Abstract
In hypertension, an increase in arterial wall thickness and loss of elasticity over time result in an increase in pulse wave velocity, a direct measure of arterial stiffness. This change is reflected in gradual fragmentation and loss of elastin fibers and accumulation of stiffer collagen fibers in the media that occurs independently of atherosclerosis. Similar results are seen with an elevated level of homocysteine (Hcy), known as hyperhomocysteinemia (HHcy), which increases vascular thickness, elastin fragmentation, and arterial blood pressure. Studies from our laboratory have demonstrated a decrease in elasticity and an increase in pulse wave velocity in HHcy cystathionine β synthase heterozygote knockout (CBS−/+) mice. Nitric oxide (NO) is a potential regulator of matrix metalloproteinase (MMP) activity in MMP-NO-TIMP (tissue inhibitor of metalloproteinase) inhibitory tertiary complex. We have demonstrated the contribustion of the NO synthase (NOS) isoforms, endothelial NOS and inducible NOS, in the activation of latent MMP. The differential production of NO contributes to oxidative stress and increased oxidative/nitrative activation of MMP resulting in vascular remodeling in response to HHcy. The contribution of the NOS isoforms, endothelial and inducible in the collagen/elastin switch, has been demonstrated. We have showed that an increase in inducible NOS activity is a key contributor to HHcy-mediated collagen/elastin switch and resulting decline in aortic compliance. In addition, increased levels of Hcy compete and suppress the γ-amino butyric acid-receptor, N-methyl-d-aspartame-receptor, and peroxisome proliferator-activated receptor. The HHcy causes oxidative stress by generating nitrotyrosine, activating the latent MMPs and decreasing the endothelial NO concentration. The HHcy causes elastinolysis and decrease elastic complicance of the vessel wall. The treatment with γ-amino butyric acid-receptor agonist (muscimol), N-methyl-d-aspartame-receptor agonist (MK-801), and peroxisome proliferator-activated receptor agonists (ciprofibrate and ciglitazone) mitigates the cardiovascular dysfunction in HHcy. Antioxid. Redox Signal. 15, 1927–1943.
Introduction
Cardiovascular disease (CVD) remains the major cause of morbidity and death among all racial and ethnic groups. Collectively, CVDs are characterized by dysfunctional conditions of the heart, arteries, and veins. The classic risk factors that have been used to describe the risk of developing CVD include family history, sex, age, tobacco consumption, high blood pressure, elevated glucose, obesity, lack of physical activity, and abnormal blood cholesterol (82, 83). Interestingly, pathologic and epidemiologic studies suggest that only one-half to two-thirds of the incidence of vascular diseases can be explained by these classic risk factors (64, 80). These findings have provoked a surge of new investigations to identify new emerging risk factors for the development of CVD. Recently, elevated plasma levels of the toxic sulfur-containing amino acid, homocysteine (Hcy), has received a great deal of interest, primarily due to its prevalence in the general population and strong correlation with the development of a number of vascular related diseases (Fig. 1).
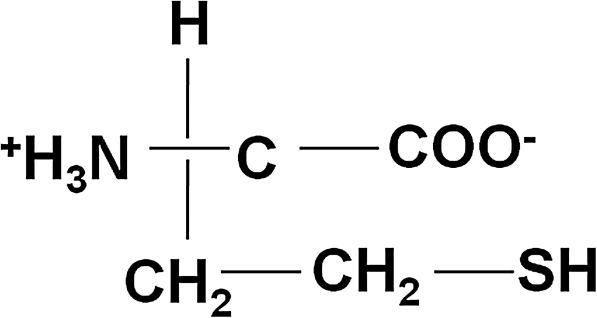
FIG. 1.
Structure of Hcy. A nonprotein amino acid containing a highly reactive sulfur molecule. Hcy, homocysteine.
Historical investigations, dating back to the early 1900s, have proven that excessive circulating plasma homoysteine concentrations, hyperhomocysteinemia (HHcy), are associated with an increased risk for CVD. Hcy was first described in 1932 by Butz and de Vigneaud when they discovered homologs of cysteine and cystine (later denoted Hcy and homocystine) and defined the compounds as having the structure of a sulfhydryl amino acid (6, 12). In 1933, the first description of homocysteinuria was reported as a case study in the New England Journal of Medicine. Researchers described the case of a hyperhomocysteinemic mentally retarded 8-year-old boy who strangely died of a massive heart attack (9). It was not until almost 30 years later that Carson and Neil discovered a biochemical association between elevated Hcy levels and mental retardation in children (8, 20). In 1964, Mudd et al. further expanded the understanding of the clinical effects of Hcy by reporting a causal relationship between thromboembolic events in progressive illness of mentally retarded patients and a deficiency in the Hcy clearing enzyme, cystathionine β synthase (CBS) (44). Soon afterward in 1969, Dr. Kilmer McCully, a Harvard physician, first described the vascular pathology of patients with homocysteinuria that included smooth muscle proliferation, progressive arterial stenosis, and hemodynamic changes. Dr. McCully noted that Hcy may have a causal role independent of any metabolic abnormalities. This was the basis of his Hcy theory of atherosclerosis, which implied that moderately elevated total plasma Hcy (tHcy) was a risk factor for CVD in the low-risk general population (41). In 1976, Wilcken and Wilcken published the first clinical study on coronary artery patients supporting Dr. McCully's theory (79). Since that time the number of clinical and mechanistic studies has increased exponentially and the field of Hcy research has reached new scientific heights.
HHcy is developed by genetic enzymatic deficiencies and/or nutritional defects that interfere with the proper metabolism of methionine and/or Hcy. The increase in extracellular Hcy is toxic to cells and tissues and can initiate a broad array of vascular complications. Recent epidemiologic studies have shown that moderately elevated plasma Hcy levels are highly prevalent in the general population and are associated with an increased risk for fatal and nonfatal CVD, independent of classic cardiovascular risk factors (13). During the past 36 years there have been multiple studies that have unequivocally established that HHcy predicts and precedes the occurrence of CVD, stroke, and thromboembolic disease. HHcy has also been implicated as a pathophysiological mechanism for other disease states that include insulin-resistant diabetes, Parkinson's disease, Alzheimer's disease, schizophrenia, and Down's syndrome.
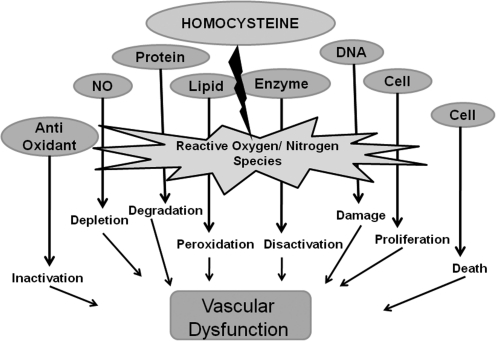
Regardless of whether HHcy is a risk marker or independent risk factor for CVD, it is clearly important to examine the role of Hcy role in mediating vascular dysfunction. This research project addressed the intricate and detrimental cascade of intracellular events caused by Hcy that eventually lead to vascular dysfunction and eventual CVD. This research addresses Hcy-mediated oxidative toxicity and the contribution of endogenous nitric oxide (NO) synthesis and matrix metalloproteinase (MMP)-mediated vascular remodeling (see Toxic Triad; Fig. 2). We believe that these three factors are the primary pathological mechanisms underlying Hcy-mediated vascular diseases. Therefore, these studies attempted to bring clarity concerning the differential production and bioavailability of NO and its potential role in initiating oxidative stress (reactive oxygen and nitrogen species [ROS/RNS]), elevated MMPs activity, and subsequently Hcy-mediated vascular dysfunction.
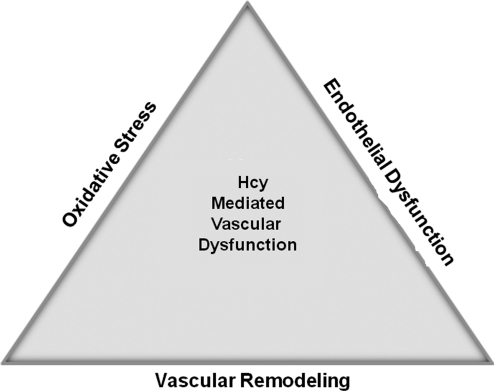
FIG. 2.
The Toxic Triad. The three pathological mechanisms—oxidative stress, endothelial dysfunction, and vascular remodeling—underlying Hcy-mediated vascular diseases.
Vessel Physiology
Homeostasis is defined as the maintenance of the internal fluid environment that bathes the body's cells by highly coordinated and regulated actions of the body systems bringing about relatively stable chemical and physical conditions. The human cardiovascular system contributes to homeostasis by transporting nutrients, oxygen, carbon dioxide, wastes, electrolytes, and hormones to and from various parts of the body for the survival of all cells. The cardiovascular system is comprised of three basic components, the heart, blood vessels, and blood.
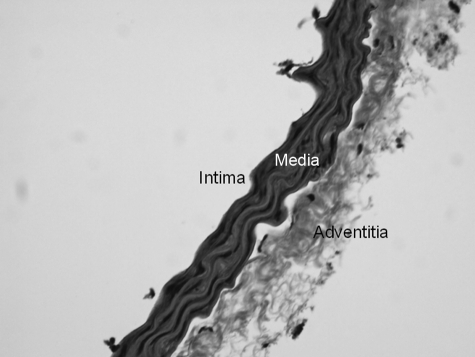
The transport of blood through the cardiovascular system can be sectioned into three divisions, an arterial distribution system, a capillary exchanges system, and a venous return system. The structure of a blood vessel wall consists of three primary layers, intima, media, and adventitia (Fig. 3). The intima is the semipermeable inner most continuous, single layer of squamous endothelial cells (ECs) lining the luminal face of blood vessels. The ECs are oriented parallel to the length of the vessel, tightly fit together, and they communicate via junctional components that allow electrical and chemical coupling. The intima is surrounded by a prominent thin layer of internal elastic lamina. The medial muscle layer, the thickest layer, consists of circular vascular smooth muscle cells (VSMCs) that serve as the contractile components of blood vessels. Smooth muscle cells do not have sarcomere like cardiac myocytes; however, they have actin and myosin filaments that are responsible for cell contraction. The outer most fibroelastic adventitial layer is the strong protective outer covering that is made of up connective tissue and fat. It is the site of neural innervations and functions to anchor vessels to the nearby tissue. Interestingly, each layer has associated elastic and collagen fibers (composing the extracellular matrix [ECM]) that allow the vessel to stretch and recoil in response to changes in intravascular pressures. Blood vessels actively engage in maintaining blood pressure and blood flow distribution. This is achieved through the coordination of the autonomic nervous system, hormonal control, and endothelial factors to mediate vascular constriction and dilation.
