Abstract
Diabetes exacerbates ischemic heart disease morbidity and mortality via incompletely understood mechanisms. Although adiponectin (APN) reduces myocardial ischemia/reperfusion (MI/R) injury in nondiabetic animals, whether APN's cardioprotective actions are altered in diabetes, a pathologic condition with endogenously reduced APN, has never been investigated. High-fat diet (HD)–induced diabetic mice and normal diet (ND) controls were subjected to MI via coronary artery ligation, and given vehicle or APN globular domain (gAPN, 2 μg/g) 10 min before reperfusion. Compared to ND mice (where gAPN exerted pronounced cardioprotection), HD mice manifested greater MI/R injury, and a tripled gAPN dose was requisite to achieve cardioprotective extent seen in ND mice (i.e., infarct size, apoptosis, and cardiac function). APN reduces MI/R injury via AMP-activated protein kinase (AMPK)–dependent metabolic regulation and AMPK-independent antioxidative/antinitrative pathways. Compared to ND, HD mice manifested significantly blunted gAPN-induced AMPK activation, basally and after MI/R (p < 0.05). Although both low- and high-dose gAPN equally attenuated MI/R-induced oxidative stress (i.e., NADPH oxidase expression and superoxide production) and nitrative stress (i.e., inducible nitric oxide synthase expression, nitric oxide production, and peroxynitrite formation) in ND mice, only high-dose gAPN efficaciously did so in HD mice. We demonstrate for the first time that HD-induced diabetes diminished both AMPK-dependent and AMPK-independent APN cardioprotection, suggesting an unreported diabetic heart APN resistance. Antioxid. Redox Signal. 15, 1779–1788.
Introduction
Ischemic cardiovascular disease is a major cause of morbidity and mortality in diabetic patients, who demonstrate enhanced vulnerability to ischemia/reperfusion insult and resultant death. Clarification of the molecular link between type-2 diabetes and cardiovascular injury may therefore help identify novel efficacious therapies attenuating postischemic myocardial injury, reducing myocardial ischemic morbidity, and ultimately decreasing diabetic mortality of cardiovascular etiology.
Adiponectin (APN) is an adipocytokine secreted from adipose tissue (11). Numerous epidemiological studies have demonstrated the correlation between reduced APN levels and increased risk of cardiovascular disease in obese and diabetic individuals (1, 19). APN deficiency is an independent risk factor for endothelial dysfunction, hypertension, coronary heart disease, myocardial infarction, and other cardiovascular complications (13). We and others have demonstrated through investigations that exogenous APN supplementation exerts significant cardiovascular protection against myocardial ischemia/reperfusion (MI/R) injury. However, to date, all studies reporting cardiovascular protection by APN were performed in nondiabetic animals (24, 25). Whether the cardioprotective actions of APN might be altered in the diabetic state, a pathologic condition where endogenous APN is significantly reduced and supplementation of APN may be most appropriate, has never been previously investigated.
Emerging evidence suggests that APN malfunction occurs in the early stage of metabolic syndrome and contributes to the development of insulin resistance, a pathologic condition known to play a key role in the etiology of diabetes mellitus and related disorders. Specifically, there is reduced APN-induced AMP-activated protein kinase (AMPK) activation in hepatic tissue from hypertensive or insulin receptor transgenic knockout animals; impaired APN stimulation of AMPK/acetyl-CoA carboxylase (ACC) and fatty acid oxidation in cultured myotubes and skeletal muscle strips isolated from obese/diabetic individuals has been documented (5, 7, 18, 21). High-fat diet (HD)–fed rats manifest loss of APN-induced acute ACC phosphorylation and stimulation of fatty acid oxidation in their muscle, and this APN resistance precedes skeletal muscle lipid accumulation and insulin resistance (17). Further, our most recent studies demonstrated that diet-induced obesity/hyperlipidemia caused significant vascular APN resistance (via AMPK/ACC phosphorylation and endothelial nitric oxide synthase [eNOS] activation) (16). However, whether and how diabetes may cause cardiac APN resistance, thus diminishing the cardioprotective effects of APN, has never been previously investigated.
Therefore, the aims of the present study were to determine whether the cardioprotective effect of globular domain of APN (gAPN) [the isoform with 20 times higher biologic activity than full length of APN (11)] against MI/R injury is altered in a HD induced diabetic model, and if so, to identify the potential underlying mechanisms for any observed altered APN function during in vivo MI/R in the diabetic state.
Materials and Methods
Adult (6-week-old) male C57BL/6J mice were randomized to receive a HD (60 kcal%) (Research Diets Inc. D12492i) or a 10 kcal% control normal diet (ND, D12450Bi) containing the same protein content as the HD. All experiments were performed in adherence with the National Institutes of Health Guidelines on the Use of Laboratory Animals, and were approved by the Thomas Jefferson University Committee on Animal Care.
Metabolic characterization
Mice were fasted overnight by removal to a clean cage without food at the end of their dark (feeding) cycle, ∼6 pm. Mice were weighed the morning after at 8 am, and 30 μl blood was obtained via tail clip to assess plasma glucose (Accu-Chek Active Blood Glucose Monitoring System; Roche Diagnostics, Indianapolis, IN), plasma insulin (enzyme-linked immunosorbent assay [ELISA]; Linco, Billerica, MA), and plasma APN (ELISA; R&D Systems, Minneapolis, MN). Weight and plasma measurements were recorded initially and weekly thereafter. The Homeostatic Model Assessment (HOMA) score, a surrogate measure of insulin resistance, was calculated initially and weekly thereafter, via HOMA calculator v2.2.2 (University of Oxford, United Kingdom).
