. However, this hypothesis is not supported by experimental findings revealing markedly increased, rather than decreased, total NO production in ischemic–reperfused tissue obtained from AdKO mice [Tao et al. (62)]. Interestingly, iNOS expression is increased in AdKO mice compared to controls, and gAd treatment effectively blocks iNOS expression in the AdKO animals (62). These novel results indicate that Ad differentially regulates NO production by both eNOS and iNOS. Under physiologic conditions, Ad stimulates NO production via eNOS phosphorylation, and thus contributes to its vasodilatory, anti-inflammatory, and vascular-protective actions through an AMPK-dependent pathway. In contrast, under pathologic conditions when iNOS is induced, Ad prevents excess NO generation by inhibiting iNOS expression and this antinitrative action of Ad is largely AMPK independent (64, 66) (Fig. 6).
FIG. 6.
Ad signaling in cardiomyocytes. Ad isoforms have been shown to exert multiple actions, such as activation of AMP kinases, CRT/CD91 activation with COX-2 involvement, suppression of TNF signaling via a COX-2-prostaglandin E2-linked cascade (26a), and reduction of oxidative and nitrative stress after MI/R injury that is associated with suppression of iNOS induction. CD91, CRT adapter protein; MI/R, myocardial ischemia-reperfusion. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
Ad Resistance as a New Risk Factor of Cardiovascular Disease
Ad metabolically mimics insulin, promoting glucose uptake and inhibiting hepatic glucose production (4). Ad exerts anti-inflammatory and antiatherogenic properties via its vascular stimulation of endothelial NO production (10). Increasing studies have demonstrated the association between resistance to Ad's metabolic/vascular effects with insulin resistance and endothelial dysfunction in cardiovascular diseases (23, 45). We review the phenomenon of Ad resistance as a novel risk factor for cardiovascular disease and pathology.
High fat (HF)-rich diets impair the insulin-signaling cascade by incremental skeletal muscle lipid accumulation (31). As mechanistically described above, a primary physiologic effect of Ad is increased fatty acid (FA) oxidation. Animals subjected to HF-diet manifest Ad resistance, demonstrating blunted FA oxidation in response to Ad administration, in addition to decreased maximal insulin-stimulated glucose transport (38). Mullen's later studies investigating HF-fed rats revealed temporal relevance and importance to Ad resistance; the loss of Ad's stimulatory effect on FA oxidation preceded the increase in plasmalemmal fatty acid transporters. Through resultant accumulation of intramuscular diacylglycerol (DAG) and ceramide, insulin signaling was consequently blunted, and maximal insulin-stimulated glucose transport in skeletal muscle impairment occurred after the onset of Ad resistance (37). In studying obese patients manifesting no significant insulin resistance, Bruce and colleagues discovered that their subjects had deficient serum Ad levels. Further, blunted AMPK activation in response to exogenous gAd in obese muscle further suggested Ad resistance development in obesity (8). Taken together, Ad system derangement likely plays a central role in obesity or HF-diet-induced insulin resistance.
We have conducted a series of studies investigating Ad resistance in the vasculature. Utilizing a rat model with HF-diet-induced obesity, we determined whether obesity/hyperlipidemia alters the vascular response to Ad (33). We noted a significant peaked plasma Ad level in HF-diet-fed animals by 8 weeks, which rapidly declined thereafter. Regardless of circulatory Ad levels, phosphorylated AMPK and eNOS in vascular tissue remained significantly reduced at all observed time points. Recombinant full-length Ad (rAd)-induced AMPK/eNOS phosphorylation and vasodilatation were significantly reduced in 16-week obese/hyperlipidemic aortic segments. Vascular AdipoR1 and AdipoR2 expression levels were significantly reduced after 16 weeks of HF diet. Our data supported that modification/inactivation of Ad by unidentified factors present in obese/hyperlipidemic plasma decreased vascular AdipoR1/R2 expression, and reduced circulating Ad levels all contribute to vascular Ad resistance at varying stages of obesity.
