Abstract
Objective
Granulocyte colony-stimulating factor (G-CSF) is a widespread therapeutic agent for stimulation of hematopoietic progenitor and stem cell (HPSC) mobilization from bone marrow (BM). Plasminogen (Plg) has been shown critical for HPSC mobilization. Here we investigated the role of Plg in G-CSF-induced HPSC mobilization and underlying mechanism.
Methods and Results
By using gene-targeted mice, our data show that Plg is required for G-CSF-induced HPSC egress to sinusoidal capillaries in BM and sequent mobilization to peripheral circulation. G-CSF induces Plg-dependent activation of matrix metalloproteinase-9 (MMP-9) in BM, and MMP-9 neutralization or deficiency suppresses HPSC migration and mobilization. Reconstitution of MMP-9 activity by BM transplantation after lentiviral overexpression rescues HPSC mobilization in Plg-deficient mice, indicating that MMP-9 activation is required for Plg-mediated HPSC mobilization. Interestingly, after G-CSF simulation, Plg down-regulates stromal cell-derived factor-1 (SDF-1) in BM and spatiotemporally regulates the expression of CXCR4 on mobilized HPSC, and reconstitution of MMP-9 activity in Plg-deficient mice reversed CXCR4 expression on HPSC in plasma and BM, suggesting that CXCR4 serves as a new downstream signal of Plg/MMP-9 in HPSC mobilization.
Conclusions
Our data elucidated a novel mechanism that Plg regulates MMP-9-dependent CXCR4 expression to facilitate HPSC mobilization in response to G-CSF.
Keywords: Plasminogen, G-CSF, hematopoietic progenitor and stem cells, MMP-9, SDF-1/CXCR4
In multiple pathological settings including stoke and myocardial infarction (MI), hematopoietic progenitor and stem cells (HPSC) are mobilized from the bone marrow (BM) to sites of injury to promote tissue repair and regeneration.1 Stimulation of HPSC mobilization by cytokine challenge has emerged as an important therapeutic strategy for treatment of ischemia heart disease.1,2 Granulocyte colony-stimulating factor (G-CSF) is the most commonly used mobilizing agent, however, impaired response to G-CSF is observed in 25% of patients and 10–20% of healthy donors.3–5 Therefore, a better understanding of the underlying mechanisms regulating G-CSF-induced HPSC mobilization may offer novel approaches for strengthening stem cell-mediated therapeutics.
Central to the regulation of HPSC mobilization is proteinases-mediated inactivation of cytokine signals, e.g., c-Kit/c-Kit ligand (c-KitL) and stromal cell-derived factor-1 (SDF-1)/CXCR4, that anchor HPSC in the BM microenvironment;6 the inactivation of these cytokine signals allows HPSC to proliferate, migrate to the sinusoid capillaries and enter the peripheral blood eventually. Plasminogen (Plg), activated by tissue- and urokinase- type plasminogen activator (tPA and uPA) to plasmin, is a critical mediator for HPSC mobilization from BM to the circulation.7–10 The underlying mechanism is poorly understood, but likely related to regulation of these cytokine signals. Previous studies 8 show that Plg regulates HPSC mobilization through plasmin cleavage of uPA receptor (uPAR) to release soluble uPAR to facilitate HPSC migration. Interestingly, uPA is not required for G-CSF-induced HPSC mobilization11, indicating that uPAR works as a downstream target of Plasmin rather than a plasminogen activator receptor for plasmin generation during G-CSF-induced HPSC mobilization. However, a 3–4 fold lower inhibition in HPSC mobilization was observed in uPAR−/− mice compared with Plg−/− mice8, suggesting there are unexplored mechanisms for Plg regulation of HPSC mobilization other than merely via uPAR cleavage. Although c-kitL is inactivated by Plg-mediated activation of matrix metalloproteinase-9 (MMP-9) during 5-fluoruracil (5-FU)-induced HPSC mobilization,7 c-kitL does not seem to be involved in G-CSF-induced HPSC mobilization since G-CSF does not affect its level.12 SDF-1/CXCR4 signal is a major chemotactic signal for stem cell mobilization,13–15 however, whether SDF-1/CXCR-4 signal contributes to Plg-mediated HPSC mobilization by G-CSF is unknown.
In addition to adhesion and chemotaxis signals, HPSC mobilization is also subjected to regulation by proteolytic enzyme-mediated matrix degradation.6,10 We have shown that MMP-9 acts downstream of Plg to regulate inflammatory cell migration.16 Consistently, MMP-9 is required for Plg-regulated hematopoietic regeneration after 5-FU-induced myeloablation,7 however, its role in Plg-regulated HPSC mobilization by G-CSF remains elusive. While there is evidence for roles of Plg, MMP-9 and SDF-1/CXCR4 in HPSC mobilization from the BM, the interaction of these pathways has not been investigated.
In the present study, we investigate the roles of SDF-1/CXCR4 and MMP-9 in Plg-mediated HPSC mobilization by G-CSF in vivo. Our findings establish a novel mechanism by which Plg regulates SDF-1/CXCR4 expression to modulate HPSC mobilization through MMP-9 activation.
Methods and Materials
Mice
The Plg−/− and MMP-9−/− mice were in the C57BL/6J background. All animal procedures were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee (see expanded methods in Data Supplement).
Stem Cell Mobilization
Mice received a daily subcutaneous injection of mouse G-CSF (200 μg/kg body weight, PeproTech Inc.) for 5 consecutive days. For some experiments, on day 4 immediately after the G-CSF injection, mouse proMMP-9 (1 μg) or human actMMP-9 (1 μg) (Calbiochem) were intravenously injected into the retro-orbital sinus. 5 days after G-CSF treatment, blood was drawn from retro-orbital sinus and the number of white blood cells (WBC) was counted with a hematocytometer. Blood, BM, femurs and tibias were harvested (see expanded methods in Data Supplement) for further experiments.
Flow Cytometry
For analysis of HPSC cells (Lin−c-kit+), blood cells from G-CSF-treated mice were incubated with a cocktail of biotin-conjugated lineage-specific monoclonal antibodies (CD5, CD45R, CD11b, Gr-1, TER119 and 7/4) (Miltenyi Biotec.) and labeled with FITC-anti-biotin IgG together with PE-anti-c-kit (BD Pharmingen.). For analysis of CXCR4 in HPSC, cells were labeled with lineage cocktail antibodies, PE-anti-ckit and FITC-anti-mouse CXCR4 antibody (clone 2B11, BD Pharmingen), which recognizes 0–63 amino acids of the N-terminal of CXCR4. CXCR4 expression on cells was expressed as mean fluorescence intensity (MFI). The FACS was done on a FACSCalibur or FACSCanto™ II (BD Biosciences). Isotype control (eBioscience) antibodies were used to exclude false positive cells.
Zymography of MMP-9
BM and blood plasma were subjected to electrophoresis and gelatin zymography as describe previously.16 The intensitiesof the bands were quantified using ImageJ software.