FIG. 3.
Mouse aorta stained with hematoxylin and eosin. 20 × magnification.
Much of the research reported in this study deals with the aorta, the largest artery in the circulatory system that serves as the central conduit from the heart to the body. It originates at the left ventricle of the heart and ascends, arches, and then descends through the chest on the left side of the vertebral column and passes into the abdominal cavity through the aortic hiatus in the diaphragm. The aorta supplies the head and neck as well as the major organs in the chest, abdomen, and legs with oxygenated blood for their nutrition. To withstand varying differences in transmural pressure, in vivo, the aorta possesses highly elastic properties allowing it to stretch.
The ability of a vessel to expand in response to increasing pressure is referred to as vessel compliance (C). It is the change in volume (ΔV) divided by the change in pressure (ΔP) (Equation 1). The elastin and collagen fibers aid in determining the compliance of the vasculature. In the vessel wall, elastin fibers account for most of the stretch and collagen the tensile strength. The use of the volume/pressure curve is a convenient method to examine the relationship of pressure to volume in a vessel. At rest, an aorta is highly compliant. In vivo, the aorta is able to passively increase its volume by increasing its radius to accommodate the increase in transmural pressure of the blood being ejected from the left ventricle of the heart, which moderately decreases compliance. The elastic properties of the vessel wall allow the vessel to take on more pressure and increase its volume by increasing the radius of the vessel wall, which in turn increases wall tension. However, if the elastic properties of the aorta are decreased, the compliance will be decreased as well, resulting in an increase in vessel wall tension, arterial pressure, and resistance, along with decreased radial and volume response. Smooth muscle cells contract to increase and maintain vascular tone but in turn reduce vascular compliance. Contraction of smooth muscle can thereby decrease arterial blood volume and increase arterial pressure. Equation 1: C = ΔV/ΔP
Table 3.
Homocysteine Autooxidation
| Equation 1: Hcy + Mn+ → M(n−1) + Hcy• |
| Equation 2: M(n−1)+ + O2 → Mn+O2•− |
| Equation 3: Hcy• + Hcy → Hcy-SS-Hcy•− |
| Equation 4: Hcy-SS-Hcy•− + O2 → Hcy-SS-Hcy + O2•− |
Hcy autooxidation is catalyzed by transition metal ions when exposed to plasma leading to the generation of superoxide in reactions 1–4. Hcy-SH, free Hcy; Mn+, transition metal; Hcy•, thiyl radical; Hcy-SS-Hcy•−, Hcy radical intermediate; O2, oxygen; O2•−, superoxide.
Homocysteine
There are more than 20 epidemiologic studies (e.g., 13, 82, 83), showing that moderately elevated plasma Hcy levels are highly prevalent in the general population and are associated with an increased risk for fatal and nonfatal CVD, independent of the classic cardiovascular risk factors (13). Over the past 36 years, there have been more than 100 clinical studies that have unequivocally established the relationship between Hcy and diseases of the coronary, cerebral, and peripheral arteries and veins (56). McCully et al. suggested that nearly 50% of the U.S. population have elevated circulating Hcy levels (40). Together, these studies undoubtedly point to Hcy as a novel and widely occurring independent risk factor that predicts and precedes the occurrence of CVD, thus giving reason to better understand Hcy and its metabolism.
Metabolism
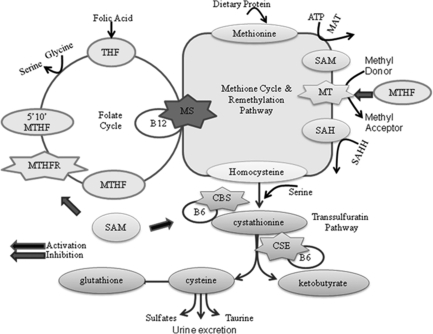
Methionine is an essential amino acid that is derived primarily from the diet. It occurs naturally in all food high in protein and is also derived from the endogenous breakdown of proteins. It is metabolized in the methionine cycle that functions to provide one carbon methyl unit for transmethylation reactions. The important methyl groups are responsible for over 400 identified biochemical reactions, including methylation and synthesis of nucleic acids (DNA and RNA), proteins, phospholipids, myelin, polysaccharides, choline, and catecholamine. Methionine also serves as a source of sulfur for the synthesis of cysteine and taurine. For these reasons optimal methionine metabolism is vital. Hcy, a toxic non-protein forming sulfur-containing amino acid, is formed as a primary intermediate during the metabolism of methionine (48). The daily dietary intake only provides a limited supply of methionine and synthesis of new methionine is considered energetically expensive. Therefore, the ability of a cell to salvage this amino acid from Hcy is extremely important for optimal cellular function and survival. The interconnected pathways of methionine and Hcy metabolism can be split into three essential pathways: methionine cycle, remethylation, and transsulfuration (Fig. 4).
FIG. 4.
Methionine-Hcy metabolism. A representation of the methionine cycle, remethylation pathway, transsulfuration pathway, and connection to the folate cycle. All Hcy found in the human body is formed during the metabolism of methionine in the methionine cycle. Hcy is metabolized in the remethylation and transsulfuration pathways. CBS, cystathionine β synthase; CSE, γ-cystathionase; MS, methionine synthase; MT, methyltransferase; MAT, methionine adenosyltransferase; MTHFR, methylene tetrahydrofolate reductase; SAHH, S-adenosylhomocysteine hydrolase; SAM, S-adenosylmethionine.
In the methionine cycle, methionine is intracellularly converted to S-adenosylmethionine (SAM) in a reaction catalyzed by ATP and the enzyme methionine adenosyltransferase. Methionine provides the one-carbon units for SAM-dependent methyl reactions (3). SAM serves as a primary intracellular methyl donor for reactions catalyzed by methyltransferases (MTs) and is the principal metabolic regulator of the flow of Hcy between the remethylation and transsulfuration metabolic pathways (16). After a series of MT reactions, SAM is converted to S-adenosylhomocysteine (SAH). This reaction is subject to competitive product inhibition because SAH has a higher affinity for the MT active site than SAM (29). SAH is then reversibly hydrolyzed by SAH hydrolase yielding adenosine and Hcy. Interestingly, the equilibrium dynamics of this reaction favors SAH production; however, the efficient rapid metabolic advancement of the pathways, in particular the transulfuration pathway, drives the reaction to Hcy synthesis (16, 18). At this point, Hcy is at the critical intersection of two metabolizing pathways: remethylation and transsulfuration.
Under conditions of negative methionine balance, Hcy is metabolized through the methionine conserving or salvaging remethylation pathway that is widely distributed in all tissues, with greater activity in the human liver and kidney (13). This pathway utilizes a folate coenzyme system (the folate cycle) to provide new methyl groups to regenerate methionine. Remethylation is achieved by the enzyme methionine synthase (MS, 5-methyltetrahydrofolate-homocysteine methyltransferase) that uses cofactor vitamin B12 (cobalamin) and 5-methyltetrahydrofolate (5-MTHF) as the methyl donor. MS transfers a methyl group from the 5-MTHF to Hcy, regenerating methionine, vitamin B12, and THF. The efficient activity of this pathway requires an adequate supply of folic acid and the enzyme MTHF reductase (MTHFR) (17). After remethylation, the newly recycled methionine can be used in the protein synthesis of taurine or again be reconverted to SAM for another round of methyl reactions. The sufficient remethylation of Hcy is essential to continuously supply diet-derived methionine and maintain Hcy and SAM at low concentrations. Accordingly, this pathway is regulated by the SAM/SAH ratio. If there is a disturbance in Hcy remethylation, SAH will accumulate, leading to a decrease in the SAM/SAH ratio that will result in the inhibition of most methionine adenosyltransferases, decreased SAM production, and decreased remethylation (29).
When methionine or cysteine synthesis is required, Hcy enters the catabolic transsulfuration pathway. This pathway occurs exclusively in the liver, kidney, small intestine, and pancreas and is not found in human cardiovascular cells and tissues. This is the only pathway capable of ridding the body of excess toxic sulfur-containing amino acids, including increased circulating Hcy (61). In the first reaction, Hcy is condensed with serine to form cystathionine in an irreversible reaction catalyzed by the vitamin B6 (pyridoxal-5′-phosphate)-dependent enzyme CBS. Cystathionine is subsequently hydrolyzed by vitamin B6-dependent γ-cystathionase to form cysteine and α-ketobutyrate. In the liver and kidney, ∼50% of Hcy is used for the production of cysteine (32), which has a central role in a number of important biosynthetic pathways, including serving as a precursor for the production of glutathione and being utilized in the synthesis of proteins such as taurine. Cysteine can also be further degraded into inorganic sulfate and excreted in the urine (17).
Under normal physiological and metabolic conditions, the two pathways are under strict balance, working synergistically to maintain low levels of intracellular and tissue concentration of Hcy. If there is a deficiency in the dietary intake of methionine, more Hcy will enter the remethylation pathway to salvage methionine. Approximately 50% of formed Hcy is remethylated to methionine (48). When there is excessive methionine intake, a larger percentage of the Hcy is degraded by the transsulfuration pathway (74). However, if there is an increase in Hcy relative to its consumption, it will build up in the cell and spill out into the plasma, urine, and tissues. This will lead to vascular toxicity resulting in the initiation of a broad array of aberrant vascular complications.