In a yet unpublished study, we examined the reduced cardioprotective action of Ad in a HF-diet-induced diabetic mouse model, and its involved mechanisms. As many studies in normal animals have demonstrated, Ad (Ad) reduces MI/R injury. However, our preliminary experiments revealed the attenuation of Ad-mediated cardioprotection in the diabetic state, where there existed already significantly reduced endogenous Ad levels. HF-diet-induced obesity/diabetic mice and age-matched control mice on normal diet (ND) were subjected to myocardial ischemia/reperfusion, and were treated with gAd before reperfusion. In comparison to ND mice, HF-diet mice endured greater MI/R injury. A nearly threefold greater gAd dose was required to achieve significant cardioprotection comparable to that observed in ND mice treated with standard Ad dose. Compared to ND mice, gAd-induced cardiac AMPK activation was significantly blunted in HF mice. Moreover, although both low and high doses of gAd were equally effective in attenuating MI/R-induced oxidative stress (NADPH oxidase expression and superoxide production) and nitrative stress (iNOS expression, NO production, and peroxynitrite formation) in ND mice, only high-dose gAd effectively reduced MI/R-induced oxidative/nitrative stress in HF mice. Taken together, our results demonstrated that HF-induced diabetes diminished both AMPK-dependent and AMPK-independent cardioprotection of gAd, suggestive of an unreported Ad resistance in the diabetic heart.
Summary and Perspective
Ad, an endogenous insulin-sensitizing hormone, is an abundantly produced adipocytokine of primarily adipose origin, and circulates in plasma as various multimeric complexes. Deficiency and/or imbalance of Ad's oligomeric forms has well-described associations with insulin resistance, atherosclerosis, endothelial dysfunction, and cardiovascular disease. Four major biologic functions have been identified for Ad: (i) metabolic regulatory function, (ii) vascular protective function, (iii) anti-inflammatory role, and (iv) cardioprotective/anti-ischemic properties. The major intracellular pathway activated by Ad includes phosphorylation of AMP-activated protein kinase, which is responsible for many of Ad's properties. Additionally, several AMPK-independent mechanisms for Ad's anti-inflammatory and cardioprotective actions have been delineated.
Pharmacologic agents (i) augmenting Ad's circulating concentrations (including novel or existing medications), (ii) increasing Ad receptor expression, or (iii) dispersing specific Ad receptor agonists capable of downstream signaling pathway induction are all potential therapeutic modalities for ameliorating disease pathologies discussed in this review. Ad's roles in disease processes via elucidated and yet unknown mechanisms provide the potential for multiple therapeutic targets with clinical applications. Increasing attention to Ad in recent years is well warranted given the potential salutary benefits of the protein in numerous studies. Further work will reveal the definitive effects and contributions of the multiple Ad oligomeric forms in the vasculature, cardiac, and metabolic systems.
Abbreviations Used
- ACC
acetyl co-A carboxylase
- Ad
adiponectin
- AdipoR1
adiponectin receptor 1
- AdipoR2
adiponectin receptor 2
- AdKO
adiponectin knock out
- AMPK
AMP-activated protein kinase
- APPL1
adapter protein
- CAMs
cell adhesion molecules
- CD91
CRT adapter protein
- COX-2
cyclo-oxygenase 2
- CPT1
carnitine palmotyltransferase 1
- CRT
calreticulin
- eNOS
endothelial nitric oxide synthase
- fAd
full-length adiponectin
- G6Pase
glucose-6-phosphatase
- gAd
globular domain of adiponectin
- HMW
high molecular weight
- Hsp90
heat shock protein 90
- IKKβ
Iκβ kinase
- iNOS
inducible nitric oxide synthase
- LMW
low molecular weight
- MI/R
myocardial ischemia/reperfusion
- NFκβ
nuclear factor κβ
- NO
nitric oxide
- NOX2
NADPH oxidase 2/gp91phox
- PEPCK
phosphoenolpyruvate carboxykinase
- PKA
protein kinase A
- ROS
reactive oxygen species
- TNFα
tumor necrosis factor alpha
Acknowledgments
This research was supported by the following grants: NIH HL-63828, HL-096686, American Diabetes Association 7-05-RA-83, and American Heart Association Grant-in-Aid 0855554D (to X.L.M.).