After 8 weeks of being fed HD or ND, 14-week-old mice were anesthetized with 2% isoflurane (total isoflurane exposure time <5 min), and MI was produced via left anterior descending coronary artery slip-knot ligation as described in our previous studies (25). Twenty minutes after MI, both HD mice and controls were randomized to one of the following groups: sham MI (n = 12), MI + vehicle (n = 13), MI + low dose gAPN (2 μg/g intraperitoneal injection IP; n = 13), and MI + high dose gAPN (6 μg/g IP; n = 13). After 30 min of MI, the slip-knot was released, and reperfusion commenced. After 3 h (for all assays except cardiac function and infarct size) or 24 h (for cardiac function and infarct size only) reperfusion, the ligature around the coronary artery was retied, and 1 ml of 2% Evans blue dye was injected into the left ventricular cavity. The isolated Evans-blue-negative stained cardiac portion (ischemic/reperfused tissue, or area-at-risk) was utilized for immunohistological, biochemical, and Western blot studies as described below.
Determination of myocardial apoptosis and myocardial injury
Myocardial apoptosis was determined by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) staining, caspase-3 activity, and DNA fragmentation as described in our previous study (27). Serum levels of the cardiac-specific isoform of troponin-I were determined utilizing ELISA from Life Diagnostics (West Chester, PA) (8). Myocardial infarct size was assessed by Evans blue—2,3,5-triphenyl tetrazolium chloride double staining methods (27).
Determination of cardiac function
Cardiac function was determined by echocardiography and left ventricular catheterization methods 24 h after reperfusion before thoracotomy, as described in our previous studies (25, 27).
Quantification of superoxide production
Myocardial superoxide content (in area-at-risk) was quantified by lucigenin-enhanced luminescence as described previously (25).
Determination of total nitric oxide and nitrotyrosine content in cardiac tissue
The tissue nitric oxide (NO) and its metabolic products (NO2 and NO3) were determined by use of a chemiluminescence NO detector (Siever 280i NO Analyzer), and MI/R cardiac tissue nitrotyrosine (NT) content was quantified by ELISA as described previously (25, 27).
Western blot analysis
Proteins were separated on sodium dodecyl sulfate–polyacrylamide gel electrophoresis gels, transferred to nitrocellulose membranes, and incubated with primary antibodies (monoclonal antibody against phosphorylated ACC, inducible NO synthase [iNOS] [Upstate, Chicago, IL], or gp91phox [Transduction Laboratories, San Jose, CA]) and horseradish peroxidase-conjugated secondary antibody. The blot was developed with a Supersignal Chemiluminescence detection kit (Pierce, Rockford, IL), and observed with a Kodak Image Station 400 (Rochester, NY).
Statistical analysis
All values in the text and figures are presented as mean ± standard error of the mean of n independent experiments. Analysis of variance was performed across all investigated groups first. Post hoc pair-wise tests for certain group pairs with assessment of statistical significance were then performed after Bonferroni correction of the overall significance level. Western blot densities were analyzed with Kruskal–Wallis test followed by Dunn post hoc test. p-values ≤0.05 were considered statistically significant. The authors had full access to and take full responsibility for data integrity. All authors have read and agreed to the article as written.
Results
Eight weeks HD induced type-2 diabetes in mice
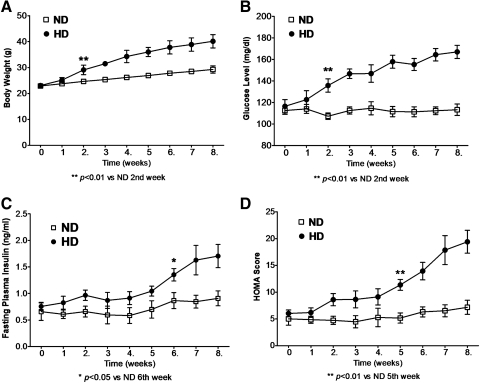
We conducted initial experiments to determine the ability of HD to induce type-2 diabetes in mice. Mice were fasted overnight, and body weight, plasma glucose, and insulin and APN levels were determined (Fig. 1). HD-fed mice had greater body weight increase than ND after 2 weeks (Fig. 1A, p < 0.01). Concomitantly, fasting plasma glucose increased steadily in HD mice after 2 weeks (Fig. 1B, p < 0.01), and fasting plasma insulin increased from 6 weeks onward (Fig. 1C, p < 0.05). After 5 weeks HD, calculated HOMA score was positively correlated with elevating insulin levels (Fig. 1D, p < 0.01).
FIG. 1.
High-fat diet (HD) for 8 weeks induced type-2 diabetes model in mice. (A) Body weight. (B) Fasting plasma glucose. (C) Fasting plasma insulin determined by enzyme-linked immunosorbent assay. (D) Fasting Homeostatic Model Assessment (HOMA) score calculated by fasting plasma glucose and insulin. n = 10–13 mice per group. *p < 0.05, **p < 0.01 versus normal diet (ND) mice at the same time point.
