Abstract
Objective
The purpose of this study was to investigate the effect of sildenafil in patients with failing Fontan physiology.
Design
A retrospective chart review was performed to compare history and available data in patients with Fontan circulations before and after starting sildenafil. The paired and unpaired Student’s t-tests were used for statistical analyses.
Patients
Six patients at our institution with Fontan physiology, persistent symptoms of cyanosis or effusion, and poor hemodynamics as measured in the catheterization laboratory were placed on sildenafil. One patient was not included in the analysis because of insufficient length of treatment. All patients had symptoms of failing Fontan hemodynamics with either persistent cyanosis or effusions. In this group, the mean pulmonary artery pressure was greater than 15 mm Hg (17.4 ± 1.5 mm Hg) with mean estimated pulmonary vascular resistance of 3.5 ± 1.0 Wood units × m2 prior to starting sildenafil.
Results
Sildenafil significantly increased the systemic arterial oxyhemoglobin saturation in this group (82.8 ± 7.3% pre-treatment vs. 91.0 ± 5.5% post-treatment, P = .017). In the four out of five patients who have had follow-up catheterizations, there was a significant decrease in pulmonary artery pressure (17.4 ± 1.5 mm Hg pre-treatment vs. 13.8 ± 2.1 mm Hg post-treatment, P = .018) and in estimated pulmonary vascular resistance pre- and post-sildenafil treatment (3.5 ± 1.0 Wood units × m2 pre-treatment vs. 2.0 ± 0.4 Wood units × m2 post-treatment, P = .031).
Conclusions
Sildenafil may be a useful adjunct to therapy in patients with failing Fontan physiology likely through its function as a pulmonary vasodilator.
Keywords: Single Ventricle, Fontan, Sildenafil, Pulmonary Vascular Resistance
Introduction
Patients with single ventricle (SV) physiology undergoing staged Fontan palliation depend on low pulmonary vascular resistance (PVR) among other factors for successful circulatory status. The complications of high PVR in Fontan physiology are numerous and can lead to failure of the intended circulation. Persistent pleural effusions, protein-losing enteropathy, formation of collateral vessels, excessive cyanosis, and failure to thrive are a few of the complications in this patient population. The acute use of nitric oxide and other pulmonary vasodilators has been reported for SV patients with Fontan physiology and low cardiac output associated with high pulmonary artery pressures (PAPs).1 However, some patients become symptomatic months to years after Fontan surgery, and with continued evidence of failure of the circulation, they eventually are referred for cardiac transplantation. To our knowledge, there have been no case series reports of the use of sildenafil to lower PVR in patients with SV physiology. We report our initial experience with the use of sildenafil in this population.
Methods
A retrospective review of our clinical and cardiac catheterization databases was undertaken to characterize the hemodynamics of patients with Fontan physiology who were treated with sildenafil. Approval was obtained from the Institutional Review Board for the review. The six patients were chosen for sildenafil administration based on persistent symptoms of failing Fontan physiology and high PVR as estimated during cardiac catheterization. All patients lived in or around Denver, Colorado, which is at an altitude of 1610 meters and all catheterizations were performed in Denver unless otherwise specified.
Analyses were performed using the paired and unpaired Student’s t-tests. A P value of <0.05 was considered significant. Statistics within the paper are reported as the mean value ± one standard deviation. The figures include box-and-whisker plots of the data as well as pre-post line graphs to illustrate results.
Results
Six patients with SV Fontan physiology were treated with sildenafil as part of their medication regimen. The goal dose was 1 mg/kg three times a day and was achieved in all patients. However, one patient developed persistent priapism 3 weeks into treatment. Weaning the dose was unsuccessful in relieving symptoms and it was subsequently discontinued. Given the insufficient length of treatment, he is not included in the analysis.
Prior to Fontan surgery, all patients had preoperative cardiac catheterizations that revealed favorable hemodynamics for proceeding with the final stage of palliation. Significant venovenous or aortopulmonary collaterals were addressed at that time. Their surgeries were performed in the age range of 18–30 months. Each patient except for Patient 4 had a fenestration created at the time of surgery. Each patient was taken to the cardiac catheterization lab because of persistent symptoms following Fontan palliation: three of the six had persistent cyanosis and the other two had persistent pleural effusions. It was ensured that all patients were free of obstructive lesions. The average time from surgery to catheterization to evaluate hemodynamics prior to starting sildenafil was 8.3 months with a range from 2 to 24 months. Patient demographics, symptoms, and cardiac catheterization data are listed in Table 1. Further details for each patient are as follows.
Table 1.