Total Hcy
Hcy exists in the human plasma in various forms (Table 1). Majority of clinical studies involving Hcy have relied on the measurement of tHcy, which includes Hcy and its derivative disulfides (mixed disulfides, Hcy thiolactone, and protein-bound Hcy) (76). In the plasma, ∼70% of the tHcy circulates bound to proteins (mainly albumin). The binding is through disulfide bonds with protein cysteine residues (43). Approximately 25% of the Hcy binds with itself to form the dimer homocystine, whereas the remainder (<5%) binds with other thiols, including cysteine, to form a Hcy-cysteine disulfides mix. This segment includes the reduced Hcy, which accounts for 1%–2% of the tHcy (53), and the oxidized form, which accounts for the remainder.
Table 1.
Homocysteine Terminology, Derivatives, and Concentrations in Normal Human Plasma
 |
Hcy, homocysteine; RSH, alkyl thiol.
There are many factors that are used to predict tHcy levels. Blood tHcy levels increase with age (49). The tHcy levels progressively increases with age, due to the decline in Hcy metabolism in the kidney (49). Women have lower tHcy than men, which could be partially due to the influence of sex hormones (estrogen and progesterone) and menstruation (49). Men have 10% higher plasma values of Hcy, which appear to be partly due to a greater muscle mass and an increased production of creatine/creatinine, a rich source of Hcy. Interestingly, the differences observed between males and female disappear after menopause (81). Lifestyle determinants such as smoking, lack of exercise, caffeine, and chronic alcohol consumption have also been associated with higher tHcy levels (47, 49). Data from the Hordaland Homocysteine Study indicate that smoking and heavy caffeine consumption are associated with elevated tHcy, whereas exercise may reduce tHcy (49). Genetic determinants such as inborn errors of Hcy metabolism, including CBS and MTHFR deficiencies, are associated with severe HHcy. Furthermore, a few prescription drugs have been shown to influence tHcy levels. Studies have shown that the antidiabetic drug metformin decreases folate and vitamin B12 levels (7). Additionally, anticonvulsive and hypolipidemic drugs have been reported to increase tHcy levels. On the preventative side, antirheumatid drug, penicillamine (60), and mucolytic drug, acetylcysteine (78), have been associated with a decrease in tHcy and have been suggested as possible treatments for elevated tHcy.
HHcy is the consequence of enzymatic deficiencies and/or nutritional defects that interfere with the proper metabolism of methionine and/or Hcy. HHcy is considered a biochemical abnormality rather than a specific disease. Normal tHcy concentration ranges from 5 to 15 μM in the fasting state (33). Higher fasting values are arbitrarily classified as mild (15–30 μM), intermediate (30–100 μM), and severe (>100 μM) HHcy (36). Extremely severe cases such as homocysteinuria are defined as extreme elevated plasma or serum tHcy level due to an inborn error in Hcy metabolism resulting in Hcy in the urine. Under these circumstances, plasma levels can reach above 400 μM and unfortunately 20% of the patients die before 30 years of age (44).
Development of HHcy
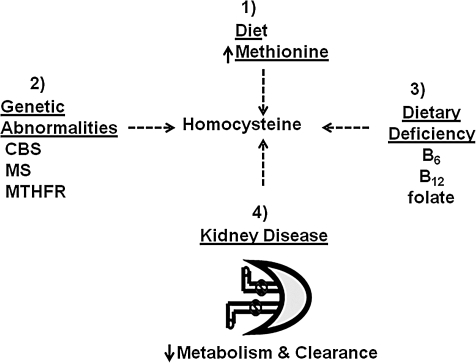
There is a complex interaction between multiple genetic, metabolic, and environmental factors that must remain in balance to keep Hcy levels low. A single or combination of changes in these factors can greatly influence an increase in tHcy. There are four major disorders that have been heavily studied that explain the development of HHcy: (i) methionine rich diet; (ii) vitamin deficiencies (B12, B6 and folate); (iii) genetic abnormalities (CBS, MS, and MTHFR); and (iv) decreased renal function (Fig. 5). Additionally, there are other genetic, physiologic, pathologic, and nutritional factors that cause elevations in Hcy, including and not limited to hypertension, diabetes, and vegetarianism.
FIG. 5.
The development of hyperhomocysteinemia. The four pathways of Hcy accumulation: (1) methionine-rich diet; (2) genetic abnormality in MTHFR, MS, CBS, and MAT; (3) dietary deficiency in vitamin cofactors folate, vitamin B6, and vitamin B12; (4) kidney disease.
Methionine-rich diet
Hcy metabolism is nutritionally regulated by controlling the dietary intake of the essential amino acid methionine that is responsible for supplying sulfur, one-carbon units, and other elements used by the body for metabolism and growth. Meat, fish, and dairy products are all good sources of methionine. The average daily intake of methionine is 2.3 g/day (15.4 mmol/day) for men and 1.6 g/day (10.7 mmol/day) for women in the United States. The typical low basal level of methionine favors several cycles of Hcy remethylation then catabolism. Interestingly, half of the dietary methionine is converted to Hcy (71), and excessive dietary intake, for example high meat intake, can be extremely toxic causing an increase in tHcy levels. The mammalian liver has an elaborate system to dispose of the excessive Hcy, but the system's activity is short lived. Increased Hcy will then result in an increase in intracellular SAM levels (3). SAM acts to both reduce Hcy remethylation by inhibiting MTHFR and to activate CBS and thus increase Hcy catabolism via the transsulfuration pathway (34). At this point there is a decreased need for Hcy remethylation due to the abundance of available methionine and the methyl donor SAM. As a result of SAM accumulation, it will also bind to MTs with higher affinity than SAH and inhibit intracellular methylation. Over time, the stressed transulfuration pathway is overwhelmed and Hcy continues to uncontrollably build up, leading to a great metabolic consequence. For these reasons, methionine loading is a reliable and sensitive method of testing for disturbances in the Hcy metabolic pathways (5). Several studies have shown that tHcy levels were elevated postmethinioine loading in subjects with normal fasting tHcy levels, suggesting problems in Hcy transulfuration (25). Animal studies suggest that diets high in methionine, in the presence of vitamin B6, B12, and folic acid deficiencies, may increase the risk for atherosclerosis and CVD by increasing blood levels of cholesterol and Hcy. It is strongly suggested that a diet rich in fruit and vegetables limits methionine and Hcy levels and reduces the risk of developing hypertension and other CVD (2).
Genetic abnormalities
Genetic abnormalities in the enzymes responsible for Hcy metabolism, MS, MTHFR, and CBS, however rare, are able to produce a more severe elevation in tHcy levels, and the severity is dependent on the site of the gene mutations. MTHFR is a cytoplasmic enzyme that reduces 5, 10-MTHF to 5-MTHF, the methyl donor for the remethylation pathway. A homozygous deficiency in MTHFR interferes with regeneration of methionine due to decreased availability of the methyl donor MTHF resulting in an increased intracellular Hcy level and HHcy (45). MTHFR deficiency is also commonly associated with impaired folate status. When left untreated, a person can experience premature vascular episodes (stroke, heart attack, and thromboembolic complications) before the age of 30 years (44).
MS is responsible for the regeneration of methionine from Hcy. Diminished activity of MS due to a genetic abnormality impairs the remethylation of Hcy and increases the intracellular Hcy levels by SAM-dependent mechanisms. Without methionine regeneration there is a reduction in SAM concentrations causing a decrease in SAM-dependent regulation of 5-MTHF levels and allosteric SAM-regulated CBS activity. A fully active MTHFR, a consequence of decreased SAM, causes 5-MTHF accumulation (3). This decreases intracellular folate levels and the distribution of folate derivatives by restricting the incorporation of MTHF from the blood into cellular folate pools, thus decreasing nucleotide biosynthesis (22). For this reason MS is known to be a major player in folate metabolism converting 5-MTHF to more useful forms such as THF (3). Moreover, a decrease in SAM concentration also decreases CBS activity, funneling Hcy into the inactive remethylation pathway.
CBS deficiency is the most common genetic and identifiable cause of severe hereditary HHcy (76) and is considered a rare recessive autosomal disease. In the transsulfuration pathway, CBS is responsible for catabolism of Hcy by condensing it with serine to form cystathionine. Upon CBS inhibition, Hcy is diverted into the remethylation pathway increasing methionine synthesis, leading to an increase in intracellular SAM that will eventually feedback and inhibit MTHFR, thus inhibiting remethylation pathway. Consequently, both pathways are impaired and Hcy is severely increased. The homozygous CBS form of this disease, congenital homocystinuria, can be associated with the presence of Hcy in urine and plasma Hcy concentration of up to 400 μM during fasting (44). Homozygous individuals are rare (1:200,000 births) and develop early childhood fatal thrombotic vascular disease (38). Heterozygote CBS typically have much less marked HHcy, with plasma Hcy concentration in a range of 20–40 μM (38). Clinical conditions include osteoporosis, skeletal abnormalities, mental retardation, and premature vascular episodes (39). In mice with genetic deficiency of CBS, HHcy is associated with impaired endothelial function and abnormal lipid metabolism.
Dietary deficiencies
There is particular interest in the nutritional deficiencies because the vitamin-derived cofactors facilitate and aid in the regulation of optimal Hcy metabolism. Dietary intake and plasma levels of folic acid, vitamin B12, and vitamin B6 are inversely related to tHcy concentration, and a deficiency in either can result in moderately elevated tHcy level. Many studies have argued that most individuals with HHcy also have inadequate concentrations of one or more of the required vitamin cofactors (54). These deficiencies are highly prevalent due to poor dietary intake and may account for the majority of moderate HHcy cases. Interestingly, vitamin therapy has been shown to reduce or normalize elevated plasma Hcy levels (75), though further evaluation of its effectiveness is warranted.