HD increased susceptibility to MI/R injury
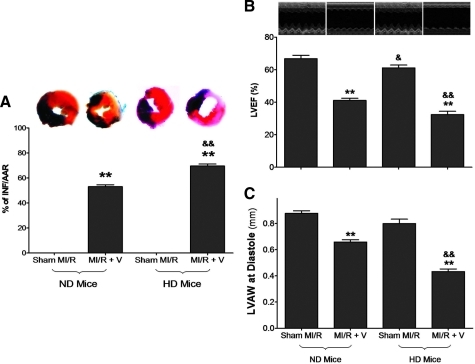
HD mice had normal basal cardiac function (Sham MI/R groups in Fig. 2). However, when subjected to in vivo MI/R injury, HD mice manifested significantly greater cardiac injury than their ND controls. Specifically, MI/R-induced infarct size was enlarged (Fig. 2A), and cardiac function was further depressed (Fig. 2B).
FIG. 2.
HD increased susceptibility of myocardial ischemia/reperfusion (MI/R) injury. (A) Myocardial infarction determined by Evans blue/2,3,5-triphenyl tetrazolium chloride double staining. (B) Left ventricular ejection fraction (LVEF) determined by echocardiography. (C) Left ventricular wall thickness (LVAW) determined by echocardiography. n = 10–13 mice per group. **p < 0.01 versus sham control in the same diet group; &p < 0.05, &&p < 0.01 between the different diet groups with the same treatment (i.e., sham MI/R or MI/R + V). (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
Cardioprotective effect of gAPN was attenuated in HD mice
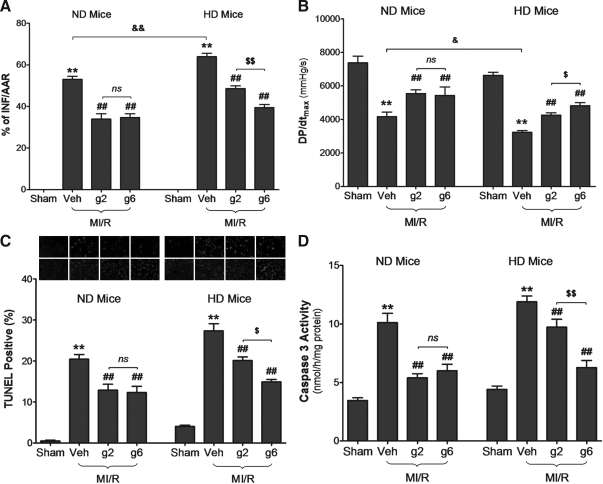
Having demonstrated that HD increased susceptibility to MI/R injury, we compared the cardioprotective effect of gAPN in HD and ND animals subjected to MI/R. Consistent with our previously published finding, treatment of ND animals with 2 μg/g gAPN 10 min before reperfusion significantly attenuated MI/R-induced injury, as evidenced by infarct size reduction (Evans blue/2,3,5-triphenyl tetrazolium chloride double staining, Fig. 3A), cardiac function improvement (dP/dtmax, Fig. 3B), and apoptosis decrease (TUNEL staining, Fig. 3C; caspase-3 activity, Fig. 3D). However, treatment of HD animals with the same dose of gAPN (i.e., 2 μg/g) only attenuated a relatively small portion of MI/R-induced injury (33.5% reduction of infarct size, 31.8% increase of dP/dtmax, 31.0% reduction of TUNEL positive cells, and 28.9% reduction of caspase-3 activity, p-value all <0.05 vs. 2 μg/g gAPN-treated ND animals), indicating that the cardioprotective actions of gAPN are reduced in HD-induced type-2 diabetic condition. To obtain more evidence supporting a hypothesis that type-2 diabetes causes cardiac APN resistance, the cardioprotective effects of a higher dose of gAPN (6 μg/g) were investigated. As summarized in Figure 3, administration of high-dose gAPN failed to further enhance its cardioprotection in ND mice, suggesting that the cardioprotective effects of gAPN against MI/R injury was already saturated at 2 μg/g. However, in HD animals, high-dose gAPN alleviated more MI/R-induced damage than low-dose gAPN (1.59-fold decrease in infarct size, 1.75-fold increase in dP/dtmax, 1.72-fold reduction in TUNEL-positive cells, and 2.59-fold decrease in caspase-3 activity, p < 0.05 vs. 2 μg/g group). These novel data suggest attenuated cardioprotective action of gAPN in the HD condition, which can be overcome with increased gAPN dosage.
FIG. 3.
Cardioprotective effect of globular domain of adiponectin (gAPN) was attenuated in HD mice. (A) Myocardial infarction determined by Evans blue/2,3,5-triphenyl tetrazolium chloride double staining. (B) Left ventricular pressure (dP/dt) determined by hemodynamic measurements. (C) Cardiomyocyte apoptosis determined by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL). (D) Caspase-3 activity. n = 10–13 mice per group. **p < 0.01 versus sham control in the same diet group; ##p < 0.01 versus vehicle-treated group in the same diet group; $p < 0.05, $$p < 0.01 versus low dose gAPN-treated animals in the same diet group; &p < 0.05, &&p < 0.01 between different diet groups with the same treatment; ns, no significant difference between compared groups.
