Abstract
Oxidative stress plays a central role in the pathogenesis of diverse chronic inflammatory disorders including diabetic complications, cardiovascular disease, aging, neurodegenerative disease, autoimmune disorders and pulmonary fibrosis. Protein misfolding can lead to chronic endoplasmic reticulum (ER) stress which can exacerbate oxidative stress. This can trigger apoptotic cascades resulting in chronic inflammatory disorders. Despite intense interest in origins and magnitude of oxidative stress, ability to quantify oxidants has been limited because they are short-lived. We have developed quantitative mass spectrometry (MS) based analytical strategies to analyze stable end-products of protein oxidation. These molecules provide quantitative and mechanistic assessment of degree of oxidative stress in cell cultures, tissues and biofluids of animal models of disease and human samples. Our studies support the hypothesis that unique reactive intermediates generated in localized microenvironments of vulnerable tissues promote end-organ damage. The ability to quantify these changes and assess response to therapies will be pivotal in understanding disease mechanisms and monitoring efficacy of therapy.
Keywords: Oxidative stress, oxidized amino acids, gas chromatography mass spectrometry, endoplasmic reticulum stress, electrospray ionization tandem mass spectrometry
1. Introduction
Oxygen forms the basis of aerobic life but is only sparingly reactive by itself. However, it is well-recognized that it can be activated by cellular metabolism to form reactive compounds termed as “reactive oxygen species” (ROS) which in turn can form a variety of intermediates including “reactive nitrogen species” (RNS). While physiological levels of such oxidants have a beneficial role in cellular signaling and host defense, excess oxidants can lead to pathological consequences.
Oxidative stress is due to the dysregulation in the synchronized balance between the oxidant generating systems and the antioxidant defense in quenching the excessive free radicals. Proteins, lipids and deoxyribonucleic acid (DNA) are the major targets of oxidative damage, eventually leading to the dysfunction of cellular physiology, as well as stimulating apoptosis. Alternatively, detrimental effects of ROS may be mediated by aberrant “redox signaling” in specific pathophysiological contexts. This latter effect may be attributed to disruption of the normal physiological roles of ROS in cell signaling (Thannickal, 2010).
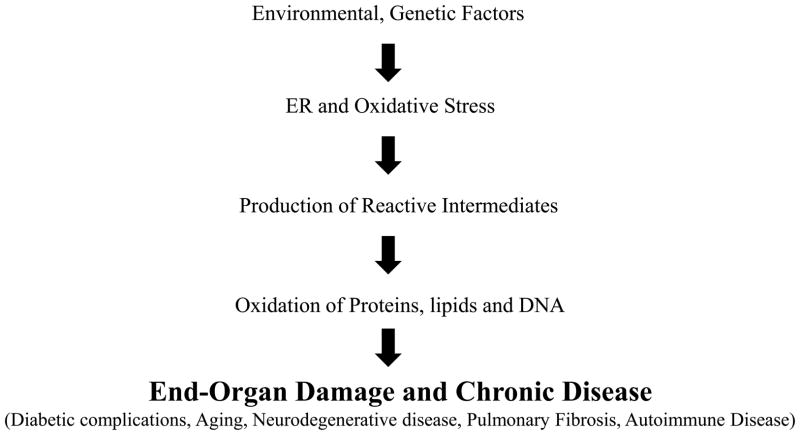
During the past two decades, considerable evidence has implicated oxidative stress in several distinct human disorders, including aging (Beckman and Ames, 1998; Muller et al., 2007), atherosclerosis (Baynes and Thorpe, 2000; Pennathur and Heinecke, 2007), neurodegenerative diseases (Butterfield, 2006; Lin and Beal, 2006), diabetes (Baynes and Thorpe, 1999; Brownlee, 2001; Houstis et al., 2006; Jay et al., 2006; Vivekanadan-Giri et al., 2008), pulmonary fibrosis (Hecker et al., 2009) and end-stage renal disease (Cottone et al., 2008; Percy et al., 2005). A major focus of research has dwelt on the importance of ER stress and its link with oxidative stress. The unfolded protein response (UPR) is a signaling pathway that is triggered in response to protein misfolding in the ER. It has been noted that UPR is activated in response to oxidative stress as an adaptive mechanism (Malhotra and Kaufman, 2007) and antioxidants reduce ER stress (Back et al., 2009; Malhotra et al., 2008; Song et al., 2008). Persistent ER stress and oxidative stress in the susceptible individual can lead to end-organ damage (Fig. 1). Oxidative stress has also been implicated in many chronic fibrotic diseases as well (Thannickal, 2010). Indeed, fibrosis is a major cause of organ failure and death accounting for roughly 45% of mortality in the United States population (Wynn, 2004). Therefore, understanding pathways of oxidative damage and designing specific and sensitive methods of detecting oxidized biomolecules is of critical importance. In this article, we will discuss our work in developing tandem mass spectrometric (MS/MS) methods to quantify specific amino acid oxidation markers that provide mechanistic information on oxidative pathways operative in vivo. We will outline sample preparation from cell cultures, tissue and other biospecimens, preparation of internal standards, liquid chromatography and MS conditions and parameters for quantitative analysis of amino acid oxidation markers.
Figure 1. Roles of oxidative stress and endoplasmic reticulum (ER) stress in chronic disease.
Interaction between environmental and genetic factors, promote ER and oxidative stress. Eventual damage to proteins, lipids and DNA can lead to end-organ damage and chronic disease.