References
- 1.Arita Y. Kihara S. Ouchi N. Maeda K. Kuriyama H. Okamoto Y. Kumada M. Hotta K. Nishida M. Takahashi M. Nakamura T. Shimomura I. Muraguchi M. Ohmoto Y. Funahashi T. Matsuzawa Y. Adipocyte-derived plasma protein adiponectin acts as a platelet-derived growth factor-BB-binding protein and regulates growth factor-induced common postreceptor signal in vascular smooth muscle cell. Circulation. 2002;105:2893–2898. doi: 10.1161/01.cir.0000018622.84402.ff. [DOI] [PubMed] [Google Scholar]
- 2.Arita Y. Kihara S. Ouchi N. Takahashi M. Maeda K. Miyagawa J. Hotta K. Shimomura I. Nakamura T. Miyaoka K. Kuriyama H. Nishida M. Yamashita S. Okubo K. Matsubara K. Muraguchi M. Ohmoto Y. Funahashi T. Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 3.Behrends M. Schulz R. Post H. Alexandrov A. Belosjorow S. Michel MC. Heusch G. Inconsistent relation of MAPK activation to infarct size reduction by ischemic preconditioning in pigs. Am J Physiol Heart Circ Physiol. 2000;279:H1111–H1119. doi: 10.1152/ajpheart.2000.279.3.H1111. [DOI] [PubMed] [Google Scholar]
- 4.Berg AH. Combs TP. Du X. Brownlee M. Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–953. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 5.Berra M. Armillotta F. D'Emidio L. Costantino A. Martorana G. Pelusi G. Meriggiola MC. Testosterone decreases adiponectin levels in female to male transsexuals. Asian J Androl. 2006;8:725–729. doi: 10.1111/j.1745-7262.2006.00204.x. [DOI] [PubMed] [Google Scholar]
- 6.Bonnard C. Durand A. Vidal H. Rieusset J. Changes in adiponectin, its receptors and AMPK activity in tissues of diet-induced diabetic mice. Diabetes Metab. 2008;34:52–61. doi: 10.1016/j.diabet.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Bottner A. Kratzsch J. Muller G. Kapellen TM. Bluher S. Keller E. Bluher M. Kiess W. Gender differences of adiponectin levels develop during the progression of puberty and are related to serum androgen levels. J Clin Endocrinol Metab. 2004;89:4053–4061. doi: 10.1210/jc.2004-0303. [DOI] [PubMed] [Google Scholar]
- 8.Bruce CR. Mertz VA. Heigenhauser GJ. Dyck DJ. The stimulatory effect of globular adiponectin on insulin-stimulated glucose uptake and fatty acid oxidation is impaired in skeletal muscle from obese subjects. Diabetes. 2005;54:3154–3160. doi: 10.2337/diabetes.54.11.3154. [DOI] [PubMed] [Google Scholar]
- 9.Cao Y. Tao L. Yuan Y. Jiao X. Lau WB. Wang Y. Christopher T. Lopez B. Chan L. Goldstein B. Ma XL. Endothelial dysfunction in adiponectin deficiency and its mechanisms involved. J Mol Cell Cardiol. 2009;46:413–419. doi: 10.1016/j.yjmcc.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen H. Montagnani M. Funahashi T. Shimomura I. Quon MJ. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J Biol Chem. 2003;278:45021–45026. doi: 10.1074/jbc.M307878200. [DOI] [PubMed] [Google Scholar]
- 11.Cnop M. Havel PJ. Utzschneider KM. Carr DB. Sinha MK. Boyko EJ. Retzlaff BM. Knopp RH. Brunzell JD. Kahn SE. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003;46:459–469. doi: 10.1007/s00125-003-1074-z. [DOI] [PubMed] [Google Scholar]
- 12.Cooper-DeHoff RM. Pepine CJ. Metabolic syndrome and cardiovascular disease: challenges and opportunities. Clin Cardiol. 2007;30:593–597. doi: 10.1002/clc.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Degawa-Yamauchi M. Dilts JR. Bovenkerk JE. Saha C. Pratt JH. Considine RV. Lower serum adiponectin levels in African-American boys. Obes Res. 2003;11:1384–1390. doi: 10.1038/oby.2003.187. [DOI] [PubMed] [Google Scholar]
- 14.Diamant M. Tushuizen ME. The metabolic syndrome and endothelial dysfunction: common highway to type 2 diabetes and CVD. Curr Diab Rep. 2006;6:279–286. doi: 10.1007/s11892-006-0061-4. [DOI] [PubMed] [Google Scholar]