Folic acid is a form of the water-soluble vitamin B9 and has the most effect on Hcy metabolism likely due to its dietary need. It is necessary for the production of new cells especially during a period of rapid cell division and growth such as during pregnancy. A deficiency in folic acid, the most common cause of mild to moderate HHcy, impairs the folate coenzyme system by decreasing MTHF synthesis and inhibiting methyl donation, thus impairing the remethylation pathway. Insufficient dietary intake of folic acid has been strongly associated with birth defects.
Vitamin B12 is important in the normal function of the brain and nervous system and is also involved in DNA and fatty acid synthesis. A deficiency in B12 renders MS inactive compromising the cell's ability to remethylate Hcy. A vitamin B12 deficiency can also cause 5-MTHF accumulation at the expense of intracellular folate pool (3). Decreased vitamin B12 has been associated with Alzheimer disease, clinical depression, and bipolar disease.
Vitamin B6 acts as a coenzyme in the metabolism of several amino acids from glucose during gluconeogenesis. Many studies have found a weak association to basal tHcy. A deficiency in vitamin B6 inhibits the activity of CBS, therefore inhibiting the catabolism of Hcy. A decrease in vitamin B6 has been associated Parkinson's disease and autism.
Impaired renal function
The kidneys are a major site of Hcy metabolism possessing all three metabolizing enzymes: MS, CBS, and γ-cystathionase (16). Deranged renal function is associated the mild HHcy; however, the causes are unclear. Studies by Guttormsen et al. demonstrated that the hepatic metabolic removal of Hcy load is greatly impaired in end stage renal disease due to impaired transsulfuration pathway (24). HHcy is also a consequence of several addition proposed mechanisms, including genetic factors associated with Hcy metabolizing enzymes, impaired vitamin cofactors, and uremic toxicity (19).The vast majority of circulating Hcy is removed by metabolic processes, whereas a small fraction remains free and small enough to be filtered by the glomerulus and minimally excreted (∼1%) in the urine daily (18). It has been proposed that HHcy is a consequence of reduced renal excretion of Hcy in a damaged kidney (76) even though other studies report the contrary (31).
Vascular effects of HHcy: cellular and molecular mechanisms
Moderate HHcy has been accepted as an independent risk factor for atherosclerosis and CVD (14, 53). If by chance there is a disruption in methionine and/or Hcy metabolism, intracellular Hcy levels will increase and significant amounts of the amino acid consequently spills out of the cell and accumulates in the surrounding tissue. Plasma and tissue Hcy concentrations then rise and over time vascular complications ensue (70). Observations in clinical and animal studies have identified potential pathophysiological targets where Hcy exerts its damaging effect. Those targets include ECs, VSMCs, connective tissue, platelets, coagulation factors, lipids, and NO signal transduction molecules. Unfortunately, there is not an established, unifying hypothesis by which Hcy evokes vascular damage. However, there are numerous biological and biomolecular mechanisms that have been heavily studied and proposed to explain the pathological changes associated with elevated tHcy levels. It has been suggested that Hcy initially attacks the vascular endothelium and starts the negative cascade of vascular complications, including the retardation of endothelium growth. There is strong documented evidence supporting the clinical relevance of Hcy as a risk factor for the development of CVD and it is therefore important to examine each major proposed molecular and cellular mechanism and their potential role in Hcy-mediated vascular dysfunction (Table 2).
Table 2.
Mechanisms of Hyperhomocysteinemia-Induced Pathogenesis
| Molecular effects of HHcy | Cellular effects of HHcy |
|---|---|
| Decreased NO production | Endothelial dysfunction |
| Decreased NO bioavailability | Impaired EC vasorelaxation |
| Oxidative stress | Mitochondrial damage |
| Lipid peroxidation | SMC proliferation |
| Coagulation | ECM degradation |
| Clotting | DNA and RNA damage |
| Inflammation | Apoptosis |
HHcy, hyperhomocysteinemia; NO, nitric oxide; EC, endothelial cell; SMC, smooth muscle cell; ECM, extracellular matrix.
Oxidative stress is possibly the most detrimental stressor in the pathogenesis of most diseases. Studies have shown that the pro-oxidative Hcy exerts direct biological damage to vascular cells and tissue through an oxidative mechanism that damages lipids, nucleic acids, and proteins (40). Hcy itself has the ability to generate potent ROS when oxidized due to its highly reactive sulfhydryl group. In the circulation, this thiol undergoes rapid metal-catalyzed sulfhydryl auto-oxidation, leading to the generation of superoxide and hydrogen peroxide. In addition to auto-oxidative ROS production, decreased Hcy clearance and resultant accumulation further increases the production of harmful oxidants that collectively spurs on the negative cascade of vascular complications. Hcy has a detrimental effect on vascular cells and tissues as a result of oxidative damage to lipids, nucleic acids, and proteins.The injurious oxidative stress exerted by Hcy is also thought to have an indirect effect on vascular redox reactions by diminishing the expression and activity of the antioxidant enzymes superoxide dismutase (SOD) and glutathione peroxidase.
The endothelium is composed of ECs that line all blood vessels (vascular endothelium) and the innermost layer of the heart (endocardial endothelium) and is responsible for managing vasomotor function, cellular growth, platelet adhesion, coagulation, and immune function. Endothelial dysfunction, defined as the impairment of normal homoeostatic properties of the vascular endothelium, is thought to be the initial and significant event in the development of vascular diseases. Direct toxic EC damage and endothelium dysfunction mediated by Hcy has been related primarily to and initiated by the generation of potent ROS (27, 46). Oxidative radicals are responsible for diminished production and bioavailability of endothelial-derived NO that renders ECs incapable of responding to various stimuli causing impaired endothelial-dependent vascular reactivity (27). In addition, radicals promote exacerbated cell proliferation and increase platelet aggregation, coagulation of blood, and formation of blood clots, all of which are associated with the progression severe vascular disorders and diseases.
As a consequence of Hcy-produced oxidative radicals, there is a critical impairment in the production of endothelium derived NO by several different mechanisms. Direct Hcy-induced EC damage or cell death would naturally decrease the endothelium's ability to produce and release NO. Studies have shown that Hcy suppresses NO production without altering NO synthase (NOS) protein levels or enzyme activity and the Hcy-induced endothelial injury and subsequent reduction in NO production are primarily associated with increased ROS levels (35). Hcy promotes the oxidation of the endothelial NOS (eNOS) cofactor, tetrahydrobiopterin (BH4), resulting in the uncoupling of the NOS enzyme, spontaneous oxygen radical synthesis, and decreased NO production. A reduction in BH4 availability and the uncoupling of eNOS mediated by Hcy are significant in Hcy-mediated oxidative stress. Hcy is also known to increase asymmetrical dimethylarginine (ADMA), an endogenous inhibitor of eNOS, thus decreasing NO production. It has also been proposed that Hcy can increase NO production by increasing the activity of neural NOS and inducible NOS. Although the increase NO can be biologically positive, the increase of NO in a highly oxidative environment, such as the case in HHcy, can lead to more peroxynitrite (ONOO−) formation.
Hcy indirectly decreases NO bioavailability by generating superoxide that rapidly consumes NO resulting in the generation of ONOO−, therefore reducing NO bioavailability (15). ONOO− is a potent RNS and at high levels can account for a significant proportion of NO deficit and cytotoxic damage mediated by Hcy. Nitrotyrosine is then formed when ONOO− nitrates tyrosine residues on proteins (67). In bovine aortic ECs, Hcy decreased NO bioavailability independent of eNOS protein expression but did decrease NO bioactivity through a mechanism involving increased ROS production (76).
In the walls blood of vessels, NO contributes to the regulation of systemic blood flow and pressure by activating intracellular signaling pathways that modulate calcium levels in VSMC resulting in vasodilation. Hcy is known to decrease vascular function by the oxidative depletion of biologically active NO. Several studies in human and animals models have demonstrated that elevated Hcy levels impaired endothelium-mediated vasodilation. In isolated aortic rings treated with Hcy, there was an attenuation of aortic relaxation to the endothelium-dependent agonist acetylcholine. NO-mediated vasodilation was impaired in a dose response manner in healthy subjects during short-term HHcy-induced by methionine loading. Tawakol et al. assessed brachial artery diameter in elderly subjects in response to reactive hyperemia (65). Researchers saw a significant decline in endothelial-dependent vasodilation in HHcy subjects compared to control. In humans, vascular reactivity can be assessed by the perfused forearm technique to measure flow-mediated endothelium-dependent dilation. In normotensive HHcy patients there was a significant reduction in vasodilation to acetylcholine compared to normo-homocysteinemic subjects. This study confirmed the previously observed impairment of endothelium-dependent vasodilation in conduit vessels of normotensive individuals (65). These impairments are associated with other cardiovascular risk factors, including hypertension and diabetes.
Hcy has also been shown to cause striking changes in vessel wall structure by inducing ECM alterations that fragment the arterial internal and medial elastic lamina (44, 46). An increase in intimal redox stress accompanied by decreased NO availability will lead to the increased activity of MMPs that are responsible for the degradation of ECM components. Hence, Hcy provokes MMP-mediated deterioration of elastic structures and increased deposition of stiff collagen causing the remodeling of the vessel wall and resultant vessel diseases (26).
Unlike the growth retardation of the endothelium, Hcy has been associated with myointimal hyperplasia and VSMC hypertrophy, prominent features in atherosclerosis (55). However, the source of stimulation is not completely known. It has been shown that Hcy stimulates VSMC proliferation by the activity of Hcy-generated oxygen radicals that activate cytokines that are active in the initial phase of the proliferative process. It has also been reported that the Hcy-mediated reduction in NO synthesis and release by injured ECs causes the release of growth factors that provoke proliferation of nearby VSMC (66). In a study by Tsai et al., there was a synergistic increase in DNA synthesis and cycling of mRNA in both rat aortic smooth muscle cells and human aortic smooth muscle cells treated with biological levels of Hcy (66).
