Abstract
Herpes simplex virus type 1 and type 2 (HSV-1 and HSV-2) -specific CD8+ T cells that reside in sensory ganglia, appears to control recurrent herpetic disease by aborting or reducing spontaneous and sporadic reactivations of latent virus. A reliable animal model is the ultimate key factor to test the efficacy of therapeutic vaccines that boost the level and the quality of sensory ganglia-resident CD8+ T cells against spontaneous herpes reactivation from sensory neurons, yet its relevance has been often overlooked. Herpes vaccinologists are hesitant about using mouse as a model in pre-clinical development of therapeutic vaccines because they do not adequately mimic spontaneous viral shedding or recurrent symptomatic diseases, as occurs in human. Alternatives to mouse models are rabbits and guinea pigs in which reactivation arise spontaneously with clinical features relevant to human disease. However, while rabbits and guinea pigs develop spontaneous HSV reactivation and recurrent ocular and genital disease none of them can mount CD8+ T cell responses specific to Human Leukocyte Antigen- (HLA-) restricted epitopes. In this review, we discuss the advantages and limitations of these animal models and describe a novel “humanized” HLA transgenic rabbit, which shows spontaneous HSV-1 reactivation, recurrent ocular disease and mounts CD8+ T cell responses to HLA-restricted epitopes. Adequate investments are needed to develop reliable preclinical animal models, such as HLA class I and class II double transgenic rabbits and guinea pigs to balance the ethical and financial concerns associated with the rising number of unsuccessful clinical trials for therapeutic vaccine formulations tested in unreliable mouse models.
Keywords: Therapeutic Vaccine, Animal model, HSV-1, HSV-2, ocular herpes, genital herpes, CD8+ T cells
1. Introduction
Herpes simplex viruses (HSV-1 & HSV-2) are nearly ubiquitous pathogens that cause fatal disseminated disease in newborns to significant morbidity and mortality in adults ranging from skin lesion (cold sores), genital ulcerations, blinding eye keratitis, and encephalitis in immunocompromised individuals [1-4]. Most importantly, HSV-2 infected individuals are at higher risk (4 fold) of HIV-1 acquisition than HSV-2 non-infected individuals [5]. HSV-1 & HSV-2 establish latency in sensory ganglia followed by sporadic and spontaneous reactivations and recurrent shedding in mucocutaneous surfaces [3, 6-10]. The natural history of HSV-1 and HSV-2 infections is illustrated in Fig. 1. At present no FDA approved therapeutic vaccines are available for herpes. Although the standard antiviral drug regimens (e.g. acyclovir) reduce/suppress recurrent symptomatic disease, asymptomatic shedding, and lateral transmission to some extent; they do not clear the infection or stop recurrent disease [3, 4, 11-13].
Fig. 1. The natural history of genital, oro-facial and ocular herpes infections.
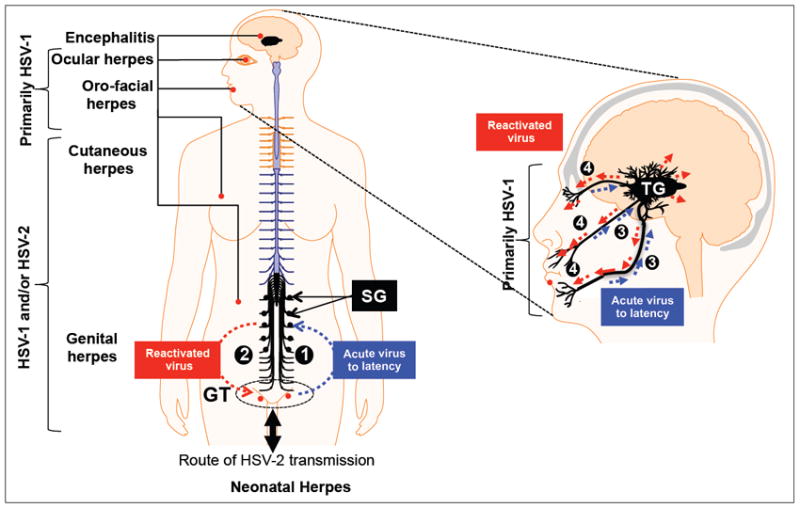
Left: (1) While most cases of genital herpes are caused by HSV-2, reports of HSV-1 genital infection are increasing. The virus is transmitted through the genital tract (GT) proliferate locally in this primary site of infection, enters the sensory nerves and travels along nerves to the lumbosacral dorsal root ganglia (sacral ganglia or SG) that innervate the GT, where it establishes a latent infection. (2). Sporadic spontaneous reactivation of HSV-2 from latently infected neurons leads to viral shedding in GT which can cause symptomatic recurrent genital herpes, one of the most prevalent sexually transmitted disease. Right: HSV-1 is transmitted through eyes, lips, mouth and cause orofacial and ocular herpes. (3) Ocular herpes is mainly caused by HSV-1, which infects the cornea and then establishes latency in sensory neurons of the trigeminal ganglia (TG). (4) Sporadic spontaneous reactivation of HSV-1 from latently infected neurons leads to viral shedding in saliva and tears which can cause symptomatic recurrent Herpes Stromal Keratitis (HSK), a blinding corneal disease.
Reliable animal models of herpes infection and disease are the key to the development of an effective therapeutic vaccine against HSV-1 and HSV-2. Because of the obvious ethical and practical considerations in assessing candidate herpes therapeutic vaccine directly in humans, a critical question is: which species would be the most appropriate animal model to investigate the protective efficacy of the growing numbers of therapeutic vaccine candidates? For most immunologists, mouse is the preferred model due to the availability of (i) unlimited inbred and transgenic strains, (ii) specific immune molecule knockout strains, and (iii) the well-characterized immunological probes to study the immune response to specific therapies. In that perspective, the majority of preclinical studies are evaluating HSV vaccine efficiencies on mouse models. Unfortunately, in contrast to humans, recurrent HSV shedding and recurrent herpetic disease does not occur in mice because HSV spontaneous reactivation is either extremely rare or does not occur in mice [14]. Therefore, although mouse studies have provided ample crucial information regarding immune response against primary HSV-1 and HSV-2 infections[15-19], the efficacy of human epitope-based therapeutic vaccines against recurrent shedding and disease cannot be assessed in mice. Several clinical trials have been carried out based on mice data, but almost none led to clinical success. HSV infection in the murine model preclude its use to study horizontal or vertical transmission or to evaluate strategies designed to prevent reactivation. The lack of an accurate animal model that translates human immune responses and diseases, certainly limits the proper preclinical assessment.
More than two third of the world’s population is infected with HSV-1 & HSV-2, and many people suffer from psychological problems related to herpes [20] even though multiple therapeutic approaches including topical and/or oral administration of anti viral drugs have been available for more than 75 years. While, a majority of human population is infected with HSV-1 and/or HSV-2, more than half of them do not show any clinical symptoms (asymptomatic individuals). Thus, the immune systems of those individuals are likely capable of controlling the clinical symptoms, but cannot prevent viral shedding. Therefore, a therapeutic HSV vaccine, which can boost the immune system of both asymptomatic and symptomatic individuals to decrease the viral shedding, transmission and disease, is highly desirable. In an ideal herpes animal model: (i) the infection would be initiated via a mucocutaneous route similar to that by which humans are commonly infected, i.e., inoculation of the ocular, oro-facial or genital mucosal epithelium; (ii) a small proportion of the animals would develop spontaneous disease symptoms similar in both pathology and severity to those seen in the minority of symptomatic humans, (iii) a large proportion of the animals, would develop immune responses after infection that would protect the animal from the disease, similar to those seen in the majority of asymptomatic humans. In these “asymptomatic” animals the immune response can be scrutinized to determine which aspects are important for protection, including innate and adaptive immune responses. The “symptomatic” animals, which cannot spontaneously control the pathogen, provide an opportunity for the investigation of potential protective therapeutic immunization. In this review, we will talk about the advantages and limitations of different animal models for herpes infection and disease that has been used in preclinical development of therapeutic vaccines. We will also provide a summary with key issues involved in the pre-clinical selection of new vaccines for clinical trials.
2. CD8+ T cells decrease HSV reactivation
Increasing neutralizing antibody titers in herpes clinical vaccine trials did not reduce viral reactivation; shedding and recurrent disease suggesting the necessity of cellular immunity [21-23]. (1) In mice, CD8+ T cells accumulate in sensory ganglia from 7-10 days following herpes infection and become the predominant T-cell type during latency [24]. HSV-specific CD8+ T-cells producing IFN-γ and GrB appear to suppress (or abort) induced viral reactivation in explanted mouse sensory ganglia [24, 25]. It has been proposed that CD8+ T-cells may similarly reduce detectable HSV reactivation in vivo [26-29]. If correct, a human therapeutic vaccine that increases HSV-specific IFN-γ(+) and GrB+ CD8+ T cells in latently infected sensory ganglia should significantly decrease HSV spontaneous reactivation (as measured by shedding in tears) and reduce recurrent eye disease, which is dependent on virus reactivation. (2) Significant numbers of activated CD8+ T cells producing IFN-γ and TNF-α, were found in latently infected TG of human cadavers indicating the existence of an antigen-driven immune response [30-32]. While HSV-specific CD8+ T-cells appear to be the key factor to protect spontaneous reactivation and recurrent herpes, a challenge for herpes immunologists is: “To find an appropriate preclinical animal model to study the efficacy of human HLA-restricted T cell epitopes based vaccine to decrease recurrent herpes disease and spontaneous viral reactivation?” The ideal animal model should able to produce an immune response specific to human HSV epitopes (such as HLA-A*0201-restricted epitopes), while mimicking all aspects of viral pathogenesis, neuropathology, neuro-invasiveness, and latency that occur in human.
3. The mouse model lacks spontaneous HSV-1 and HSV-2 reactivation
The mouse is the premier mammalian system used for modeling human disease and understanding the immune mechanism of specific genes in normal and diseased states. However, in the case of HSV-1 and HSV-2, sporadic spontaneous reactivation and recurrent disease do not occur in mice. During primary herpes infection, the virus: (i) infects mucocutaneous surfaces of skin, mouth, genital tract, and eyes, (ii) invades the local sensory nerves by propagating via neurons, and (iii) establishes life-long latency in the neuron bodies of sensory ganglia (sacral ganglia for genital herpes and trigeminal ganglia for ocular and oral herpes). Factors such as hormonal changes, stress, and ultraviolet (UV) exposure trigger virus reactivation. The reactivated viruses travel back to re-infect the primary site of infection (i.e. the skin, the mouth, the genital tract or the eyes). A representative diagram of HSV infection, development of latency and the subsequent viral reactivation are shown in Fig. 2. A good example of recurrent herpes disease is Herpes Stromal Keratitis (HSK), an inflammatory disease of the cornea, which may result in corneal opacity and blindness. HSK in human occurs long after the primary infection, when reactivated virus returns to the eye from latency. The destructive consequences of ocular HSV-1 infection appear to be immune related [33-42]. The immune system of the infected individual but not the virus causes the majority of corneal damages [43].
Fig. 2.
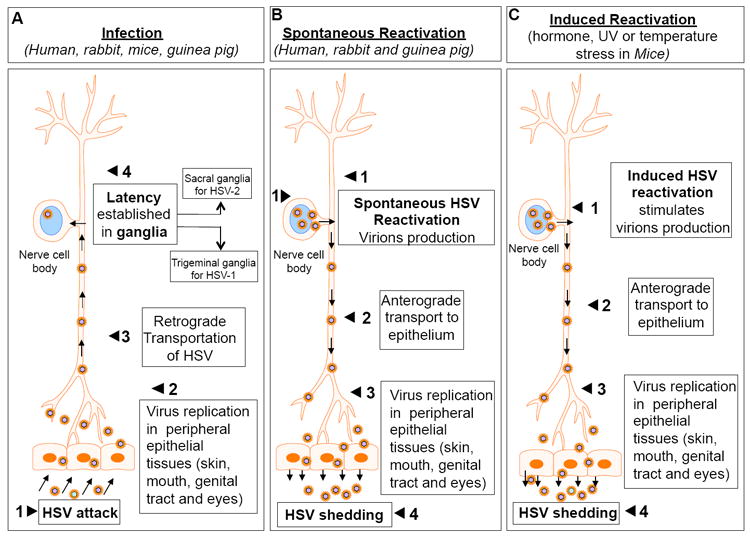
A simple step-by-step representation showing the route of HSV attack (A), establishment of latency (A), spontaneous reactivation (B) and hormone induced reactivation (C). Panel B shows the “spontaneous reactivation” of HSV from latency and the path of anterograde transport from nerve ganglia to primary site of infection. Panel C shows the hormone induced “artificial reactivation” of HSV from latency and the path of anterograde transport from nerve ganglia to primary site of infection. Spontaneous HSV reactivation in mice is a rare event. Statistically, about 1/100 eyes or less shows “spontaneous reactivation” upon a period of extended sampling. Also <1% of TG have infectious virus but too low for useful analysis. Using RT-PCR 1 neuron/10 TG has lytic transcripts detectable.