2. Experimental Procedures
2.1 Isotope dilution MS is a highly sensitive and specific method for detecting oxidized biomolecules in vivo
ROS and RNS intermediates are difficult to detect in vivo because they are extremely short-lived due to their high reactivity with endogenous substrates; however, these oxidized substrates may serve as biomarkers for the activation of relevant oxidative stress pathways. Immunohistochemistry and dihydroethidium fluorescence have been extensively used to study oxidation-specific epitopes and oxidant production. These techniques are highly sensitive, and their ability to provide epitope-specific structural data can localize oxidative events to cell types or to subcellular locations. However, they are nonspecific as antibodies can bind to structurally similar compounds and, at best, only semiquantitative. The major drawback of high performance liquid chromatography (HPLC) based methods, is the appearance of co-eluting structurally similiar compounds (Shigenaga et al., 1997). Spectrophotometric or flurometric detection of 3-nitrotyrosine can be confounded by contribution from tryptophan oxidation or other protein modifications (Guptasarma et al., 1992). In contrast, MS offers a highly sensitive and specific approach to quantify oxidative biomarkers. When combined with isotope dilution, in which a labeled internal standard which is identical to the target analyte except for the heavy isotope is added to the mixture, accurate quantitation can be achieved to low femtomole to attomole levels. Addition of the internal standard also allows normalization for loss of analytes during sample preparation.
2.2 Oxidized amino acid content correlates with degree of oxidative stress in vivo
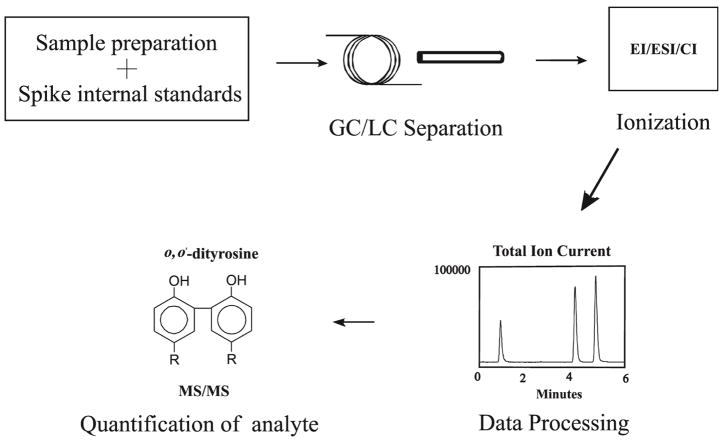
To understand the molecular mechanisms that promote oxidative stress in vivo, we first identified the patterns of oxidation products that are formed by well-characterized oxidant-generating model systems. We then characterized patterns of products in tissue and plasma derived from animal models of disease and human samples. Since lipid peroxidation products readily undergo subsequent chain-propagating reactions and lose their initial oxidant imprint, we chose aromatic amino acids in proteins to study stable end-products of oxidation. Using a combination of free radical generating systems in vitro and studying biospecimens from animal models of disease and humans, we and others defined patterns of these oxidative markers that accurately indicate pathways of oxidation that are activated (Back et al., 2009; Bergt et al., 2004; Brennan and Hazen, 2003; Hazen et al., 1997; Leeuwenburgh et al., 1997a; Leeuwenburgh et al., 1997b; Malhotra et al., 2008; Okamura et al., 2009; Pennathur et al., 2005; Pennathur et al., 1999; Pennathur et al., 2001; Pop-Busui et al., 2009; Shishehbor et al., 2003; Shu et al., 2009; Song et al., 2008; Vincent et al., 2009; Wiggin et al., 2008; Zhang et al., 2008; Zheng et al., 2004). We have utilized this molecular fingerprinting strategy to identify tissue-specific pathways of oxidation in vivo. The phenylalanine residues in proteins when subjected to glycoxidation or hydroxyl radical damage forms ortho-tyrosine and meta-tyrosine. Oxidation reactions of tyrosine residues include dimerization (to form o,o′-dityrosine; mediated by tyrosine radicals, ROS and RNS), chlorination (to form 3-chlorotyrosine; catalyzed by myeloperoxidase) and nitration (to from 3-nitrotyrosine; mediated by RNS). Quantifying these unnatural amino acids characterizes the underlying oxidant pathway operative in target tissue. The overall scheme for analysis for oxidative amino acids is outlined in Fig. 2. The analytical strategy can be divided into four parts: 1) sample preparation, 2) separation of analytes 3) detection of analytes and data processing, 4) quantification of analytes.
Figure 2. Workflow for tandem mass spectrometry based oxidized amino acid analysis.
Biological samples are processed and internal standards are added. Subsequently the sample is subjected to either gas (GC) or liquid (LC) chromatography separation. The eluent is ionized by one of several modes of ionization such as EI, CI, or ESI. Following ionization, the resultant mass spectra are derived from the mass analyzers and the analytes of interest are identified. EI, electron impact ionization; CI, chemical ionization; ESI, electrospray ionization; MS/MS, tandem mass spectrometry;
2.3 Sample Preparation
Oxidized amino acids can be measured from proteins derived from a variety of different samples including tissue, biofluids (plasma, urine, cerebrospinal fluid), and cell culture. In general, plasma and urine measurements provide systemic levels of oxidant stress and tissue determinations provide local levels of oxidant stress. During procurement, it is important to ensure that samples are acquired in a sterile and uniform fashion. It is extremely important to prevent ex-vivo oxidation as it will confound interpretation. Sample preparation typically entails isolation of proteins, hydrolysis to liberate individual amino acids, sample clean-up with solid phase extraction and processing prior to further analysis. Processing the samples is a double-edged sword because each step will result in some degree of analyte loss. It is imperative to add internal standards prior to processing to account for these losses.