The erratic activity of the tightly regulated process of inflammation is considered to have a key role in the pathogenesis and progression of atherosclerosis. Studies have indicated that Hcy initiates and enhances chronic vascular inflammation of the vessel wall through the enhanced expression and activity of various inflammatory mediators along the inflammatory cascade. Hcy has been shown to initiate the process by increasing the expression and plasma levels of the inflammatory cytokine, tumor necrosis factor alpha (TNF-α), and enhancing the activation of a redox-sensitive nuclear inflammatory transcription factor, nuclear factor-kappa B (NFκB), in the vasculature. Hcy stimulates interleukin-6 (IL-6) expression in rat VSMC through the activation of NFκB, and this increase in IL-6 exacerbates homeostatic inflammation and subsequent cellular dysfunction. The inflammatory process is further promoted by increasing the expression of the receptor for advanced glycation end- products (RAGE) as a result of the formation and accumulation of its signal-transducing ligand, EN-RAGE (30).
It has long been believed that Hcy may cause lipid peroxidiation by an oxidation-dependent pathway. Hcy generates oxidative radicals that initiate oxidative degradation of lipids on the EC surface (28), which causes loss of membrane function and increased permeability (27). Clinical data have shown that patients with HHcy have an increase in end products of lipid peroxidation such as F2-isoprostanes and malondialdehyde. After supplementation of folic acid and antioxidants, Racek et al. reported a significant reduction in plasma malondialdehyde in HHcy patients (52). Hcy-generated oxidative radicals are also capable of oxidizing low-density lipoproteins in the plasma. Heinecke et al. observed that Hcy formed sulfur-centered radicals that directly modified low-density lipoproteins in cultured human skin fibroblasts incubated with thiol (28).
Endothelial cells also possess several antithrombotic mechanisms to protect against intravascular thrombosis. However, elevated plasma Hcy levels have been reported to cause an imbalance in coagulant and clotting properties toward a prothrombotic state in coronary and peripheral disease that is primarily mediated by the endothelial dysfunction (11). A decrease in NO bioavailability influences platelet activation and increased von Willebrand factor expression that promotes platelet adhesion (57). Hcy also increases platelet activation and aggregation by increasing thromboxane formation and activity on the cell surface, resulting in enhanced interaction between platelets and the vessel wall. At the same time, oxidatively perturbed ECs further initiate pro-coagulant activity by increasing the release of tissue factors, such as factor V, (32) that converts prothrombin to thrombin, and inhibiting thrombomodulin cell surface expression that activates thrombin and decreases protein C activation (57).
Finally, HHcy also causes prolonged vascular damage by inducing programmed cell death in many cell types by activating various death signaling pathways. The mechanisms by which Hcy induces cell death are varied. In ECs, Hcy has been shown to activate the Fas cell death pathway, the p53/Noxa pathway, and the cytochrome-C activated caspase 3 and 9 pathway. Hcy has also been found to have adverse affects in the heart. Levrand et al. found that in adult rat ventricular cardiomyocytes, Hcy toxicity activated the caspase-3/poly (ADP-ribose) polymerase (PARP) pathway leading to DNA damage and cell death (36). They also observed the ONOO−-mediated activation of extracellular signal-regulated kinase/c-Jun NH2-terminal kinase death pathway. Interestingly, Hcy is also known to interfere with endoplasmic reticulum processing of proteins resulting in activation of unfolded proteins and endoplasmic reticulum stress followed by apoptosis (32). A study reported that Hcy-induced oxidative apoptosis was partially attenuated by blocking nicotinamide adenine dinucleotide phosphate-oxidase (NADPH oxidase) in human umbilical vein ECs.
Hcy-Mediated Oxidative Stress
Many common and life-threatening human diseases, including atherosclerosis, Alzheimers disease, cancer, and aging, have free radical reactions as an underlying mechanism of injury. A free radical is defined as any atomic or molecular species capable of independent existence that contains one or more unpaired electrons, causing the molecule to be unstable and reactive. The intracellular source of free radicals includes but is not limited to, normal products of mitochondrial respiration, NADPH oxidase, NOS, cycloxygenases, lipoxygenases, cytochrome P-450, monooxygenases, and xanthine oxidase. Oxygen is utilized by various enzymes as an electron acceptor and reactions lead to the formation of a series of ROS such as superoxide anion (O2−), hydrogen peroxide (H2O2), and hydroxyl radical (•OH) (77). These highly unstable molecules tend to react rapidly with adjacent molecules, donating, abstracting, or even sharing their outer orbital electrons. This process starts a chain reaction that continues the generation of more radicals that react with new targets that eventually disable the protective antioxidant systems and diminish their ability to detoxify the reactive intermediates and/or repair ROS-mediated damage. The resulting oxidative stress subsequently damages proteins, lipids, and DNA that leads to cell death and disease. Although it is clearly understandable that ROS are harmful, they, however, have a contrasting beneficial role. Phagocytic cells of the immune system use the oxidizing power of ROS to attack and kill infectious pathogens. ROS also play an important role in cell signaling by acting as messengers to help maintain cellular and tissue homeostasis. Nevertheless, oxidative stress is considered the primary biochemical mechanism responsible for Hcy-induced cellular injury and dysfunction.
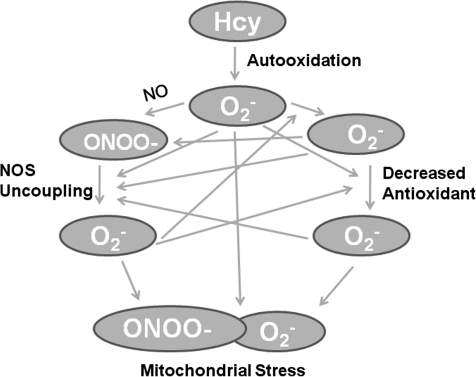
It is now well accepted that HHcy is an independent risk factor for coronary artery disease, cerebrovascular disease, and peripheral vascular occlusive disease (13). Although there is convincing research supporting the tenet that Hcy-mediated radical production underlies the pathology of vascular injury, studies have not been able to completely elucidate the exact mechanisms linking Hcy, oxidative stress, and cellular/tissue damage. Accumulating evidence suggests that Hcy instigates ROS production by several mechanisms independent of the conventional Hcy auto-oxidation (Fig. 6).
FIG. 6.
Generation and amplification of Hcy-mediated oxidative stress. ONOO−, peroxynitrite; NOS, nitric oxide synthase.
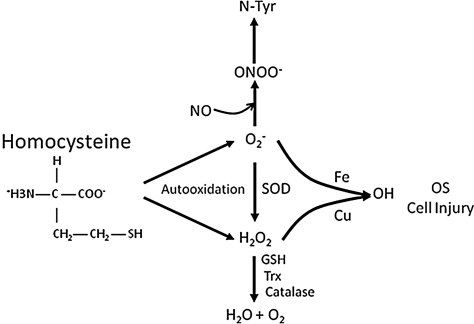
A very small fraction of Hcy remains in its reduced form, whereas ∼98% is present in the oxidized forms that include homocystine, mixed disulfides, and homocysteine thiolactone. The auto-oxidation of Hcy is one of the more accepted mechanisms of ROS formation. Hcy contains a reactive sulfhydryl group and, like most thiol-containing amino acids, can undergo rapid auto-oxidization in the presence of oxygen and metal ions (iron and copper) generating potent ROS, superoxide, and hydrogen peroxide. In this reaction, Hcy reduces a transition metal ion generating a thiyl radical (Equation 1 in (Table 3). The reduced metal ion reacts with oxygen-generating superoxide (Equation 2 in Table 3). The thiyl radical then reacts with Hcy to generate a free radical intermediate (Equation 3 in Table 3) that reduces oxygen to superoxide (Equation 4 in Table 3). The net reaction is the formation of two superoxides and homocystine. The liberated ROS from Hcy auto-oxidation is proatherogenic (Fig. 7) and alters endothelial function, causes endothelial injury, exposes the underlying matrix, mediates smooth muscle cell proliferation, and promotes the activation of platelets and leukocytes. Interestingly, cysteine, the most abundant low-molecular-weight thiol in the plasma, has a higher concentration (∼250 μM) than Hcy, yet does not cause endothelial injury (74). It has been suggested that cysteine, at low concentrations, is an important extracellular anti-oxidant acting as a precursor to glutathione, whereas Hcy is an extracellular oxidant.
FIG. 7.
Hcy-mediated OS. Amplified reactive oxygen species and reactive nitrogen species generation after Hcy autooxidation. OS, oxidative stress; SOD, superoxide dismutase.
Mammalian cells, including ECs, VSMCs, myocytes, fibroblasts, neutrophils, and macrophages, have NADPH oxidase as a major source of superoxide production. HHcy-mediated superoxide production from NADPH oxidase is a critical biochemical mechanism in the pathogenesis of various vascular diseases, including hypertension and atherosclerosis. NADPH oxidase is a plasma membrane-associated enzyme that generates O2− via the single-electron reduction of molecular oxygen. In general, the complex is composed of two plasma membrane-associated components, glycoprotein gp91phox and p22phox, and several cytosolic components, p40phox, p47phox, p67phox, and Rac. Flavin and heme prosthetic groups are also associated with this complex. Recent studies have discovered a family of nonphagocytic NADPH oxidases that are based on seven different homologs of the gp91phox or NOX (for NADPH oxidase). Under basal conditions, these cardiovascular NADPH oxidases are moderately constitutive and function as an oxygen sensory mechanism. NADPH oxidase activity in nonphagocytic cells is mediated by diverse stimuli such as cytokines, hormones, local metabolic changes, hemodynamic forces, and signaling pathways and can also be regulated at the gene level of oxidase subunits. In VSMC, oxidase activity is driven by angiotensin II, thrombin, and TNF-α. Metabolic changes such as increased lactate and reoxygenation activate NADPH oxidase in cardiac myocytes. In ECs, mechanical forces increase NADPH oxidase-mediated superoxide production.