HSV-1 infection in mice eye can lead to a localized infection followed by infection of sensory neurons of the trigeminal ganglia (TG) and virus spread in the central nervous system (CNS) and ultimately to the brain. Mice survived from lethal HSV-1 infection, maintain latency mainly in the sensory nerve of TG. During this latent infection period, no infectious virus can be recovered from the nerve tissue but if the nerve tissues are explanted and maintained on “feeder layer” of cultured cells, virus will eventually appear to replicate. This observation indicates that the viral genome remains intact during latency. However, evidence of spontaneous reactivation of HSV-1 does not occur in mice [14] (Fig. 2c). In 2002 Feldman et al and in 2006 Margolis et al showed the episode of HSV-1 spontaneous “molecular reactivation” from latency in mice TG [44, 45]. However, the amount of infectious virus they detected in latently infected TG was at the lower limit of the sensitivity. Statistically, about 1/100 TG or less shows “spontaneous reactivation” upon a period of extended sampling and RT-PCR able to detect only 1 neuron/10 TG with lytic transcripts [45-48]. It is possible that the mouse model is deficient in effective transport of the virus to the periphery. Besides that, the systemic immune responses (passively transferred neutralizing antibody, adoptively transferred CD8+ T cells) do protect mouse eye, but not human eye, against ocular HSV-1 disease. Therefore, while the mice model is quite useful to study the genetic parameters during latent infection, the physiological process of reactivation cannot be effectively studied in mice. Hence, mice cannot be used to assess the efficacy of candidate therapeutic vaccines against spontaneous virus reactivation. The “adaptation” of the “defense mechanism” and “pathogenic mechanism” of herpes virus in mouse models are more effective than human. For instance, it has been proposed that immune responses to ocular herpes infection in susceptible 129 strain mice lead to central nervous system immunopathology and fatal encephalitis [49-51]. Whether, the mechanism reported in these models applies to humans is yet to be proven.
A hallmark statement made by a herpes immunologist, that the validity of HSK mouse models “relies on the assumption (however great) that similar events occur in the human condition” [52]. In mice, HSK is a one-time event, which initiates within ~14-21 days of the primary ocular infection. Initial ocular infection is followed by leukocyte infiltration, virus clearance, and a second phase of leukocyte infiltration and chronic inflammation in the absence of live virus [19, 53]. Thus mouse HSK is a (delayed) response to the acute (primary) ocular infection rather than a recurrent disease due to return of reactivated virus from the TG. In contrast, human HSK can recur numerous times over decades, associated with reactivation of HSV-1 from latency, and thus appears to be a truly recurrent disease. In addition, mouse T cells do not recognize human leukocyte antigen- (HLA-) restricted epitopes [27] and therefore the immunogenicity and protective efficacy of human T cell epitope-based vaccine and immunotherapy cannot be assessed in normal strains of mice. This problem can be solved by using Human leukocyte antigen (HLA) transgenic mice that develop T-cell responses against HLA-restricted T cell epitopes [15-19]. In fact, we have recently used HLA transgenic mice to study the immunogenicity and protective efficacy of several HLA-A*020-restricted epitopes against primary HSV-1 infection and disease [17, 54]. Nonetheless, the HLA transgenic mice model still do not resemble the human scenario and cannot be used to assess therapeutic efficacy of HLA-restricted human T cell epitopes.
Additional disadvantages of mouse model are: (i) Disparity in appearance of mouse vs. human disease by clinical exam; (ii) HSV-1 Ag is not detected in mouse cornea during HSK whereas viral Ag has been detected in 74% of human HSK cornea although the specific Ag(s) remain unknown. The detection of viral Ag is higher in those corneas that have current active episodes of HSK [55]; (iii) In mice, HSK can only be induced by molecular mimicry [56-58] but in human it is a natural event [19]. These differences make mouse a non-reliable experimental model to evaluate and relate to human HSK. The severity of HSV-1 corneal disease in mouse model is affected by (i) host strain, (ii) virus strain and (iii) host immune system. It is well documented that strains of inbred mice differ in their susceptibility to corneal HSV1 infection [59, 60]. For example, while C57BL6 mice being most resistant, DBA/2 mice being most susceptible, and BALB/C mice being intermediate. This order of resistance matches the resistance to latency and susceptibility to encephalitis [61, 62]. Thus, although mouse studies have provided plenty of useful and important information regarding ocular HSV-1 infection and despite the tremendous amount of knowledge gained about mouse immunology in general, the mouse is not an ideal model to extrapolate the mechanisms of recurrent-HSK in humans.
The mouse genital infection model is derived from Parr et al. [63-67] where female mice are treated with medroxyprogesterone to thin the genital epithelium lining and make the mice uniformly susceptible to HSV-2 vaginal infection. However, spontaneous reactivation of the virus from sensory ganglia and shedding of the virus in genital tract do not seem to occur in mice (as opposed to guinea pigs and humans) [68]. Therefore, the potential effect of vaccination against HSV recurrences in mice can only be inferred from the levels of latent viral DNA within neurons [69], since these levels have been shown to correlate with the number of ex vivo recurrence rates in mice [70-74].
4. Rabbit model closely mimics human ocular HSV-1 infection and disease
In contrast to mice, rabbits are relatively better choice for studying ocular HSV-1 infection, disease and immunity. Similar to humans, rabbit can develop primary ocular disease, undergoes latent infection and establishes life-long latency in the neuron of TG (Fig. 2A & B). Rabbits, HSV-1 intermittently reactivates from latently infected primary sensory neurons, leads to peripheral shedding of infectious virus and recurrent corneal disease similar to humans [75]. Increasing evidence suggests that in most humans, HSV sheds spontaneously (~35% of the time for HSV-1 and 15% of the time for HSV-2) [76-80]. In rabbit ocular model, HSV-1 sheds through tears both spontaneously and in response to local or systemic stimuli [81-83]. Certainly, this pattern of viral reactivation in rabbit closely resembles that in humans. In addition, most HSV-1 strains cause acute ocular infection, latency in TG and spontaneous shedding in rabbit. Many researchers use rabbit as a recurrent model of HSV1 infection and eye disease bearing in mind that: (i) numerous similarities exist between rabbit and human ocular mucosal immune system [84-86]; (ii) several T-cell-mediated ocular diseases, including herpetic conjunctivitis and recurrent corneal herpetic stromal keratitis (HSK), have been reported to be similar in rabbits and humans [87-89]; (iii) compared to mice, rabbit conjunctiva associate lymphoid tissue (CALT) resembles human CALT [90-93]. Microanatomy and immuno-histological studies indicate that rabbit conjunctival mucosa is comparable to that of humans and has a typical follicular ultra-structure with an abundance of “conjunctival lymphoid follicles” (CLF), whereas no lymphoid tissue was identified in mice [93-97]; (iv) from a practical standpoint, rabbits possess a relatively large cornea and conjunctival surface offering abundant mucosal associated lymphoid tissue for in vitro studies; (v) finally, the recent availability of many monoclonal and polyclonal antibodies specific to rabbit immune cell CD markers, cytokines and growth factors provide useful immunological tools for an unprecedented phenotypic and functional analysis of rabbit T cell repertoire and function.
5. Circulating HSV-specific CD8+ T cells do access HSV-1 infected trigeminal ganglia in mice and rabbits
We have reproducibly demonstrated that circulating HSV-1 CD8+ T cells induced following parenteral and mucosal immunizations can efficiently migrate from the periphery to sensory ganglia and protect from acute infection following an ocular challenge in mice [13, 98-101] and rabbits [102]. An important goal of a therapeutic HSV-1 vaccine would be to enhance HSV-specific CD8+ T cells population that would migrate to latently infected TG and stop or reduce virus reactivation. Whether the size and the function of CD8+ memory T cell pool can be increased in relevant animal models of spontaneous latency/reactivation and humans following therapeutic vaccines remains to be determined. Mice can be infected with HSV via several routes including footpad, flank, ocular, intravaginal, and each route has its own unique properties. HSV infection at any of these sites initiates with local viral replication, which leads to the development of lesions, establishment of latent infection within sensory neurons and persistence of latent viral DNA within local sensory ganglia and in some cases, central nervous disease. Mice can certainly mount CD8+ T-cell responses in TG following acute infection, but spontaneous reactivation of HSV-1 and recurrent corneal disease are either rare or absent making the mouse an unreliable model to assess therapeutic vaccines [14]. While spontaneous reactivation of the virus from sensory ganglia and shedding of the virus in tears or in genital tract do not seem to occur in mice (as opposed to rabbits, guinea pigs and humans), reactivation of HSV from latent infection is readily observed in vitro when ganglia are explanted in culture [68]. HSV reactivation in mice can be induced to a limited extent by ultraviolet irradiation [103] or elevated body temperature or hormone [104, 105]. A recent study used an artificial restraint “stress” mouse model of virus reactivation [106]. In this model, mice are subjected to stress by restraining them in aerated plastic tubes for 12 hrs and depriving them of food and water for the same period of time. If this condition is translated to humans, it would be equivalent to a 12 hours stress caused by a “9-scale earthquake” similar to what recently happened in Japan. Obviously, this protocol of extreme and acute stress does not represent the daily “physiological” stress situations in humans (be that a physical or chemical stress). Interestingly, the authors recognized that “Exposure of HSV-1 latently infected mice to restraint stress diminishes the TG-resident CD8+ T cell population, compromises the function of the remaining CD8+ T cells [107-110], and induces HSV-1 reactivation from latency [111].” The study also claimed that exposing latently infected mice to restraint stress and treating them with TAK-779 blocked CD8+ T cell migration from blood to TG and conclude “Our findings suggest that augmenting the number of circulating HSV-specific CD8+ T cells is not sufficient to bolster the HSV-specific memory T cell population in latently infected sensory ganglia”. One cannot expect CD8+ T cells to migrate from the blood to latently infected TG if the number of these same CD8+ T cells is reduced in the first place, in both the blood and TG following the restraint stress itself [107-110]. Therefore, the mouse may not represent an appropriate animal model to investigate whether therapeutic vaccines will/or will nor boost the number and function of CD8+ T cells in the TG that would reduce spontaneous virus reactivation.
6. Human Leukocyte Antigen (HLA) transgenic rabbits: a “humanized” animal model that develops spontaneous recurrent ocular herpes and responds to human T cell epitopes
HLA transgenic rabbit model of ocular herpes has been proposed recently in our laboratory [112, 113]. Like their wild type counterparts, “humanized” HLA-A*0201 transgenic rabbits: (i) develop acute corneal disease; (ii) develop spontaneous reactivation; (iii) produces recurrent HSK, which is clinically similar to human HSK. In addition, “humanized” HLA-A*0201 transgenic rabbits (i) expresses human HLA class I molecules. One major component of the rabbit immune system is replaced by the identical component taken from human counterpart (i.e. HLA-A*0201 class I molecules) [112]; (ii) recognize and mount immune responses to HSV-1 human CD8+ T cell epitopes [114]. (iii) Although the state of the art in rabbit immunology still lags behind that of the mouse and human, several monoclonal and polyclonal antibodies specific to rabbit CD markers, cytokines, chemokines, and growth factors are now commercially available. Anti-rabbit CD8+ T cell mAbs and human tetramers have proven success to analyze HSV-specific CD8+ T cell infiltrates in TG and conjunctiva of acutely infected HLA Tg rabbits [12].
Taken together, the striking similarities between HLA Tg rabbit and human in terms of HSV-1 infection and immunity suggests that the HLA Tg rabbit is a preferred model to study the role of “symptomatic” vs. “asymptomatic” CD8+ T cells in exacerbating and controlling spontaneous HSV-1 reactivation and recurrent HSK. To our knowledge, this HLA Tg rabbit model is the only animal model with spontaneous HSV-1 reactivation that can develop “humanized” CD8+ T cell responses to human HSV epitopes [114]. This HLA Tg rabbit model will allow us for the first time to investigate the role of human specific CD8+ T-cells in reducing HSV-1 spontaneous reactivation. It will now allow for the first time to test the hypothesis that a therapeutic vaccine encompassing asymptomatic human CD8+ T-cell epitopes from frequently recognized HSV-1 antigens (such as ICP-0, ICP-4, gB, gD, VP11/12, and VP13/14) can decrease the effects of spontaneous reactivation (virus shedding in eyes and recurrent herpetic eye disease). A schematic diagram of HSV-1 spontaneous reactivation from the TGs and the possible role of “asymptomatic” CD8+ T cells (specific to human “asymptomatic” HSV-1 CD8+ T cell epitopes) in decreasing ocular herpes infection and disease in HLA-Tg rabbit are shown in Fig. 4. In addition, the availability of an HSV-1 mutant virus (CJLAT) that produces high levels of recurrent HSK in rabbits (up to 70% of eyes, compared to ~1-2% of wild type HSV-1 infected eyes) [115, 116] will allow for the first time to assess the therapeutic efficacy of human CD8+ T-cell epitope-based vaccines against recurrent HSK.
Fig. 4.