2.3.1 Buffers and solutions required for sample preparation
Antioxidant buffer (Buffer A): consists of 100 μM diethylenetriaminepentaacetic acid (DTPA), 50 μM butylated hydroxyl toluene (BHT) in 1% (v/v) ethanol, and 10 mM 3-amino-1,2,4-triazole in 50 mM sodium phosphate buffer, pH 7.4. DTPA is a metal-chelator and prevents metal-catalyzed oxidation reactions; BHT is a lipid soluble antioxidant; aminotriazole is a peroxidase inhibitor. Fresh antioxidant buffer is prepared prior at the start of the experiment.
Delipidation buffer (Buffer B) consists of water, methanol and water saturated ether in 1:3:7 (v/v/v) ratio.
2.3.2 Tissue collection and protein isolation
The animals are perfused with antioxidant Buffer A prior to harvesting the tissue samples. This step ensures the removal of red blood cells and prevents ex-vivo oxidation. The tissue samples are immediately stored in Buffer A in −80°C until analysis. Prior to the start analytical procedure, the tissue samples are thawed, minced and washed several times in aliquots of freshly prepared Buffer A. The samples are then homogenized with a hand tissue homogenizer. It is important to make sure that no residual tissue pieces are present. The tissue lysates, are once again sonicated using stainless steel probe sonicator (Omni ruptor-250, Omni International, Marietta, GA). The tissue lysate is centrifuged at 1000g for 10 minutes at 4°C. The supernatant containing soluble proteins is aliquoted and stored for further processing.
2.3.3. Protein isolation from cell cultures
Prior to harvesting attached cells, the plates are washed several times with Buffer A. Following treatment with trypsin, the cells are diluted with Buffer A and spun briefly for 5 minutes at 1000g. The supernatant is discarded and the cell pellet is overlaid with fresh Buffer A and then resuspended and the process is repeated two more times. Subsequently, the cell suspension is subjected to sonication using stainless steel probe sonicator. The cell lysate is centrifuged at 1000g for 10 minutes at 4°C. The supernatant containing soluble proteins is aliquoted and stored for further processing.
2.3.4. Protein precipitation, delipidation and hydrolysis of lysates derived from biological samples
All subsequent procedures are carried out at 4°C. Tissue or cell lysates, are taken in pyrolyzed hydrolysis vials and the volume is adjusted to 1ml with 50mM phosphate buffer, pH 7.4. For plasma, 5μl is diluted with 50mM phosphate buffer (pH 7.4) to a final volume of 1 mL in pyrolyzed hydrolysis vials. Subsequently the protein is precipitated by addition of trichloroacetic acid (10% final concentration). After centrifugation at 3000g for 10 minutes at 4°C, the protein precipitate is delipidated with Buffer B. This step ensures removal of lipids which can interfere with sample analysis. Following centrifugation, the lipid fraction is discarded. The protein pellet is dried in the fume hood under nitrogen to remove traces of the solvent. 4N Methanesulfonic acid pretreated with benzoic acid (10mg/ml) is added to the delipidated protein sample. Known concentration of isotope labeled internal standards of ortho-tyrosine and meta-tyrosine, o,o′-dityrosine, 3-chlorotyrosine, 3-nitrotyrosine, 13C6 phenylalanine and 13C6 tyrosine are spiked into the samples for absolute quantification of the modified amino acids and their precursors found in the biological samples. An alternate labeled precursor amino acid, [13C9, 15N1]tyrosine, is added to monitor potential artifact formation which usually is a problem mainly for tyrosine halogenation, dimerization and nitration products. The samples are hydrolyzed at 100°C in sand bath for 24 hours.
2.3.5. Solid phase extraction of oxidized amino acids from biological samples
Chrom-P columns (ENVI-ChromP, 3 mL, Supelco., Bellefonte, PA) are activated with 100% methanol. Methanol is allowed to flow through gravity in the columns, to remove any trapped air bubbles. The column is preconditioned four times with 2ml of 0.1% trifluroacetic acid (TFA) in water at pH 4. The acid hydrolysate is subjected to solid phase extraction and washed with 2ml of 0.1% TFA of pH 4. The amino acids bound to the column are eluted with 50% methanol. The eluent is collected in clean glass vial and dried at 60°C under vacuum. The dried samples are resolubilized in 50μl of the 0.1% TFA.