Several studies have clearly shown that Hcy significantly increases the expression and activity of NADPH oxidase; however, the mechanism of induction remains unknown. Nevertheless, there are several proposed mechanisms by which Hcy might stimulate oxidase-mediated superoxide production in both immune and vascular cells, which strongly suggest its involvement in Hcy-induced oxidative stress, endothelial dysfunction, and vascular wall damage. Hcy increased the activation of NADPH oxidase and production of O2− radicals from human neutrophils. They further observed that Hcy directly mobilized the cytosolic subunits p47phox and p67phox to the cell membrane activating the NADPH oxidase. Hcy increased superoxide generation in phagocytic monocytes via the phosphorylation of cytosolic subunits p47phox and p67phox via protein kinase C β (PKCβ) activation that promoted the assembly of the active oxidase enzyme. These observations prove that phagocytic cells are significant sources of O2− contributing to the inflammatory responses progression of atherosclerosis. It was shown that Hcy greatly enhanced ROS generation and apoptosis in human umbilical vein ECs, possibly through an NADPH oxidase and/or c-Jun NH2-terminal kinase signaling pathway-dependent manner. The role of NAPDH oxidase in Hcy-induced oxidative stress and cell death was further confirmed after they observed a reduction in ROS production and partial attenuation of cell death after blocking oxidase activity with apocynin. In our laboratory, we demonstrated a dose- and time-dependent increase in NADPH oxidase mRNA accompanied by a dose-dependent increase in ROS production in Hcy-treated microvascular ECs. This correlates with the upregulation of protease-activated receptor-4 that is involved in inflammatory processes (69). In a recent study using ECs we have shown that Hcy increases NOX-4, and causes its translocation to the mitochondria. The translocation increased ROS generation and this increase was ameliorated by activating peroxisome proliferator-activated receptor gamma (PPAR-γ) with ciglitazone (68).
Mammalian cells synthesize NO by a family of NOS isoforms. There has been substantial attention and accumulating evidence that a vital source of in vivo ROS generation is eNOS and the oxygen-derived radicals are pathophysiological mediators in many cardiovascular disorders, including atherosclerosis. Active NOS enzymes are homodimers. Each NOS monomer catalyzes the flavin-mediated electron transport of electrons from the N-terminal-bound NADPH donor of the reductase domain to the C-terminal prosthetic heme group of the oxidase domain of the other monomer. The enzyme requires BH4 for homodimerization and modulation of redox state during electron transfer. The heme iron and the BH4 at the active site catalyze the reaction of oxygen with L-arginine resulting in NO synthesis.
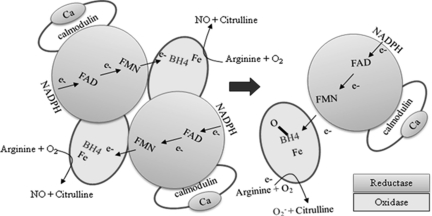
Uncoupling of NOS causes electrons to directly reduce molecular oxygen, thus generating O2−. Several mechanisms are potentially responsible for NOS uncoupling. Studies have shown that suboptimal concentrations of BH4 and/or L-arginine are partially responsible for superoxide generation. It has been suggested that ONOO−, the byproduct of the reaction between NO and O2−, oxidizes BH4 leading to the uncoupling of eNOS. The reduction in BH4 allows the NOSs to convert to an uncoupled state, thus disrupting the flow of electrons resulting in O2− synthesis (Fig. 8). In addition, BH4 can also function as a scavenger of radicals being converted to the BH3 radical. Ascorbate treatment in HHcy has been shown to improve endothelial function. These studies confirmed that a step in BH4 reduction is involved in Hcy-induced oxidative stress. The transport of L-arginine into the EC is important for NO production. A decrease in intracellular L-arginine availability allows NOS to utilize molecular oxygen as a substrate to produce O2−. NOS uncoupling has also been shown when eNOS is dephosphorylated at the threonine residue 495 and when eNOS is redistributed to the cytosolic fraction of the cell.
FIG. 8.
Schematic representation of Hcy-mediated nitric oxide synthase uncoupling. Hcy-produced radicals oxidize BH4 uncoupling the NOS dimer, which interferes with the flow of elections from the reductase domain to the oxidase domain. The lost electrons reduce molecular oxygen and produce superoxide (O2−). Hcy-mediated depletion of L-arginine uncouples the NOS, allowing it to use molecular oxygen to produce O2−. NADPH, nicotinamide adenine dinucleotide phosphate (reduced); BH4, tetrahydrobiopterin.
Hcy has been shown to have a role in altering NOS activity in favor of O2− formation. Data have suggested that Hcy-generated oxidative stress in ECs results in oxidation of BH4, eNOS uncoupling, and subsequent ROS production (84). Jin et al. reported that Hcy limits the cellular supply of arginine by impairing arginine uptake by the cell, therefore uncoupling eNOS (35). This mechanism could be responsible for the decrease in endothelium-mediated vasodilator response seen in HHcy patients. It has been shown that exogenous BH4 improves endothelial function in chronic smokers and augments NO bioactivity in hypercholesterolemic humans.
It is well established that NO is inherently cardio-protective and mediates key vascular functions. However, under certain physiological and pathological conditions, it forms secondary oxidants that serve a role in vascular cytotoxicity. Under conditions when NO and O2− production are elevated, they rapidly react generating toxic levels of ONOO− that is a major concern in the bioactivity of NO and endothelial function (15). ONOO− is a reactive but stable, potent agent that oxidizes and nitrates a variety of target molecules. ONOO− has been identified as a mediator of cellular damage and vascular injury, by oxidizing and nitrating cellular DNA, proteins, and lipids. (67).
ONOO− has been proven to be a potent mediator in the oxidative and nitrosative mechanisms mediated by Hcy. Superoxide is elevated during HHcy and is partly responsible for scavenging of NO and increased generation of ONOO− (46, 84). It is capable of nitrating tyrosine residues in proteins, generating its most identifiable product, nitrotyrosine (4). Our lab has observed a significant increase in nitrotyrosine in HHcy-treated cells with no change in basal NO production (69). This finding was consistent with the theory that Hcy impacts NO bioavailability to a greater degree by NO scavenging than by the reduction of NO production. The ONOO−-mediated reduction in NO bioavailability and bioactivity then causes endothelial dysfunction and has been implicated in the pathogenesis of human atherosclerosis.
The biological activity and availability of ROS is dependent on their relative balance in relation to the antioxidant systems. Cells are usually able to defend themselves against ROS damage by the activity of numerous built-in enzymatic defense mechanisms that act to neutralize the harmful effects of toxic oxygen molecules. These endogenous defense systems include the availability effective antioxidants like glutathione, ascorbic acid, and α-tocopherol (Vitamin E) or the detoxification of oxidizing agents by enzymes like SOD or catalase (77). However, in a disease state, the overproduction and activity of ROS become a burden for endogenous antioxidant systems, tipping the scale toward atherogenesis.
Hcy-increased radical generation and oxidative injury to the vascular wall can also be attributed to the diminished expression and activity of catalase and SOD, key antioxidant enzymes. There can also be decreased activity of glutathione peroxidase, SOD, and catalase that promotes the generation of oxidative stress (27). The decreased ability to scavenge harmful radicals leaves cells and tissues defenseless against oxidative damage.
Vascular effects of Hcy-mediated oxidative stress
Although there is no direct method to measure oxidative stress in humans, indirect estimates and side effects support the belief that ROS-mediated damage is the underlying molecular mechanism of Hcy-mediated vascular dysfunction (Fig. 9). The inability of the tissue to scavenge harmful oxidants results in lipid peroxidation, protein modifications, endothelial damage, decreasing ability to synthesize NO, limited normal vasodilation, cell death, and alterations in tissue morphology. In addition to oxidative damage, oxygen radicals can activate signaling molecules such as NFκB that amplify the oxidative response. It will be important to develop specific therapeutic strategies and approaches to treat or regulate oxidative mediated vascular dysfunction in HHcy patients. Currently, there are no data showing whether vascular dysfunction in HHcy human subjects is improved with antioxidant therapy.
FIG. 9.
Molecular effects of Hcy-mediated oxidative stress. Hyperhomocysteinemia-generated reactive oxygen and nitrogen species evoke damaging molecular effects that underlie vascular dysfunction.
Biochemical and Vascular Effects of Hcy-Mediated NO Depletion
Hcy induces endothelial dysfunction and clinical manifestations of CVD by altering virtually every component of NO metabolism, including NOS expression, localization, activation, and activity (Table 4 and Fig. 10). There is not one decisive mechanism by which Hcy decreases NO production and bioavailability, yet there are convincing data supporting several proposed biochemical mechanisms. Most studies point to Hcy-induced oxidative stress as the primary mediator in this process.
Table 4.
Differential Properties of Nitric Oxide Synthase Isoforms
| nNOS | iNOS | eNOS | |
|---|---|---|---|
| Type | I | II | III |
| Origin | Neurons | Macrophage | Endothelium |
| Other site of expression | VSMC | Endothelium, VSMC | VSMC, neurons |
| Expression | Constitutive | Inducible | Constitutive |
| Molecular mass (kDa) | 140 | 130 | 130 |
| mRNA size (kb) | 10 | 4.0–5.0 | 4.4 |
| Ca/CaM independent | |||
| Regulation | Ca/CaM | Transcription | Ca/CaM |
| Activation | Stimuli | Injury | Shear stress |
| Activator | Glutamate | Cytokines | Acetylcholine |
| Phosphorylation | PKA | PKA | PKA |
| N-myristoylation site | NO | No | Yes |
| NO Production | Moderate (nM to μM) | High (μM) | low (pM to nM) |
| Function | Signaling | Toxin/immune defense | Signaling |
Designations and characteristics of each NOS isoform: NOS, nitric oxide synthase; nNOS, neuronal NOS; iNOS, inducible NOS; eNOS, endothelial NOS.