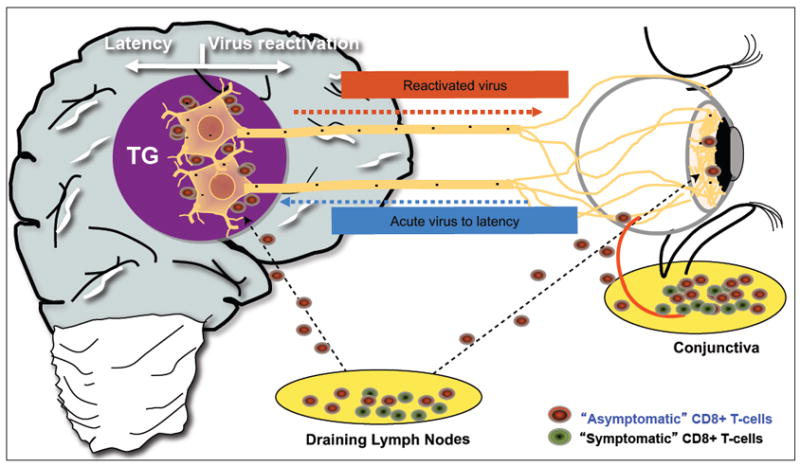
A schematic representation showing the possible role of asymptomatic CD8+ T cells (specific to human HSV-1 CD8+ T cell epitope) in decreasing ocular herpes infection and disease in the newly identified HLA-A*0201 transgenic (HLA Tg) rabbit model of spontaneous reactivation, recurrent corneal disease. Unlike what happens in the mouse model, in the rabbit model, similar to humans, circulating HSV-1-specific CD8+ T cells do access HSV-1 latently infected trigeminal ganglia where they encounter newly made antigens made following spontaneous reactivation of the virus from latency.
A recent publication by Chentoufi et al. from our laboratory [112] showed that HSV-1 (McKrae) infected HLA Tg rabbits recognize human CD8+ T cell epitopes from HSV-1 gD. The frequencies of CD8+ T-cells specific to a panel of 10 potential HLA-A*0201-restricted human gD epitopes in the draining lymph nodes (DLN) of HLA Tg rabbits have been measured by standard HLA-A*0201 tetramer-staining assays. In parallel, the frequencies of CD8+ T-cells specific to the same 10 peptide panel in the PBMC of HSV-1 asymptomatic HLA-A*0201(+) human individuals are also compared. Three gD epitopes (gD53-61, gD70-78, and gD278-286) are highly recognized by both humans and HLA Tg rabbits but not by non-transgenic (wild type New Zealand rabbits). These results conveyed the message that HLA-A*0201 Tg rabbits and HLA-A*0201(+) human individuals share similar CD8+ T-cell responses when the epitopes were presented by the same HLA-A*0201 molecule. This highlights the potential of HLA-A*0201 Tg rabbits as a valuable animal model for the pre-clinical assessment of human HLA-A*0201-restricted epitope-based therapeutic vaccines.
When HLA Tg rabbits latently infected with wild type HSV-1, only 2% of rabbit eyes develop HSK which is virtually identical to human HSK by clinical exam. Interestingly, HSV-1 mutant (CJLAT), which was constructed from HSV-1 strain McKrae, showed significantly higher spontaneous reactivation rate than other HSV-1 viruses [117]. Over 70% of CJLAT-infected rabbit eyes develop severe HSK [115] which are indistinguishable from human HSK. HSK in CJLAT infected rabbits are evidenced by: (i) Clinical exam [115] (ii) histopathology [115]; (iii) Confocal microscopy. Therefore, the CJLAT-infected HLA Tg rabbit model would represent a realistic animal model for systematic study of recurrent-HSK.
7. Guinea pig model closely mimics human genital HSV-2 infection and disease
The guinea pig represents the gold standard and the most clinically relevant animal model for evaluation of therapeutic vaccine candidates against genital herpes caused by HSV-2 infection [118-120]. Many clinical and pathologic features of acute and recurrent genital disease of guinea pigs inoculated with low doses of HSV-2 are similar to those seen in human infection. Intravaginal inoculation of HSV-2 into guinea pigs leads to acute replication and disease at the site of infection, establishment of a latent viral reservoir within the innervating sensory neurons, and periodic reactivation leading to viral shedding and even recurrent clinical lesions [73, 74, 121, 122]. The development of both acute and recurrent disease provides the opportunity to test both the prophylactic and the therapeutic effects of potential vaccines in guinea pig model. Since late-1980s, the guinea pig model of genital herpes has allowed investigators to evaluate several vaccine and immunotherapeutic strategies for the control of recurrent herpes virus infections [123]. The guinea pig model is useful in testing therapeutic vaccine candidates against recurrent HSV infection for several reasons [124]. (i) This animal model mimics human infection, with self-limited primary vulvovaginitis developing after inoculation with HSV. (ii) Despite a full range of host immune responses, guinea pigs infected intravaginally with HSV-2 develop primary genital disease and establish latency in sacral ganglia (SG) (Fig. 2A & B). After recovery from primary illness, animals exhibit both spontaneous and UV induced recurrent disease, and establish latent infection in the lumbosacral dorsal root ganglia [125]. (iii) Periodic reactivation from latency produces recurrent genital infections, similar to human genital herpes. The infectious virus is usually detected in the genital tract along with high levels of recurrent genital disease [126, 127]. However, the disadvantages are the lack of inbred strains and limited availability of immunologic reagents to follow the immune responses. Due to the current paucity of immunological reagents for the guinea pig, the full range of immune effector mechanisms cannot be determined [73, 74, 128-130]. In addition, guinea pig T cells do not recognize human leukocyte antigen- (HLA-) restricted T cell epitopes and therefore the immunogenicity and protective efficacy of human T cell epitope-based vaccine and immunotherapy cannot be assessed in guinea pigs. The development of HLA transgenic guinea pig would be able to generate T-cell responses specific to HLA-restricted T cell epitopes. Moreover, with the development of defined immunologic reagents, this model should prove useful for exploring the immune responses that are important in the control of primary, latent, and recurrent HSV-2 infection and disease in humans.
Lack of a HLA transgenic guinea pig model has been a significant impediment in the development therapeutic CD8+ T-cell based vaccine against genital herpes. The development of a “humanized” guinea pig model using a transgenic approach has long been awaited. The technical challenges including: (i) a longer gestation time (60–75 days) relative to that of mice (20–30 days); (ii) a smaller average litter size (4 vs. ≥7); (iii) a limited number of litters/ year (5 vs. 10); (iv) a difficulty in obtaining fertilizable eggs; and (v) the unavailability of embryonic stem cells, certainly obstruct the progress in making transgenic guinea pigs. In efforts to address these issues, our lab is applying novel transgenic technologies, such as physical and chemical delivery methods of naked plasmid DNA directly to spermatogonium, to develop a guinea pig model carrying HLA class I and class II (HLA-A*0201 and HLA-DR) genes. A transgenic guinea pig over-expressing the human HLA-A*0201 and HLA-DR would serve as a valuable research model to test the efficacy of T-cell based therapeutic human vaccines against genital herpes. Cotton rat as an alternative model for HSV-2 infection and disease has been proposed by others to test the efficacy and safety of candidate therapeutic vaccine [131]. The salient feature of this model is that, unlike mice, vaginal infection does not require hormonal manipulation. Primary infection of cotton rat is associated with spread of virus to other organs such as the liver. Rats recover from primary infection are prone to spontaneous clinical recurrences that mimic human disease and infection. Additional advantages include the availability of immunological reagents to study the immune response to HSV in rats.
8. Protective immune mechanisms against herpes in various animal models versus human
The herpes studies on mouse model have absorbed decades of research and considerable amounts of resources, yet cannot guarantee the efficacy of vaccines in humans. Some advantages and disadvantages of preclinical animal models used for the evaluation of herpes therapeutic vaccine study are listed in Table I. Fig. 3 illustrates the HSV infection-reactivation cycle and the steps where a therapeutic vaccine can be targeted to interrupt virus cycle. Immunization with gD induces full protection against primary herpes infection and disease in various mouse models [132-134]. However, therapeutic immunization with gD induce a 74% protection against genital herpes in HSV-1 seronegative women, while failing to induce significant protection in men [135]. The reason for this partial protection and the immune mechanism behind it remains to be elicited. Usually, human individuals need higher number of doses to elicit protection. For example, while transient moderate protection was inconsistently achieved after 3 doses of vaccination lasting over 6 months in humans, often a single dose of vaccination is sufficient to induce strong protection in mice [133, 136]. Immunization schedules that are effective in mice (either BALB/c or C57BL/6) failed to protect rabbits and guinea pigs [137]. A series of 2 immunizations with gB or gD provided protection in only a fraction of immunized rabbits and guinea pigs and did not prevent death [138, 139]. Unlike mice, both rabbits and guinea pigs are extremely difficult to protect [138, 140, 141]. Rabbits and guinea pigs are exquisitely susceptible to infection since < 102 pfu could induce ocular and genital infection respectively [138, 139]. Rabbits and guinea pigs develop latent infection and shedding of virus at low density [138]. The same is observed in humans. In contrast, in mice, high virus loads and death are the most frequent outcome following herpes infection [133, 136]. The similarity of herpes infection between rabbits, guinea pigs and humans appears as a valuable advantage, as compared to mouse models. Therefore, the mouse model of herpes infection is quite different from human/ rabbit and guinea pig in terms of the spontaneous herpes reactivation issue (Fig. 2A, 2B & 2C). This suggests that a distinct pattern of protective immune mechanisms might play in mouse. This is well illustrated in the recent history of success of pre-clinical herpes vaccine using mouse models that were failed in clinical trials [4]. Initially it was thought that the protection mechanism was operated by the induction of virus neutralizing antibodies (based on mAb passive transfer experiments in mice) rather than by the induction of HSV-specific CD8 T cells. Later reports however have claimed IFN-gamma-producing and granzyme B (GrB) positive CD8+ T cells as a critical component of defense [24, 142]. Recent pre-clinical and clinical vaccine studies clearly suggest that the immune mechanisms of protection in murine models differ from those in humans.
Table I.
A list of advantages and disadvantages for preclinical animal models generally used for herpes vaccine research.
| Animal | Advantages | Disadvantages |
|---|---|---|
| Mouse |
|
|
| Guinea Pig |
|
|
| Rabbit |
|
|
| Cotton Rat |
|
|
Fig. 3.
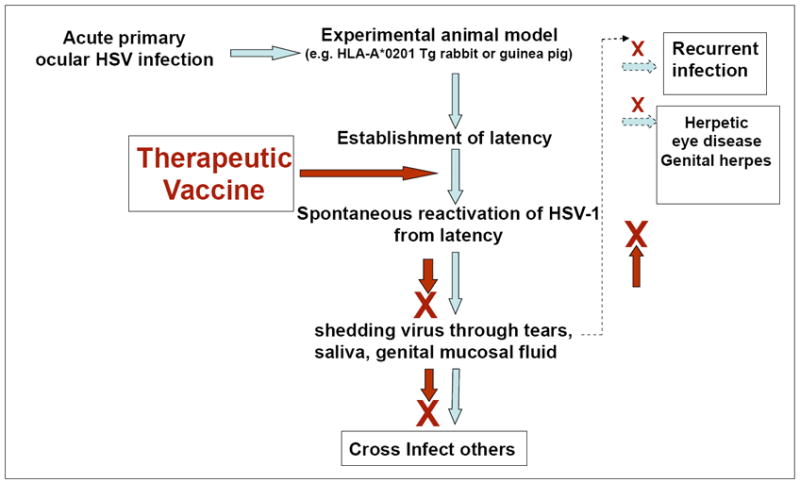
Schematic flow chart representing the HSV infection-reactivation cycle and the potential targeted step where therapeutic vaccine should intervene this cycle. The blue arrow indicates infections, latency, spontaneous reactivation, viral shedding, recurrent infection and eye disease. The brown arrow and the brown cross -indicated inhibition of spontaneous reactivation, viral shedding, recurrent infection and eye disease.
Since we have limitations to study the human immunity against herpes, we have to rely on the best fitting animal models that reflect the desired pattern of immune responses. Table II provides the list of all experimental animal models used so far for HSV infection. Models are considered valuable until the test results have been ascertained using the same virus in its natural host. The first and fore most criteria of any successful vaccination study at the pre clinical level is to choose the appropriate animal model to demonstrate the safety, immunogenicity, and protective efficacy of the candidate therapeutic vaccine. Table III offers a recent update of preclinical animal models used for herpes vaccine research. We recently uncover that the HLA-Tg rabbits are the candid animal model for studying therapeutic vaccines against HSV-1 mediated ocular infection and disease. In the same note, we anticipate that HLA transgenic guinea pig would be the other perfect model to study the recurrent genital herpes shedding and disease. However, HLA transgenic guinea pig model is not currently available but the concept certainly could be a future objective to study recurrent genital herpes.
Table II.
Current list of experimental laboratory animals and non-human primates used to study HSV-1 and HSV-2 infection and disease.
| Experimental animals used for HSV infection
| ||||
|---|---|---|---|---|
| Animals | HSV-1 | HSV-2 | Route of infection | Disease |
| Mice | HSV-1 (all strains) | HSV-2 (all strains) | All possible routes | |
| Cotton Rat | N/A | HSV-2 (strain G) | Topical through vagina | Genital herpes |
| Wister Rat | HSV-1(strain F & 16) | N/A | Topical through eyes | |
| Wister Rat | HSV-1 (strain F) | Intra nasal | ||
| Lewis Rat | HSV-1 strain McKrae) | Topical through eyes | ||
| Rabbit | HSV-1 (strain McKrae) | N/A | Topical through eyes | Herpetic keratitis |
| Guinea Pig | N/A | HSV-2(strain 17) | Topical through vagina | Genital herpes |
| Zebra fish* | HSV-1 (strain KOS) | N/A | Intra Peritonial | Central nervous system |
|
| ||||
| Non-human Primates | ||||
| Rhesus Macaques | N/A | HSV-2 (strain G) | ||
| Owl Monkey** | HSV-1 (strain F) | Intracerebral injections | ||
Zebrafish. 2008, Burgos JS;
Gene Ther. 2003, Deisboeck TS
Table III.