2.4. Preparation of authentic and isotope labeled standards
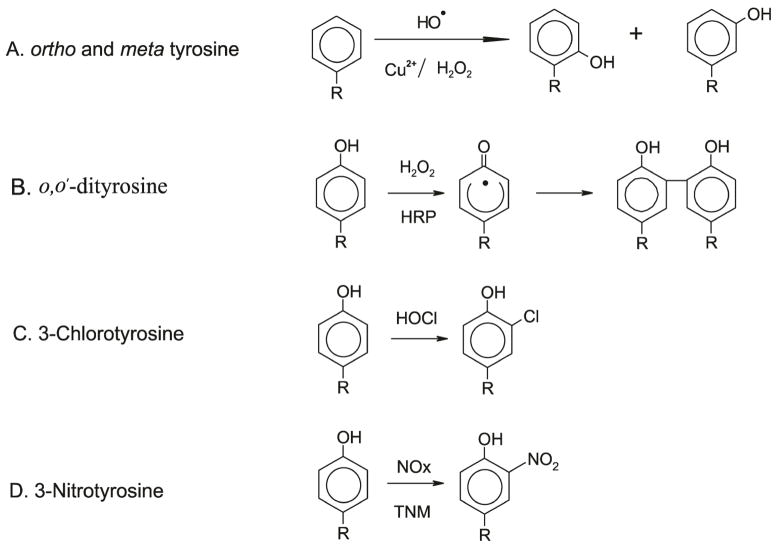
Isotope labeled L-[13C6] phenylalanine and L-[13C6] tyrosine are obtained from Cambridge Isotope Laboratories (Andover, MA) and are used to synthesize all isotopically labeled standards. The overall synthetic strategy is outlined in Fig. 3. Isotopically labeled ortho-tyrosine and meta-tyrosine were synthesized using [13C6] phenylalanine, copper, and hydrogen peroxide (H2O2) as described previously (Huggins et al., 1992). o,o′-[13C12] dityrosine was prepared with - [13C6] tyrosine, horseradish peroxidase (HRP), and H2O2 as described previously (Leeuwenburgh et al., 1997b; Pennathur et al., 2005; Pennathur et al., 1999)). L-3-Chloro[13C6] tyrosine was synthesized by reacting L-[13C6] tyrosine with an equimolar concentration of hypochlorous acid (HOCl) in the presence of 100 mM Cl− in 20 mM phosphate buffer (pH 4.0). Reactions were carried out for 60 min at 37°C. They were initiated by adding HOCl and terminated with 0.1 mM methionine (Gaut et al., 2002). Excess of tetranitromethane (TNM) was used to synthesize [13C6] 3-nitrotyrosine from 2mM L-[13C6] tyrosine in 50mM Tris buffer (pH 8.0). The mixture was vortexed for 1 hour at room temperature (Gaut et al., 2002). The resulting amino acids were isolated using solid-phase extraction with a 3-mL C18 column (Supelco Inc, Bellefonte, PA). Concentrations of amino acids were determined by comparison with authentic standards using HPLC and monitoring of A276. Further confirmation is obtained by verifying characteristic MS spectra by electrospray ionization tandem mass spectrometry (ESI/MS/MS)
Figure 3. Structures and synthesis of oxidized amino acids.
Oxidized amino acids are synthesized from the precursors as described in experimental procedures. H2O2, hydrogen peroxide; HOCl, hypochlorous acid; HRP, horse radish peroxidase; TNM, tetranitromethane
2.5 Separation and detection of analytes
The two main separation platforms used are either gas chromatography (GC) or liquid chromatography (LC) coupled with MS.
2.5.1. Gas Chromatography/Mass Spectrometry (GC/MS)
GC/MS is a commonly used platform for measuring oxidized amino acids. As separation in GC occurs in an oven at high temperatures, analytes need to be volatile and thermally stable and it is therefore necessary to derivatize samples prior to analysis. The amino acids are converted to either heptafluorobutyryl derivatives or trimethylsilyl derivatives prior to analysis. The analytes entering the source in GC/MS are ionized by electron impact (EI) or chemical ionization (CI) and the mass analyzer is usually a single quadrupole. While necessary, it is important to keep in mind that derivatization is additional sample processing that can result in analyte loss and artifact formation and this is one of the major drawbacks of GC/MS. Additionally, GC/MS has limited mass range and therefore not suitable for larger molecular weight compounds such as o,o′-dityrosine. For these reasons, we no longer employ GC/MS for routine analysis of oxidized amino acids.
2.5.2 Liquid Chromatography/Mass Spectrometry (LC/MS)
Biomedical mass spectrometry has recently been revolutionized by the advent of ionization methods that permit introduction of large, complex biomolecules into the mass spectrometer in liquid solutions. ESI is perhaps the most powerful of the new ionization methods for coupling LC with MS. In ESI/MS, large, complex, ionic molecules are introduced into the ion source as liquid solutions as a fine spray of charged droplets. This permits direct MS analysis of intact, simple and complex analytes. Since high temperatures and need for volatility are not involved, sample derivatization is not needed.
The triple Quadrupole (QQQ) is a versatile mass analyzer. The basic subunit consists of 4 parallel metal rods – hence the term quadrupole. A radiofrequency voltage is applied to the rods and only ions within a particular mass to charge ratio (m/z) range will be able to traverse through the quadrupole to the detector. Ions outside the defined m/z range spiral out of control and are destroyed when they hit a rod. A QQQ combines 3 quadrupoles in series and allows MS/MS to be performed. In MS/MS, the first quadrupole is used to scan across a preset m/z range and select a user-defined parent ion of interest which is then fragmented in the second quadrupole by colliding with an inert gas such as nitrogen through the process of collision-induced dissociation (CID). The fragment daughter ions are sorted in the third quadrupole for analysis. Several functional groups (e.g. H2O, COOH, etc) characteristically fragment during CID, and thus analysis of the daughter ions can yield structural information about the parent ion. In addition, as compounds have unique fragmentation patterns, QQQ with multiple reaction monitoring (MRM) can be used to experimentally confirm potential analytes. QQQ is very sensitive and provides the most precise quantitation in the MRM mode and therefore ideally suited for analysis of oxidized amino acids.
3. Results
3.1 LC/MS/MS detection of oxidized amino acids using authentic standards
Oxidized amino acids are quantified by LC/ESI/MS/MS in the MRM mode. MS experiments were performed using an Agilent 6410 Triple Quadrapole mass spectrometer coupled with an Agilent 1200 HPLC system (Agilent Technologies, New Castle, DE), equipped with a multimode source. C-18 column from Agilent with 1.8μm particle size and 4.6 × 50mm dimension is used for the HPLC separation. The amino acid hydrolysate is subjected to reverse phase separation utilizing the following parameters. The mobile phase is 0.1% formic acid (Solvent A) and 0.1% formic acid in acetonitrile (Solvent B). The column was first equilibrated with 100% Solvent A. The gradient for the HPLC run is as follows: isocratic 2% acetonitrile for 0.5 to 3 minutes, and then a linear gradient of 10% to 95% acetonitrile, for 3 to 7 minutes with a flow rate of 0.6ml/min. Under these chromatography conditions, authentic compounds and isotopically labeled standards exhibited retention times identical to those of analytes derived from tissue samples. The limit of detection (signal/noise >10) was < 100 fmol for all of the amino acids.