PKA, protein kinase A; VSMC, vascular smooth muscle cell.
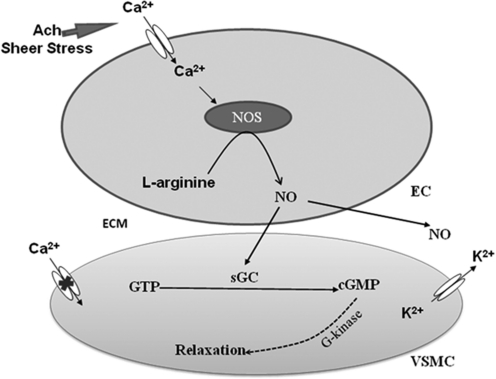
FIG. 10.
Induction of SMC relaxation via NO. VSMC, vascular smooth muscle cell; EC, endothelial cell; ECM, extracellular matrix.
There is conflicting data on whether HHcy affects the gene and protein expression of NOS enzymes. Nevertheless, studies have shown that treating ECs with pathophysiological concentrations of Hcy significantly decrease eNOS protein expression in a dose-dependent manner. This finding, however, is contradictory to a previous study that found that HHcy does not affect the protein expression or gene transcription of eNOS. Tyagi et al. reported an increase in inducible NOS (iNOS) activity in Hcy-treated ECs that led to oxidative stress (69). Conversely, Zhang et al. reported no change in eNOS expression or induction of iNOS expression in Hcy-treated ECs (84). A recent study reported the most comprehensive and supportive data on the potential effects of Hcy on NOS gene expression. They reported that Hcy caused significant DNA hypermethylation in the iNOS gene, increasing iNOS expression, which influenced atherogenesis. They showed that PPAR-α/γ ligands decreased the aberrant methylation and gene expression, and had an anti-atherosclerotic effect. The inconsistency in these findings suggests that HHcy alters NO bioavailability through alternative mechanisms.
Modifications in the subcellular localization of eNOS have also been shown to change NOS expression.The caveolin–eNOS interactions may be modulated by an oxidative environment. It has been proposed that ROS profoundly affects the structure and function of caveolae, dissociating the regulatory interaction and subsequent decrease in eNOS, without altering NO production. Accordingly, Hcy-generated ROS can also mediate these same changes found in hypercholesterolemia.
HHcy has been shown to mediate changes in substrate and cofactor availability and binding that can disrupt NO production. The intracellular level of L-arginine is in the micromolar range, and the arginine paradox states that there must be a sufficient and continual supply of L-arginine for NO synthesis (35). Hcy is capable of indirectly oxidizing L-arginine transporters as a result decreasing intracellular L-arginine availability without altering NOS activity. NOS will instead use molecular oxygen to generate O2− (35). It has been shown that exogenous L-arginine supplementation could decrease ONOO− formation (35). Hcy can interfere with substrate binding and decrease NO synthesis. Hcy inhibits dimethylarginine dimethylaminohydrolase, an enzyme that is responsible for degrading the endogenous inhibitor of NO production, ADMA. When ADMA accumulates in the cell, it competes with L-arginine for binding to NOS, therefore decreasing NOS activity and NO production (62). A higher plasma level of ADMA has been related to Hcy-mediated oxidative stress, and L-arginine supplementation has been shown to improve endothelium-dependent vasodilation in HHcy patients (10). Hcy generated O2− and ONOO− have been shown to oxidize sulfhydryl groups in eNOS and to oxidize NOS cofactor BH4. Both of these agents are responsible for NOS uncoupling, additional H2O2 and O2− formation, reduction in eNOS activity, and subsequent decrease in NO synthesis (84). A decrease in BH4 availability can also lead to the dissociation of NADPH oxidation that too contributes to the uncoupling of the NOS homodyne.
NOS activation is directly mediated by changes in intracellular calcium. Neural NOS is co-localized and functionally coupled to the N-methyl-d-aspartate (NMDA) receptor. The NMDA receptor is known to participate in Hcy-induced oxidative excitotoxicity and neurotoxicity. Hcy has been shown to bind to the NMDA receptor with high affinity, causing an increase in intracellular calcium (63). The excessive influx of calcium and prolonged activation eventually renders the NOS enzyme desensitized and stimulates ROS production. Stimulation of the overproduction of NO by the NMDA receptor has been implicated in calcium dysregulation and neurodegeneration of neurons.
The biosynthesis of NO can be regulated at the transcriptional and posttranslational level. Hcy likely increases iNOS-derived NO by activating different transcriptional factors, which enhances vascular inflammation. In VSMCs, Hcy has been shown to increase the expression of iNOS by NFκB-dependent transcriptional activation and IL-1β-induced increased mRNA that collectly disrupts homeostatic anti-inflammatory mechanisms. During this process, Hcy activates TNFα, which in turn facilitates the translocation of NFκB from the cytosol to the nucleus, where it induces iNOS and other inducible genes (1). The increase in iNOS-induced NO production in VSMCs is partly a compensatory response for the absence of eNO synthesis. However, the increase in iNOS activity may contribute to the promotion of atherogensis by increasing the production of oxidative products.
NO synthesis can also be regulated by modification of phosphorylation. Hcy has been shown to modify eNOS and iNOS activity via a PKC-dependent pathway, and this may represent an important signaling pathway in Hcy-mediated development of CVD. HHcy increased redox-sensitive, PKC-mediated phosphorylation of threonine 495 located in the CaM-binding domain, thus decreasing the catalytic activity of eNOS.
Lastly, Hcy can directly and indirectly disrupt the biological function of NO. NO readily reacts with sulfhydryl groups to form nitrosothiols that act to stabilize and transport NO. Initially, upon exposure, ECs are able to defend themselves and detoxify Hcy by increasing the synthesis and release of NO, which in turn leads to the formation of S-nitroso-homocysteine, a potent vasodilator. Unfortunately, this defense mechanism is limited and chronic exposure to Hcy ultimately leads to impaired basal NO production, radical formation, and subsequent endothelial injury. Hcy can also decrease the bioavailability and bioactivity of NO through the oxidative degradation of NO forming ONOO− (84). Depending on the redox state of the environment, the unpaired electron of NO makes it a highly reactive free radical species and can quickly react with O2−. This explains the observation of a decrease in NO detection as a result of limited bioavailability rather than suppressed production. Both the formation of S-nitroso-homocysteine and ONOO− reduced the amount of active NO available to modulate vessel function.
In summary, a decrease in NO homeostasis is a mediating aspect in alterations in hemodynamic factors, inflammation, thrombogenicity, and cell death. It is clear that the previously mentioned mechanisms of Hcy-mediated NO depletion and endothelial dysfunction have a significant role in the atherogenesis in the coronary, cerebral, and peripheral vasculature (discussed in detail later). Although available evidence demonstrates that folic acid supplementation and vitamin therapy could improve Hcy-mediated impairment of NO metabolism by reducing intracellular O2− generation, these possibilities still need further investigation.
Vascular Remodeling-Role of MMPs
Vascular remodeling underlies the pathogenesis of major CVDs, including atherosclerosis and restenosis, and is defined as the enduring change in the size and composition of blood vessels and tissues (21). Physiological and pathological vascular remodeling involves the breakdown and synthesis of the ECM. This process is initiated by a family of specialized proteases, MMPs (Fig. 11). Currently, there are many proposed mechanisms of Hcy-mediated vascular remodeling, yet the process of vascular rearrangement is still unclear. In HHcy, it has been shown that Hcy induces the production of ROS and reduces the bioavailability of NO, the first two legs of the Toxic Triad. These findings support previous studies that suggest that Hcy-mediated activation of MMPs is potentiated by oxidative stress and by decreased NO bioavailability (73).
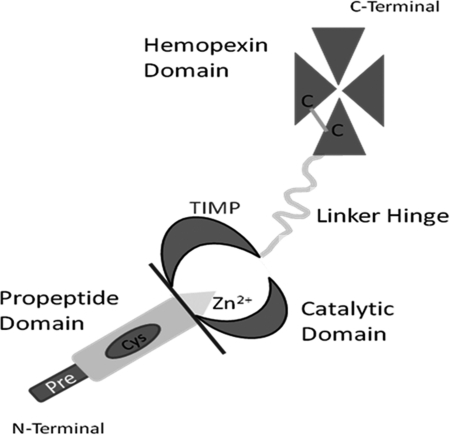
FIG. 11.
Schematic of the basic structure of an MMP. MMP, matrix metalloproteinase; TIMP, tissue inhibitor of metalloproteinase.
Hcy, MMPs, and vascular remodeling
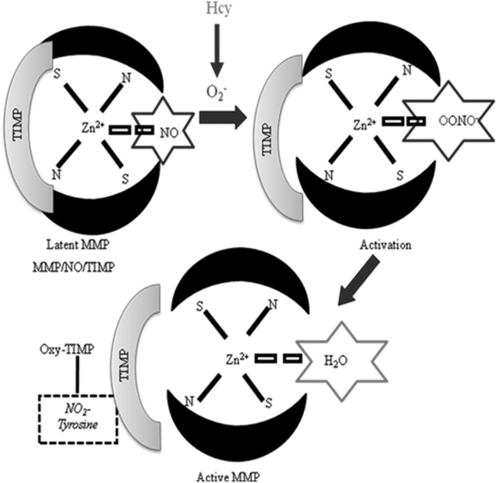
The process of abnormal vascular remodeling is an underlying component of many vascular pathologies in which the ECM has gross composition, disorganization, and altered geometry (72). Studies have suggested that Hcy can instigate this process and exerts its atherogenic effect by increasing the expression and activity of latent resident MMPs, by several mechanisms (73). It is well known that Hcy promotes a highly toxic oxidative environment within the vasculature that can trigger the activation of MMPs (59) (Fig. 12). The cysteine residues within the MMP propeptide domain are highly sensitive to changes in the redox environment. Hcy-generated O2− mediates the oxidation of those cysteine residues activating latent MMPs and tissue inhibitor of metalloproteinases (TIMPs) (70). NO is known to be coordinated in the activation site of MMPs, having a role in its latency. Hcy-generated O2− quenches the coordinated NO generating ONOO−, which exposes the active site and increases the activity of MMPs (51). The Hcy-generated ONOO− can lead to nitration of tyrosine residues (nitrotyrosine) within MMPs and also contributing to their activation (Fig. 12).