A recent update summarizing the list of preclinical animal models used to study the efficacy of herpes vaccine in treating primary ocular, primary genital and recurrent genital infections.
| Recent Preclinical animal models used for herpes vaccine study | |||||
|---|---|---|---|---|---|
| Animal | Transgenic | Virus | Ocular/genital | Infection | Outcome |
| Mice1 | RAG2(-/-) gamma (-/-) | HSV-2 | Genital | Primary | 1. Protective innate immune response |
| 2. Reduced viral replication in genital tract | |||||
| Rabbit2 | HLA-A2 | HSV-1 | Ocular | Primary | 1. Reduced HSV-1 replication in tears |
| 2. Reduced corneal eye disease | |||||
| Guinea Pig 3 | Wild type | HSV-2 | Genital | Primary | 1. Less genital skin disease |
| 2. Reduced viral replication in genital tract | |||||
| Guinea Pig4 | Wild Type | HSV-2 | Genital | Recurrent | 1. Reduced recurrent genital lesions |
Kwant-Mitchell, A. 2009, J. Virol;
Chentoufi, AA. 2010, J. Immunol.;
Fotouhi, F. 2008, FEMS Immunol Med. Microbiol.
Rose, WA 2nd, 2008, Int. J. Antimicrob Agents
9. Pre-clinical investigation of viral vectors for prime-boost based therapeutic ocular herpes vaccines in rabbits
The concept of using an unrelated recombinant virus to deliver viral antigens for the purpose of therapeutic vaccination was developed more than 25 years ago [143]. In 1983, a vaccinia virus (VACV) recombinant for the hepatitis B surface antigen (HBsAg) was the first viral vector shown to successfully induce protective immunity against HBV [143]. Since then, a number of unrelated viral-vector vaccines using adenovirus, HSV, vaccinia virus, retrovirus, New-castle disease virus, vesicular stomatitis virus, measles virus, poliovirus and West Nile virus, have been designed and tested in both preclinical and clinical trials (reviewed in [144]). Among the advantages of adenoviral vectors are: (i) their natural tropism for mucosal surfaces, which makes them ideal for the purpose of mucosal vaccination against HSV-1 ocular infection for which parenteral immunization strategies have failed to confer protection [145-147]; and (ii) their remarkable ability to significantly boost T cell responses that were primed by a sub-unit vaccine (e.g. DNA and peptides) [148]. Recombinant rAd5 vector-based vaccines have been used successfully in rabbit eyes [149], and thus are suitable for the prime-boost pre-clinical studies against ocular (in rabbits) and genital (in guinea pigs) herpes. Currently there are six active clinical trial studies in the US that use rAd5 vector-based vaccines for non-herpes conditions (ClinicalTrial.gov. To minimize the potential rAd5-induced keratoconjunctivitis in rabbits, we have recently constructed replication incompetent rAd5 vectors expressing human “asymptomatic” CD4+ and CD8+ T cell epitopes from gB and gD (BenMohamed. in preparation). While the replication incompetent rAd5 is a promising clinical vaccine vector (reviewed in [148, 150, 151]), safety and pre-existing immunity are still concerns, and may require adoption of additional measures: (1) Of the 58 different human adenovirus (Adv) types belonging to species A-G, Adv8, -19, -37, -53 -54 and -56 (all within species D) are associated with acute keratoconjunctivitis in humans [152-159]. Some adenovirus strains present specific tropism to the eyes others are restricted [160, 161]. While the majority of serotypes utilize coxsackievirus-adenovirus receptor (CAR) as their primary attachment receptor, the subgroup D Adv19 and -37 associated with epidemic keratoconjunctivitis in humans use CD46 receptor (known as membrane cofactor protein) [162-164]. Adv37 also uses sialic acid linked to galactose via alpha2,3 glycosidic bonds as a cellular receptor [160]. In rabbits eyes, subgroup C (Adv1, -2, -5, and -6) appeared to be the most successful as vectors [165]. Topical ocular delivery of live Ad5, uses CD46 receptor that is ubiquitously expressed by a variety of cells from rabbit ocular surface [162-164], and may induce keratoconjunctivitis [161, 166]. Therefore, caution may be necessary when applying rAdv5 to rabbit eyes. (2) In addition, the majority of adults in developed world have inherent systemic Ad5 nAbs that may dampen its high transduction efficiency and immunogenicity when delivered parenterally [167, 168]. Replication-incompetent rAd5 vectors elicit potent T-cell responses in humans, but they have been shown to be substantially less immunogenic when administered intramuscularly to seropositive individuals who have preexisting anti Ad5 nAbs [168]. However, such limitation may not apply when the topical ocular route is used to deliver the rAd5 vaccine [169]. Indeed, pre-existing systemic anti-Ad5 nAbs did not seem to block transgene expression in the eye [149, 170-173]. Moreover, repeated ocular or mucosal administrations of rAd5 vectors result in efficient transgene delivery, expression and immunogenicity, even in the presence of preexisting systemic anti-Ad5 nAbs [169, 174-178]. Together, the above reports suggest that even with preexisting systemic anti-Ad5 nAbs, topical ocular mucosal route in HLA transgenic rabbits may still induce strong local HSV-specific CD8+ T cell responses. Once a replication incompetent rAd5 vector-based vaccine induced immunity in test-of-concept pre-clinical studies in humanized HLA Tg rabbits, future rAd vector-based clinical vaccines will be constructed using a vector derived from an Ad serotype that is rare in the developing world and distinct from Ad5, such as Ad26 and Ad35. This may effectively circumvent the problem linked to pre-existing anti-Ad5 nAbs. Those clinical studies will also very likely consider heterologous rAd35/rAd26 prime-boost regimens since this combination elicited remarkably potent T cell responses in non-human primates [150, 179-182]. Ad26 and Ad35 vectors infect a variety of human cells that ubiquitously express CD46, their primary receptor [167]. Furthermore, rAd26 and rAd35 efficiently transduce in the presence of anti-Ad5 antibodies, and seroprevalence of Ad35 in adults is much lower than that of Ad5 [148, 150, 167, 183].
10. Summary
Current concepts of sub-unit based prophylactic vaccines for ocular and genital herpes are promising but not successful in therapeutic settings [6, 7]. To achieve a successful therapeutic vaccine against recurrent virus shedding and recurrent disease, we are currently working on a novel prime-boost homologous and heterologous vaccine strategy that uses “asymptomatic” lipopeptide epitopes priming followed by replication incompetent recombinant adenovirus vector 5, 26 or 35 (rAd5, rAd26 and/or rAd35) boosting, with the lipopeptides and the rAds delivering the same “asymptomatic” CD8+ T cells epitopes.
Development of new and improved therapeutic vaccines and their delivery systems is constantly being pursued for the treatment of human herpes diseases. However, prior to their use in humans, all new vaccine candidates must undergo pre-clinical evaluation in a reliable animal model. The scientific community must overcome the challenge of finding such reliable animal model for pre-clinical studies that are important not only to establish the safety, immunogenicity and protective efficacy of the vaccine compound, but also to plan protocols for subsequent clinical trials from which safety and efficacy can be evaluated.
The mouse is the premier mammalian system for modeling human diseases and understanding the immune mechanism of specific genes in normal and diseased states. Mouse studies have provided plenty of useful and important information regarding ocular HSV-1 infection and despite the tremendous amount of knowledge gained about mouse immunology in general; the mouse is not an ideal model to extrapolate the mechanisms of recurrent HSV-1 and HSV-2 in humans. Spontaneous reactivations, sporadic shedding of the virus in the eyes and GT and recurrent disease do not occur in mice. Therefore, circulating HSV-specific CD8+ T cells may not access HSV latently infected sensory ganglia simply because there is no spontaneous antigens expressed as occurred in humans, rabbits and guinea pigs. Thus mouse in not a reliable model for assessing the efficacy of therapeutic vaccine against spontaneous reactivation or recurrent herpetic disease.
Besides the availability of numerous mouse specific immunological tools and mouse strains such as knock-outs, knock-ins and transgenic mice, the other advantages of mice are their faster breeding process and cheaper housing that made them praised as prime experimental animal model. But an increasing number of studies have revealed substantial differences between the immune responses induced by a vaccine candidate of mice and humans. Therapeutic vaccine candidates that show promise in a given mouse strain might fail in outbreed human population.
There has been a tendency to ignore differences in herpes infection and immunity between mice and humans, and in many cases, perhaps, make the assumption that what is true in mice is necessarily true in humans. By making such assumptions we run the risk of overlooking aspects of human herpes infection and immunity that do not occur, or cannot be modeled, in mice.
Persistent effort on developing herpes therapeutic vaccine using mouse as a preclinical model only increase the failure rate pointlessly. It is worth considering the possibility that any given immune response in a mouse may not occur in precisely the same way in humans.
So the current gamble is in between rabbit and guinea pig versus mouse model for the continuation of herpes therapeutic vaccine study at the preclinical level. While the HLA-transgenic rabbit and HLA-transgenic guinea pig models are difficult to generate, the easily available mouse model generate plentiful of data but may not be extrapolated to human.
We hope that the newly introduced HLA transgenic rabbit model (and maybe one day HLA transgenic guinea pigs one day) with spontaneous reactivation and recurrent herpes disease that mount a T-cell response to HLA-restricted human CD8+ T-cell epitopes will solve many hurdles associated with the preclinical phase of herpes vaccine development. These two animal models will help to address two questions (i) Can spontaneous herpes shedding and recurrent ocular or genital disease be reduced by induction of a vigorous “protective” HSV-specific CD8+ T cells in the sensory ganglia induced by “asymptomatic” epitopes? (ii) Conversely, can recurrent herpetic disease and spontaneous shedding be exacerbated by “pathogenic” CD8+ T-cells induced by “symptomatic” epitopes?
Acknowledgments
This work was supported by NIH Grants RO1 EY14900 and RO1 EY019896 and The Discovery Eye Foundation.