Patient Characteristics and Cardiac Catheterization Data
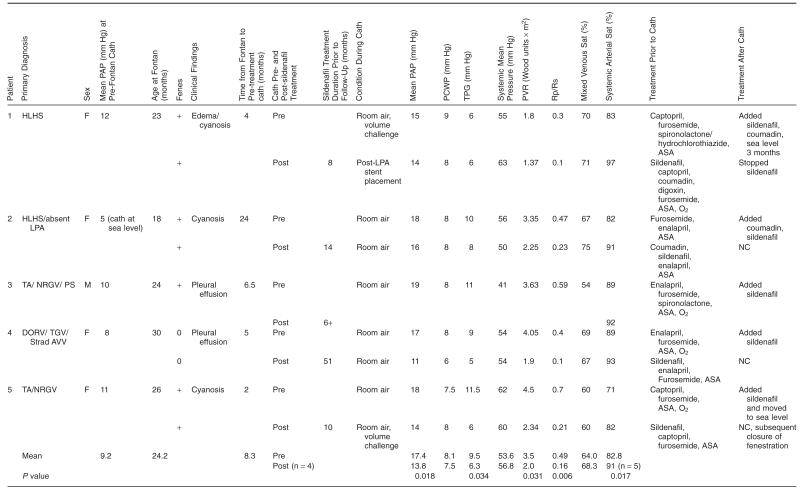
|
ASA, aspirin; Cath, catheterization; F, female; Fenes, fenestration; HLHS, hypoplastic left heart syndrome; LPA, left pulmonary artery; M, male; NC, no change; O2, oxygen; PAP, pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; Rp/Rs, resistance ratio; Sat, saturation; TA/NRGV/PS, tricuspid atresia/ normally related great vessels/ pulmonary stenosis; DORV/TGV/strad AVV, double outlet right ventricle, transposition of the great vessels, straddling atrioventricular valve; TPG, transpulmonary gradient.
Patient 1 was taken for cardiac catheterization 4 months after surgery. Mild left pulmonary artery stenosis was treated with balloon angioplasty and subsequent stent placement. She was also started on sildenafil and low dose coumadin at the time of the first catheterization because of persistent cyanosis, elevated PAPs, and demonstrated vasoreactivity to oxygen and nitric oxide. Clinically, she improved with decreased edema and improved saturations in the presence of a patent fenestration. The follow-up catheterization was repeated in Denver approximately 8 months after spending 3 months at sea level.
Patient 2 had persistent cyanosis and catheterization performed 2 years after surgery revealed elevated PAP and transpulmonary gradient. The estimated PVR was 3.3 Wood units × m2 which decreased to 2.2 Wood units × m2 after 15 months of sildenafil therapy with an increase in saturations to 91% even with a patent fenestration.
Patient 3 had a postoperative course complicated by spontaneous closure of the fenestration, junctional rhythm, and persistent pleural and pericardial effusions for which he underwent pacemaker insertion and pericardiectomy. Catheterization performed 5 months later revealed caval and PAPs of 19 mm Hg with a PVR of 3.6 Wood units × m2 on room air. He was placed on sildenafil and 1 year later is asymptomatic. Subsequent catheterization has not been performed, as there is no clinical indication.
Patient 4 had a postoperative course complicated by pleural effusions for which she underwent right pleural decortication. Catheterization 6 months after surgery revealed elevated PAP and PVR. Pulmonary vasoreactivity was demonstrated with oxygen and nitric oxide administration and she was started on sildenafil.
Patient 5 had no complications postoperatively but had low saturations. Catheterization 2 months after surgery revealed PAPs of 16–19 mm Hg with transpulmonary gradient of 8.5 mm Hg. Several small veno-venous collateral vessels were coil-occluded at that time. She was treated with sildenafil and concomitantly moved to sea level for approximately 6 months time. When she returned, her baseline saturation was 90% and catheterization data revealed PAPs of 14 mm Hg. She then remained at altitude on sildenafil and 7 months later, she continued to have low resistances, suggesting a sildenafil effect. At that time, the fenestration was device-occluded percutaneously.
The data show a significant improvement in systemic saturations (Figure 1). In the four out of five patients who have had follow-up catheterization, there was a significant decrease in PAP (Figure 2) and in estimated PVR (Figure 3). Change in cardiac index was not part of the analysis in this study. Although difficult to quantify, persistent pleural effusions requiring aggressive diuretic therapy were present in Patients 3 and 4. With follow-up after sildenafil therapy, diuretics were weaned with no further formation of pleural effusion.
Figure 1.
