While the early stages of melanoma are eminently curable with adequate surgery, patients with metastatic melanoma have a dismal prognosis. None of the currently administered cytotoxic or biological therapies have convincingly been shown to improve overall survival, an experience again re-affirmed by the latest results of the large, randomized phase III trial 18991 of the European Organization for Research and Treatment of Cancer (EORTC) (Eggermont et al., 2008). This growing number of ineffective, but often highly toxic, treatment modalities and the emerging success and addition of molecular targeted agents to the treatment armentarium of other cancers has raised great expectations for similar progress in genotype- and pathway-directed targeted therapy in malignant melanoma.
Microphtalmia-associated transcription factor (MITF) is known to be a major ‘hub’ for the activation of oncogenic survival and proliferation signaling in malignant melanoma. MITF activates multiple downstream mediators critical for normal pigment cell physiology, melanocyte survival, cell cycle regulation, and growth including proto-oncogene Bcl-2, which enhances melanocyte survival, TBX2, a suppressor of senescence, c-met and CDK2, all involved in growth-related signal transduction. MITF also plays a central role in the development and progression of melanoma, either through re-activation of early developmental pathways or dysregulation secondary due to oncogenic signal activation. Overexpression of MITF in conjunction with BRAFV600 mutations leads to malignant transformation of melanocytes, and reduction of MITF activity sensitizes melanoma cells to chemotherapeutic agents (Garraway et al., 2005). The MITF locus on chromosome 3p is amplified in 10 – 20 % of melanoma, a genetic alteration not seen in benign nevi. Amplifications occured twice as common in metastatic tumor compared to primary lesions, and are associated with a poorer prognosis. Furthermore, 20% of metastatic melanoma and 14% of primary tumors harbor somatic mutations in the MITF pathway altering MITF function during melanomagenesis (Cronin et al., 2009).
With MITF playing such a critical role, what are the upstream regulators of this ‘master’ regulator? Multiple signaling pathways are known to regulate expression levels of MITF. These include Notch/Delta, Mc1R/MSH, endothelin receptor B/endothelin 3, c-Kit/SCF, Ras-Raf-Mek-Erk, and Frizzled/Wnt β-Catenin pathways and there are binding sites in the MITF-M promoter for the transcription factors Pax3, Sox10, LEF1, and CREB (Mitra and Fisher, 2009). .There is extensive crosstalk and synergistic signaling between the various pathways and MITF activation. For example, activated Erk is able to both directly phosphorylate MITF which recruits a co-factor to MITF enhancing its effect on downstream targets, as well as to upregulate MITF activity by CREB-mediated promoter activation. Now, a novel pathway has to be added:
In the latest issue of the Proceedings of the National Academy of Sciences, Zhu et al. report on the TAM receptor kinase TYRO3 as a novel upstream regulator of MITF. The authors performed a genome-wide gain-of-function screen by co-transfecting MITF-M promoter-driven luciferase reporter gene as a readout and cDNA expressing (Mammalian Gene Collection) plasmids into B16-F0 mouse melanoma cells. From 263 initial hits, 23 genes showed specificity for the MITF-M promoter but only the TYRO3 gene was exclusively elevated in human melanoma cells and not in other human cancer lines. TYRO3 expression levels were overexpressed (over 3-fold) in 50 percent of 40 tested melanoma samples, and TYRO3 expression levels correlated well with increased MITF expression levels which prompted the authors to hypothesize that TYRO3 is a major upstream oncogenic regulator of MITF in melanoma.