- 15.Dyck JR. The ischemic heart: starving to stimulate the adiponectin-AMPK signaling axis. Circulation. 2007;116:2779–2781. doi: 10.1161/CIRCULATIONAHA.107.742023. [DOI] [PubMed] [Google Scholar]
- 16.Fang X. Sweeney G. Mechanisms regulating energy metabolism by adiponectin in obesity and diabetes. Biochem Soc Trans. 2006;34:798–801. doi: 10.1042/BST0340798. [DOI] [PubMed] [Google Scholar]
- 17.Frystyk J. Berne C. Berglund L. Jensevik K. Flyvbjerg A. Zethelius B. Serum adiponectin is a predictor of coronary heart disease: a population-based 10-year follow-up study in elderly men. J Clin Endocrinol Metab. 2007;92:571–576. doi: 10.1210/jc.2006-1067. [DOI] [PubMed] [Google Scholar]
- 18.Goldstein BJ. Scalia R. Adipokines and vascular disease in diabetes. Curr Diab Rep. 2007;7:25–33. doi: 10.1007/s11892-007-0006-6. [DOI] [PubMed] [Google Scholar]
- 19.Goldstein BJ. Scalia RG. Ma XL. Protective vascular and myocardial effects of adiponectin. Nat Clin Pract Cardiovasc Med. 2009;6:27–35. doi: 10.1038/ncpcardio1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonon AT. Widegren U. Bulhak A. Salehzadeh F. Persson J. Sjoquist PO. Pernow J. Adiponectin protects against myocardial ischaemia-reperfusion injury via AMP-activated protein kinase, Akt, and nitric oxide. Cardiovasc Res. 2008;78:116–122. doi: 10.1093/cvr/cvn017. [DOI] [PubMed] [Google Scholar]
- 21.Hattori Y. Nakano Y. Hattori S. Tomizawa A. Inukai K. Kasai K. High molecular weight adiponectin activates AMPK and suppresses cytokine-induced NF-kappaB activation in vascular endothelial cells. FEBS Lett. 2008;582:1719–1724. doi: 10.1016/j.febslet.2008.04.037. [DOI] [PubMed] [Google Scholar]
- 22.Hattori Y. Suzuki M. Hattori S. Kasai K. Globular adiponectin upregulates nitric oxide production in vascular endothelial cells. Diabetologia. 2003;46:1543–1549. doi: 10.1007/s00125-003-1224-3. [DOI] [PubMed] [Google Scholar]
- 23.Hotta K. Funahashi T. Arita Y. Takahashi M. Matsuda M. Okamoto Y. Iwahashi H. Kuriyama H. Ouchi N. Maeda K. Nishida M. Kihara S. Sakai N. Nakajima T. Hasegawa K. Muraguchi M. Ohmoto Y. Nakamura T. Yamashita S. Hanafusa T. Matsuzawa Y. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595–1599. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 24.Hu E. Liang P. Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271:10697–10703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- 25.Hug C. Wang J. Ahmad NS. Bogan JS. Tsao TS. Lodish HF. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc Natl Acad Sci U S A. 2004;101:10308–10313. doi: 10.1073/pnas.0403382101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hulver MW. Saleh O. MacDonald KG. Pories WJ. Barakat HA. Ethnic differences in adiponectin levels. Metabolism. 2004;53:1–3. doi: 10.1016/j.metabol.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 26a.Ikeda Y. Ohashi K. Shibata R. Pimental DR. Kihora S. Ouchi N. Walsh K. Cyclooxygenase-2 induction by adiponectin is regulated by a sphingosine kinase-1 dependent mechanism in cardiac myocytes. FEBS Lett. 2008;582:1147–1150. doi: 10.1016/j.febslet.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kobayashi H. Ouchi N. Kihara S. Walsh K. Kumada M. Abe Y. Funahashi T. Matsuzawa Y. Selective suppression of endothelial cell apoptosis by the high molecular weight form of adiponectin. Circ Res. 2004;94:e27–e31. doi: 10.1161/01.RES.0000119921.86460.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kojima S. Funahashi T. Sakamoto T. Miyamoto S. Soejima H. Hokamaki J. Kajiwara I. Sugiyama S. Yoshimura M. Fujimoto K. Miyao Y. Suefuji H. Kitagawa A. Ouchi N. Kihara S. Matsuzawa Y. Ogawa H. The variation of plasma concentrations of a novel, adipocyte derived protein, adiponectin, in patients with acute myocardial infarction. Heart. 