Positive LC/ESI/tandem MS was performed using following parameters: capillary spray voltage 4000 V, drying gas flow 11 L/min, drying gas temperature 350°C and nebulizer pressure 45 psi. Flow injection analysis was used to optimize the fragmentor voltage. Optimal fragmentor voltage for each amino acid in MS2 scan mode was obtained. Mass range between m/z 100 and m/z 400 was scanned to obtain full scan mass spectra.
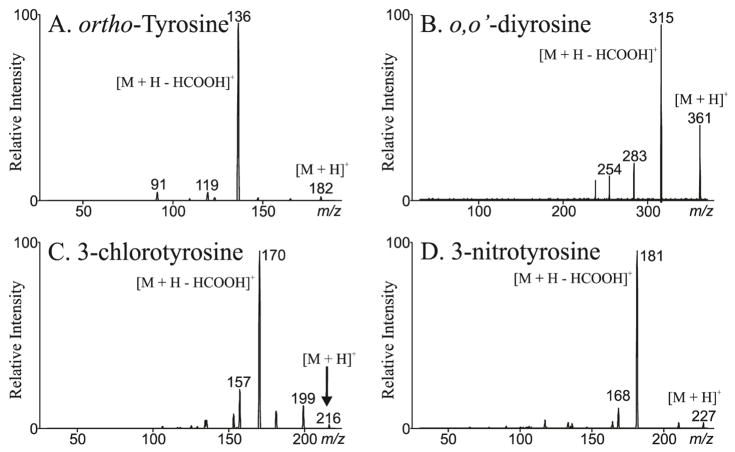
Figure 4 depicts the MS/MS spectrum from ortho-tyrosine (Panel A), o,o′-dityrosine (Panel B), 3-chlorotyrosine (Panel C) and 3-nitrotyrosine (Panel D). meta-tyrosine has an identical MS/MS spectrum as ortho-tyrosine but can be distinguished due to differences in retention time (Table 1). The molecular ion [M + H]+ of ortho-tyrosine, o,o′-dityrosine, 3-chlorotyrosine and 3-nitrotyrosine are m/z 182, 361, 216 and 227 respectively. The neutral loss of m/z 46 is the most important product ion for all the oxidized amino acids. This yields an intense ion at m/z 136, 315, 170 and 181 respectively. These transitions form ideal candidates for MRM analysis.
Figure 4. Liquid chromatography electrospray ionization tandem mass spectrometry of oxidized amino acids.
The samples were separated by reverse phase HPLC and subjected to ESI/MS as described in methods. MS/MS spectra reveal [M + H]+ of ortho-tyrosine (Panel A), o,o′-dityrosine (Panel B), 3-chlorotyrosine (Panel C) and 3-nitrotyrosine (Panel D) are m/z 182, 361, 216 and 227. The neutral loss of m/z 46 is the most intense product ion for all the oxidized amino acids.
Table 1. LC/ESI/MS/MS analysis of oxidized amino acids.
The samples were separated by reverse phase HPLC and subjected to ESI/MS as described in Methods. The amino acid analytes, ion transitions utilized for MRM analysis and the retention time are summarized.
| Amino acid | Ion Transition | Retention Time |
|---|---|---|
| 13C6 ortho-tyrosine | 188 → 142 | 3.55 |
| 12C6 ortho-tyrosine | 182 → 136 | 3.55 |
| 13C6 meta-tyrosine | 188 → 142 | 2.95 |
| 12C6 meta-tyrosine | 182 → 136 | 2.95 |
| 13C6 o,o′-dityrosine | 373 → 327 | 2.25 |
| 12C6 o,o′-dityrosine | 361 → 315 | 2.25 |
| 13C6 chlorotyrosine | 222 → 176 | 4.22 |
| 12C6 chlorotyrosine | 216 → 170 | 4.22 |
| 13C6 nitrotyrosine | 233 → 187 | 4.88 |
| 12C6 nitrotyrosine | 227 → 181 | 4.88 |
| 13C915N1 o,o′-dityrosine (dimerization artifact) | 381 → 334 | 2.25 |
| 13C915N1 chlorotyrosine (chlorination artifact) | 226 → 179 | 4.22 |
| 13C915N1 nitrotyrosine (nitration artifact) | 237 → 190 | 4.88 |
| 13C6 phenylalanine | 172 → 126 | 3.75 |
| 12C6 phenylalanine | 166 → 120 | 3.75 |
| 13C6 tyrosine | 188 → 142 | 1.90 |
| 12C6 tyrosine | 182 → 136 | 1.90 |
| 13C915N1 tyrosine | 192 → 145 | 1.90 |
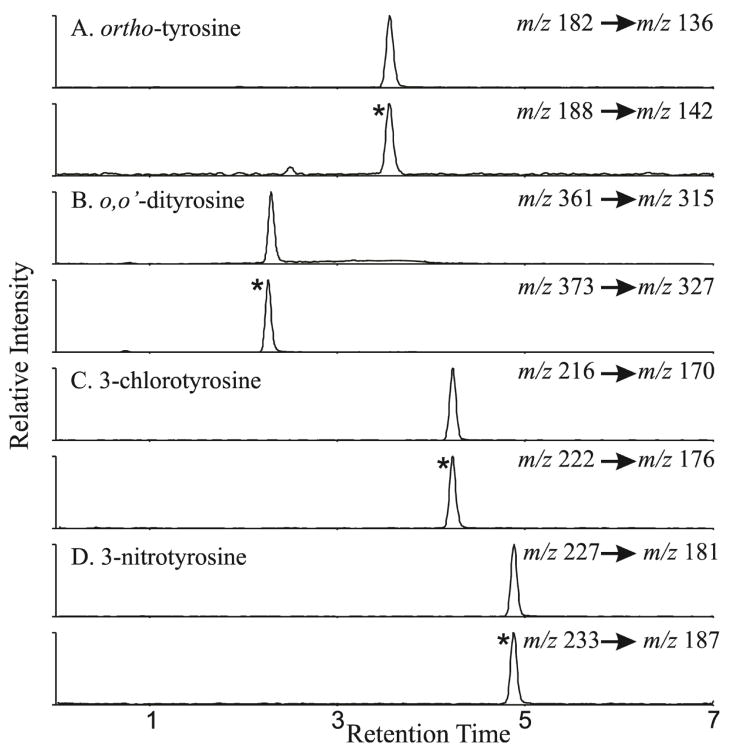
3.2. Quantitation of oxidized amino acids by MRM analysis
The amino acids are quantified using isotope dilution LC/MS/MS in the MRM mode. The retention times and the MRM transitions utilized for quantification of the authentic compounds, internal standards and artifact monitoring analytes are outlined in Table 1. Extracted ion chromatograms are derived from the MRM transitions are shown in Fig. 5. The ion at m/z 136 and its respective isotopically labeled internal standard ion at m/z 142 were used to quantify ortho-tyrosine and meta-tyrosine. o,o′-dityrosine is analyzed using the m/z 315 ion and its isotopically labeled internal standard ion at m/z 327. 3-Chlorotyrosine is quantified using ion at 170 m/z and its respective isotopically labeled internal standard ion at m/z 176. 