Abbreviations
- HLA
Human Leukocyte Antigen
- HSV
Herpes Simplex Virus
- IFN-γ
Interferon gamma
- Tg
Transgenic
- TG
Trigeminal ganglia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Morrison LA. Vaccines against genital herpes: progress and limitations. Drugs. 2002;62(8):1119–29. doi: 10.2165/00003495-200262080-00001. [DOI] [PubMed] [Google Scholar]
- 2.Wilson KM, Triantafilou K, Morrison IE, Cherry RJ, Fernandez N. Single particle imaging of cell-surface HLA-DR tetramers. Biochem Soc Trans. 1997 May;25(2):360S. doi: 10.1042/bst025360s. [DOI] [PubMed] [Google Scholar]
- 3.Dasgupta G, Nesburn AB, Wechsler SL, BenMohamed L. Developing an asymptomatic mucosal herpes vaccine: the present and the future. Future Microbiol. 2010 Jan;5(1):1–4. doi: 10.2217/fmb.09.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dasgupta G, Chentoufi AA, Nesburn AB, Wechsler SL, BenMohamed L. New concepts in herpes simplex virus vaccine development: notes from the battlefield. Expert Rev Vaccines. 2009 Aug;8(8):1023–35. doi: 10.1586/erv.09.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rubbo PA, Tuaillon E, Nagot N, Chentoufi AA, Bollore K, REYNES J, et al. HIV-1 infection impairs HSV-specific CD4+ and CD8+ T cell response by reducing Th1 cytokines and CCR5 ligand secretion. AIDS. 2011 doi: 10.1097/QAI.0b013e318224d0ad. In press. [DOI] [PubMed] [Google Scholar]
- 6.Vagvala SP, Thebeau LG, Wilson SR, Morrison LA. Virus-encoded b7-2 costimulation molecules enhance the protective capacity of a replication-defective herpes simplex virus type 2 vaccine in immunocompetent mice. J Virol. 2009 Jan;83(2):953–60. doi: 10.1128/JVI.02022-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morrison LA. Replication-defective virus vaccine-induced protection of mice from genital herpes simplex virus 2 requires CD4 T cells. Virology. 2008 Jun 20;376(1):205–10. doi: 10.1016/j.virol.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chentoufi AA, Kritzer E, Tran M, Dasgupta G, EA R, Xianzhi J, et al. The Herpes Simplex Virus-1 Encoded Latency-Associated Transcript Promotes Dysfunctional Virus-Specific CD8+ T Cells in Latently Infected Trigeminal Ganglia: A Novel Immune Evasion Mechanism. J Virology. 2011 doi: 10.1128/JVI.00587-11. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allen SR, Hamrah P, Gate DM, Mott KR, Mantopoulos D, Zheng L, et al. The role of LAT in increased CD8+ T cell exhaustion in trigeminal ganglia of mice latently infected with herpes simplex virus type 1. J Virology. 2011 doi: 10.1128/JVI.02290-10. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang X, Chentoufi AA, Hsiang C, Carpenter D, Osorio N, Benmohamed L, et al. The herpes simplex virus type 1 latency associated transcript (LAT) can protect neuronal derived C1300 and Neuro2A cells from Granzyme B induced apoptosis and CD8 T-cell killing. J Virol. 2010 Dec 22; doi: 10.1128/JVI.01791-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chentoufi AA, Dasgupta G, Nesburn AB, Bettahi I, Binder NR, Choudhury ZS, et al. Nasolacrimal duct closure modulates ocular mucosal and systemic CD4(+) T-cell responses induced following topical ocular or intranasal immunization. Clin Vaccine Immunol. 2010 Mar;17(3):342–53. doi: 10.1128/CVI.00347-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chentoufi AA, Dasgupta G, Christensen ND, Hu J, Choudhury ZS, Azeem A, et al. A Novel Human Leukocyte Antigen (HLA-A*0201) Transgenic Rabbit Model for Pre-clinical Evaluation of Human CD8+ T-Cell Epitope-Based Vaccines Against Ocular Herpes. J Immunol. 2009 Jan 1; doi: 10.4049/jimmunol.0902322. In press(00):00- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang X, Chentoufi AA, Dasgupta G, Nesburn AB, Wu M, Zhu X, et al. A genital tract peptide epitope vaccine targeting TLR-2 efficiently induces local and systemic CD8+ T cells and protects against herpes simplex virus type 2 challenge. Mucosal Immunol. 2009 Mar;2(2):129–43. doi: 10.1038/mi.2008.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gebhardt BM, Halford WP. Evidence that spontaneous reactivation of herpes virus does not occur in mice. Virol J. 2005 Aug 18;2:67. doi: 10.1186/1743-422X-2-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mott KR, Chentoufi AA, Carpenter D, BenMohamed L, Wechsler S, Ghiasi H. A glycoprotein K (gK) CD8+ T-cell epitope of herpes simplex virus types 1 and 2 increases ocular virus replication and pathogenicity. Invest Ophthalmol Vis Sci. 2009 Jan 24; doi: 10.1167/iovs.08-2957. [DOI] [PubMed] [Google Scholar]
- 16.Zhang X, Castelli FA, Zhu X, Wu M, Maillere B, BenMohamed L. Gender-dependent HLA-DR-restricted epitopes identified from herpes simplex virus type 1 glycoprotein D. Clin Vaccine Immunol. 2008 Sep;15(9):1436–49. doi: 10.1128/CVI.00123-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chentoufi AA, Zhang X, Lamberth K, Dasgupta G, Bettahi I, Nguyen A, et al. HLA-A*0201-restricted CD8+ cytotoxic T lymphocyte epitopes identified from herpes simplex virus glycoprotein D. J Immunol. 2008 Jan 1;180(1):426–37. doi: 10.4049/jimmunol.180.1.426. [DOI] [PubMed] [Google Scholar]
- 18.Taneja V, David CS. HLA class II transgenic mice as models of human diseases. Immunol Rev. 1999 Jun;169:67–79. doi: 10.1111/j.1600-065x.1999.tb01307.x. [DOI] [PubMed] [Google Scholar]
- 19.Koelle DM, Reymond SN, Chen H, Kwok WW, McClurkan C, Gyaltsong T, et al. Tegument-specific, virus-reactive CD4 T cells localize to the cornea in herpes simplex virus interstitial keratitis in humans. J Virol. 2000 Dec;74(23):10930–8. doi: 10.1128/jvi.74.23.10930-10938.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tobian AA, Quinn TC. Herpes simplex virus type 2 and syphilis infections with HIV: an evolving synergy in transmission and prevention. Curr Opin HIV AIDS. 2009 Jul;4(4):294–9. doi: 10.1097/COH.0b013e32832c1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corey L, Langenberg AG, Ashley R, Sekulovich RE, Izu AE, Douglas JM, Jr, et al. Recombinant glycoprotein vaccine for the prevention of genital HSV-2 infection: two randomized controlled trials. Chiron HSV Vaccine Study Group. Jama. 1999 Jul 28;282(4):331–40. doi: 10.1001/jama.282.4.331. [DOI] [PubMed] [Google Scholar]
- 22.Hartley C. Immunologic strategies for herpes vaccination. Jama. 2000 Feb 9;283(6):746. [PubMed] [Google Scholar]
- 23.Friedman HM. Immunologic strategies for herpes vaccination. Jama. 2000 Feb 9;283(6):746. doi: 10.1001/jama.283.6.746. [DOI] [PubMed] [Google Scholar]
- 24.Sheridan BS, Cherpes TL, Urban J, Kalinski P, Hendricks RL. Reevaluating the CD8 T-cell response to herpes simplex virus type 1: involvement of CD8 T cells reactive to subdominant epitopes. J Virol. 2009 Mar;83(5):2237–45. doi: 10.1128/JVI.01699-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu T, Khanna KM, Chen X, Fink DJ, Hendricks RL. CD8(+) T cells can block herpes simplex virus type 1 (HSV-1) reactivation from latency in sensory neurons. J Exp Med. 2000 May 1;191(9):1459–66. doi: 10.1084/jem.191.9.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu T, Khanna KM, Carriere BN, Hendricks RL. Gamma interferon can prevent herpes simplex virus type 1 reactivation from latency in sensory neurons. J Virol. 2001 Nov;75(22):11178–84. doi: 10.1128/JVI.75.22.11178-11184.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Divito S, Cherpes TL, Hendricks RL. A triple entente: virus, neurons, and CD8+ T cells maintain HSV-1 latency. Immunol Res. 2006;36(1-3):119–26. doi: 10.1385/IR:36:1:119. [DOI] [PubMed] [Google Scholar]
- 28.Khanna KM, Lepisto AJ, Hendricks RL. Immunity to latent viral infection: many skirmishes but few fatalities. Trends Immunol. 2004 May;25(5):230–4. doi: 10.1016/j.it.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 29.Knickelbein JE, Khanna KM, Yee MB, Baty CJ, Kinchington PR, Hendricks RL. Noncytotoxic lytic granule-mediated CD8+ T cell inhibition of HSV-1 reactivation from neuronal latency. Science. 2008 Oct 10;322(5899):268–71. doi: 10.1126/science.1164164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verjans GM, Hintzen RQ, van Dun JM, Poot A, Milikan JC, Laman JD, et al. Selective retention of herpes simplex virus-specific T cells in latently infected human trigeminal ganglia. Proc Natl Acad Sci U S A. 2007 Feb 27;104(9):3496–501. doi: 10.1073/pnas.0610847104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Theil D, Arbusow V, Derfuss T, Strupp M, Pfeiffer M, Mascolo A, et al. Prevalence of HSV-1 LAT in human trigeminal, geniculate, and vestibular ganglia and its implication for cranial nerve syndromes. Brain Pathol. 2001 Oct;11(4):408–13. doi: 10.1111/j.1750-3639.2001.tb00408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Derfuss T, Segerer S, Herberger S, Sinicina I, Hufner K, Ebelt K, et al. Presence of HSV-1 immediate early genes and clonally expanded T-cells with a memory effector phenotype in human trigeminal ganglia. Brain Pathol. 2007 Oct;17(4):389–98. doi: 10.1111/j.1750-3639.2007.00088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bauer D, Wasmuth S, Hennig M, Baehler H, Steuhl KP, Heiligenhaus A. Amniotic membrane transplantation induces apoptosis in T lymphocytes in murine corneas with experimental herpetic stromal keratitis. Invest Ophthalmol Vis Sci. 2009 Jul;50(7):3188–98. doi: 10.1167/iovs.08-3041. [DOI] [PubMed] [Google Scholar]
- 34.Komatsu K, Miyazaki D, Morohoshi K, Kuo CH, Kakimaru-Hasegawa A, Komatsu N, et al. Pathogenesis of herpetic stromal keratitis in CCR5- and/or CXCR3-deficient mice. Curr Eye Res. 2008 Sep;33(9):736–49. doi: 10.1080/02713680802344716. [DOI] [PubMed] [Google Scholar]
- 35.Kaye S, Choudhary A. Herpes simplex keratitis. Prog Retin Eye Res. 2006 Jul;25(4):355–80. doi: 10.1016/j.preteyeres.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 36.Banerjee K, Biswas PS, Rouse BT. Elucidating the protective and pathologic T cell species in the virus-induced corneal immunoinflammatory condition herpetic stromal keratitis. J Leukoc Biol. 2005 Jan;77(1):24–32. doi: 10.1189/jlb.0904486. [DOI] [PubMed] [Google Scholar]
- 37.Stumpf TH, Case R, Shimeld C, Easty DL, Hill TJ. Primary herpes simplex virus type 1 infection of the eye triggers similar immune responses in the cornea and the skin of the eyelids. J Gen Virol. 2002 Jul;83(Pt 7):1579–90. doi: 10.1099/0022-1317-83-7-1579. [DOI] [PubMed] [Google Scholar]
- 38.Keadle TL, Morris JL, Pepose JS, Stuart PM. CD4(+) and CD8(+) cells are key participants in the development of recurrent herpetic stromal keratitis in mice. Microb Pathog. 2002 Jun;32(6):255–62. doi: 10.1006/mpat.2002.0506. [DOI] [PubMed] [Google Scholar]
- 39.Bauer D, Schmitz A, Van Rooijen N, Steuhl KP, Heiligenhaus A. Conjunctival macrophage-mediated influence of the local and systemic immune response after corneal herpes simplex virus-1 infection. Immunology. 2002 Sep;107(1):118–28. doi: 10.1046/j.1365-2567.2002.01477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verjans GM, Remeijer L, Mooy CM, Osterhaus AD. Herpes simplex virus-specific T cells infiltrate the cornea of patients with herpetic stromal keratitis: no evidence for autoreactive T cells. Invest Ophthalmol Vis Sci. 2000 Aug 9;41:2607–12. [PubMed] [Google Scholar]
- 41.Chang E, Galle L, Maggs D, Estes DM, Mitchell WJ. Pathogenesis of herpes simplex virus type 1-induced corneal inflammation in perforin-deficient mice. J Virol. 2000 Dec;74(24):11832–40. doi: 10.1128/jvi.74.24.11832-11840.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gangappa S, Manickan E, Rouse BT. Control of herpetic stromal keratitis using CTLA4Ig fusion protein. Clin Immunol Immunopathol. 1998 Jan;86(1):88–94. doi: 10.1006/clin.1997.4460. [DOI] [PubMed] [Google Scholar]
- 43.Streilein JW, Dana MR, Ksander BR. Immunity causing blindness: five different paths to herpes stromal keratitis. Immunol Today. 1997 Sep;18(9):443–9. doi: 10.1016/s0167-5699(97)01114-6. [DOI] [PubMed] [Google Scholar]
- 44.Gussow AM, Giordani NV, Tran RK, Imai Y, Kwiatkowski DL, Rall GF, et al. Tissue-specific splicing of the herpes simplex virus type 1 latency-associated transcript (LAT) intron in LAT transgenic mice. J Virol. 2006 Oct;80(19):9414–23. doi: 10.1128/JVI.00530-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feldman LT, Ellison AR, Voytek CC, Yang L, Krause P, Margolis TP. Spontaneous molecular reactivation of herpes simplex virus type 1 latency in mice. Proc Natl Acad Sci U S A. 2002 Jan 22;99(2):978–83. doi: 10.1073/pnas.022301899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ellison AR, Yang L, Voytek C, Margolis TP. Establishment of latent herpes simplex virus type 1 infection in resistant, sensitive, and immunodeficient mouse strains. Virology. 2000 Mar 1;268(1):17–28. doi: 10.1006/viro.1999.0158. [DOI] [PubMed] [Google Scholar]