2003;89:667. doi: 10.1136/heart.89.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kubota N. Terauchi Y. Yamauchi T. Kubota T. Moroi M. Matsui J. Eto K. Yamashita T. Kamon J. Satoh H. Yano W. Froguel P. Nagai R. Kimura S. Kadowaki T. Noda T. Disruption of adiponectin causes insulin resistance and neointimal formation. J Biol Chem. 2002;277:25863–25866. doi: 10.1074/jbc.C200251200. [DOI] [PubMed] [Google Scholar]
- 30.Kumada M. Kihara S. Sumitsuji S. Kawamoto T. Matsumoto S. Ouchi N. Arita Y. Okamoto Y. Shimomura I. Hiraoka H. Nakamura T. Funahashi T. Matsuzawa Y Osaka CAD Study Group. Coronary artery disease. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol. 2003;23:85–89. doi: 10.1161/01.atv.0000048856.22331.50. [DOI] [PubMed] [Google Scholar]
- 31.Lee JS. Pinnamaneni SK. Eo SJ. Cho IH. Pyo JH. Kim CK. Sinclair AJ. Febbraio MA. Watt MJ. Saturated, but not n-6 polyunsaturated, fatty acids induce insulin resistance: role of intramuscular accumulation of lipid metabolites. J Appl Physiol. 2006;100:1467–1474. doi: 10.1152/japplphysiol.01438.2005. [DOI] [PubMed] [Google Scholar]
- 32.Li R. Wang WQ. Zhang H. Yang X. Fan Q. Christopher TA. Lopez BL. Tao L. Goldstein BJ. Gao F. Ma XL. Adiponectin improves endothelial function in hyperlipidemic rats by reducing oxidative/nitrative stress and differential regulation of eNOS/iNOS activity. Am J Physiol Endocrinol Metab. 2007;293:E1703–E1708. doi: 10.1152/ajpendo.00462.2007. [DOI] [PubMed] [Google Scholar]
- 33.Li R. Xu M. Wang X. Wang Y. Lau WB. Yuan Y. Yi W. Wei X. Lopez BL. Christopher TA. Wang XM. Ma XL. Reduced vascular responsiveness to adiponectin in hyperlipidemic rats-mechanisms and significance. J Mol Cell Cardiol. 2010;49:508–515. doi: 10.1016/j.yjmcc.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lochhead PA. Salt IP. Walker KS. Hardie DG. Sutherland C. 5-aminoimidazole-4-carboxamide riboside mimics the effects of insulin on the expression of the 2 key gluconeogenic genes PEPCK and glucose-6-phosphatase. Diabetes. 2000;49:896–903. doi: 10.2337/diabetes.49.6.896. [DOI] [PubMed] [Google Scholar]
- 35.MacMurray AJ. Moralejo DH. Kwitek AE. Rutledge EA. Van Yserloo B. Gohlke P. Speros SJ. Snyder B. Schaefer J. Bieg S. Jiang J. Ettinger RA. Fuller J. Daniels TL. Pettersson A. Orlebeke K. Birren B. Jacob HJ. Lander ES. Lernmark A. Lymphopenia in the BB rat model of type 1 diabetes is due to a mutation in a novel immune-associated nucleotide (Ian)-related gene. Genome Res. 2002;12:1029–1039. doi: 10.1101/gr.412702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maeda K. Okubo K. Shimomura I. Funahashi T. Matsuzawa Y. Matsubara K. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (adipose most abundant gene transcript 1) Biochem Biophys Res Commun. 1996;221:286–289. doi: 10.1006/bbrc.1996.0587. [DOI] [PubMed] [Google Scholar]
- 37.Mullen KL. Pritchard J. Ritchie I. Snook LA. Chabowski A. Bonen A. Wright D. Dyck DJ. Adiponectin resistance precedes the accumulation of skeletal muscle lipids and insulin resistance in high-fat-fed rats. Am J Physiol Regul Integr Comp Physiol. 2009;296:R243–R251. doi: 10.1152/ajpregu.90774.2008. [DOI] [PubMed] [Google Scholar]
- 38.Mullen KL. Smith AC. Junkin KA. Dyck DJ. Globular adiponectin resistance develops independently of impaired insulin-stimulated glucose transport in soleus muscle from high-fat-fed rats. Am J Physiol Endocrinol Metab. 2007;293:E83–E90. doi: 10.1152/ajpendo.00545.2006. [DOI] [PubMed] [Google Scholar]