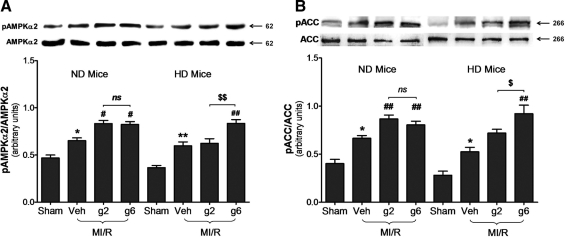
HD impaired gAPN-induced AMPK/ACC activation in cardiomyocytes, both basally and post-MI/R
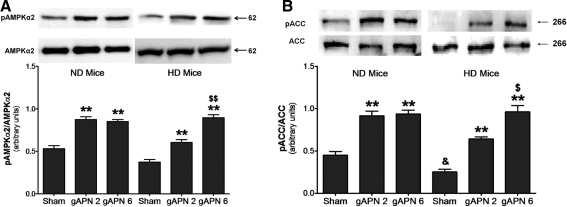
APN delivers much of its metabolic-regulatory effect via the AMPK-ACC signaling axis (28). Previous studies have demonstrated impairment in the ability of APN to stimulate AMPK/ACC phosphorylation in skeletal myocytes, hepatocytes, and endothelial cells cultured in hyperglycemic/hyperlipidemic conditions. In consistent fashion, we observed a 1.6- and 2.0-fold increase in AMPK phosphorylation and ACC phosphorylation, respectively, in ND hearts after either dose of gAPN treatment (Fig. 4A, B, left panels). However, low-dose gAPN was only partially effective in doing so (phosphorylating cardiac AMPK 44.1% and ACC 54.9% of high-dose gAPN), and a higher-dose gAPN (i.e., 6 μg/g) was required to stimulated cardiac AMPK/ACC phosphorylation to the same level in HD mice compared to ND animals.
FIG. 4.
Reduced APN stimulated AMP-activated protein kinase (AMPK)/acetyl-CoA carboxylase (ACC) activation occurred in HD mice. (A) Effect of adiponectin treatment on AMPKα2 phosphorylation determined by representative Western blots. (B) Effect of adiponectin treatment on ACC phosphorylation determined by representative Western blots. n = 5–8 hearts per group. **p < 0.01 versus sham control in the same diet group; $p < 0.05, $$p < 0.01 versus low dose gAPN-treated animals in the same diet group; &p < 0.05 between different diet groups with the same treatment.
We next determined whether HD impaired AMPK/ACC activation following MI/R. A 1.39-fold and 1.65-fold increase in AMPK and ACC phosphorylation, respectively, was observed in ND hearts post-MI/R. Both gAPN doses further enhanced AMPK (low-dose: 1.27-fold increase and high-dose: 1.26-fold increase) and ACC (low-dose: 1.30-fold increase and high-dose: 1.21-fold increase) phosphorylation modestly (Fig. 5A, B, left panel). However, low-dose gAPN failed to further activate cardiac AMPK/ACC phosphorylation in HD mice. A tripled dose of gAPN was required to achieve significant AMPK/ACC phosphorylation comparable to that induced by 2 μg/g in ND animals (Fig. 5A, B, right panel).
FIG. 5.
Cardiac adiponectin resistance to stimulate AMPK/ACC activation occurred upon MI/R injury in HD mice. (A) Effect of adiponectin treatment on AMPKα2 phosphorylation determined by representative Western blots. (B) Effect of adiponectin treatment on ACC phosphorylation determined by representative Western blots. n = 5–8 hearts per group. *p < 0.05, **p < 0.01 versus sham control in the same diet group; #p < 0.05, ##p < 0.01 versus vehicle-treated group in the same diet group; $p < 0.05, $$p < 0.01 versus low dose gAPN-treated animals in the same diet group.
Impaired APN-mediated antioxidative protection in HD mice signified cardiac resistance to APN
Recently, we reported that gAPN exerts its cardioprotective effects via AMPK-dependent metabolic regulation and AMPK-independent antioxidative/antinitrative actions (25). Having demonstrated that AMPK/ACC activation by gAPN was significantly blunted in HD animals, we further investigated whether the antioxidant/antinitrative action of gAPN is also altered, thus contributing to its diminished cardioprotective effects in diabetic animals. Two series of experiments were performed.
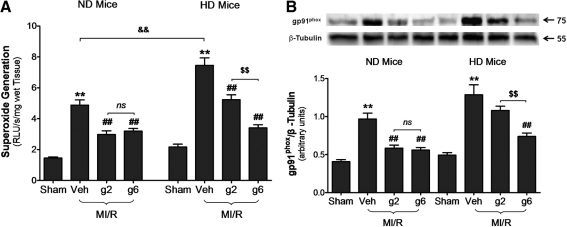
In the first series of experiments, we measured MI/R-induced oxidative stress. As illustrated in Figure 6A, there was no significant difference between superoxide overproduction in ND mice and HD mice at basal conditions. However, superoxide production was enhanced by MI/R injury in HD mice (p < 0.001 vs. ND MI/R heart). gAPN (either dose) significantly inhibited MI/R-induced superoxide overproduction to similar levels seen in ND animals (low/high-dose gAPN, respectively, reduced 55.3%/49.0% superoxide production in ND animals, Fig. 6A). However, although high-dose gAPN attenuated superoxide overproduction in HD mice to levels seen in ND animals (54.3% reduction of superoxide production, p < 0.01), low-dose gAPN reduced superoxide production by only 29.8% (p < 0.01), a 55.1% loss of efficacy compared to high-dose gAPN in reducing superoxide overproduction in HD animals (Fig. 6A).
FIG. 6.
Cardiac adiponectin resistance in HD mice impaired its antioxidative protection. (A) Production of superoxide (n = 8–10 hearts per group). (B) gp91phox determined by representative Western blots (n = 5–8 hearts per group). **p < 0.01 versus sham control in the same diet group; ##p < 0.01 versus vehicle-treated group in the same diet group; $$p < 0.01 versus low dose gAPN-treated animals in the same diet group; &&p < 0.01 between different diet groups with the same treatment.