TYRO3 regulates MITF transcription by nuclear translocation of its upstream mediator Sox10 as the authors demonstrate by Sox10 knockdown and various co-localization studies, and Sox10 nuclear translocation and MITF upregulation are dependent on the intact kinase domain of TYRO3. Using TYRO3 knockdown and Ingenuity Pathway Analysis, TYRO3 abolition in melanoma affects common oncogenic pathways like PI3K/Akt, Wnt/β catenin, Erk/MAPK, SPAK/Jnk, Jak/Stat, or TGFβ signalining. The authors show that TYRO3 acts as a major oncogene in melanoma by demonstrating that stably reduced TYRO3 levels in the melanoma line A2058 led a concomitant significant reduction in proliferation of these cells. TYRO3 knockdown led to markedly reduced caspase 3 levels suggesting that TYRO3 has antiapoptotic effects in melanoma. When TYRO3 knockdown was coupled with administration of the cisplatin or docetaxol a significant increase in caspase 3 activity with both agents was observed suggesting that TYRO3 knockdown sensitizes melanoma cells to these chemotherapeutic agents. Co-expression of TYRO3 with the BRAFV600 mutant in primary human melanocytes, which alone induces senescence but not transformation, resulted in new colony formation indicating that TYRO3 overexpression can bypass BRAFV600 induced senescence, a finding in line with the oncogenic synergism of MITF and BRAFV600 in melanoma. Conversely, TYRO3 knockdown in A2058 melanoma cells repressed tumor formation. Finally, the authors reproduced these impressive results in an in-vivo xenograft model: melanoma cells where TYRO3 had been knocked out showed no detectable tumor compared to A2058 cells with wild type TYRO3 expression.
Where do we go from here? As the authors point out, there are still a few ‘gaps’ to be filled in. The exact mechanisms of elevated TYRO3 expression in melanoma remain to be elucidated. It certainly would be interesting to test whether TYR03 is amplified in melanoma cells and indeed it is amplifies, whether these amplifications are found in a mutually exclusive fashion with MITF amplification/somatic mutation. Also, similar to other receptor cell signaling families, there might exist additional upstream regulators of TYRO3 as the recent discovery of cross-talk between TYRO3’s sister receptor Axl and the interleukin-15 receptor or the interaction between Src-family non-receptor tyrosine kinases and Axl1 and TYRO3 suggest (Budagian et al., 2005). Furthermore, it remains to be determined if Sox10 is phosphorylated directly by TYRO3 or if Sox10 nuclear translocation and MITF-M transcription are influenced by other parallel pathways activated by TYRO3. In this line, it also remains to be determined if the observed antiapoptotic effects are directly mediated by TYRO3 activation or also influenced by concomitant activation of the PI3K/Akt pathway or induction of senescence due to increased dominance of BRAFV600 mediated signaling.
Direct inhibition of transcription factors like MITF is difficult although recent data have shown that histone deacetylase inhibitors (HDACi) can suppress MITF levels as well cell proliferation (Yokoyama et al., 2008). A small molecule inhibitor of one of the other three members of the TAM receptor tyrosine kinase family, Axl is currently under preclinical and clinical evaluation (Mahadevan et al., 2007). MP470 reduces the metabolic activity of an Axl-expressing, drug resistant gastrointestinal stromal tumor. Its effect on Mer and TYRO3 receptors are not known, and autoimmune toxicity because of interruption of cell-cell signaling function mediated by TAM receptors is a concern. Nevertheless, rapid progress towards selective inhibitors of TAM receptors either in the form of small molecules, soluble receptors, or antibodies can be expected, and the results of Zhu et al. support the development of therapeutic agents against TYRO3 in melanoma.
Currently, more than 40 major oncogenic pathways have been identified in malignant melanoma (Mitra and Fisher, 2009). On average, these pathways contain between one to three components which are amenable to pharmacologic inhibition. Often there are several compounds for one target available. Considering the improved efficacy of combinational multitarget approaches in knocking down oncogenic signal flow and preventing the development of escape pathways, a plethora of therapeutic opportunities has now arisen which is unprecedented. However, as of September 21, 2009 there are a total 280 trials registered under melanoma (http://www.cancer.gov/clinicaltrials) but only 19 of them are investigating a single agent and only 7 a combination of molecular agent therapies in a phase I/II clinical trial design which are truly novel and are making use of vast molecular information and new taxonomy of cancer which is now available. These numbers fall far short of the therapeutic opportunities we have created. This raises the question if we in fact have the translational and clinical infrastructure to not only get a return from our public research investments but more importantly if we are able to fulfill the obligations and promise we have created to our patients? If we don’t, it is an empty promise.