SDF-1 ELISA
SDF-1 concentration in BM and blood plasma was determined by using a SDF-1 ELISA kit (R&D Systems.) following the manufacturer’s instructions.
Isolation of BM-derived Lin− c-kit+ Cells and Chemotaxis Assay
Lin− c-kit+ cells were isolated from BM cells by immunomagnetic separation using MACs beads (Miltenyi Biotec). A migration assay was preformed using a modified Boyden’s Chamber (see expanded methods in the Data Supplement).
Lentivirus Production
Murine MMP-9 cDNAs, wild-type (WT), or G100L mutant (a gift from Dr. Elaine W. Raines, University of Washington) were amplified by PCR and subcloned into pLVX (Clontech.). All clones were verified by double enzyme digestion and sequencing. Lentivirus was generated by transfection of HEK293T cells with pLVX-MMP-9 and packaging vectors following the Lenti-X system instructions (Clontech). Virus titer was determined by p24 ELISA following the manufacturer’s instructions (Clontech).
Bone Marrow Transduction and Transplantation
BM cells were harvested from 6- to 8-week-old Plg+/+ donor mice and cultured in DMEM containing 15% FBS (Invitrogen) for 24 hours at 37°C. Cells were transduced with lentivirus (multiplicity of infection = 5) in the culture medium containing 8 g/L polybrene (Sigma) overnight. Age- and sex-matched Plg+/+ or Plg−/− recipient mice were lethally irradiated (10.5 Gy single dose) and injected with 2 × 106 infected BM cells via retro-orbital sinus. Four weeks later, mice were injected with G-CSF for 5 consecutive days. BM and blood cells were collected for FACS analysis.
Histological Studies
The femur and tibia were harvested on Day 4 after G-CSF injection. Bone sections were stained with Hematoxylin & Eosin (H&E). For immunohistochemistry, the antibodies used included c-kit (clone c-19, Santa Cruz) or CXCR4 (TP503, Torrey Pines Biolabs), which recognizes 2–38 amino acids of the N-terminal of CXCR4. A peroxidase DAB detection system was applied according to the manufacturer’s instructions (Vector Labs). No background staining was seen with either an irrelevant isotype-matched mAb or in the absence of a primary antibody.
Statistical Analysis
All the data in the text and figures were expressed as mean ± SEM and analyzed using a t-test and ANOVA with a Newman-Keuls post-test. A P-value less than 0.05 was considered significant.
Results
Plg is Required for Mobilization of HPSC from BM to the Circulation
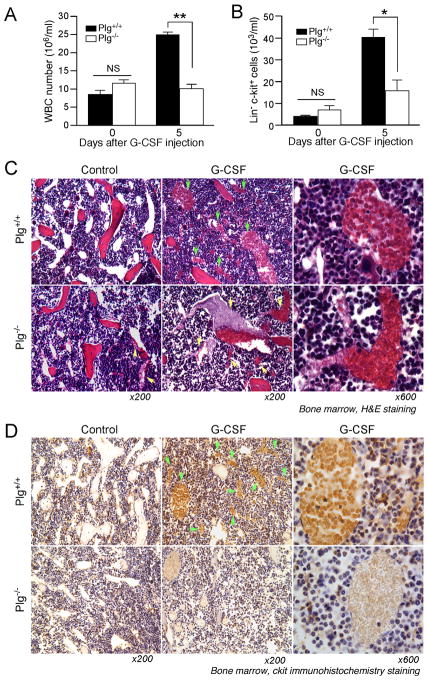
To understand the mechanism underlying stem cell mobilization, we investigated whether Plg is required for egress of cells from BM after cytokine challenge. Mice were injected with G-CSF for 5 days and a 4-fold increase in WBC counts was detected in Plg+/+ mice, but not in Plg−/− mice (Figure 1A). G-CSF significantly increased HPSC (Lin− c-kit+) cell number by 8 fold in Plg+/+ mice while Plg deficiency blocked this increase (Figure 1B). The difference in HPSC number of Plg+/+ and Plg−/− mice (33.9 ± 8.0 vs 17.0 ± 3.5 103/ml) remained significant after longer G-CSF treatment (6 days). These data confirm a previous study 8 showing that Plg is necessary for HPSC mobilization from BM to the circulation. Furthermore, our data show that administration of aprotinin, a plasminogen inhibitor targeting the catalytic site, inhibits G-CSF-induced HPSC mobilization, suggesting an important role of plasmin proteolytic function in Plg-dependent HPSC mobilization by G-CSF (Supplemental data Figure 1A, B).
Figure 1.
Plg is required for HPSC mobilization from BM to peripheral blood. A and B, WBC and blood HPSC were counted at 0 or 5 days after daily G-CSF treatment (200 μg/kg) of Plg+/+ and Plg−/− mice (n=8–11). (A) WBC counts. (B) Blood HPSC (Lin−c-kit+) counted by FACS. Bars indicate mean ± SEM. *P < 0.05; **P < 0.01; NS, P>0.05. C and D, Four days after daily G-CSF or control PBS injections, femurs and tibias were harvested, sectioned and stained, representative sections of 3 mice per treatment. (C) H&E staining. Red: bone. Dark blue: BM cells. White circles: sinusoids. Green arrow: HPSC in sinusoids. Yellow arrow: thrombi in sinusoids. (D) Immunohistochemistry staining with anti-c-kit antibody (ckit: brown color). Green arrow: HPSC in sinusoids. Magnification: ×200 or ×600.
HPSC Mobilization is Impaired in the BM in Plg−/− Mice after G-CSF Injection
A critical process for HPSC mobilization is the release of cells from the endosteal niche and migration to the sinusoidal capillaries in BM. To test whether Plg regulates HPSC egress to the sinusoidial capillaries, the femur and tibia bone sections were stained with H&E or immunostained with c-kit antibody. Sinusoids (white circles), a specific circulation system in BM, were characterized by H&E staining in Plg+/+ and Plg−/− mice (Figure 1C). Before G-CSF treatment, hematopoietic cells (dark blue) were present in BM matrix and no cells were observed in sinusoids of either Plg+/+ or Plg−/− mice. Notably, a few sinusoids containing a thrombus associated with a large number of erythrocytes were observed in Plg−/− mice (yellow arrows in Figure 1C), a typical feature of Plg deficiency.17, 18 Four days after G-CSF injection, in Plg+/+ mice, the dilated lumen of the sinusoids was filled with numerous hematopoietic cells, confirmed as c-kit+ HPSC (Figure 1D). In Plg−/− mice, G-CSF did not induce dilation of the sinusoid lumen (Figure 1C). Some sinusoids were filled with erythrocytes associated with thrombi, but not HPSC, as characterized by their morphology and negative c-kit antigen expression (Figure 1D). These results substantiate the dependence of G-CSF-induced HPSC mobilization upon Plg, and suggest that Plg regulates HPSC entry to sinusoids in BM to modulate cell mobilization.