- 47.Margolis TP, Elfman FL, Leib D, Pakpour N, Apakupakul K, Imai Y, et al. Spontaneous reactivation of herpes simplex virus type 1 in latently infected murine sensory ganglia. J Virol. 2007 Oct;81(20):11069–74. doi: 10.1128/JVI.00243-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang L, Voytek CC, Margolis TP. Immunohistochemical analysis of primary sensory neurons latently infected with herpes simplex virus type 1. J Virol. 2000 Jan;74(1):209–17. doi: 10.1128/jvi.74.1.209-217.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Openshaw H, Cantin EM. Corticosteroids in herpes simplex virus encephalitis. J Neurol Neurosurg Psychiatry. 2005 Nov;76(11):1469. doi: 10.1136/jnnp.2005.071837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Han X, Lundberg P, Tanamachi B, Openshaw H, Longmate J, Cantin E. Gender influences herpes simplex virus type 1 infection in normal and gamma interferon-mutant mice. J Virol. 2001 Mar;75(6):3048–52. doi: 10.1128/JVI.75.6.3048-3052.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lundberg P, Welander P, Openshaw H, Nalbandian C, Edwards C, Moldawer L, et al. A locus on mouse chromosome 6 that determines resistance to herpes simplex virus also influences reactivation, while an unlinked locus augments resistance of female mice. J Virol. 2003 Nov;77(21):11661–73. doi: 10.1128/JVI.77.21.11661-11673.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carr DJ, Harle P, Gebhardt BM. The immune response to ocular herpes simplex virus type 1 infection. Exp Biol Med (Maywood) 2001 May;226(5):353–66. doi: 10.1177/153537020122600501. [DOI] [PubMed] [Google Scholar]
- 53.Polcicova K, Biswas PS, Banerjee K, Wisner TW, Rouse BT, Johnson DC. Herpes keratitis in the absence of anterograde transport of virus from sensory ganglia to the cornea. Proc Natl Acad Sci U S A. 2005 Aug 9;102(32):11462–7. doi: 10.1073/pnas.0503230102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chentoufi AA, Binder NR, Berka N, Durand G, Nguyen A, Bettahi I, et al. Asymptomatic Human CD4+ Cytotoxic T-Cell Epitopes Identified from Herpes Simplex Virus Glycoprotein B. Journal Virology. 2008 doi: 10.1128/JVI.00692-08. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaye SB, Baker K, Bonshek R, Maseruka H, Grinfeld E, Tullo A, et al. Human herpesviruses in the cornea. Br J Ophthalmol. 2000 Jun;84(6):563–71. doi: 10.1136/bjo.84.6.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gangappa S, Deshpande SP, Rouse BT. Bystander activation of CD4+ T cells accounts for herpetic ocular lesions. Invest Ophthalmol Vis Sci. 2000 Feb;41(2):453–9. [PubMed] [Google Scholar]
- 57.Gangappa S, Deshpande SP, Rouse BT. Bystander activation of CD4(+) T cells can represent an exclusive means of immunopathology in a virus infection. Eur J Immunol. 1999 Nov;29(11):3674–82. doi: 10.1002/(SICI)1521-4141(199911)29:11<3674::AID-IMMU3674>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 58.Zhao ZS, Granucci F, Yeh L, Schaffer PA, Cantor H. Molecular mimicry by herpes simplex virus-type 1: autoimmune disease after viral infection. Science. 1998 Feb 27;279(5355):1344–7. doi: 10.1126/science.279.5355.1344. [DOI] [PubMed] [Google Scholar]
- 59.Metcalf JF, Michaelis BA. Herpetic keratitis in inbred mice. Invest Ophthalmol Vis Sci. 1984 Oct;25(10):1222–5. [PubMed] [Google Scholar]
- 60.Stulting RD, Kindle JC, Nahmias AJ. Patterns of herpes simplex keratitis in inbred mice. Invest Ophthalmol Vis Sci. 1985 Oct;26(10):1360–7. [PubMed] [Google Scholar]
- 61.Kastrukoff LF, Lau AS, Puterman ML. Genetics of natural resistance to herpes simplex virus type 1 latent infection of the peripheral nervous system in mice. J Gen Virol. 1986 Apr;67(Pt 4):613–21. doi: 10.1099/0022-1317-67-4-613. [DOI] [PubMed] [Google Scholar]
- 62.Lopez C. Genetics of natural resistance to herpesvirus infections in mice. Nature. 1975 Nov 13;258(5531):152–3. doi: 10.1038/258152a0. [DOI] [PubMed] [Google Scholar]
- 63.McLean CS, Erturk M, Jennings R, Challanain DN, Minson AC, Duncan I, et al. Protective vaccination against primary and recurrent disease caused by herpes simplex virus (HSV) type 2 using a genetically disabled HSV-1. J Infect Dis. 1994 Nov;170(5):1100–9. doi: 10.1093/infdis/170.5.1100. [DOI] [PubMed] [Google Scholar]
- 64.McLean GW, Abbotts AP, Parry ME, Marsden HS, Stow ND. The herpes simplex virus type 1 origin-binding protein interacts specifically with the viral UL8 protein. J Gen Virol. 1994 Oct;75(Pt 10):2699–706. doi: 10.1099/0022-1317-75-10-2699. [DOI] [PubMed] [Google Scholar]
- 65.Lecuru F, Taurelle R, Bernard JP, Parrat S, Lafay-pillet MC, Rozenberg F, et al. Varicella zoster virus infection during pregnancy: the limits of prenatal diagnosis. Eur J Obstet Gynecol Reprod Biol. 1994 Jul;56(1):67–8. doi: 10.1016/0028-2243(94)90156-2. [DOI] [PubMed] [Google Scholar]
- 66.Reddig PJ, Grinstead LA, Monahan SJ, Johnson PA, Parris DS. The essential in vivo function of the herpes simplex virus UL42 protein correlates with its ability to stimulate the viral DNA polymerase in vitro. Virology. 1994 May 1;200(2):447–56. doi: 10.1006/viro.1994.1208. [DOI] [PubMed] [Google Scholar]
- 67.Parr MB, Kepple L, McDermott MR, Drew MD, Bozzola JJ, Parr EL. A mouse model for studies of mucosal immunity to vaginal infection by herpes simplex virus type 2. Lab Invest. 1994 Mar;70(3):369–80. [PubMed] [Google Scholar]
- 68.Stevens DA, Merigan TC. Approaches to the control of viral infections in man. Ration Drug Ther. 1971 Sep;5(9):1–5. [PubMed] [Google Scholar]
- 69.Hoshino Y, Qin J, Follmann D, Cohen JI, Straus SE. The number of herpes simplex virus-infected neurons and the number of viral genome copies per neuron correlate with the latent viral load in ganglia. Virology. 2008 Mar 1;372(1):56–63. doi: 10.1016/j.virol.2007.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hoshino Y, Pesnicak L, Cohen JI, Straus SE. Rates of reactivation of latent herpes simplex virus from mouse trigeminal ganglia ex vivo correlate directly with viral load and inversely with number of infiltrating CD8+ T cells. J Virol. 2007 Aug;81(15):8157–64. doi: 10.1128/JVI.00474-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thompson RL, Sawtell NM. The herpes simplex virus type 1 latency-associated transcript gene regulates the establishment of latency. J Virol. 1997 Jul;71(7):5432–40. doi: 10.1128/jvi.71.7.5432-5440.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sawtell NM. Comprehensive quantification of herpes simplex virus latency at the single-cell level. J Virol. 1997 Jul;71(7):5423–31. doi: 10.1128/jvi.71.7.5423-5431.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dudek TE, Torres-Lopez E, Crumpacker C, Knipe DM. Evidence for differences in immunologic and pathogenesis properties of herpes simplex virus 2 strains from the United States and South Africa. J Infect Dis. 2011 May;203(10):1434–41. doi: 10.1093/infdis/jir047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dudek T, Knipe DM. Replication-defective viruses as vaccines and vaccine vectors. Virology. 2006 Jan 5;344(1):230–9. doi: 10.1016/j.virol.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 75.De Felipe C, Herrero JF, O’Brien JA, Palmer JA, Doyle CA, Smith AJ, et al. Altered nociception, analgesia and aggression in mice lacking the receptor for substance P. Nature. 1998 Mar 26;392(6674):394–7. doi: 10.1038/32904. [DOI] [PubMed] [Google Scholar]
- 76.Lafferty WE, Downey L, Celum C, Wald A. Herpes simplex virus type 1 as a cause of genital herpes: impact on surveillance and prevention. J Infect Dis. 2000 Apr;181(4):1454–7. doi: 10.1086/315395. [DOI] [PubMed] [Google Scholar]
- 77.Straus SE, Wald A, Kost RG, McKenzie R, Langenberg AG, Hohman P, et al. Immunotherapy of recurrent genital herpes with recombinant herpes simplex virus type 2 glycoproteins D and B: results of a placebo-controlled vaccine trial. J Infect Dis. 1997 Nov;176(5):1129–34. doi: 10.1086/514103. [DOI] [PubMed] [Google Scholar]
- 78.Wald A, Corey L, Cone R, Hobson A, Davis G, Zeh J. Frequent genital herpes simplex virus 2 shedding in immunocompetent women. Effect of acyclovir treatment. J Clin Invest. 1997 Mar 1;99(5):1092–7. doi: 10.1172/JCI119237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wald A, Koutsky L, Ashley RL, Corey L. Genital herpes in a primary care clinic. Demographic and sexual correlates of herpes simplex type 2 infections. Sex Transm Dis. 1997 Mar;24(3):149–55. doi: 10.1097/00007435-199703000-00005. [DOI] [PubMed] [Google Scholar]
- 80.Wald A, Schacker T, Corey L. HSV-2 and HIV: consequences of an endemic opportunistic infection. STEP Perspect. 1997 Winter;9(3):2–4. [PubMed] [Google Scholar]
- 81.Hill JM, Dudley JB, Shimomura Y, Kaufman HE. Quantitation and kinetics of induced HSV-1 ocular shedding. Curr Eye Res. 1986 Mar;5(3):241–6. doi: 10.3109/02713688609020049. [DOI] [PubMed] [Google Scholar]
- 82.Hill TJ, Yirrell DL, Blyth WA. Infection of the adrenal gland as a route to the central nervous system after viraemia with herpes simplex virus in the mouse. J Gen Virol. 1986 Feb;67(Pt 2):309–20. doi: 10.1099/0022-1317-67-2-309. [DOI] [PubMed] [Google Scholar]
- 83.Laibson PR, Kibrick S. Recurrence of herpes simplex virus in rabbit eyes: results of a three-year study. Invest Ophthalmol. 1969 Jun;8(3):346–50. [PubMed] [Google Scholar]
- 84.Chodosh J, Kennedy RC. The conjunctival lymphoid follicle in mucosal immunology. DNA Cell Biol. 2002 May-Jun;21(5-6):421–33. doi: 10.1089/10445490260099719. [DOI] [PubMed] [Google Scholar]
- 85.Romanowski EG, Araullo-Cruz T, Gordon YJ. Topical corticosteroids reverse the antiviral effect of topical cidofovir in the Ad5-inoculated New Zealand rabbit ocular model. Invest Ophthalmol Vis Sci. 1997 Jan;38(1):253–7. [PubMed] [Google Scholar]
- 86.Romanowski EG, Gordon YJ, Araullo-Cruz T, Yates KA, Kinchington PR. The antiviral resistance and replication of cidofovir-resistant adenovirus variants in the New Zealand White rabbit ocular model. Invest Ophthalmol Vis Sci. 2001 Jul;42(8):1812–5. [PubMed] [Google Scholar]
- 87.Trousdale MD, Dunkel EC, Nesburn AB. Effect of acyclovir on acute and latent herpes simplex virus infections in the rabbit. Invest Ophthalmol Vis Sci. 1980 Nov;19(11):1336–41. [PubMed] [Google Scholar]
- 88.Nesburn AB. Common viral eye diseases and latent infections. Ophthalmology. 1980 Dec;87(12):1202–7. doi: 10.1016/s0161-6420(80)35103-8. [DOI] [PubMed] [Google Scholar]
- 89.Metcalf MF, McNeill JI, Kaufman HE. Experimental disciform edema and necrotizing keratitis in the rabbit. Invest Ophthalmol. 1976 Dec;15(12):979–85. [PubMed] [Google Scholar]
- 90.Knop N, Knop E. Ultrastructural anatomy of CALT follicles in the rabbit reveals characteristics of M-cells, germinal centres and high endothelial venules. J Anat. 2005 Oct;207(4):409–26. doi: 10.1111/j.1469-7580.2005.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fukushima A, Yamaguchi T, Fukuda K, Sumi T, Kumagai N, Nishida T, et al. CD8+ T cells play disparate roles in the induction and the effector phases of murine experimental allergic conjunctivitis. Microbiol Immunol. 2006;50(9):719–28. doi: 10.1111/j.1348-0421.2006.tb03845.x. [DOI] [PubMed] [Google Scholar]
- 92.Fukushima A, Sumi T, Fukuda K, Kumagai N, Nishida T, Yagita H, et al. Interleukin 10 and transforming growth factor beta contribute to the development of experimentally induced allergic conjunctivitis in mice during the effector phase. Br J Ophthalmol. 2006 Dec;90(12):1535–41. doi: 10.1136/bjo.2006.100180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gamache DA, Dimitrijevich SD, Weimer LK, Lang LS, Spellman JM, Graff G, et al. Secretion of proinflammatory cytokines by human conjunctival epithelial cells. Ocul Immunol Inflamm. 1997 Jun;5(2):117–28. doi: 10.3109/09273949709085060. [DOI] [PubMed] [Google Scholar]
- 94.Wotherspoon AC, Hardman-Lea S, Isaacson PG. Mucosa-associated lymphoid tissue (MALT) in the human conjunctiva. J Pathol. 1994 Sep;174(1):33–7. doi: 10.1002/path.1711740106. [DOI] [PubMed] [Google Scholar]