We further determined the molecular sources potentially responsible for the impaired antioxidant actions of APN in the HD heart. There was significantly increased MI/R-induced overexpression of gp91phox in HD hearts compared to ND (Fig. 6B; lane 2 vs. lane 6, p < 0.05), consistent with enhanced cardiac superoxide overproduction trends in HD mice (Fig. 6A). Treatment of ND mice with gAPN (either dose) significantly inhibited gp91phox expression. However, only high dose of gAPN was effective in inhibiting gp91phox expression in HD animals (Fig. 6B, right panel), although the absolute level of gp91phox expression remained greater in high-dose gAPN-treated HD hearts than gAPN-treated ND hearts. Together with the data presented in Figure 6A, these results demonstrated that MI/R-induced oxidative stress was increased in HD mice, and gAPN's antioxidant effect was impaired, requiring increased APN dosage to maintain antioxidant cardioprotective efficacy under diabetic conditions.
Impaired APN-mediated antinitrative protection in HD mice further demonstrated cardiac resistance to APN
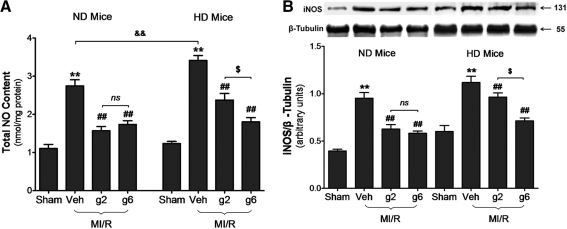
In the second series of experiments, we assessed MI/R-induced myocardial nitrative stress. As illustrated in Figure 7A, no significant difference was seen between total NO content in ND and HD mice at basal conditions, but NO production was further enhanced by MI/R injury in HD mice (p < 0.01 vs. ND MI/R heart). gAPN (either dose) significantly inhibited MI/R-induced NO overproduction in ND heart (low/high-gAPN dose reduced 42.7%/36.8%, respectively, NO overproduction, p < 0.01). In HD animals, high-dose gAPN reversed NO overproduction to similar levels seen in ND animals (47.1% reduction of NO overproduction, p < 0.01), but low-dose gAPN reduced NO overproduction by only 30.4% (p < 0.01), a 35.5% loss of efficacy compared to high-dose gAPN in decreasing NO overproduction in HD animals (Fig. 7A).
FIG. 7.
Cardiac adiponectin resistance impaired antinitrative protection. (A) Production of NOx content (n = 10–13 hearts per group). (B) Inducible nitric oxide synthase (iNOS) expression (n = 5–7/group) by representative Western blots. **p < 0.01 versus sham control in the same diet group; ##p < 0.01 versus vehicle-treated group in the same diet group; $p < 0.05 versus low dose gAPN-treated animals in the same diet group; &&p < 0.01 between different diet groups with the same treatment.
Next, we determined the molecular sources potentially responsible for the impaired antinitrative function of APN observed in the HD condition. MI/R-induced overexpression of iNOS was significantly increased in HD compared to ND hearts (Fig. 7B; lane 2 vs. lane 6, p < 0.05). These data are consistent with results in Figure 7A, showing that MI/R-induced cardiac NO overproduction was further enhanced in HD mice. Treatment of ND mice with gAPN (either dose) significantly inhibited iNOS expression (Fig. 7B; low/high-gAPN dose reduced 34.3%/36.3%, respectively, iNOS expression compared to vehicle, p < 0.01). Treatment of HD mice with high-dose gAPN similarly inhibited iNOS expression (Fig. 7B; high-gAPN dose reduced 36.3% iNOS expression compared to vehicle, p < 0.01). However, low-dose gAPN reduced iNOS expression only 13.8% compared to vehicle in HD mice (p < 0.01 vs. high dose). Together with the data presented in Figure 7A, these results demonstrated that MI/R-induced nitrative stress was increased in HD mice, and gAPN's antinitrative effect was impaired, requiring increased APN dosage to maintain antinitrative cardioprotective efficacy.
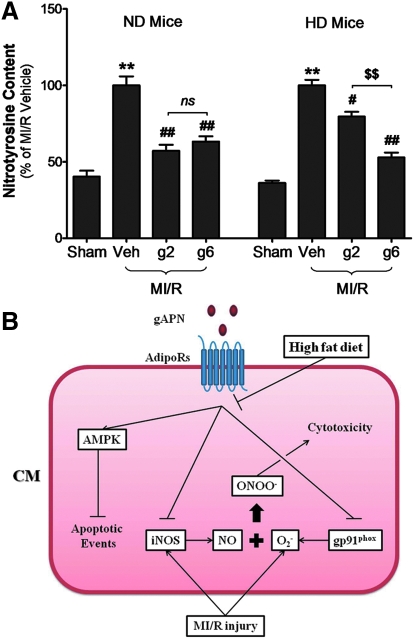
Currently, it is generally accepted that the conversion of the cytoprotective molecule NO to a cytotoxic molecule, peroxynitrite, is the major mechanism responsible for superoxide toxicity. As illustrated in Figure 8, NT content, the footprint of peroxynitrite production, was increased by MI/R injury in ND mice (p < 0.01 vs. ND MI/R heart). gAPN (either dose) significantly inhibited MI/R-induced NT generation in ND heart (low/high-dose reduced 42.8%/36.7%, respectively, of NT generation in ND heart). Whereas high-dose gAPN attenuated NT production in HD mice to levels seen in ND littermates (47.2% reduction of NT production), low-dose gAPN decreased NT production in HD animals only 20.4%, a 56.7% loss of efficacy compared to high-dose gAPN in decreasing NT overproduction in HD animals (Fig. 8).