G-CSF induces a Plg-dependent MMP-9 Activation and HPSC Mobilization is Impaired in MMP-9−/− mice
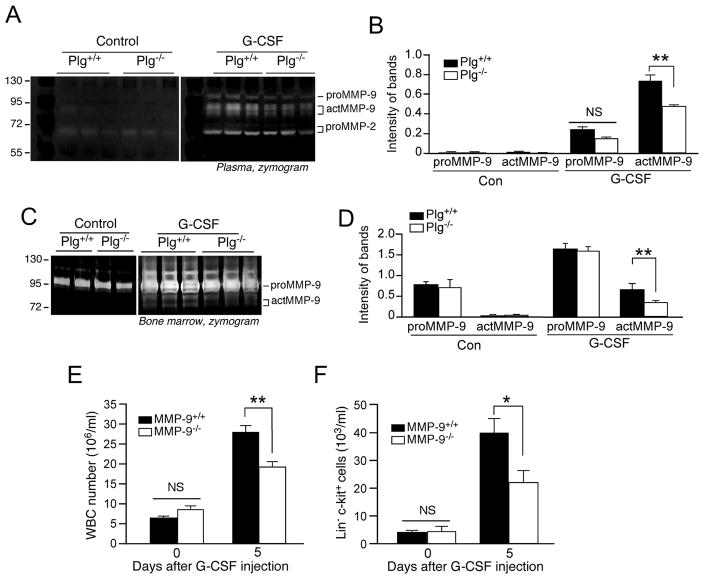
Matrix metalloproteinases, including MMP-3, MMP-9 and MMP-13, serve as downstream targets of plasmin activity19 and MMP-9 has been shown to be critical for HSPC recruitment.12, 20–22 To test whether MMP-9 are involved in Plg-mediated HPSC mobilization, Plg-regulated proteolysis in BM was investigated by gelatin zymography. No detectable MMP-9 activity was observed in the BM of either Plg+/+ or Plg−/− mice before G-CSF injection, but G-CSF significantly induced MMP-9 activation in both blood and BM of Plg+/+ mice (Figure 2A, B, C, D). In contrast, Plg deficiency significantly attenuated G-CSF-stimulated MMP-9 activation by 40%, but not proMMP-9 expression. Consistently, aprotinin significantly inhibited MMP-9 activation both in plasma and BM (Supplemental data Figure 1C, D). Together, these results indicate that G-CSF induces Plg-dependent MMP-9 activation during HPSC mobilization. Extensive studies have established the essential role of MMP-9 in stem cell mobilization, however, the results of HPSC mobilization generated from MMP-9−/− mice are still controversial.23, 24 To investigate whether MMP-9 is required for HPSC mobilization by G-CSF stimulation, stem cell mobilization was assessed in MMP-9−/− mice with a C57BL/6J background. G-CSF stimulated a marked mobilization of HPSC in MMP-9+/+ mice. MMP-9 deficiency inhibited G-CSF-induced stimulation of WBC generation and Lin− c-kit+ cell mobilization by 30% and 40%, respectively (Figure 2E, F), indicating that MMP-9 is critical for G-CSF-induced HPSC mobilization.
Figure 2.
G-CSF induces Plg-dependent MMP-9 activation and impaired HPSC mobilization in MMP-9−/− mice. A through D, Plg+/+ and Plg−/− mice were injected with G-CSF for 5 days, and protease activity in BM and blood was analyzed by gelatin zymography. Mouse proMMP-9 (105 kDa) and actMMP-9 (95 and 88 kDa) were identified by molecular weight relative to markers. A and C, Representative images of zymographs of Plasma or BM plasma. B and D, Quantitative data of the intensity of MMP-9 bands are shown (3 independent assays). E and F, MMP-9+/+ and MMP-9−/− mice were treated daily with G-CSF for 5 days. Blood WBC and HPSC (Lin− c-kit+) were counted (n = 12). (E) WBC counts. (F) HPSC counts analyzed by FACS. Bars indicate mean ± SEM. *P<0.05; **P<0.01; NS, P>0.05.
MMP-9 Activation is Required for Plg-regulated HPSC Mobilization from BM to the Peripheral Blood
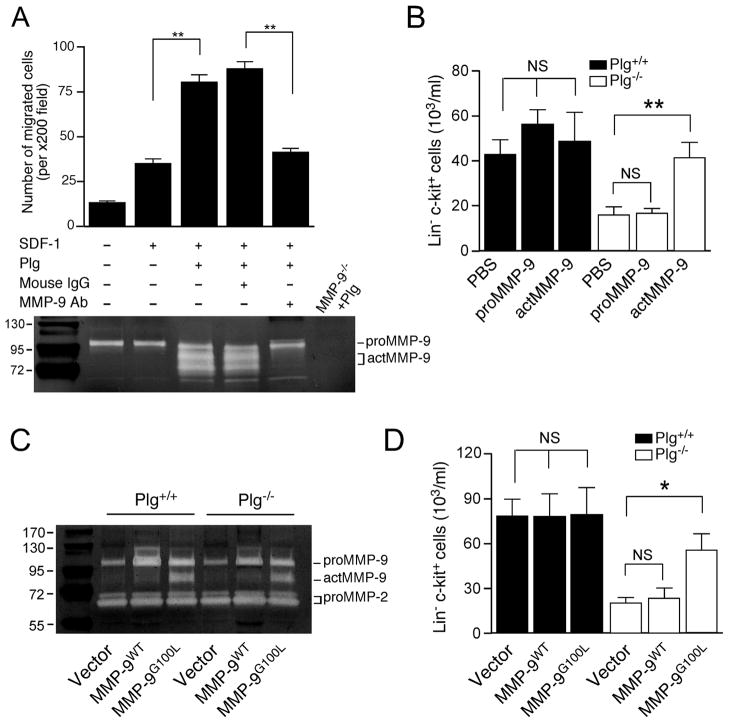
MMP-9 is required for Plg-regulated hematopoietic regeneration after 5-FU-induced myeloablation,7 however, its role in Plg-regulated HPSC mobilization by G-CSF remains unknown. BM-derived HPSC (Lin− c-kit+) cells express both MMP-9 and Plg activators like uPAR7,8, implicating a proteolytic Plg/MMP-9 activation system by HPSC. To test whether MMP-9 activation is required for Plg-regulated HPSC migration, we determined the migratory capacity of BM-derived Lin− c-kit+ cells in response to SDF-1 gradient. Lin− c-kit+ cells were isolated from the BM with a purity of over 90% (Supplemental data Figure 2). Plg is mainly synthesized by liver and released into the circulation at a high level (2 μM)25. Since no plasmin activity was detected in HPSC (Lin− c-kit+ cells) isolated from Plg+/+ or Plg−/− mice (Supplemental data Figure 3), in our in vitro migration system, Lin− c-kit+ cells isolated from Plg+/+ mice were used and an exogenous Plg protein was introduced to test the effect of Plg on HPSC migration. Lin− c-kit+ cells were seeded and exposed to SDF-1 gradient in a modified Boyden’s chamber. Plg (20 μg/ml) was added into the upper chamber and MMP-9 in medium was neutralized with an antibody that is able to efficiently block MMP-9 activation as we previously showed.16. Zymography results confirm in vitro MMP-9 activation by Plg and the inhibition of MMP-9 activity with this antibody (Figure 3A, lower panel). Plg significantly stimulated Lin− c-kit+ cells migration in response to SDF-1 (Figure 3A, upper panel). However, MMP-9 neutralization successfully abolished this stimulation, indicating a requirement of MMP-9 activation in Plg-stimulated HPSC migration in vitro.