- 95.Knop E, Knop N. The role of eye-associated lymphoid tissue in corneal immune protection. J Anat. 2005 Mar;206(3):271–85. doi: 10.1111/j.1469-7580.2005.00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Knop E, Knop N. Eye-associated lymphoid tissue (EALT) is continuously spread throughout the ocular surface from the lacrimal gland to the lacrimal drainage system. Ophthalmologe. 2003 Nov;100(11):929–42. doi: 10.1007/s00347-003-0936-6. [DOI] [PubMed] [Google Scholar]
- 97.Liu H, Meagher CK, Moore CP, Phillips TE. M cells in the follicle-associated epithelium of the rabbit conjunctiva preferentially bind and translocate latex beads. Invest Ophthalmol Vis Sci. 2005 Nov;46(11):4217–23. doi: 10.1167/iovs.05-0280. [DOI] [PubMed] [Google Scholar]
- 98.Bettahi I, Nesburn AB, Yoon S, Zhang X, Mohebbi A, Sue V, et al. Protective immunity against ocular herpes infection and disease induced by highly immunogenic self-adjuvanting glycoprotein D lipopeptide vaccines. Invest Ophthalmol Vis Sci. 2007 Oct;48(10):4643–53. doi: 10.1167/iovs.07-0356. [DOI] [PubMed] [Google Scholar]
- 99.Zhang X, Issagholian A, Berg EA, Fishman JB, Nesburn AB, BenMohamed L. Th-cytotoxic T-lymphocyte chimeric epitopes extended by Nepsilon-palmitoyl lysines induce herpes simplex virus type 1-specific effector CD8+ Tc1 responses and protect against ocular infection. J Virol. 2005 Dec;79(24):15289–301. doi: 10.1128/JVI.79.24.15289-15301.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bettahi I, Zhang X, Afifi RE, BenMohamed L. Protective immunity to genital herpes simplex virus type 1 and type 2 provided by self-adjuvanting lipopeptides that drive dendritic cell maturation and elicit a polarized Th1 immune response. Viral Immunol. 2006 Summer;19(2):220–36. doi: 10.1089/vim.2006.19.220. [DOI] [PubMed] [Google Scholar]
- 101.Zhu X, Ramos TV, Gras-Masse H, Kaplan BE, BenMohamed L. Lipopeptide epitopes extended by an Nepsilon-palmitoyl-lysine moiety increase uptake and maturation of dendritic cells through a Toll-like receptor-2 pathway and trigger a Th1-dependent protective immunity. Eur J Immunol. 2004 Nov;34(11):3102–14. doi: 10.1002/eji.200425166. [DOI] [PubMed] [Google Scholar]
- 102.Nesburn AB, Ramos TV, Zhu X, Asgarzadeh H, Nguyen V, BenMohamed L. Local and systemic B cell and Th1 responses induced following ocular mucosal delivery of multiple epitopes of herpes simplex virus type 1 glycoprotein D together with cytosine-phosphate-guanine adjuvant. Vaccine. 2005 Jan 4;23(7):873–83. doi: 10.1016/j.vaccine.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 103.Laycock KA, Lee SF, Brady RH, Pepose JS. Characterization of a murine model of recurrent herpes simplex viral keratitis induced by ultraviolet B radiation. Invest Ophthalmol Vis Sci. 1991 Sep;32(10):2741–6. [PubMed] [Google Scholar]
- 104.Sawtell NM, Thompson RL. Herpes simplex virus type 1 latency-associated transcription unit promotes anatomical site-dependent establishment and reactivation from latency. J Virol. 1992 Apr;66(4):2157–69. doi: 10.1128/jvi.66.4.2157-2169.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sawtell NM, Thompson RL. Rapid in vivo reactivation of herpes simplex virus in latently infected murine ganglionic neurons after transient hyperthermia. J Virol. 1992 Apr;66(4):2150–6. doi: 10.1128/jvi.66.4.2150-2156.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Himmelein S, St Leger AJ, Knickelbein JE, Rowe A, Freeman ML, Hendricks RL. Circulating herpes simplex type 1 (HSV-1)-specific CD8+ T cells do not access HSV-1 latently infected trigeminal ganglia. Herpesviridae. 2011;2(1):5. doi: 10.1186/2042-4280-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bonneau RH, Zimmerman KM, Ikeda SC, Jones BC. Differential effects of stress-induced adrenal function on components of the herpes simplex virus-specific memory cytotoxic T-lymphocyte response. J Neuroimmunol. 1998 Mar 1;82(2):191–9. doi: 10.1016/s0165-5728(97)00200-2. [DOI] [PubMed] [Google Scholar]
- 108.Hunzeker JT, Elftman MD, Mellinger JC, Princiotta MF, Bonneau RH, Truckenmiller ME, et al. A marked reduction in priming of cytotoxic CD8+ T cells mediated by stress-induced glucocorticoids involves multiple deficiencies in cross-presentation by dendritic cells. J Immunol. 2011 Jan 1;186(1):183–94. doi: 10.4049/jimmunol.1001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Elftman MD, Hunzeker JT, Mellinger JC, Bonneau RH, Norbury CC, Truckenmiller ME. Stress-induced glucocorticoids at the earliest stages of herpes simplex virus-1 infection suppress subsequent antiviral immunity, implicating impaired dendritic cell function. J Immunol. 2010 Feb 15;184(4):1867–75. doi: 10.4049/jimmunol.0902469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sheridan JF, Feng NG, Bonneau RH, Allen CM, Huneycutt BS, Glaser R. Restraint stress differentially affects anti-viral cellular and humoral immune responses in mice. J Neuroimmunol. 1991 Mar;31(3):245–55. doi: 10.1016/0165-5728(91)90046-a. [DOI] [PubMed] [Google Scholar]
- 111.Freeman ML, Sheridan BS, Bonneau RH, Hendricks RL. Psychological stress compromises CD8+ T cell control of latent herpes simplex virus type 1 infections. J Immunol. 2007 Jul 1;179(1):322–8. doi: 10.4049/jimmunol.179.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chentoufi AA, Dasgupta G, Christensen ND, Hu J, Choudhury ZS, Azeem A, et al. A novel HLA (HLA-A*0201) transgenic rabbit model for preclinical evaluation of human CD8+ T cell epitope-based vaccines against ocular herpes. J Immunol. 2010 Mar 1;184(5):2561–71. doi: 10.4049/jimmunol.0902322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hu J, Peng X, Schell TD, Budgeon LR, Cladel NM, Christensen ND. An HLA-A2.1-transgenic rabbit model to study immunity to papillomavirus infection. J Immunol. 2006 Dec 1;177(11):8037–45. doi: 10.4049/jimmunol.177.11.8037. [DOI] [PubMed] [Google Scholar]
- 114.Chentoufi AA, Dasgupta G, Christensen ND, Hu J, Choudhury ZS, Azeem A, et al. A Novel Human Leukocyte Antigen (HLA-A*0201) Transgenic Rabbit Model for Pre-clinical Evaluation of Human CD8+ T-Cell Epitope-Based Vaccines Against Ocular Herpes. J Immunol. 2010 Jan 1; doi: 10.4049/jimmunol.0902322. In press(00):00- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Barsam CA, Brick DJ, Jones C, Wechsler SL, Perng GC. A viral model for corneal scarring and neovascularization following ocular infection of rabbits with a herpes simplex virus type 1 (HSV-1) mutant. Cornea. 2005 May;24(4):460–6. doi: 10.1097/01.ico.0000138833.34865.39. [DOI] [PubMed] [Google Scholar]
- 116.Mott KR, Osorio N, Jin L, Brick DJ, Naito J, Cooper J, et al. The bovine herpesvirus-1 LR ORF2 is critical for this gene’s ability to restore the high wild-type reactivation phenotype to a herpes simplex virus-1 LAT null mutant. J Gen Virol. 2003 Nov;84(Pt 11):2975–85. doi: 10.1099/vir.0.19421-0. [DOI] [PubMed] [Google Scholar]
- 117.Perng GC, Maguen B, Jin L, Mott KR, Osorio N, Slanina SM, et al. A gene capable of blocking apoptosis can substitute for the herpes simplex virus type 1 latency-associated transcript gene and restore wild-type reactivation levels. J Virol. 2002;76(3):1224–35. doi: 10.1128/JVI.76.3.1224-1235.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Scriba M, Tatzber F. Pathogenesis of Herpes simplex virus infections in guinea pigs. Infect Immun. 1981 Dec;34(3):655–61. doi: 10.1128/iai.34.3.655-661.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Armerding D, Mayer P, Scriba M, Hren A, Rossiter H. In-vivo modulation of macrophage functions by herpes simplex virus type 2 in resistant and sensitive inbred mouse strains. Immunobiology. 1981;160(2):217–27. doi: 10.1016/S0171-2985(81)80049-6. [DOI] [PubMed] [Google Scholar]
- 120.Scriba M. Persistence of herpes simplex virus (HSV) infection in ganglia and peripheral tissues of guinea pigs. Med Microbiol Immunol. 1981;169(2):91–6. doi: 10.1007/BF02171776. [DOI] [PubMed] [Google Scholar]
- 121.Bourne N, Stanberry LR, Connelly BL, Kurawadwala J, Straus SE, Krause PR. Quantity of latency-associated transcript produced by herpes simplex virus is not predictive of the frequency of experimental recurrent genital herpes. J Infect Dis. 1994 May;169(5):1084–7. doi: 10.1093/infdis/169.5.1084. [DOI] [PubMed] [Google Scholar]
- 122.Krause PR, Stanberry LR, Bourne N, Connelly B, Kurawadwala JF, Patel A, et al. Expression of the herpes simplex virus type 2 latency-associated transcript enhances spontaneous reactivation of genital herpes in latently infected guinea pigs. J Exp Med. 1995 Jan 1;181(1):297–306. doi: 10.1084/jem.181.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bernstein DI. Potential for immunotherapy in the treatment of herpesvirus infections. Herpes. 2001 Mar;8(1):8–11. [PubMed] [Google Scholar]
- 124.Stanberry LR. The concept of immune-based therapies in chronic viral infections. J Acquir Immune Defic Syndr. 1994;7(Suppl 1):S1–5. [PubMed] [Google Scholar]
- 125.Stanberry LR. Evaluation of herpes simplex virus vaccines in animals: the guinea pig vaginal model. Rev Infect Dis. 1991 Nov-Dec;13(Suppl 11):S920–3. doi: 10.1093/clind/13.supplement_11.s920. [DOI] [PubMed] [Google Scholar]
- 126.Boursnell ME, Entwisle C, Blakeley D, Roberts C, Duncan IA, Chisholm SE, et al. A genetically inactivated herpes simplex virus type 2 (HSV-2) vaccine provides effective protection against primary and recurrent HSV-2 disease. J Infect Dis. 1997 Jan;175(1):16–25. doi: 10.1093/infdis/175.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Scriba M. Protection of guinea pigs against primary and recurrent genital herpes infections by immunization with live heterologous or homologous Herpes simplex virus: implications for a herpes virus vaccine. Med Microbiol Immunol (Berl) 1978 Nov 17;166(1-4):63–9. doi: 10.1007/BF02121135. [DOI] [PubMed] [Google Scholar]
- 128.Bourne N, Pyles RB, Bernstein DI, Stanberry LR. Modification of primary and recurrent genital herpes in guinea pigs by passive immunization. J Gen Virol. 2002 Nov;83(Pt 11):2797–801. doi: 10.1099/0022-1317-83-11-2797. [DOI] [PubMed] [Google Scholar]
- 129.Bourne N, Bravo FJ, Francotte M, Bernstein DI, Myers MG, Slaoui M, et al. Herpes simplex virus (HSV) type 2 glycoprotein D subunit vaccines and protection against genital HSV-1 or HSV-2 disease in guinea pigs. J Infect Dis. 2003 Feb 15;187(4):542–9. doi: 10.1086/374002. [DOI] [PubMed] [Google Scholar]
- 130.Stanberry LR, Bernstein DI, Burke RL, Pachl C, Myers MG. Vaccination with recombinant herpes simplex virus glycoproteins: protection against initial and recurrent genital herpes. J Infect Dis. 1987 May;155(5):914–20. doi: 10.1093/infdis/155.5.914. [DOI] [PubMed] [Google Scholar]
- 131.Yim KC, Carroll CJ, Tuyama A, Cheshenko N, Carlucci MJ, Porter DD, et al. The cotton rat provides a novel model to study genital herpes infection and to evaluate preventive strategies. J Virol. 2005 Dec;79(23):14632–9. doi: 10.1128/JVI.79.23.14632-14639.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hoshino Y, Dalai SK, Wang K, Pesnicak L, Lau TY, Knipe DM, et al. Comparative efficacy and immunogenicity of replication-defective, recombinant glycoprotein, and DNA vaccines for herpes simplex virus 2 infections in mice and guinea pigs. J Virol. 2005 Jan;79(1):410–8. doi: 10.1128/JVI.79.1.410-418.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Richards CM, Case R, Hirst TR, Hill TJ, Williams NA. Protection against recurrent ocular herpes simplex virus type 1 disease after therapeutic vaccination of latently infected mice. J Virol. 2003 Jun;77(12):6692–9. doi: 10.1128/JVI.77.12.6692-6699.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ghiasi H, Kaiwar R, Nesburn AB, Slanina S, Wechsler SL. Expression of seven herpes simplex virus type 1 glycoproteins (gB, gC, gD, gE, gG, gH, and gI): comparative protection against lethal challenge in mice. J Virol. 1994 Apr;68(4):2118–26. doi: 10.1128/jvi.68.4.2118-2126.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bernstein DI, Aoki FY, Tyring SK, Stanberry LR, St-Pierre C, Shafran SD, et al. Safety and immunogenicity of glycoprotein D-adjuvant genital herpes vaccine. Clin Infect Dis. 2005 May 1;40(9):1271–81. doi: 10.1086/429240. [DOI] [PubMed] [Google Scholar]
- 136.Dalai SK, Pesnicak L, Miller GF, Straus SE. Prophylactic and therapeutic effects of human immunoglobulin on the pathobiology of HSV-1 infection, latency, and reactivation in mice. J Neurovirol. 2002 Feb;8(1):35–44. doi: 10.1080/135502802317247794. [DOI] [PubMed] [Google Scholar]