- 39.Nakano Y. Tobe T. Choi-Miura NH. Mazda T. Tomita M. Isolation and characterization of GBP28, a novel gelatin-binding protein purified from human plasma. J Biochem. 1996;120:803–812. doi: 10.1093/oxfordjournals.jbchem.a021483. [DOI] [PubMed] [Google Scholar]
- 40.Ohashi K. Ouchi N. Sato K. Higuchi A. Ishikawa TO. Herschman HR. Kihara S. Walsh K. Adiponectin promotes revascularization of ischemic muscle through a cyclooxygenase 2-dependent mechanism. Mol Cell Biol. 2009;29:3487–3499. doi: 10.1128/MCB.00126-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ouchi N. Kihara S. Arita Y. Maeda K. Kuriyama H. Okamoto Y. Hotta K. Nishida M. Takahashi M. Nakamura T. Yamashita S. Funahashi T. Matsuzawa Y. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation. 1999;100:2473–2476. doi: 10.1161/01.cir.100.25.2473. [DOI] [PubMed] [Google Scholar]
- 42.Ouchi N. Kihara S. Arita Y. Nishida M. Matsuyama A. Okamoto Y. Ishigami M. Kuriyama H. Kishida K. Nishizawa H. Hotta K. Muraguchi M. Ohmoto Y. Yamashita S. Funahashi T. Matsuzawa Y. Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte-derived macrophages. Circulation. 2001;103:1057–1063. doi: 10.1161/01.cir.103.8.1057. [DOI] [PubMed] [Google Scholar]
- 43.Ouchi N. Kihara S. Arita Y. Okamoto Y. Maeda K. Kuriyama H. Hotta K. Nishida M. Takahashi M. Muraguchi M. Ohmoto Y. Nakamura T. Yamashita S. Funahashi T. Matsuzawa Y. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation. 2000;102:1296–1301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]
- 44.Ouchi N. Kobayashi H. Kihara S. Kumada M. Sato K. Inoue T. Funahashi T. Walsh K. Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J Biol Chem. 2004;279:1304–1309. doi: 10.1074/jbc.M310389200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ouchi N. Ohishi M. Kihara S. Funahashi T. Nakamura T. Nagaretani H. Kumada M. Ohashi K. Okamoto Y. Nishizawa H. Kishida K. Maeda N. Nagasawa A. Kobayashi H. Hiraoka H. Komai N. Kaibe M. Rakugi H. Ogihara T. Matsuzawa Y. Association of hypoadiponectinemia with impaired vasoreactivity. Hypertension. 2003;42:231–234. doi: 10.1161/01.HYP.0000083488.67550.B8. [DOI] [PubMed] [Google Scholar]
- 46.Ouedraogo R. Gong Y. Berzins B. Wu X. Mahadev K. Hough K. Chan L. Goldstein BJ. Scalia R. Adiponectin deficiency increases leukocyte-endothelium interactions via upregulation of endothelial cell adhesion molecules in vivo. J Clin Invest. 2007;117:1718–1726. doi: 10.1172/JCI29623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ouedraogo R. Wu X. Xu SQ. Fuchsel L. Motoshima H. Mahadev K. Hough K. Scalia R. Goldstein BJ. Adiponectin suppression of high-glucose-induced reactive oxygen species in vascular endothelial cells: evidence for involvement of a cAMP signaling pathway. Diabetes. 2006;55:1840–1846. doi: 10.2337/db05-1174. [DOI] [PubMed] [Google Scholar]
- 48.Pajvani UB. Hawkins M. Combs TP. Rajala MW. Doebber T. Berger JP. Wagner JA. Wu M. Knopps A. Xiang AH. Utzschneider KM. Kahn SE. Olefsky JM. Buchanan TA. Scherer PE. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem. 2004;279:12152–12162. doi: 10.1074/jbc.M311113200. [DOI] [PubMed] [Google Scholar]
- 49.Pischon T. Girman CJ. Hotamisligil GS. Rifai N. Hu FB. Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004;291:1730–1737. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- 50.Resmini E. Andraghetti G. Rebora A. Cordera R. Vera L. Giusti M. Minuto F. Ferone D. Leptin, ghrelin, and adiponectin evaluation in transsexual subjects during hormonal treatments. J Androl. 2008;29:580–585. doi: 10.2164/jandrol.108.004952. [DOI] [PubMed] [Google Scholar]