Figure 3.
MMP-9 activation is required for Plg-regulated HPSC mobilization. (A) HPSC (Lin− c-kit+ cells) were isolated from BM of Plg+/+ mice, and subjected to in vitro cell migration assay with SDF-1 as the chemoattractant. MMP-9 activity in culture medium of cell migration chambers was analyzed by gelatin zymography. Culture medium of cells isolated from MMP-9−/−mice treated with Plg was loaded as a negative control. Upper panel: number of migrated cells. Lower panel: zymograph. (B) Plg+/+ and Plg−/− mice were treated daily with G-CSF for 5 days and intravenously administered PBS, proMMP-9 or actMMP-9 on day 4. HPSC (Lin−c-kit+ cells) in peripheral blood from G-CSF-treated Plg+/+ and Plg−/− mice were analyzed by FACS (n = 5–8). C and D, BM cells isolated from Plg+/+ mice and lentivirally transduced to overexpress WT (proMMP-9), or G100L MMP-9 (actMMP-9). Transduced BM cells were transplanted (BMT) into lethally irradiated recipient Plg+/+ and Plg−/− mice. Four weeks later, BMT mice were injected daily with G-CSF for 5 days (n=4). (C) BMT mouse BM plasma was subjected to gelatin zymography. Mouse proMMP-9 (105 kDa) and actMMP-9 (95 kDa, 88 kDa), as well as pro MMP-2 (72 kDa and 69 kDa), were identified by molecular weight relative to markers. (D) HPSC (Lin−c-kit+ cells) in blood isolated from BMT mice with G-CSF injection were analyzed by FACS. Bars indicate mean ± SEM. *P<0.05; NS, P>0.05.
To assess whether MMP-9 activation is necessary for Plg-regulated HPSC mobilization in vivo, MMP-9 activity in Plg−/− mice was restored by administration of the active form of MMP-9 (actMMP-9), and HPSC mobilization was measured. ActMMP-9, rather than proMMP-9, almost fully rescued HPSC mobilization in Plg−/− mice (Figure 3B) to a level comparable with the circulating HPSC number in Plg+/+ mice, thus suggesting a necessary role of Plg-induced MMP-9 activation in HPSC mobilization. To further verify this, BM cells were isolated and lentivirus transduced ex vivo to express WT or mutated MMP-9 (G100L), followed by BMT to lethally irradiated recipients. G100L mutation efficiently weakens the interaction between the prodomain and the catalytic subunit, inducing the autolytic cleavage and production of active MMP-9.26 Transduction BM cells with G100L mutant increased MMP-9 activity by 3-fold in BM plasma after BMT (Figure 3C). Lentiviral treatment induced an increase in basal HPSC mobilization in both Plg+/+ and Plg−/− mice, likely due to a non-specific effect of transduction (Figure 3D). Importantly, overexpression of MMP-9G100L, but not MMP-9WT, rescued HPSC mobilization in Plg−/− mice, thus confirming that Plg-required MMP-9 activation is necessary for HPSC mobilization induced by G-CSF.
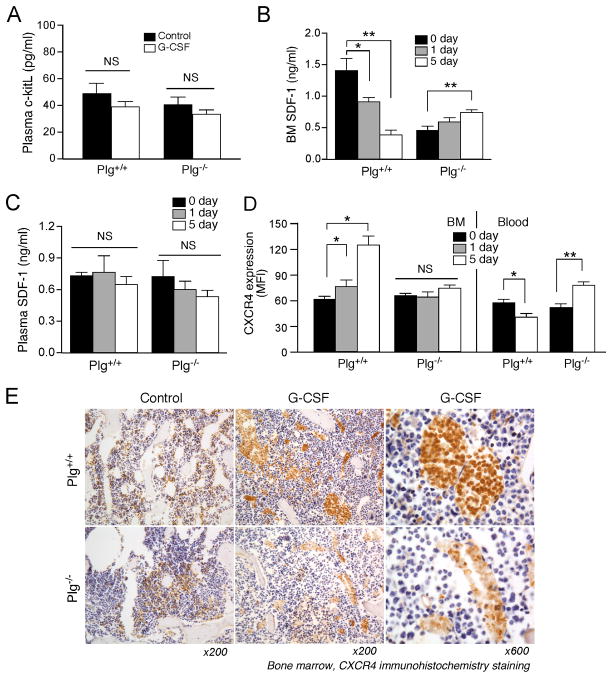
Plg Regulates Expression of BM SDF-1 but not c-kitL during HPSC Mobilization
C-kit/c-kitL and SDF-1/CXCR4, are the major cytokine signals for chemotaxis in cytokine-induced HPSC mobilization.6 Recently c-kitL has been shown as a downstream target of Plg/MMP-9 during myeloablation-induced HPSC mobilization.7 Our results indicate that G-CSF did not affect c-kitL expression in the plasma of Plg+/+ or Plg−/− mice 5 days after G-CSF injection (Figure 4A), suggesting no involvement of c-kitL in G-CSF-induced HPSC mobilization, which is consistent with a previous study.12
Figure 4.
Plg regulates SDF-1 and CXCR4 expression during G-CSF-induced HPSC mobilization. (A) Plg+/+ and Plg−/− mice were injected daily with G-CSF or PBS (control) for 5 days. c-kitL in blood was measured by ELISA (n=6–7). B through D, Mice were injected with daily G-CSF or PBS for 1 or 5 days, SDF-1 in BM and blood were measured by ELISA, and CXCR4 expression on HPSC in BM and blood analyzed by FACS and expressed as mean fluorescence intensity (MFI). B and C, Plasma and BM SDF-1 levels determined by ELISA (n=6–7). (D) CXCR4 expression (n=4). Bars indicate mean ± SEM. *P<0.05; **P<0.01; NS, P>0.05. (E) Mice were injected daily with G-CSF for 4 days, and bone sections of femurs and tibias subjected to CXCR4 immunohistochemistry staining. Brown color: CXCR4+ cells. Represenative sections of 3 mice per treatment. Magnification: ×200 or ×600
SDF-1 is a key chemoattractant for HPSC mobilization from BM. Previous studies 13 reported that G-CSF induces a significant decrease in SDF-1 in BM to a level lower than that in blood, therefore generating a gradient between BM and peripheral blood to induce HPSC egress into blood. Consistently, G-CSF treatment induced a SDF-1 gradient in Plg+/+ mice, as indicated by a lower concentration of 0.39 ± 0.08 ng/ml in BM compared to unaffected concentration of 0.65 ± 0.07 ng/ml in plasma, 5 days after injection (Figure 4B, C). After G-CSF injection, a slight increase in BM SDF-1 (0.74 ± 0.04 ng/ml) compared to no change and lower concentration in blood SDF-1 (0.54 ± 0.06 ng/ml) (Figure 4B, C) were observed in Plg−/− mice, preventing the formation of a SDF-1 gradient between the BM and blood. In addition, G-CSF did not affect SDF-1 in plasma of either Plg+/+ or Plg−/− mice, therefore suggesting a BM-specific regulation of SDF-1 by Plg. Together, these results indicate that Plg may regulate G-CSF-induced HPSC mobilization through modulation of SDF-1, but not c-kitL. Interestingly, SDF-1 baseline (without G-CSF treatment) was lower in Plg−/− mice (Figure 4B) compared to Plg+/+mice, but no increase in cell number of circulating HPSC was observed, suggesting SDF-1 gradient alone may not be sufficient to induce HPSC mobilization.