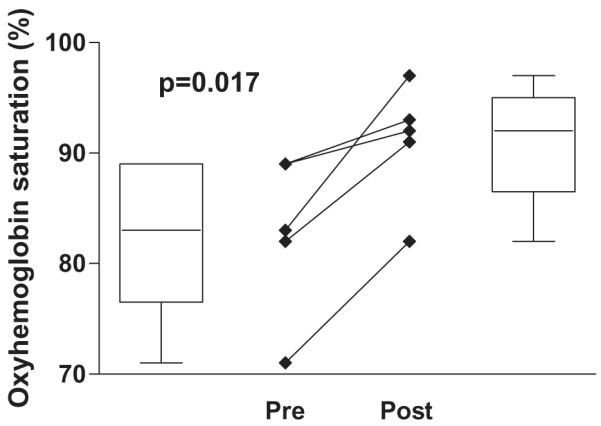
Oxyhemoglobin saturation pre-and postsildenafil treatment.
Figure 2.
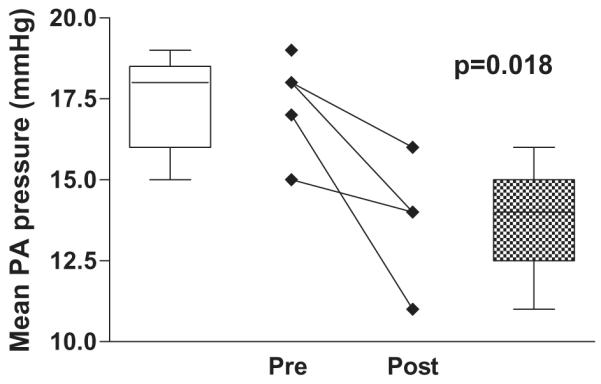
Mean pulmonary artery pressure pre- and postsildenafil treatment.
Figure 3.
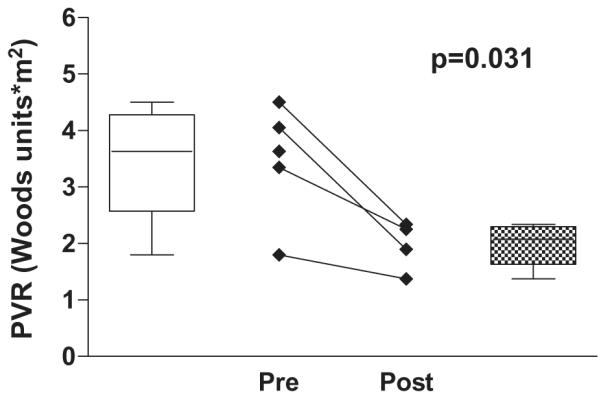
Pulmonary vascular resistance pre- and postsildenafil treatment.
Discussion
We found that in a small group of patients with symptoms of Fontan “failure,” sildenafil treatment decreased pleural effusions and increased systemic saturations. This study is interesting because patients who do not tolerate this type of circulation are frequently referred for cardiac transplantation. Although the substrate for increased PVR in this specific group has yet to be identified, it is likely a combination of endothelial dysfunction leading to decreased nitric oxide production as well as elevated activity of phosphodiesterase type 5. Sildenafil functions through selective inhibition of phosphodiesterase type 5 and subsequent increase in cyclic guanosine monophosphate to promote pulmonary vasodilation. Its use has been well documented in pediatric patients with idiopathic pulmonary hypertension and with congenital heart disease who have high PVR.2,3 Our data show that sildenafil may have a promising role as adjunct therapy to modulate PVR in this group of patients.
Although the altitude of 1610 meters in Denver separates our patient population from most others, we have no reason to believe that this set of patients is any different from other Fontan patients who are asymptomatic and doing well at this altitude. There is some evidence that the early and intermediate results of the Fontan at moderately high altitude are similar to results reported at sea level although they may have a decrease in exercise tolerance.4
Part of Fontan’s criteria for a successful operation in the original description included low transpulmonary gradient and near normal PVR defined as less than 2.5 Wood units × m2.5 The measurement of pulmonary and systemic blood flow as well as PVR is difficult in this population because of the potential for multiple sources of pulmonary blood flow and right to left shunts. Nevertheless, in order to support the passive circulation, the PAP must be low. Therefore, in this population, the standard definition of pulmonary hypertension should be modified and recent literature has suggested that the “ideal” Fontan has a mean PAP of <15 mm Hg.4,6 Previous studies have shown that elevated PAPs and PVR in these patients can lead to increased morbidity and mortality, including failure of the circulation subsequently requiring heart transplantation.7–9
The successful use of pulmonary vasodilators has been reported in postoperative Fontan patients. Yoshimura et al. have reported the significant benefit of nitric oxide to central venous pressure and transpulmonary gradient after Fontan type operations.1 To prevent the rebound effects of inhaled nitric oxide, epoprostenol has also been used in combination for postoperative patients.10 There have been various case reports of the successful treatment with sildenafil of patients with findings of protein-losing enteropathy and plastic bronchitis.11–13
To our knowledge, this is the first case series of patients with SV physiology and Fontan palliation that have been treated with sildenafil as a result of elevated PVR. Although we have a small series of patients, the data suggest that sildenafil lowers PAP and PVR in this population. Sildenafil appears to have a promising therapeutic application for patients with “failing” Fontan circulations and further investigation with larger trials and at sea level is warranted.