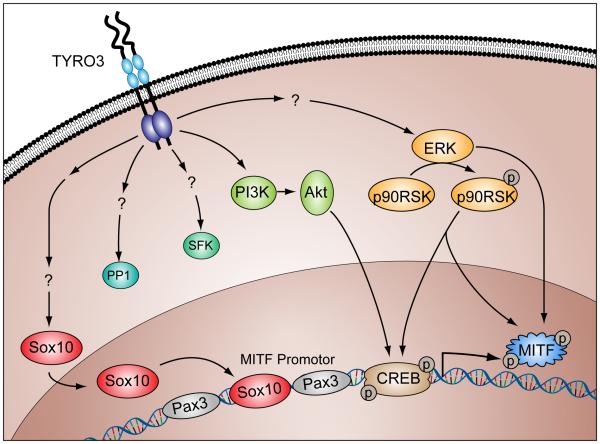
Figure 1.
Multiple TYRO3-activated pathways signaling pathways converge with transcription or activation of MITF (reproduced from Mitra et al (Mitra and Fisher, 2009)), an oncogenic transcription factor often dysregulated in malignant melanoma.
References
- Budagian V, Bulanova E, Orinska Z, Thon L, Mamat U, Bellosta P, Basilico C, Adam D, Paus R, Bulfone-Paus S. A promiscuous liaison between IL-15 receptor and Axl receptor tyrosine kinase in cell death control. Embo J. 2005;24:4260–70. doi: 10.1038/sj.emboj.7600874. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Cronin JC, Wunderlich J, Loftus SK, Prickett TD, Wei X, Ridd K, Vemula S, Burrell AS, Agrawal NS, Lin JC, Banister CE, Buckhaults P, Rosenberg SA, Bastian BC, Pavan WJ, Samuels Y. Frequent mutations in the MITF pathway in melanoma. Pigment Cell Melanoma Res. 2009;22:435–44. doi: 10.1111/j.1755-148X.2009.00578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermont AM, Suciu S, Santinami M, Testori A, Kruit WH, Marsden J, Punt CJ, Sales F, Gore M, Mackie R, Kusic Z, Dummer R, Hauschild A, Musat E, Spatz A, Keilholz U. Adjuvant therapy with pegylated interferon alfa-2b versus observation alone in resected stage III melanoma: final results of EORTC 18991, a randomised phase III trial. Lancet. 2008;372:117–26. doi: 10.1016/S0140-6736(08)61033-8. [DOI] [PubMed] [Google Scholar]
- Garraway LA, Widlund HR, Rubin MA, Getz G, Berger AJ, Ramaswamy S, Beroukhim R, Milner DA, Granter SR, Du J, Lee C, Wagner SN, Li C, Golub TR, Rimm DL, Meyerson ML, Fisher DE, Sellers WR. Integrative genomic analyses identify MITF as a lineage survival oncogene amplified in malignant melanoma. Nature. 2005;436:117–22. doi: 10.1038/nature03664. [DOI] [PubMed] [Google Scholar]
- Mahadevan D, Cooke L, Riley C, Swart R, Simons B, Della Croce K, Wisner L, Iorio M, Shakalya K, Garewal H, Nagle R, Bearss D. A novel tyrosine kinase switch is a mechanism of imatinib resistance in gastrointestinal stromal tumors. Oncogene. 2007;26:3909–19. doi: 10.1038/sj.onc.1210173. [DOI] [PubMed] [Google Scholar]
- Mitra D, Fisher DE. Transcriptional regulation in melanoma. Hematol Oncol Clin North Am. 2009;23:447–65. viii. doi: 10.1016/j.hoc.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Yokoyama S, Feige E, Poling LL, Levy C, Widlund HR, Khaled M, Kung AL, Fisher DE. Pharmacologic suppression of MITF expression via HDAC inhibitors in the melanocyte lineage. Pigment Cell Melanoma Res. 2008;21:457–63. doi: 10.1111/j.1755-148X.2008.00480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]