Plg Regulates Expression of CXCR4 during HPSC Mobilization
CXCR4, the major receptor of SDF-1, is critical for G-CSF-induced stem cell mobilization. CXCR4 interaction with SDF-1 requires an N-terminal region on CXCR4 that can be proteolytically cleaved to inactivate the CXCR4/SDF-1 function.27, 28 We investigated whether Plg regulates the expression of functional, intact CXCR4 in mobilizing HPSC by using an antibody specifically recognizing the ligand binding site of CXCR4 (N-terminal 0–63 amino acids). In Plg+/+ mice, our data show that G-CSF induced an oscillation of CXCR4 expression on HPSC mobilized from BM to the circulation consistent with previous work.13 Namely, G-CSF gradually increased CXCR4 expression on BM HPSCs (Figure 4D) indicating that high CXCR4 expression may favor HPSC mobilizing from BM to the peripheral blood, where SDF-1 is higher. Five days after daily G-CSF injection, CXCR4 expression on circulating HPSCs was significantly decreased (by 67%) compared with CXCR4 expression on BM HPSCs (Figure 4D). This indicated that CXCR4 expression was reduced after HPSC mobilization to the circulation and this reduction may be necessary for preventing HPSC migrating back to the BM from the blood. However, both Plg deficiency (Figure 4D) and plasmin inhibition by aprotinin (Supplement data Figure 4) completely abrogated this oscillation with neither an increase in CXCR4 expression in the BM nor down-regulation in circulation, indicating that Plg, particularly its enzyme activity, is essential for the regulation of CXCR4 expression during egress of HPSC from BM to the peripheral blood.
Furthermore, CXCR4 immunostaining indicated that in control mice, functional CXCR4+ cells were selectively distributed in the endosteal region in the vicinity of trabecular bone, where the most primitive HPSC reside (Figure 4E). After G-CSF stimulation, CXCR4 expression was enhanced on BM cells and the majority of CXCR4+ cells were present in the sinusoid lumen, suggesting that HPSC with high expression of intact CXCR4 were recruited from the endosteal site to the sinusoids. In contrast, after G-CSF injection, the CXCR4 expression on BM cells of Plg−/− mice was much less than that of WT mice, indicating that Plg deficiency inhibited G-CSF-induced CXCR4 expression in BM and result in fewer hematopoietic cells in sinusoids. These data suggested that Plg is required for the regulation of CXCR4 in G-CSF-induced stem cell mobilization.
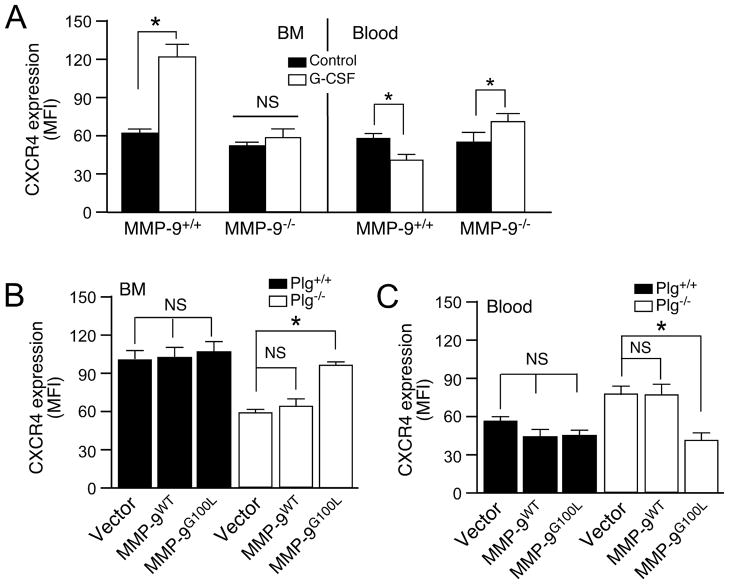
Plg Regulates CXCR4 Expression through MMP-9 activation during G-CSF-induced HPSC Mobilization
Previous studies have shown that MMP-9 degrades extracellular matrix in BM and cleaves cytokines and their receptors (c-kit/c-kitL, SDF-1/CXCR4 and vascular cell adhesion molecule, VCAM-1/α4β1 integrin) to promote HPSC egress from BM to the circulation.20, 29, 30 We investigated whether MMP-9 regulates CXCR4 expression in Plg-mediated HPSC mobilization by using MMP-9−/− mice. MMP-9 deficiency reversed G-CSF-induced up-regulation of CXCR4 expression in BM HPSC and down-regulation of CXCR4 expression in circulating HPSCs (Figure 5A). In G-CSF treated mice, BM transplantation with lentiviral overexpression of G100L MMP-9 reconstitution of MMP-9 activity in Plg−/− mice significantly increased CXCR4 expression on BM HPSCs and decreased CXCR4 expression in circulating HPSCs (Figure 5B,C). Compared to the vector alone, this led to the successful rescue of HPSC mobilization in Plg−/− mice (Figure 3D), indicating that CXCR4 expression is regulated by MMP-9 during HPSC mobilization by G-CSF. In summary, these results revealed that Plg regulates G-CSF-induced HPSC mobilization through MMP-9- mediated CXCR4 expression.
Figure 5.
MMP-9 regulates CXCR4 expression during HPSC mobilization. (A) MMP-9+/+ and MMP-9−/− mice were injected daily with G-CSF, and BM and blood cells subjected to FACS analysis for CXCR4 expression (n=8–9). B and C, BM cells from Plg+/+ mice were lentivirally transduced to overexpress WT (proMMP-9), or G100L MMP-9 (actMMP-9), and transplanted into recipient Plg+/+ or Plg−/− mice. Four weeks later, recipient mice were injected daily with G-CSF for 5 days, and CXCR4 expression on BM or blood cells determined by FACS (n=4). (B) CXCR4 expression on BM cells. (C) CXCR4 expression on blood cells. Bars indicate mean±SEM. *P<0.05; NS, P>0.05.