Footnotes
Conflict of interest: None.
References
- 1.Yoshimura N, Yamaguchi M, Oka S, et al. Inhaled nitric oxide therapy after Fontan-type operations. Surg Today. 2005;35:31–35. doi: 10.1007/s00595-004-2887-1. [DOI] [PubMed] [Google Scholar]
- 2.Schulze-Neick I, Hartenstein P, Li J, et al. Intravenous sildenafil is a potent pulmonary vasodilator in children with congenital heart disease. Circulation. 2003;108(suppl 1):II167–II173. doi: 10.1161/01.cir.0000087384.76615.60. [DOI] [PubMed] [Google Scholar]
- 3.Rashid A, Ivy D. Pulmonary hypertension in children. Curr Paediatr. 2006;16:237–247. [Google Scholar]
- 4.Day RW, Orsmond GS, Sturtevant JE, Hawkins JA, Doty DB, McGough EC. Early and intermediate results of the Fontan procedure at moderately high altitude. Ann Thorac Surg. 1994;57:170–176. doi: 10.1016/0003-4975(94)90388-3. [DOI] [PubMed] [Google Scholar]
- 5.Choussat A, Fontan F, Besse F. Selection Criteria for Fontan’s Procedure. Churchill Livingstone; Edinburgh: 1978. pp. 559–566. [Google Scholar]
- 6.Beghetti M, Aggoun Y, Da Cruz E. Nitric oxide precursors and congenital cardiac surgery: a randomized controlled trial of oral citrulline. Definition of pulmonary hypertension in Fontan circulation? J Thorac Cardiovasc Surg. 2006;132:1501–1502. doi: 10.1016/j.jtcvs.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 7.Fontan F, Kirklin JW, Fernandez G, et al. Outcome after a “perfect” Fontan operation. Circulation. 1990;81:1520–1536. doi: 10.1161/01.cir.81.5.1520. [DOI] [PubMed] [Google Scholar]
- 8.Mitchell MB, Campbell DN, Ivy D, et al. Evidence of pulmonary vascular disease after heart transplantation for Fontan circulation failure. J Thorac Cardiovasc Surg. 2004;128:693–702. doi: 10.1016/j.jtcvs.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 9.Gewillig M. The Fontan circulation. Heart. 2005;91:839–846. doi: 10.1136/hrt.2004.051789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyaji K, Nagata N, Miyamoto T, Kitahori K. Combined therapy with inhaled nitric oxide and intravenous epoprostenol (prostacyclin) for critical pulmonary perfusion after the Fontan procedure. J Thorac Cardiovasc Surg. 2003;125:437–439. doi: 10.1067/mtc.2003.199. [DOI] [PubMed] [Google Scholar]
- 11.Apostolopoulou SC, Papagiannis J, Rammos S. Bosentan induces clinical, exercise and hemodynamic improvement in a pre-transplant patient with plastic bronchitis after Fontan operation. J Heart Lung Transplant. 2005;24:1174–1176. doi: 10.1016/j.healun.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 12.Haseyama K, Satomi G, Yasukochi S, Matsui H, Harada Y, Uchita S. Pulmonary vasodilation therapy with sildenafil citrate in a patient with plastic bronchitis after the Fontan procedure for hypoplastic left heart syndrome. J Thorac Cardiovasc Surg. 2006;132:1232–1233. doi: 10.1016/j.jtcvs.2006.05.067. [DOI] [PubMed] [Google Scholar]
- 13.Uzun O, Wong JK, Bhole V, Stumper O. Resolution of protein-losing enteropathy and normalization of mesenteric Doppler flow with sildenafil after Fontan. Ann Thorac Surg. 2006;82:e39–e40. doi: 10.1016/j.athoracsur.2006.08.043. [DOI] [PubMed] [Google Scholar]