FIG. 8.
Peroxynitrite was significantly blunted in HD-induced diabetic heart. (A) Nitrotyrosine content determined by ELISA (n = 10–13/group). **p < 0.01 versus sham control in the same diet group; #p < 0.05, ##p < 0.01 versus vehicle-treated group in the same group; $$p < 0.01 versus low dose gAPN-treated animals in the same diet group. (B) A schematic illustration of the hypothesis tested in the current study. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
Discussion
Several important observations have been made in the present study. First, our results demonstrated that 8-week HD-induced diabetes diminished, but did not fully abrogate the cardioprotective action of gAPN in a clinically relevant animal model of in vivo regional MI/R. gAPN doses >2 μg/g body weight offered no additional cardioprotective benefit against MI/R injury in ND mice, but this dose only reversed a minimal portion of ischemia-induced myocardial apoptosis, infarct size, and cardiac dysfunction in HD mice. This finding provides the first evidence that a resistance to the cardioprotective effects of APN develops in the diabetic condition in vivo. Second, we have provided the first direct mechanistic evidence underlying this impaired response to APN cardioprotection, which is partly via AMPK-ACC activation resistance as well as diminished AMPK-independent antioxidative/antinitrative protection. Finally, we have demonstrated that, although APN resistance impaired its cardioprotection, increased gAPN dosage can still attenuate MI/R-induced myocardial apoptosis, reduce infarct size, and salvage cardiac function in the diabetic condition, suggestive of a cautiously positive future clinical application of APN.
APN is an abundant circulating adipocytokine secreted from adipose tissue, which has at least three major functions, including an insulin sensitization/metabolic-regulatory function (in the liver and muscle), an anti-inflammatory/vascular protective function, and an anti-ischemic/cardioprotective function. Numerous epidemiological studies reveal the correlation between reduced APN levels and increased morbidity/mortality of cardiovascular ischemic diseases and diabetes mellitus (3, 10, 12, 15, 22, 29). Conversely, a higher plasma APN concentration is associated with a lower risk of ischemic heart disease in men (9, 15, 20). Moreover, Walsh's group (24) and our group (25) have recently demonstrated markedly increased MI/R injury in APN-knockout mice, and exogenous APN supplementation can significantly decrease myocardial apoptosis, infarct size, and impaired cardiac function. Together, these exciting results support the role of APN as a directly cardioprotective molecule against MI/R injury, suggesting that APN might be a novel therapeutic molecule in the treatment of diabetic cardiovascular injury.
In obesity and type-2 diabetes, tissue response to insulin is significantly blunted, and this insulin resistance is known to play a key role in the pathogenesis of diabetes mellitus and related disorders (26). Numerous studies have demonstrated that APN shares many biological functions with insulin, including lipid oxidation, glucose uptake, and cardiovascular protection, and that there exists APN and insulin signaling cross-talk at multiple levels (4). However, whether obesity and type-2 diabetes may also alter tissue response to APN has just begun to be recognized and the potential impact of APN resistance on diabetic cardiovascular injury remains largely unknown. Specifically, although the anti-ischemic/cardioprotective effects of APN have been well-recognized, there has been no investigation regarding the cardioprotective effects of APN in the obesity/diabetic animal, a pathologic condition where supplementation of APN is needed. Here, we demonstrate that a HD-induced diabetic model attenuates the cardioprotective actions of gAPN in response to regional MI/R in vivo, providing the first direct evidence of cardiac resistance to APN.
The majority of APN's metabolic regulatory function occurs via the AMPK/ACC signaling axis (28), whereas its inhibition of the inflammatory response and elicitation of vasodilatation/vasculoprotection occurs largely through the AMPK/eNOS axis (6, 23). Consistent with these studies, our present data demonstrated gAPN further activated AMPK and phosphorylated ACC downstream, effecting cardioprotection against MI/R injury in ND mice. Importantly, HD weakened this AMPK-ACC phosphorylation and impaired cardioprotection of gAPN, giving novel mechanistic evidence that observed APN resistance in cardioprotection occurs partly via impaired AMPK activation.
Although the AMPK pathway is critical to APN's metabolic-regulatory, anti-inflammatory, and vasculoprotective function, we have recently provided direct evidence of AMPK-independent gAPN-mediated cardioprotection in the intact animal (27). Overproduction of reactive oxygen species is a central cause of reperfusion-induced endoplasmic reticulum stress and apoptosis, and we recently demonstrated that APN inhibited gp91phox (the essential membrane component of NADPH oxidase responsible for a large portion of superoxide production in the MI/R heart) overexpression, thus attenuating oxidative stress-induced tissue injury (25). Presently, we demonstrate increased gp91phox expression and enhanced superoxide production in diabetic hearts. Although gAPN significantly reversed MI/R-induced gp91phox over-expression and superoxide overproduction in ND mice, the same gAPN dose could only attenuate a small percentage of MI/R-induced oxidative stress, and a tripled gAPN dose was required to halt MI/R-induced superoxide generation and diminish gp91phox overexpression in HD mice to levels observed in ND littermates. Together, these results show impairment of gAPN's antioxidant effect, and increased APN dosage is required to sustain its antioxidant cardioprotective efficacy.