Discussion
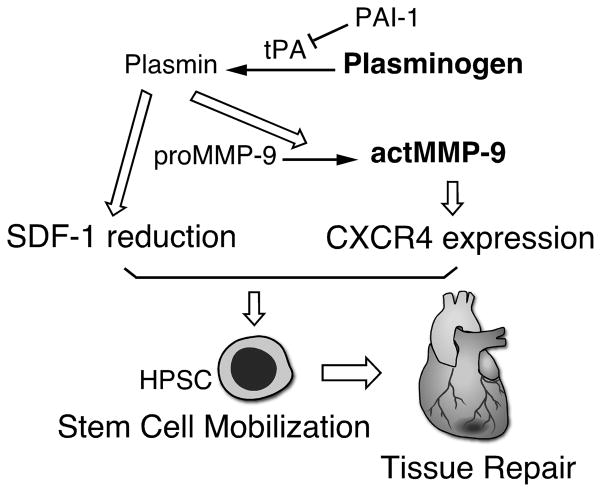
G-CSF is a cytokine produced by a variety of cells, and clinically the recombinant form is used as a therapeutic agent to enhance HPSC mobilization in chemotherapy recovery, HPSC transplantation and ischemic cerebral and heart damage. In the BM G-CSF and other mobilizing agents induce changes in the release of proteases and modulation of SDF-1/CXCR4, c-kit/c-kitL and adhesive molecules to enhance HPSC mobilization6. The present study highlights a role of Plg in the G-CSF-induced HPSC mobilization and identifies a novel underlying mechanism via MMP-9-mediated SDF-1/CXCR4 expression. Our findings show that MMP-9 activation is required for Plg-mediated HPSC mobilization by G-CSF. Furthermore, Plg regulates HPSC mobilization through SDF-1/CXCR4, the major chemotaxis signaling pathway in stem cell mobilization. Interestingly, CXCR4 works downstream of Plg-dependent MMP-9 activation during G-CSF-induced HPSC mobilization. Thus, our data elucidate the molecular mechanism of Plg-mediated HPSC mobilization by activation of MMP-9 and regulation of SDF-1/CXCR-4 signals. (Figure 6).
Figure 6.
A schematic model for Plg regulation of HPSC mobilization by G-CSF. Plg regulates SDF-1 in BM and MMP-9-dependent CXCR4 expression on HPSC to facilitate HPSC mobilization from BM to circulation, which may contribute to tissue repair after injuries including myocardial ischemia.
Consistent with a recent study by Tjwa et al.8, our data identify a critical role of Plg in HPSC mobilization by G-CSF. Their mechanistic study shows that cleavage of uPAR is involved in the Plg-regulated HPSC mobilization by G-CSF. However, uPAR deficiency inhibits much less HPSC mobilization in response to G-CSF than Plg deficiency, suggesting the presence of other unidentified pathways for Plg-mediated HPSC mobilization. The novel mechanism revealed by our study indicates uPAR is not the only signal responsible for Plg-mediated HPSC mobilization. The newly identified MMP-9-mediated SDF-1/CXCR4 signal seems to be a critical mediator for G-CSF-induced HPSC mobilization, as indicated by marked suppression in HPSC mobilization by Plg and MMP-9 deficiency and significant rescue of HPSC mobilization in Plg−/− mice by overexpression of active MMP-9 in BM cells.
Proteolytic enzymes such as neutrophil elastase, cathepsin G and MMP-9 play important roles in HPSC mobilization.13, 14, 31 Previous studies show that an elevated MMP-9 in BM in clinical and experimental animal therapies is induced by G-CSF,31, 32 as well as by other stem cell recruitment agents, such as IL-8, 5-FU, and Groβ.12, 20–22 Gene loss of MMP-9 in mice severely impairs HPSC recruitment to blood by G-CSF.20, 21 Neutralization with anti-MMP-9 antibody inhibits IL-8- or GROβ-induced HPSC mobilization by 90% and G-CSF-induced mobilization by 40% in mice,12,22 indicating that MMP-9 is critical for cytokine-induced stem cell mobilization. Controversially, other studies show that MMP-9 deficiency in mice fails to affect stem cell mobilization by cytokines23, 24, likely due to difference in mouse background and dose of G-CSF treatment: i) MMP-9−/− mice with different background including C57BL/B6 (in our study), 129 20, 23, 24, and FVB 21 were used. Our previous studies 33 indicate that different mouse strains have different phenotype, e.g., inflammatory cell recruitment, even though all of them are WT. ii) Mice were treated with G-CSF at different doses, such as daily at 50 μg/kg/day 20, daily at 250 μg/kg/day 23, or twice daily at a dose of 250 μg/kg/day 24. Our study found MMP-9 deficiency significantly inhibited HPSC mobilization by G-CSF, suggesting a critical role of MMP-9 in stem cell mobilization. Incomplete inhibition of HPSC mobilization may be due to: 1) MMP-9 is a crucial but not the sole factor required for G-CSF-induced stem cell mobilization; 2) Other MMPs may compensate the function of MMP-9 for HPSC mobilization in MMP-9 knockout mice 34. Our data for the first time establishes a necessary role of MMP-9 activation in Plg-regulated stem cell mobilization by a rescue experiment that reconstitution MMP-9 activity in Plg−/− mice restores HPSC mobilization in our G-CSF model. During stem cell mobilization, protease activation cleaves cytokines that regulate HPSC migration, including c-kitL and SDF-1/CXCR4 in BM.20,31 Accumulating evidence indicates that activation of the CXCR4 is critical for MMP-9-mediated HPSC mobilization. Recent studies15 show that G-CSF injection fails to induce stem cell mobilization in CXCR4−/− mice, regardless of the fact that G-CSF markedly stimulates MMP-9 activity in BM, thus suggesting that MMP-9-mediated HPSC mobilization may be dependent on CXCR4. Furthermore, transplantation of CXCR4+ VEGFR+ cells into MMP-9−/− mice rescues the severe defects in ischemic revascularization,35. However, the direct evidence for MMP-9 regulating CXCR4 expression in HPSC mobilization is lacking. In our study, the results show that both G-CSF treatment and Plg deficiency do not affect c-kitL level, a verified downstream cytokine for Plg/MMP-9 during 5-FU-induced HPSC mobilization.7 While, G-CSF induces a Plg/MMP-9-dependent CXCR4 expression in BM, and actMMP-9 treatment in Plg−/− mice restores the CXCR4 expression to the level in G-CSF-treated WT mice, suggesting that CXCR4, instead of c-kitL, acts as a new downstream signal of Plg/MMP-9 in G-CSF-induced HPSC mobilization.
SDF-1/CXCR4 is critical for cell chemotaxis in BM during HPSC mobilization and CXCR4 is expressed on the majority of circulating G-CSF-mobilized HPSC.36 To date the precise role of CXCR4 in HPSC mobilization has not been well defined. Previous studies 13, 35, 37, 38 show that acute inhibition of CXCR4 with the non-peptide antagonist AMD3100 promotes HPSC mobilization, where chronic inhibition with AMD3100 or neutralizing antibody impairs mobilization. Previous studies13–15 suggest that G-CSF induces a spatiotemporal regulation of CXCR4 expression to facilitate HPSC mobilization: CXCR4 expression is transiently decreased immediately after G-CSF injection, which may facilitate HPSC detachment from stromal cells, but gradually increases and peaks during stem cell mobilization in BM and may stimulate cell chemotaxis in response to a SDF-1 gradient. CXCR4 expression on mobilized cells in the circulation is significantly lower than that in BM, which may prevent cells from homing back to the BM. Our results show this mobilization-favorable oscillation of CXCR4 expression can be abolished by Plg/MMP-9 deficiency, indicating that Plg/MMP-9 may spatiotemporally regulate CXCR4 expression to facilitate stem cell mobilization. Of great interest, whether MMP-9 regulates CXCR4 expression directly or indirectly remains to be determined.