3-Nitrotyrosine is quantified using the ion at m/z 181 and its respective isotopically labeled internal standard ion at m/z 187. To quantify phenylalanine and tyrosine (the precursor amino acids), the sample was diluted 1 in 1000 and analyzed in a separate injection as these precursor amino acids are typically present 1000 to 10,000 fold excess to their oxidized counterparts. Phenylalanine, the precursor amino acid for ortho-tyrosine and meta-tyrosine, was quantified using the ion at m/z 126 and the isotopically labeled internal standard ion at m/z 132. The ion at m/z 136 and the isotopically labeled internal standard ion at m/z 142 were used to quantify tyrosine, the precursor amino acid for o,o′-dityrosine, 3-chlorotyrosine and 3-nitrotyrosine. We constructed an external calibration curve that used each amino acid as a standard and the corresponding isotopically labeled amino acid as an internal standard. The ratio of ion currents for each amino acid divided by that of the internal standard was a linear function of unlabeled amino acid for all ranges over which the amino acids were measured. The limit of detection (signal/noise >10) was <100 fmol for all the amino acids. Potential artifact during sample work-up is usually an issue with o,o′-dityrosine, 3-chlorotyrosine and 3-nitrotyrosine determination. Therefore, we routinely add L-[13C9, 15N]tyrosine, a isotopically distinct tyrosine. We monitor artifact formation by analyzing appearance of m/z 334 (dimerization: o,o′-dityrosine artifact), m/z 179 (chlorination artifact), and m/z 190 (nitration artifact). The amount of artifact is then subtracted from the measured oxidized amino acid to correct for this issue. We do not routinely monitor for artifact formation for ortho-tyrosine and meta-tyrosine. If this is an issue, an isotopically distinct phenylalanine can be added to monitor this possibility. Finally, we normalize the oxidation markers to the precursor amino acids so that changes in tissue volume, amount of protein or extraction efficiency are accounted for.
Figure 5. Quantification of oxidized amino acids in the multiple reaction monitoring mode utilizing extracted ion chromatograms.
The samples were separated by reverse phase HPLC and subjected to ESI/MS as described in methods. Extracted ion chromatograms were derived from the MRM transitions for ortho-tyrosine (Panel A), o,o′-dityrosine (Panel B), 3-chlorotyrosine (Panel C) and 3-nitrotyrosine (Panel D) as shown figure. The ratio of ion currents for each amino acid compared with the internal standard (depicted by *) was utilized to quantify the levels of the oxidized amino acids. Note co-elution of authentic oxidized amino acid to the corresponding isotopically labeled internal standard.
4. Conclusions
Many lines of evidence implicate oxidative stress in chronic diseases. However, accurate measurement of oxidant generation in vivo is challenging. The power of tandem mass spectrometry offers a unique opportunity to quantify low levels of oxidized biomolecules from complex bio specimens. We have utilized this approach to precisely assess oxidative stress in biological samples ranging from cell cultures, biofluids and tissue from animal models of disease and humans. This analytical approach thus has a broad applicability to study a variety of disorders in which oxidative stress plays a causal role. More studies are needed to evaluate the utility of these markers to predict disease progression and to determine efficacy of potential interventions.
Acknowledgments
This work is supported in part by grants from the National Institutes of Health (HL094230, DK089503 and DK082841), the Doris Duke Foundation Clinical Scientist Development Award the American College of Rheumatology Within our Reach Award, and by the Molecular Phenotyping Core of the Michigan Nutrition and Obesity Research Center (DK089503).