APN is known to stimulate NO production via AMPK-mediated eNOS phosphorylation. However, both iNOS expression and total NO production are markedly enhanced in IR tissue from APN-KO mice (25). By itself, NO is not toxic and does not cause significant tissue injury, even at very high concentration in physiological conditions (14). However, in the presence of O2 − (which occurs during ischemia/reperfusion) NO reacts to form peroxynitrite, an extremely toxic molecule causative of rampant oxidative/nitrative tissue injury (2, 25). We previously demonstrated the antinitrative effects of gAPN in MI/R hearts are not AMPK-mediated (27). Presently, we show that the diabetic state unfettered gAPN's inhibition of iNOS overexpression, thus increasing NO production and peroxynitrite formation. However, gAPN's antinitrative properties could be regained with increased gAPN dosage.
Collectively, our data demonstrated that diabetes blunted gAPN cardioprotection by impairing both AMPK-dependent and AMPK-independent pathways through which gAPN protects ischemic/reperfused heart. These results further indicate that the APN resistance is developed at high levels before the divergence of gAPN cardioprotective signaling. The detailed mechanisms underlying how the diabetic condition impairs the cardiomyocyte response to APN concerning activation of AMPK and antioxidative/nitrative effect is currently under investigation. Moreover, the cardioprotection of gAPN was blunted but no abrogated, and a higher concentration remains to be effective in diabetic animals. These results may potentially translate to differential clinical treatment plans for diabetic versus nondiabetic patients in the face of myocardial ischemic injury.
Abbreviations Used
- ACC
acetyl-CoA carboxylase
- AMPK
AMP-activated protein kinase
- APN
adiponectin
- ELISA
enzyme-linked immunosorbent assay
- eNOS
endothelial nitric oxide synthase
- gAPN
globular domain of adiponectin
- HD
high-fat diet
- HOMA
Homeostatic Model Assessment
- LVAW
left ventricular wall thickness
- LVEF
left ventricular ejection fraction
- MI/R
myocardial ischemia/reperfusion
- ND
normal diet
- NO
nitric oxide
- NT
nitrotyrosine
- TUNEL
terminal deoxynucleotidyl transferase–mediated dUTP nick-end labeling
Acknowledgments
This research was supported by the following grants: NIH HL-63828, HL-096686, American Diabetes Association 7-05-RA-83, and American Heart Association Grant-in-Aid 0855554D (X.L.M.).
Author Disclosure Statement
No competing financial interests exist for any of the authors of this article.
References
- 1.Basu R. Pajvani UB. Rizza RA. Scherer PE. Selective downregulation of the high molecular weight form of adiponectin in hyperinsulinemia and in type 2 diabetes: differential regulation from nondiabetic subjects. Diabetes. 2007;56:2174–2177. doi: 10.2337/db07-0185. [DOI] [PubMed] [Google Scholar]
- 2.Beckman JS. Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 3.Behrends M. Schulz R. Post H. Alexandrov A. Belosjorow S. Michel MC. Heusch G. Inconsistent relation of MAPK activation to infarct size reduction by ischemic preconditioning in pigs. Am J Physiol Heart Circ Physiol. 2000;279:H1111–H1119. doi: 10.1152/ajpheart.2000.279.3.H1111. [DOI] [PubMed] [Google Scholar]
- 4.Berg AH. Combs TP. Du X. Brownlee M. Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–953. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 5.Bruce CR. Mertz VA. Heigenhauser GJ. Dyck DJ. The stimulatory effect of globular adiponectin on insulin-stimulated glucose uptake and fatty acid oxidation is impaired in skeletal muscle from obese subjects. Diabetes. 2005;54:3154–3160. doi: 10.2337/diabetes.54.11.3154. [DOI] [PubMed] [Google Scholar]
- 6.Chen H. Montagnani M. Funahashi T. Shimomura I. Quon MJ. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J Biol Chem. 2003;278:45021–45026. doi: 10.1074/jbc.M307878200. [DOI] [PubMed] [Google Scholar]
- 7.Chen MB. McAinch AJ. Macaulay SL. Castelli LA. O'Brien PE. Dixon JB. Cameron-Smith D. Kemp BE. Steinberg GR. Impaired activation of AMP-kinase and fatty acid oxidation by globular adiponectin in cultured human skeletal muscle of obese type 2 diabetics. J Clin Endocrinol Metab. 2005;90:3665–3672. doi: 10.1210/jc.2004-1980. [DOI] [PubMed] [Google Scholar]
- 8.Elrod JW. Calvert JW. Morrison J. Doeller JE. Kraus DW. Tao L. Jiao X. Scalia R. Kiss L. Szabo C. Kimura H. Chow CW. Lefer DJ. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci U S A. 2007;104:15560–15565. doi: 10.1073/pnas.0705891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frystyk J. Berne C. Berglund L. Jensevik K. Flyvbjerg A. Zethelius B. Serum adiponectin is a predictor of coronary heart disease: a population-based 10-year follow-up study in elderly men. J Clin Endocrinol Metab. 2007;92:571–576. doi: 10.1210/jc.2006-1067. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein BJ. Scalia R. Adipokines and vascular disease in diabetes. Curr Diab Rep. 2007;7:25–33. doi: 10.1007/s11892-007-0006-6. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein BJ. Scalia RG. Ma XL. Protective vascular and myocardial effects of adiponectin. Nat Clin Pract Cardiovasc Med. 2009;6:27–35. doi: 10.1038/ncpcardio1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hotta K. Funahashi T. Arita Y. Takahashi M. Matsuda M. Okamoto Y. Iwahashi H. Kuriyama H. Ouchi N. Maeda K. Nishida M. Kihara S. Sakai N. Nakajima T. Hasegawa K. Muraguchi M. Ohmoto Y. Nakamura T. Yamashita S. Hanafusa T. Matsuzawa Y. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595–1599. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 13.Kadowaki T. Yamauchi T. Kubota N. Hara K. Ueki K. Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116:1784–1792. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim YM. Bombeck CA. Billiar TR. Nitric oxide as a bifunctional regulator of apoptosis. Circ Res. 1999;84:253–256. doi: 10.1161/01.res.84.3.253. [DOI] [PubMed] [Google Scholar]
- 15.Kumada M. Kihara S. Sumitsuji S. Kawamoto T. Matsumoto S. Ouchi N. Arita Y. Okamoto Y. Shimomura I. Hiraoka H. Nakamura T. Funahashi T. Matsuzawa Y. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol. 2003;23:85–89. doi: 10.1161/01.atv.0000048856.22331.50. [DOI] [PubMed] [Google Scholar]
- 16.Li R. Xu M. Wang X. Wang Y. Lau WB. Yuan Y. Yi W. Wei X. Lopez BL. Christopher TA. Wang XM. Ma XL. Reduced vascular responsiveness to adiponectin in hyperlipidemic rats-mechanisms and significance. J Mol Cell Cardiol. 2010;49:508–515. doi: 10.1016/j.yjmcc.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mullen KL. Pritchard J. Ritchie I. Snook LA. Chabowski A. Bonen A. Wright D. Dyck DJ. Adiponectin resistance precedes the accumulation of skeletal muscle lipids and insulin resistance in high-fat-fed rats. Am J Physiol Regul Integr Comp Physiol. 2009;296:R243–R251. doi: 10.1152/ajpregu.90774.2008. [DOI] [PubMed] [Google Scholar]
- 18.Mullen KL. Smith AC. Junkin KA. Dyck DJ. Globular adiponectin resistance develops independently of impaired insulin-stimulated glucose transport in soleus muscle from high-fat-fed rats. Am J Physiol Endocrinol Metab. 2007;293:E83–E90. doi: 10.1152/ajpendo.00545.2006. [DOI] [PubMed] [Google Scholar]
- 19.Ouchi N. Shibata R. Walsh K. Cardioprotection by adiponectin. Trends Cardiovasc Med. 2006;16:141–146. doi: 10.1016/j.tcm.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pischon T. Girman CJ. Hotamisligil GS. Rifai N. Hu FB. Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004;291:1730–1737. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez A. Catalan V. Becerril S. Gil MJ. Mugueta C. Gomez-Ambrosi J. Fruhbeck G. Impaired adiponectin-AMPK signalling in insulin-sensitive tissues of hypertensive rats. Life Sci. 2008;83:540–549. doi: 10.1016/j.lfs.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 22.Shibata R. Numaguchi Y. Matsushita K. Sone T. Kubota R. Ohashi T. Ishii M. Kihara S. Walsh K. Ouchi N. Murohara T. Usefulness of adiponectin to predict myocardial salvage following successful reperfusion in patients with acute myocardial infarction. Am J Cardiol. 2008;101:1712–1715. doi: 10.1016/j.amjcard.2008.02.057. [DOI] [PubMed] [Google Scholar]
- 23.Shibata R. Ouchi N. Kihara S. Sato K. Funahashi T. Walsh K. Adiponectin stimulates angiogenesis in response to tissue ischemia through stimulation of amp-activated protein kinase signaling. J Biol Chem. 2004;279:28670–28674. doi: 10.1074/jbc.M402558200. [DOI] [PubMed] [Google Scholar]
- 24.Shibata R. Sato K. Pimentel DR. Takemura Y. Kihara S. Ohashi K. Funahashi T. Ouchi N. Walsh K. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med. 2005;11:1096–1103. doi: 10.1038/nm1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tao L. Gao E. Jiao X. Yuan Y. Li S. Christopher TA. Lopez BL. Koch W. Chan L. Goldstein BJ. Ma XL. Adiponectin cardioprotection after myocardial ischemia/reperfusion involves the reduction of oxidative/nitrative stress. Circulation. 2007;115:1408–1416. doi: 10.1161/CIRCULATIONAHA.106.666941. [DOI] [PubMed] [Google Scholar]
- 26.Venables MC. Jeukendrup AE. Physical inactivity and obesity: links with insulin resistance and type 2 diabetes mellitus. Diabetes Metab Res Rev. 2009;25(Suppl 1):S18–S23. doi: 10.1002/dmrr.983. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y. Gao E. Tao L. Lau WB. Yuan Y. Goldstein BJ. Lopez BL. Christopher TA. Tian R. Koch W. Ma XL. AMP-activated protein kinase deficiency enhances myocardial ischemia/reperfusion injury but has minimal effect on the antioxidant/antinitrative protection of adiponectin. Circulation. 2009;119:835–844. doi: 10.1161/CIRCULATIONAHA.108.815043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamauchi T. Kamon J. Minokoshi Y. Ito Y. Waki H. Uchida S. Yamashita S. Noda M. Kita S. Ueki K. Eto K. Akanuma Y. Froguel P. Foufelle F. Ferre P. Carling D. Kimura S. Nagai R. Kahn BB. Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 29.Zhu M. Miura J. Lu LX. Bernier M. DeCabo R. Lane MA. Roth GS. Ingram DK. Circulating adiponectin levels increase in rats on caloric restriction: the potential for insulin sensitization. Exp Gerontol. 2004;39:1049–1059. doi: 10.1016/j.exger.2004.03.024. [DOI] [PubMed] [Google Scholar]