In conclusion, we elucidate a distinct mechanism underlying Plg-mediated stem cell mobilization in response to G-CSF. In this newly identified pathway, Plg regulates MMP-9 activation and SDF-1/CXCR4 signaling, particularly MMP-9-dependent CXCR4 expression, to modulate HPSC mobilization, contributing a better understanding of the role of Plg in G-CSF-induced stem cell mobilization. Targeting these pathways may offer a therapeutic opportunity to enhance G-CSF-induced stem cell mobilization for treatment of ischemic disease.
Supplementary Material
Acknowledgments
Funding Sources
This study was funded in part by grants from American Heart Association (AHA0625331B and 09BGIA2050157) and the National Institutes of Health, National Heart, Lung, and Blood Institute (R01HL078701).
Footnotes
Disclosures
None
Reference List
- 1.Grigoropoulos NF, Mathur A. Stem cells in cardiac repair. Curr Opin Pharmacol. 2006;6:169–75. doi: 10.1016/j.coph.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci U S A. 2001;98:10344–9. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stiff P, Gingrich R, Luger S, Wyres MR, Brown RA, LeMaistre CF, Perry J, Schenkein DP, List A, Mason JR, Bensinger W, Wheeler C, Freter C, Parker WRL, Emmanouilides C. A randomized phase 2 study of PBPC mobilization by stem cell factor and filgrastim in heavily pretreated patients with Hodgkin’s disease or non-Hodgkin’s lymphoma. Bone Marrow Transplant. 2000;26:471–81. doi: 10.1038/sj.bmt.1702531. [DOI] [PubMed] [Google Scholar]
- 4.Holm M. Not all healthy donors mobilize hematopoietic progenitor cells sufficiently after G-CSF administration to allow for subsequent CD34 purification of the leukapheresis product. J Hematother. 1998;7:111–3. doi: 10.1089/scd.1.1998.7.111. [DOI] [PubMed] [Google Scholar]
- 5.Anderlini P, Przepiorka D, Seong C, Smith TL, Huh YO, Lauppe J, Champlin R, Korbling M. Factors affecting mobilization of CD34+ cells in normal donors treated with filgrastim. Transfusion. 1997;37:507–12. doi: 10.1046/j.1537-2995.1997.37597293882.x. [DOI] [PubMed] [Google Scholar]
- 6.Nervi B, Link DC, DiPersio JF. Cytokines and hematopoietic stem cell mobilization. J Cell Biochem. 2006;99:690–705. doi: 10.1002/jcb.21043. [DOI] [PubMed] [Google Scholar]
- 7.Heissig B, Lund LR, Akiyama H, Ohki M, Morita Y, Romer J, Nakauchi H, Okumura K, Ogawa H, Werb Z, Dano K, Hattori K. The plasminogen fibrinolytic pathway is required for hematopoietic regeneration. Cell Stem Cell. 2007;1:658–70. doi: 10.1016/j.stem.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tjwa M, Sidenius N, Moura R, Jansen S, Theunissen K, Andolfo A, De MM, Dewerchin M, Moons L, Blasi F, Verfaillie C, Carmeliet P. Membrane-anchored uPAR regulates the proliferation, marrow pool size, engraftment, and mobilization of mouse hematopoietic stem/progenitor cells. J Clin Invest. 2009;119:1008–18. doi: 10.1172/JCI36010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tjwa M, Janssens S, Carmeliet P. Plasmin therapy enhances mobilization of HPCs after G-CSF. Blood. 2008;112:4048–50. doi: 10.1182/blood-2008-07-166587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tjwa M, Moura R, Moons L, Plaisance S, De MM, Jansen S, Dewerchin M, Verfaillie C, Carmeliet P. Fibrinolysis-independent role of plasmin and its activators in the haematopoietic recovery after myeloablation. J Cell Mol Med. 2009;13:4587–95. doi: 10.1111/j.1582-4934.2008.00521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carmeliet P, Tjwa M. Means and methods for the recruitment and identification of stem cells. 2010/0178297 A1. patent US. 2010 Jul 15;
- 12.Pelus LM, Bian H, King AG, Fukuda S. Neutrophil-derived MMP-9 mediates synergistic mobilization of hematopoietic stem and progenitor cells by the combination of G-CSF and the chemokines GRObeta/CXCL2 and GRObetaT/CXCL2delta4. Blood. 2004;103:110–9. doi: 10.1182/blood-2003-04-1115. [DOI] [PubMed] [Google Scholar]
- 13.Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, Habler L, Ponomaryov T, Taichman RS, Renzana-Seisdedos F, Fujii N, Sandbank J, Zipori D, Lapidot T. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3:687–94. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 14.Levesque JP, Hendy J, Takamatsu Y, Simmons PJ, Bendall LJ. Disruption of the CXCR4/CXCL12 chemotactic interaction during hematopoietic stem cell mobilization induced by GCSF or cyclophosphamide. J Clin Invest. 2003;111:187–96. doi: 10.1172/JCI15994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Christopher MJ, Liu F, Hilton MJ, Long F, Link DC. Suppression of CXCL12 production by bone marrow osteoblasts is a common and critical pathway for cytokine-induced mobilization. Blood. 2009;114:1331–9. doi: 10.1182/blood-2008-10-184754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gong Y, Hart E, Shchurin A, Hoover-Plow J. Inflammatory macrophage migration requires MMP-9 activation by plasminogen in mice. J Clin Invest. 2008;118:3012–24. doi: 10.1172/JCI32750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bugge TH, Flick MJ, Daugherty CC, Degen JL. Plasminogen deficiency causes severe thrombosis but is compatible with development and reproduction. Genes Dev. 1995;9:794–807. doi: 10.1101/gad.9.7.794. [DOI] [PubMed] [Google Scholar]
- 18.Ploplis VA, Carmeliet P, Vazirzadeh S, Van Vlaenderen I, Moons L, Plow EF, Collen D. Effects of disruption of the plasminogen gene in mice on thrombosis, growth and health. Circulation. 1995;92:2585–93. doi: 10.1161/01.cir.92.9.2585. [DOI] [PubMed] [Google Scholar]
- 19.Lijnen HR. Plasmin and matrix metalloproteinases in vascular remodeling. Thromb Haemost. 2001;86:324–33. [PubMed] [Google Scholar]