Abbreviations
- BHT
butylated hydroxytoulene
- CI
chemical ionization
- CID
collision-induced dissociation
- DNA
deoxyribonucleic acid
- DTPA
diethylenetriaminepentaacetic acid
- EI
electron impact ionization, ESI, electrospray ionization
- ER
endoplasmic reticulum
- GC/MS
gas chromatography/mass spectrometry
- HPLC
high performance liquid chromatography
- LC/MS
liquid chromatography/mass spectrometry
- MRM
multiple reaction monitoring
- MS
mass spectrometry
- MS/MS
tandem mass spectrometry
- m/z
mass to charge ratio
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- QQQ
triple quadrupole
- HRP
horse radish peroxidase
- TNM
tetranitromethane
- TFA
trifluroacetic acid
- UPR
unfolded protein response
References
- Back SH, Scheuner D, Han J, Song B, Ribick M, Wang J, Gildersleeve RD, Pennathur S, Kaufman RJ. Translation attenuation through eIF2alpha phosphorylation prevents oxidative stress and maintains the differentiated state in beta cells. Cell Metab. 2009;10:13–26. doi: 10.1016/j.cmet.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baynes JW, Thorpe SR. Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes. 1999;48:1–9. doi: 10.2337/diabetes.48.1.1. [DOI] [PubMed] [Google Scholar]
- Baynes JW, Thorpe SR. Glycoxidation and lipoxidation in atherogenesis. Free Radic Biol Med. 2000;28:1708–1716. doi: 10.1016/s0891-5849(00)00228-8. [DOI] [PubMed] [Google Scholar]
- Beckman KB, Ames BN. The free radical theory of aging matures. Physiol Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- Bergt C, Pennathur S, Fu X, Byun J, O’Brien K, McDonald TO, Singh P, Anantharamaiah GM, Chait A, Brunzell J, Geary RL, Oram JF, Heinecke JW. The myeloperoxidase product hypochlorous acid oxidizes HDL in the human artery wall and impairs ABCA1-dependent cholesterol transport. Proc Natl Acad Sci U S A. 2004;101:13032–13037. doi: 10.1073/pnas.0405292101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan ML, Hazen SL. Amino acid and protein oxidation in cardiovascular disease. Amino Acids. 2003;25:365–374. doi: 10.1007/s00726-003-0023-y. [DOI] [PubMed] [Google Scholar]
- Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- Butterfield DA. Oxidative stress in neurodegenerative disorders. Antioxid Redox Signal. 2006;8:1971–1973. doi: 10.1089/ars.2006.8.1971. [DOI] [PubMed] [Google Scholar]
- Cottone S, Lorito MC, Riccobene R, Nardi E, Mule G, Buscemi S, Geraci C, Guarneri M, Arsena R, Cerasola G. Oxidative stress, inflammation and cardiovascular disease in chronic renal failure. J Nephrol. 2008;21:175–179. [PubMed] [Google Scholar]
- Gaut JP, Byun J, Tran HD, Heinecke JW. Artifact-free quantification of free 3-chlorotyrosine, 3-bromotyrosine, and 3-nitrotyrosine in human plasma by electron capture-negative chemical ionization gas chromatography mass spectrometry and liquid chromatography-electrospray ionization tandem mass spectrometry. Anal Biochem. 2002;300:252–259. doi: 10.1006/abio.2001.5469. [DOI] [PubMed] [Google Scholar]
- Guptasarma P, Balasubramanian D, Matsugo S, Saito I. Hydroxyl radical mediated damage to proteins, with special reference to the crystallins. Biochemistry. 1992;31:4296–4303. doi: 10.1021/bi00132a021. [DOI] [PubMed] [Google Scholar]
- Hazen SL, Crowley JR, Mueller DM, Heinecke JW. Mass spectrometric quantification of 3-chlorotyrosine in human tissues with attomole sensitivity: a sensitive and specific marker for myeloperoxidase-catalyzed chlorination at sites of inflammation. Free Radic Biol Med. 1997;23:909–916. doi: 10.1016/s0891-5849(97)00084-1. [DOI] [PubMed] [Google Scholar]
- Hecker L, Vittal R, Jones T, Jagirdar R, Luckhardt TR, Horowitz JC, Pennathur S, Martinez FJ, Thannickal VJ. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med. 2009;15:1077–1081. doi: 10.1038/nm.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- Huggins TG, Staton MW, Dyer DG, Detorie NJ, Walla MD, Baynes JW, Thorpe SR. o-Tyrosine and dityrosine concentrations in oxidized proteins and lens proteins with age. Ann N Y Acad Sci. 1992;663:436–437. doi: 10.1111/j.1749-6632.1992.tb38692.x. [DOI] [PubMed] [Google Scholar]
- Jay D, Hitomi H, Griendling KK. Oxidative stress and diabetic cardiovascular complications. Free Radic Biol Med. 2006;40:183–192. doi: 10.1016/j.freeradbiomed.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Leeuwenburgh C, Hardy MM, Hazen SL, Wagner P, Oh-ishi S, Steinbrecher UP, Heinecke JW. Reactive nitrogen intermediates promote low density lipoprotein oxidation in human atherosclerotic intima. J Biol Chem. 1997a;272:1433–1436. doi: 10.1074/jbc.272.3.1433. [DOI] [PubMed] [Google Scholar]
- Leeuwenburgh C, Rasmussen JE, Hsu FF, Mueller DM, Pennathur S, Heinecke JW. Mass spectrometric quantification of markers for protein oxidation by tyrosyl radical, copper, and hydroxyl radical in low density lipoprotein isolated from human atherosclerotic plaques. J Biol Chem. 1997b;272:3520–3526. doi: 10.1074/jbc.272.6.3520. [DOI] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal. 2007;9:2277–2293. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- Malhotra JD, Miao H, Zhang K, Wolfson A, Pennathur S, Pipe SW, Kaufman RJ. Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proc Natl Acad Sci U S A. 2008;105:18525–18530. doi: 10.1073/pnas.0809677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller FL, Lustgarten MS, Jang Y, Richardson A, Van Remmen H. Trends in oxidative aging theories. Free Radic Biol Med. 2007;43:477–503. doi: 10.1016/j.freeradbiomed.2007.03.034. [DOI] [PubMed] [Google Scholar]