- 51.Retnakaran R. Hanley AJ. Raif N. Connelly PW. Sermer M. Zinman B. Hypoadiponectinaemia in South Asian women during pregnancy: evidence of ethnic variation in adiponectin concentration. Diabet Med. 2004;21:388–392. doi: 10.1111/j.1464-5491.2004.1151.x. [DOI] [PubMed] [Google Scholar]
- 52.Ruan H. Lodish HF. Insulin resistance in adipose tissue: direct and indirect effects of tumor necrosis factor-alpha. Cytokine Growth Factor Rev. 2003;14:447–455. doi: 10.1016/s1359-6101(03)00052-2. [DOI] [PubMed] [Google Scholar]
- 53.Scherer PE. Williams S. Fogliano M. Baldini G. Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 54.Shibata R. Numaguchi Y. Matsushita K. Sone T. Kubota R. Ohashi T. Ishii M. Kihara S. Walsh K. Ouchi N. Murohara T. Usefulness of adiponectin to predict myocardial salvage following successful reperfusion in patients with acute myocardial infarction. Am J Cardiol. 2008;101:1712–1715. doi: 10.1016/j.amjcard.2008.02.057. [DOI] [PubMed] [Google Scholar]
- 55.Shibata R. Ouchi N. Kihara S. Sato K. Funahashi T. Walsh K. Adiponectin stimulates angiogenesis in response to tissue ischemia through stimulation of amp-activated protein kinase signaling. J Biol Chem. 2004;279:28670–28674. doi: 10.1074/jbc.M402558200. [DOI] [PubMed] [Google Scholar]
- 56.Shibata R. Sato K. Pimentel DR. Takemura Y. Kihara S. Ohashi K. Funahashi T. Ouchi N. Walsh K. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med. 2005;11:1096–1103. doi: 10.1038/nm1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shimabukuro M. Higa N. Asahi T. Oshiro Y. Takasu N. Tagawa T. Ueda S. Shimomura I. Funahashi T. Matsuzawa Y. Hypoadiponectinemia is closely linked to endothelial dysfunction in man. J Clin Endocrinol Metab. 2003;88:3236–3240. doi: 10.1210/jc.2002-021883. [DOI] [PubMed] [Google Scholar]
- 58.Shinmura K. Tamaki K. Saito K. Nakano Y. Tobe T. Bolli R. Cardioprotective effects of short-term caloric restriction are mediated by adiponectin via activation of AMP-activated protein kinase. Circulation. 2007;116:2809–2817. doi: 10.1161/CIRCULATIONAHA.107.725697. [DOI] [PubMed] [Google Scholar]
- 59.Takahashi A. Kureishi Y. Yang J. Luo Z. Guo K. Mukhopadhyay D. Ivashchenko Y. Branellec D. Walsh K. Myogenic Akt signaling regulates blood vessel recruitment during myofiber growth. Mol Cell Biol. 2002;22:4803–4814. doi: 10.1128/MCB.22.13.4803-4814.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takemura Y. Ouchi N. Shibata R. Aprahamian T. Kirber MT. Summer RS. Kihara S. Walsh K. Adiponectin modulates inflammatory reactions via calreticulin receptor-dependent clearance of early apoptotic bodies. J Clin Invest. 2007;117:375–386. doi: 10.1172/JCI29709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tan KC. Xu A. Chow WS. Lam MC. Ai VH. Tam SC. Lam KS. Hypoadiponectinemia is associated with impaired endothelium-dependent vasodilation. J Clin Endocrinol Metab. 2004;89:765–769. doi: 10.1210/jc.2003-031012. [DOI] [PubMed] [Google Scholar]
- 62.Tao L. Gao E. Jiao X. Yuan Y. Li S. Christopher TA. Lopez BL. Koch W. Chan L. Goldstein BJ. Ma XL. Adiponectin cardioprotection after myocardial ischemia/reperfusion involves the reduction of oxidative/nitrative stress. Circulation. 2007;115:1408–1416. doi: 10.1161/CIRCULATIONAHA.106.666941. [DOI] [PubMed] [Google Scholar]
- 63.Waki H. Yamauchi T. Kamon J. Ito Y. Uchida S. Kita S. Hara K. Hada Y. Vasseur F. Froguel P. Kimura S. Nagai R. Kadowaki T. Impaired multimerization of human adiponectin mutants associated with diabetes. Molecular structure and multimer formation of adiponectin. J Biol Chem. 2003;278:40352–40363. doi: 10.1074/jbc.M300365200. [DOI] [PubMed] [Google Scholar]