- 137.Nesburn AB, Ghiasi H, Wechsler SL. Ocular safety and efficacy of an HSV-1 gD vaccine during primary and latent infection. Invest Ophthalmol Vis Sci. 1990 Aug;31(8):1497–502. [PubMed] [Google Scholar]
- 138.Bravo FJ, Bourne N, Harrison CJ, Mani C, Stanberry LR, Myers MG, et al. Effect of antibody alone and combined with acyclovir on neonatal herpes simplex virus infection in guinea pigs. J Infect Dis. 1996 Jan;173(1):1–6. doi: 10.1093/infdis/173.1.1. [DOI] [PubMed] [Google Scholar]
- 139.Willey DE, Smith MD, Nesburn AB, Trousdale MD. Sequential analysis of antibody responses in serum, aqueous humor and tear film during latent and induced recurrent HSV infections. Curr Eye Res. 1985 Dec;4(12):1235–40. doi: 10.3109/02713688509017681. [DOI] [PubMed] [Google Scholar]
- 140.Yoshikawa T, Hill JM, Stanberry LR, Bourne N, Kurawadwala JF, Krause PR. The characteristic site-specific reactivation phenotypes of HSV-1 and HSV-2 depend upon the latency-associated transcript region. J Exp Med. 1996 Aug 1;184(2):659–64. doi: 10.1084/jem.184.2.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bravo FJ, Myers MG, Stanberry LR. Neonatal herpes simplex virus infection: pathogenesis and treatment in the guinea pig. J Infect Dis. 1994 May;169(5):947–55. doi: 10.1093/infdis/169.5.947. [DOI] [PubMed] [Google Scholar]
- 142.Knickelbein JE, Hendricks RL, Charukamnoetkanok P. Management of herpes simplex virus stromal keratitis: an evidence-based review. Surv Ophthalmol. 2009 Mar-Apr;54(2):226–34. doi: 10.1016/j.survophthal.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 143.Smith GL, Mackett M, Moss B. Infectious vaccinia virus recombinants that express hepatitis B virus surface antigen. Nature. 1983 Apr 7;302(5908):490–5. doi: 10.1038/302490a0. [DOI] [PubMed] [Google Scholar]
- 144.Draper SJ, Heeney JL. Viruses as vaccine vectors for infectious diseases and cancer. Nat Rev Microbiol. 2010 Jan;8(1):62–73. doi: 10.1038/nrmicro2240. [DOI] [PubMed] [Google Scholar]
- 145.Nesburn AB, Slanina S, Burke RL, Ghiasi H, Bahri S, Wechsler SL. Local periocular vaccination protects against eye disease more effectively than systemic vaccination following primary ocular herpes simplex virus infection in rabbits. J Virol. 1998 Oct;72(10):7715–21. doi: 10.1128/jvi.72.10.7715-7721.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nesburn AB, Burke RL, Ghiasi H, Slanina SM, Wechsler SL. Therapeutic periocular vaccination with a subunit vaccine induces higher levels of herpes simplex virus-specific tear secretory immunoglobulin A than systemic vaccination and provides protection against recurrent spontaneous ocular shedding of virus in latently infected rabbits. Virology. 1998 Dec 5;252(1):200–9. doi: 10.1006/viro.1998.9454. [DOI] [PubMed] [Google Scholar]
- 147.Nesburn AB, Burke RL, Ghiasi H, Slanina SM, Wechsler SL. A therapeutic vaccine that reduces recurrent herpes simplex virus type 1 corneal disease. Invest Ophthalmol Vis Sci. 1998 Jun;39(7):1163–70. [PubMed] [Google Scholar]
- 148.Paris RM, Kim JH, Robb ML, Michael NL. Prime-boost immunization with poxvirus or adenovirus vectors as a strategy to develop a protective vaccine for HIV-1. Expert Rev Vaccines. 2010 Sep;9(9):1055–69. doi: 10.1586/erv.10.106. [DOI] [PubMed] [Google Scholar]
- 149.Brandsma JL, Shlyankevich M, Zhang L, Slade MD, Goodwin EC, Peh W, et al. Vaccination of rabbits with an adenovirus vector expressing the papillomavirus E2 protein leads to clearance of papillomas and infection. J Virol. 2004 Jan;78(1):116–23. doi: 10.1128/JVI.78.1.116-123.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Barouch DH. Novel adenovirus vector-based vaccines for HIV-1. Curr Opin HIV AIDS. 2010 Sep;5(5):386–90. doi: 10.1097/COH.0b013e32833cfe4c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Patterson S, Papagatsias T, Benlahrech A. Use of adenovirus in vaccines for HIV. Handb Exp Pharmacol. 2009;188:275–93. doi: 10.1007/978-3-540-71029-5_13. [DOI] [PubMed] [Google Scholar]
- 152.Walsh MP, Chintakuntlawar A, Robinson CM, Madisch I, Harrach B, Hudson NR, et al. Evidence of molecular evolution driven by recombination events influencing tropism in a novel human adenovirus that causes epidemic keratoconjunctivitis. PLoS One. 2009;4(6):e5635. doi: 10.1371/journal.pone.0005635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Smith JG, Wiethoff CM, Stewart PL, Nemerow GR. Adenovirus. Curr Top Microbiol Immunol. 2010;343:195–224. doi: 10.1007/82_2010_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hanna L, Jawetz E, Mitsui Y, Thygeson P, Kimura SJ, Nicholas C. Continuing studies on the association of adenovirus type 8 with epidemic keratoconjunctivitis. Am J Ophthalmol. 1957 Oct;44(4, Part 2):66–74. doi: 10.1016/0002-9394(57)90433-6. [DOI] [PubMed] [Google Scholar]
- 155.Butt AL, Chodosh J. Adenoviral keratoconjunctivitis in a tertiary care eye clinic. Cornea. 2006 Feb;25(2):199–202. doi: 10.1097/01.ico.0000170693.13326.fb. [DOI] [PubMed] [Google Scholar]
- 156.Ishiko H, Shimada Y, Konno T, Hayashi A, Ohguchi T, Tagawa Y, et al. Novel human adenovirus causing nosocomial epidemic keratoconjunctivitis. J Clin Microbiol. 2008 Jun;46(6):2002–8. doi: 10.1128/JCM.01835-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Aoki K, Ishiko H, Konno T, Shimada Y, Hayashi A, Kaneko H, et al. Epidemic keratoconjunctivitis due to the novel hexon-chimeric-intermediate 22,37/H8 human adenovirus. J Clin Microbiol. 2008 Oct;46(10):3259–69. doi: 10.1128/JCM.02354-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chintakuntlawar AV, Chodosh J. Cellular and tissue architecture of conjunctival membranes in epidemic keratoconjunctivitis. Ocul Immunol Inflamm. 2010 Oct;18(5):341–5. doi: 10.3109/09273948.2010.498658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ishiko H, Aoki K. Spread of epidemic keratoconjunctivitis due to a novel serotype of human adenovirus in Japan. J Clin Microbiol. 2009 Aug;47(8):2678–9. doi: 10.1128/JCM.r00313-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Olofsson S, Kumlin U, Dimock K, Arnberg N. Avian influenza and sialic acid receptors: more than meets the eye? Lancet Infect Dis. 2005 Mar;5(3):184–8. doi: 10.1016/S1473-3099(05)01311-3. [DOI] [PubMed] [Google Scholar]
- 161.Trousdale MD, Nobrega R, Wood RL, Stevenson D, dos Santos PM, Klein D, et al. Studies of adenovirus-induced eye disease in the rabbit model. Invest Ophthalmol Vis Sci. 1995 Dec;36(13):2740–8. [PubMed] [Google Scholar]
- 162.Iacobelli-Martinez M, Nemerow GR. Preferential activation of Toll-like receptor nine by CD46-utilizing adenoviruses. J Virol. 2007 Feb;81(3):1305–12. doi: 10.1128/JVI.01926-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wu E, Trauger SA, Pache L, Mullen TM, von Seggern DJ, Siuzdak G, et al. Membrane cofactor protein is a receptor for adenoviruses associated with epidemic keratoconjunctivitis. J Virol. 2004 Apr;78(8):3897–905. doi: 10.1128/JVI.78.8.3897-3905.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Trauger SA, Wu E, Bark SJ, Nemerow GR, Siuzdak G. The identification of an adenovirus receptor by using affinity capture and mass spectrometry. Chembiochem. 2004 Aug 6;5(8):1095–9. doi: 10.1002/cbic.200400037. [DOI] [PubMed] [Google Scholar]
- 165.Gordon YJ, Araullo-Cruz T, Romanowski E, Myers B, Santora D, Lin M, et al. Replication of ocular isolates of human adenovirus is serotype-dependent in rabbit corneal organ culture. Curr Eye Res. 1991 Mar;10(3):267–71. doi: 10.3109/02713689109003449. [DOI] [PubMed] [Google Scholar]
- 166.Trousdale MD, Nobrega R, Stevenson D, Nakamura T, dos Santos PM, Labree L, et al. Role of adenovirus type 5 early region 3 in the pathogenesis of ocular disease and cell culture infection. Cornea. 1995 May;14(3):280–9. doi: 10.1097/00003226-199505000-00009. [DOI] [PubMed] [Google Scholar]
- 167.Sakurai F. Development of a replication-incompetent adenovirus vector derived from subgroup B adenovirus serotype 35. Yakugaku Zasshi. 2008 Dec;128(12):1751–61. doi: 10.1248/yakushi.128.1751. [DOI] [PubMed] [Google Scholar]
- 168.Barouch DH, Nabel GJ. Adenovirus vector-based vaccines for human immunodeficiency virus type 1. Hum Gene Ther. 2005 Feb;16(2):149–56. doi: 10.1089/hum.2005.16.149. [DOI] [PubMed] [Google Scholar]
- 169.Song K, Bolton DL, Wei CJ, Wilson RL, Camp JV, Bao S, et al. Genetic immunization in the lung induces potent local and systemic immune responses. Proc Natl Acad Sci U S A. 2010 Dec 21;107(51):22213–8. doi: 10.1073/pnas.1015536108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ahi YS, Bangari DS, Mittal SK. Adenoviral Vector Immunity: Its Implications and Circumvention Strategies. Curr Gene Ther. 2011 Apr 1; doi: 10.2174/156652311796150372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Shiver JW, Fu TM, Chen L, Casimiro DR, Davies ME, Evans RK, et al. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature. 2002 Jan 17;415(6869):331–5. doi: 10.1038/415331a. [DOI] [PubMed] [Google Scholar]
- 172.Dhaliwal DK, Barnhorst DA, Jr, Romanowski E, Rehkopf PG, Gordon YJ. Efficient reactivation of latent herpes simplex virus type 1 infection by excimer laser keratectomy in the experimental rabbit ocular model. Am J Ophthalmol. 1998 Apr;125(4):488–92. doi: 10.1016/s0002-9394(99)80189-5. [DOI] [PubMed] [Google Scholar]
- 173.Romanowski EG, Araullo-Cruz T, Gordon YJ. Multiple adenoviral serotypes demonstrate host range extension in the New Zealand rabbit ocular model. Invest Ophthalmol Vis Sci. 1998 Mar;39(3):532–6. [PubMed] [Google Scholar]
- 174.Borras T, Gabelt BT, Klintworth GK, Peterson JC, Kaufman PL. Non-invasive observation of repeated adenoviral GFP gene delivery to the anterior segment of the monkey eye in vivo. J Gene Med. 2001 Sep-Oct;3(5):437–49. doi: 10.1002/jgm.210. [DOI] [PubMed] [Google Scholar]
- 175.Yu MJ, Shen WY, Lai MC, Constable IJ, Papadimitriou JM, Rakoczy PE. The role of vascular endothelial growth factor (VEGF) in abnormal vascular changes in the adult rat eye. Growth Factors. 2000;17(4):301–12. doi: 10.3109/08977190009028973. [DOI] [PubMed] [Google Scholar]
- 176.Hamilton MM, Brough DE, McVey D, Bruder JT, King CR, Wei LL. Repeated administration of adenovector in the eye results in efficient gene delivery. Invest Ophthalmol Vis Sci. 2006 Jan;47(1):299–305. doi: 10.1167/iovs.05-0731. [DOI] [PubMed] [Google Scholar]
- 177.Lemckert AA, Grimbergen J, Smits S, Hartkoorn E, Holterman L, Berkhout B, et al. Generation of a novel replication-incompetent adenoviral vector derived from human adenovirus type 49: manufacture on PER.C6 cells, tropism and immunogenicity. J Gen Virol. 2006 Oct;87(Pt 10):2891–9. doi: 10.1099/vir.0.82079-0. [DOI] [PubMed] [Google Scholar]
- 178.Havenga M, Vogels R, Zuijdgeest D, Radosevic K, Mueller S, Sieuwerts M, et al. Novel replication-incompetent adenoviral B-group vectors: high vector stability and yield in PER.C6 cells. J Gen Virol. 2006 Aug;87(Pt 8):2135–43. doi: 10.1099/vir.0.81956-0. [DOI] [PubMed] [Google Scholar]
- 179.Nkolola JP, Peng H, Settembre EC, Freeman M, Grandpre LE, Devoy C, et al. Breadth of neutralizing antibodies elicited by stable, homogeneous clade A and clade C HIV-1 gp140 envelope trimers in guinea pigs. J Virol. 2010 Apr;84(7):3270–9. doi: 10.1128/JVI.02252-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Liu J, O’Brien KL, Lynch DM, Simmons NL, La Porte A, Riggs AM, et al. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature. 2009 Jan 1;457(7225):87–91. doi: 10.1038/nature07469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Barouch DH, Liu J, Lynch DM, O’Brien KL, La Porte A, Simmons NL, et al. Protective efficacy of a single immunization of a chimeric adenovirus vector-based vaccine against simian immunodeficiency virus challenge in rhesus monkeys. J Virol. 2009 Sep;83(18):9584–90. doi: 10.1128/JVI.00821-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Barouch DH, O’Brien KL, Simmons NL, King SL, Abbink P, Maxfield LF, et al. Mosaic HIV-1 vaccines expand the breadth and depth of cellular immune responses in rhesus monkeys. Nat Med. 2010 Mar;16(3):319–23. doi: 10.1038/nm.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hamilton MM, Byrnes GA, Gall JG, Brough DE, King CR, Wei LL. Alternate serotype adenovector provides long-term therapeutic gene expression in the eye. Mol Vis. 2008;14:2535–46. [PMC free article] [PubMed] [Google Scholar]