- 20.Heissig B, Hattori K, Dias S, Friedrich M, Ferris B, Hackett NR, Crystal RG, Besmer P, Lyden D, Moore MA, Werb Z, Rafii S. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625–37. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cramer DE, Wagner S, Li B, Liu J, Hansen R, Reca R, Wu W, Surma EZ, Laber DA, Ratajczak MZ, Yan J. Mobilization of hematopoietic progenitor cells by yeast-derived beta-glucan requires activation of matrix metalloproteinase-9. Stem Cells. 2008;26:1231–40. doi: 10.1634/stemcells.2007-0712. [DOI] [PubMed] [Google Scholar]
- 22.Pruijt JF, Fibbe WE, Laterveer L, Pieters RA, Lindley IJ, Paemen L, Masure S, Willemze R, Opdenakker G. Prevention of interleukin-8-induced mobilization of hematopoietic progenitor cells in rhesus monkeys by inhibitory antibodies against the metalloproteinase gelatinase B (MMP-9) Proc Natl Acad Sci U S A. 1999;96:10863–8. doi: 10.1073/pnas.96.19.10863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levesque JP, Liu F, Simmons PJ, Betsuyaku T, Senior RM, Pham C, Link DC. Characterization of hematopoietic progenitor mobilization in protease-deficient mice. Blood. 2004;104:65–72. doi: 10.1182/blood-2003-05-1589. [DOI] [PubMed] [Google Scholar]
- 24.Robinson SN, Pisarev VM, Chavez JM, Singh RK, Talmadge JE. Use of matrix metalloproteinase (MMP)-9 knockout mice demonstrates that MMP-9 activity is not absolutely required for G-CSF or Flt-3 ligand-induced hematopoietic progenitor cell mobilization or engraftment. Stem Cells. 2003;21:417–27. doi: 10.1634/stemcells.21-4-417. [DOI] [PubMed] [Google Scholar]
- 25.Raum D, Marcus D, Alper CA, Levey R, Taylor PD, Starzl TE. Synthesis of human plasminogen by the liver. Science. 1980;208:1036–7. doi: 10.1126/science.6990488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gough PJ, Gomez IG, Wille PT, Raines EW. Macrophage expression of active MMP-9 induces acute plaque disruption in apoE-deficient mice. J Clin Invest. 2006;116:59–69. doi: 10.1172/JCI25074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valenzuela-Fernandez A, Planchenault T, Baleux F, Staropoli I, Le-Barillec K, Leduc D, Delaunay T, Lazarini F, Virelizier JL, Chignard M, Pidard D, Arenzana-Seisdedos F. Leukocyte elastase negatively regulates Stromal cell-derived factor-1 (SDF-1)/CXCR4 binding and functions by amino-terminal processing of SDF-1 and CXCR4. J Biol Chem. 2002;277:15677–89. doi: 10.1074/jbc.M111388200. [DOI] [PubMed] [Google Scholar]
- 28.Brelot A, Heveker N, Montes M, Alizon M. Identification of residues of CXCR4 critical for human immunodeficiency virus coreceptor and chemokine receptor activities. J Biol Chem. 2000;275:23736–44. doi: 10.1074/jbc.M000776200. [DOI] [PubMed] [Google Scholar]
- 29.McQuibban GA, Butler GS, Gong JH, Bendall L, Power C, Clark-Lewis I, Overall CM. Matrix metalloproteinase activity inactivates the CXC chemokine stromal cell-derived factor-1. J Biol Chem. 2001;276:43503–8. doi: 10.1074/jbc.M107736200. [DOI] [PubMed] [Google Scholar]
- 30.Fiore E, Fusco C, Romero P, Stamenkovic I. Matrix metalloproteinase 9 (MMP- 9/gelatinase B) proteolytically cleaves ICAM-1 and participates in tumor cell resistance to natural killer cell-mediated cytotoxicity. Oncogene. 2002;21:5213–23. doi: 10.1038/sj.onc.1205684. [DOI] [PubMed] [Google Scholar]
- 31.Jin F, Zhai Q, Qiu L, Meng H, Zou D, Wang Y, Li Q, Yu Z, Han J, Li Q, Zhou B. Degradation of BM SDF-1 by MMP-9: the role in G-CSF-induced hematopoietic stem/progenitor cell mobilization. Bone Marrow Transplant. 2008;42:581–8. doi: 10.1038/bmt.2008.222. [DOI] [PubMed] [Google Scholar]
- 32.Saito T, Usui N, Asai O, Dobashi N, Yano S, Osawa H, Takei Y, Takahara S, Ogasawara Y, Otsubo H, Yamaguchi Y, Minami J, Hoshi Y, Kataoka M, Aiba K. Elevated serum levels of human matrix metalloproteinase-9 (MMP-9) during the induction of peripheral blood stem cell mobilization by granulocyte colony-stimulating factor (G-CSF) J Infect Chemother. 2007;13:426–8. doi: 10.1007/s10156-007-0553-4. [DOI] [PubMed] [Google Scholar]
- 33.Hoover-Plow JL, Gong Y, Shchurin A, Busuttil SJ, Schneeman TA, Hart E. Strain and model dependent differences in inflammatory cell recruitment in mice. Inflamm Res. 2008;57:457–63. doi: 10.1007/s00011-008-7062-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ducharme A, Frantz S, Aikawa M, Rabkin E, Lindsey M, Rohde LE, Schoen FJ, Kelly RA, Werb Z, Libby P, Lee RT. Targeted deletion of matrix metalloproteinase-9 attenuates left ventricular enlargement and collagen accumulation after experimental myocardial infarction. J Clin Invest. 2000;106:55–62. doi: 10.1172/JCI8768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jin DK, Shido K, Kopp HG, Petit I, Shmelkov SV, Young LM, Hooper AT, Amano H, Avecilla ST, Heissig B, Hattori K, Zhang F, Hicklin DJ, Wu Y, Zhu Z, Dunn A, Salari H, Werb Z, Hackett NR, Crystal RG, Lyden D, Rafii S. Cytokine-mediated deployment of SDF-1 induces revascularization through recruitment of CXCR4+ hemangiocytes. Nat Med. 2006;12:557–67. doi: 10.1038/nm1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rutella S, Pierelli L, Bonanno G, Scambia G, Leone G, Rumi C. Homogeneous expression of CXC chemokine receptor 4 (CXCR4) on G-CSF-mobilized peripheral blood CD34+ cells. Blood. 2000;95:4015–6. [PubMed] [Google Scholar]
- 37.Broxmeyer HE, Orschell CM, Clapp DW, Hangoc G, Cooper S, Plett PA, Liles WC, Li X, Graham-Evans B, Campbell TB, Calandra G, Bridger G, Dale DC, Srour EF. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005;201:1307–18. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liles WC, Broxmeyer HE, Rodger E, Wood B, Hubel K, Cooper S, Hangoc G, Bridger GJ, Henson GW, Calandra G, Dale DC. Mobilization of hematopoietic progenitor cells in healthy volunteers by AMD3100, a CXCR4 antagonist. Blood. 2003;102:2728–30. doi: 10.1182/blood-2003-02-0663. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.