- 64.Wang Y. Gao E. Tao L. Lau WB. Yuan Y. Goldstein BJ. Lopez BL. Christopher TA. Tian R. Koch W. Ma XL. AMP-activated protein kinase deficiency enhances myocardial ischemia/reperfusion injury but has minimal effect on the antioxidant/antinitrative protection of adiponectin. Circulation. 2009;119:835–844. doi: 10.1161/CIRCULATIONAHA.108.815043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang Y. Lau WB. Gao E. Tao L. Yuan Y. Li R. Wang X. Koch WJ. Ma XL. Cardiomyocyte-derived adiponectin is biologically active in protecting against myocardial ischemia/reperfusion injury. Am J Physiol Endocrinol Metab. 2010;298:E663–E670. doi: 10.1152/ajpendo.00663.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Y. Tao L. Yuan Y. Lau WB. Li R. Lopez BL. Christopher TA. Tian R. Ma XL. Cardioprotective effect of adiponectin is partially mediated by its AMPK-independent antinitrative action. Am J Physiol Endocrinol Metab. 2009;297:E384–E391. doi: 10.1152/ajpendo.90975.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu X. Mahadev K. Fuchsel L. Ouedraogo R. Xu SQ. Goldstein BJ. Adiponectin suppresses IkappaB kinase activation induced by tumor necrosis factor-alpha or high glucose in endothelial cells: role of cAMP and AMP kinase signaling. Am J Physiol Endocrinol Metab. 2007;293:E1836–E1844. doi: 10.1152/ajpendo.00115.2007. [DOI] [PubMed] [Google Scholar]
- 68.Xi W. Satoh H. Kase H. Suzuki K. Hattori Y. Stimulated HSP90 binding to eNOS and activation of the PI3-Akt pathway contribute to globular adiponectin-induced NO production: vasorelaxation in response to globular adiponectin. Biochem Biophys Res Commun. 2005;332:200–205. doi: 10.1016/j.bbrc.2005.04.111. [DOI] [PubMed] [Google Scholar]
- 69.Xu SQ. Mahadev K. Wu X. Fuchsel L. Donnelly S. Scalia RG. Goldstein BJ. Adiponectin protects against angiotensin II or tumor necrosis factor alpha-induced endothelial cell monolayer hyperpermeability: role of cAMP/PKA signaling. Arterioscler Thromb Vasc Biol. 2008;28:899–905. doi: 10.1161/ATVBAHA.108.163634. [DOI] [PubMed] [Google Scholar]
- 70.Yamauchi T. Kamon J. Ito Y. Tsuchida A. Yokomizo T. Kita S. Sugiyama T. Miyagishi M. Hara K. Tsunoda M. Murakami K. Ohteki T. Uchida S. Takekawa S. Waki H. Tsuno NH. Shibata Y. Terauchi Y. Froguel P. Tobe K. Koyasu S. Taira K. Kitamura T. Shimizu T. Nagai R. Kadowaki T. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–769. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 71.Yamauchi T. Kamon J. Minokoshi Y. Ito Y. Waki H. Uchida S. Yamashita S. Noda M. Kita S. Ueki K. Eto K. Akanuma Y. Froguel P. Foufelle F. Ferre P. Carling D. Kimura S. Nagai R. Kahn BB. Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 72.Yamauchi T. Kamon J. Waki H. Terauchi Y. Kubota N. Hara K. Mori Y. Ide T. Murakami K. Tsuboyama-Kasaoka N. Ezaki O. Akanuma Y. Gavrilova O. Vinson C. Reitman ML. Kagechika H. Shudo K. Yoda M. Nakano Y. Tobe K. Nagai R. Kimura S. Tomita M. Froguel P. Kadowaki T. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 73.Yokota T. Oritani K. Takahashi I. Ishikawa J. Matsuyama A. Ouchi N. Kihara S. Funahashi T. Tenner AJ. Tomiyama Y. Matsuzawa Y. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood. 2000;96:1723–1732. [PubMed] [Google Scholar]
- 74.Zhu M. Miura J. Lu LX. Bernier M. DeCabo R. Lane MA. Roth GS. Ingram DK. Circulating adiponectin levels increase in rats on caloric restriction: the potential for insulin sensitization. Exp Gerontol. 2004;39:1049–1059. doi: 10.1016/j.exger.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 75.Ziemke F. Mantzoros CS. Adiponectin in insulin resistance: lessons from translational research. Am J Clin Nutr. 2010;91:258S–261S. doi: 10.3945/ajcn.2009.28449C. [DOI] [PMC free article] [PubMed] [Google Scholar]