- Okamura DM, Pennathur S, Pasichnyk K, Lopez-Guisa JM, Collins S, Febbraio M, Heinecke J, Eddy AA. CD36 regulates oxidative stress and inflammation in hypercholesterolemic CKD. J Am Soc Nephrol. 2009;20:495–505. doi: 10.1681/ASN.2008010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennathur S, Heinecke JW. Oxidative stress and endothelial dysfunction in vascular disease. Curr Diab Rep. 2007;7:257–264. doi: 10.1007/s11892-007-0041-3. [DOI] [PubMed] [Google Scholar]
- Pennathur S, Ido Y, Heller JI, Byun J, Danda R, Pergola P, Williamson JR, Heinecke JW. Reactive carbonyls and polyunsaturated fatty acids produce a hydroxyl radical-like species: a potential pathway for oxidative damage of retinal proteins in diabetes. J Biol Chem. 2005;280:22706–22714. doi: 10.1074/jbc.M500839200. [DOI] [PubMed] [Google Scholar]
- Pennathur S, Jackson-Lewis V, Przedborski S, Heinecke JW. Mass spectrometric quantification of 3-nitrotyrosine, ortho-tyrosine, and o,o′-dityrosine in brain tissue of 1-methyl-4-phenyl-1,2,3, 6-tetrahydropyridine-treated mice, a model of oxidative stress in Parkinson’s disease. J Biol Chem. 1999;274:34621–34628. doi: 10.1074/jbc.274.49.34621. [DOI] [PubMed] [Google Scholar]
- Pennathur S, Wagner JD, Leeuwenburgh C, Litwak KN, Heinecke JW. A hydroxyl radical-like species oxidizes cynomolgus monkey artery wall proteins in early diabetic vascular disease. J Clin Invest. 2001;107:853–860. doi: 10.1172/JCI11194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percy C, Pat B, Poronnik P, Gobe G. Role of oxidative stress in age-associated chronic kidney pathologies. Adv Chronic Kidney Dis. 2005;12:78–83. doi: 10.1053/j.ackd.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Pop-Busui R, Oral E, Raffel D, Byun J, Bajirovic V, Vivekanandan-Giri A, Kellogg A, Pennathur S, Stevens MJ. Impact of rosiglitazone and glyburide on nitrosative stress and myocardial blood flow regulation in type 2 diabetes mellitus. Metabolism. 2009;58:989–994. doi: 10.1016/j.metabol.2009.02.020. [DOI] [PubMed] [Google Scholar]
- Shigenaga MK, Lee HH, Blount BC, Christen S, Shigeno ET, Yip H, Ames BN. Inflammation and NO(X)-induced nitration: assay for 3-nitrotyrosine by HPLC with electrochemical detection. Proc Natl Acad Sci U S A. 1997;94:3211–3216. doi: 10.1073/pnas.94.7.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shishehbor MH, Brennan ML, Aviles RJ, Fu X, Penn MS, Sprecher DL, Hazen SL. Statins promote potent systemic antioxidant effects through specific inflammatory pathways. Circulation. 2003;108:426–431. doi: 10.1161/01.CIR.0000080895.05158.8B. [DOI] [PubMed] [Google Scholar]
- Shu L, Park JL, Byun J, Pennathur S, Kollmeyer J, Shayman JA. Decreased nitric oxide bioavailability in a mouse model of Fabry disease. J Am Soc Nephrol. 2009;20:1975–1985. doi: 10.1681/ASN.2008111190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song B, Scheuner D, Ron D, Pennathur S, Kaufman RJ. Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. J Clin Invest. 2008;118:3378–3389. doi: 10.1172/JCI34587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thannickal VJ. Aging, antagonistic pleiotropy and fibrotic disease. Int J Biochem Cell Biol. 2010;42:1398–1400. doi: 10.1016/j.biocel.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent AM, Hayes JM, McLean LL, Vivekanandan-Giri A, Pennathur S, Feldman EL. Dyslipidemia-induced neuropathy in mice: the role of oxLDL/LOX-1. Diabetes. 2009;58:2376–2385. doi: 10.2337/db09-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivekanadan-Giri A, Wang JH, Byun J, Pennathur S. Mass spectrometric quantification of amino acid oxidation products identifies oxidative mechanisms of diabetic end-organ damage. Rev Endocr Metab Disord. 2008;9:275–287. doi: 10.1007/s11154-008-9093-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggin TD, Kretzler M, Pennathur S, Sullivan KA, Brosius FC, Feldman EL. Rosiglitazone treatment reduces diabetic neuropathy in streptozotocin-treated DBA/2J mice. Endocrinology. 2008;149:4928–4937. doi: 10.1210/en.2008-0869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol. 2004;4:583–594. doi: 10.1038/nri1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Saha J, Byun J, Schin M, Lorenz M, Kennedy RT, Kretzler M, Feldman EL, Pennathur S, Brosius FC., 3rd Rosiglitazone reduces renal and plasma markers of oxidative injury and reverses urinary metabolite abnormalities in the amelioration of diabetic nephropathy. Am J Physiol Renal Physiol. 2008;295:F1071–1081. doi: 10.1152/ajprenal.90208.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Nukuna B, Brennan ML, Sun M, Goormastic M, Settle M, Schmitt D, Fu X, Thomson L, Fox PL, Ischiropoulos H, Smith JD, Kinter M, Hazen SL. Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J Clin Invest. 2004;114:529–541. doi: 10.1172/JCI21109. [DOI] [PMC free article] [PubMed] [Google Scholar]