FIG. 12.
A plausible mechanism of Hcy-induced activation of latent resident MMPs. Hcy-generated oxidative radicals oxidize NO in the catalytic domain active site of the latent MMP generating ONOO−. Generated superoxide and ONOO− can subsequently oxidize cysteine residues within the TIMP, generate nitrotyrosine, and result in the cleavage of TIMPs rendering the MMP active.
Our laboratory has extensively investigated the various signaling pathways associated with the Hcy-mediated MMP activation and the potential roles of MMPs in several vascular pathologies. We have proposed a novel mitochondrial mechanism for Hcy-induced MMP activation. Moshal et al. demonstrated that Hcy-activated calcium-dependent cystiene protease, calpain-1, mediated intramitochondrial oxidative stress and subsequent MMP-9 activation (42). Our lab has also investigated the role of γ-amino butyric acid (GABA), a major inhibitory neurotransmitter, and its receptors, GABA-A/B, in Hcy-meditated microvascular remodeling. Investigations of the central nervous system have shown that Hcy competitively binds to the GABA-A receptor and thus acts as an excitatory neurotransmitter (23). Our lab observed an increase in MMP-2 activity, TIMP-3 expression, decreased TIMP-4, and constrictive collagen remodeling in Hcy-treated microvascular ECs that was ameliorated by GABA receptor agonist, further confirming the role of GABA receptors in Hcy-mediated cerebrovascular remodeling. Moreover, we have demonstrated that amelioration of Hcy-mediated constrictive remodeling was due in part to the induction of NO generation by GABA receptor agonists (58).
In a diabetic mouse model, we observed glomerulosclerosis that was mediated by increased tissue Hcy, oxidative reduction of NO bioavailability, and increased MMP-2 activity. The observed renal arteriole contractile and relaxation dysfunction was ameliorated after the activation of PPARγ. It is believed that HHcy-dependent matrix accumulation may have a role in arterial hypertrophy seen in hypertension. The ECM accumulation and arterial remodeling observed in an animal model of HHcy-induced arterial hypertension is in part due to increased MMP-2 and MMP-9 activation (51). Authors reported a substantial decrease in systolic blood pressure after the administration of 3-deazaadenosine, a potent SAH hydrolase inhibitor, that decreases Hcy formation. Lominadze et al. demonstrated that acute HHcy increased brain microvascular leakage that could potentiate stroke (37). The observed increase in microvascular permeability was partly mediated by increased MMP activity and alterations were significantly ameliorated by MMP inhibition.
Conclusion
The HHcy contributes to generate ROS, RNS, and reactive thiol species. This decreases the bioavailability of the NO. These processes activate the latent MMPs, and inactive the TIMP. This leads to adverse cardiovascular remodeling.
Abbreviations Used
- ADMA
asymmetric dimethyl arginine
- BH4
tetrahydrobiopterin
- CBS
cystathionine β synthase
- CSE
γ-cystathionase
- CVD
cardiovascular disease
- ECM
extracellular matrix
- ECs
endothelial cells
- eNOS
endothelial nitric oxide synthase
- GABA
γ-amino butyric acid receptor
- Hcy
homocysteine
- HHcy
hyperhomocysteinemia
- IL
interleukin
- iNOS
inducible NOS
- MAT
methionine adenosyltransferase
- MMP
matrix metalloproteinase
- MS
methionine synthase
- MT
methyltransferase
- MTHFR
methylene tetrahydrofolate reductase
- NADPH
nicotinamide adenine dinucleotide phosphate (reduced)
- NFκB
nuclear factor-kappa B
- NMDA
N-methyl-d-aspartate
- NO
nitric oxide
- NOS
nitric oxide synthase
- ONOO−
peroxynitrite
- OS
oxidative stress
- PKA
protein kinase A
- PKC
protein kinase C
- PPAR
peroxisome proliferators activated receptor
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SAHH
S-adenosylhomocysteine hydrolase
- SAM
S-adenosylmethionine
- SOD
superoxide dismutase
- TIMP
tissue inhibitor of metalloproteinase
- tHcy
total plasma Hcy
- TNF-α
tumor necrosis factor alpha
- VSMCs
vascular smooth muscle cells
Acknowledgments
A part of this study was supported by NIH grants HL-71010, HL-74185, HL-88012, and NS-51568.
References
- 1.Aktan F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004;75:639–653. doi: 10.1016/j.lfs.2003.10.042. [DOI] [PubMed] [Google Scholar]
- 2.Appel LJ. Moore TJ. Obarzanek E. Vollmer WM. Svetkey LP. Sacks FM. Bray GA. Vogt TM. Cutler JA. Windhauser MM. Lin PH. Karanja N. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1999;336:1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 3.Banerjee RV. Matthews RG. Cobalamin-dependent methionine synthase. FASEB J. 1990;4:1450–1459. doi: 10.1096/fasebj.4.5.2407589. [DOI] [PubMed] [Google Scholar]
- 4.Beckman JS. Oxidative damage and tyrosine nitration from peroxynitrite. Chem Res Toxicol. 1996;9:836–844. doi: 10.1021/tx9501445. [DOI] [PubMed] [Google Scholar]
- 5.Bostom AG. Roubenoff R. Dellaripa P. Nadeau MR. Sutherland P. Wilson PW. Jacques PF. Selhub J. Rosenberg IH. Validation of abbreviated oral methionine-loading test. Clin Chem. 1995;41:948–949. [PubMed] [Google Scholar]
- 6.Butz LW. Du Vigneaud V. The formation of a homologue of cystine by the decomposition of methioinine with sulfuric acid. J Biol Chem. 1932;99:135–142. [Google Scholar]
- 7.Carlsen SM. Fulling I. Grill V. Bjerve KS. Schneede J. Refsum H. Metformin increases total serum homocysteine levels in non-diabetic male patients with coronary heart disease. Scand J Clin Lab Invest. 1997;57:521–527. doi: 10.3109/00365519709084603. [DOI] [PubMed] [Google Scholar]
- 8.Carson NAJ. Neill DW. Metabolic abnormalities detected in a survey of mentally backward individuals in Northern Ireland. Arch Dis Child. 1962;37:505–513. doi: 10.1136/adc.37.195.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Case records of the Massachusets General Hospital. Marked cerebral symptoms following a limp of three months' duration. New Engl J Med. 1933;209:1063–1066. [Google Scholar]
- 10.Chen H. Fitzgerald R. Brown AT. Qureshi I. Breckenridge J. Kazi R. Wang Y. Wu Y. Zhang X. Mukunyadzi P. Eidt J. Moursi MM. Identification of a homocysteine receptor in the peripheral endothelium and its role in proliferation. J Vasc Surg. 2005;41:853–860. doi: 10.1016/j.jvs.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 11.Coppola A. Davi G. De Stefano V. Mancini FP. Cerbone AM. Di Minno G. Homocysteine, coagulation, platelet function, and thrombosis. Semin Thromb Hemost. 2000;26:243–254. doi: 10.1055/s-2000-8469. [DOI] [PubMed] [Google Scholar]
- 12.Du Vigneaud V. Dyer HM. Harmon J. The growth-promoting properties of homocystine when added to a cystine-deficient diet and the proof ofstructure of homocystine. J Biol Chem. 1933;101:719–726. [Google Scholar]
- 13.Eikelboom JW. Lonn E. Genest J., Jr. Hankey G. Yusuf S. Homocyst(e)ine and cardiovascular disease: a critical review of the epidemiologic evidence. Ann Intern Med. 1999;131:363–375. doi: 10.7326/0003-4819-131-5-199909070-00008. [DOI] [PubMed] [Google Scholar]
- 14.Elfering SL. Sarkela TM. Giulivi C. Biochemistry of mitochondrial nitric-oxide synthase. J Biol Chem. 2002;277:38079–38086. doi: 10.1074/jbc.M205256200. [DOI] [PubMed] [Google Scholar]
- 15.Faraci FM. Hyperhomocysteinemia, a million ways to lose control. Arterioscler Thromb Vasc Biol. 2003;23:371–373. doi: 10.1161/01.ATV.0000063607.56590.7F. [DOI] [PubMed] [Google Scholar]
- 16.Finkelstein JD. Methionine metabolism in mammals. J Nutr Biochem. 1990;1:228–237. doi: 10.1016/0955-2863(90)90070-2. [DOI] [PubMed] [Google Scholar]
- 17.Finkelstein JD. The metabolism of homocysteine: pathways and regulation. Eur J Pediatr. 1998;157:40–44. doi: 10.1007/pl00014300. [DOI] [PubMed] [Google Scholar]
- 18.Friedman AN. Bostom AG. Selhub J. Levey AS. Rosenburg H. The kidney and homocysteine metabolism. J Am Soc Nephrol. 2001;12:2181–2189. doi: 10.1681/ASN.V12102181. [DOI] [PubMed] [Google Scholar]
- 19.Geisel J. Zimbelmann I. Schorr H. Knapp JP. Bodis M. Hubner U. Herrmann W. Genetic defects as important factors for moderate hyperhomocysteinemia. Clin Chem Lab Med. 2001;39:698–704. doi: 10.1515/CCLM.2001.115. [DOI] [PubMed] [Google Scholar]
- 20.Gerritsen T. Vaughn JG. Weisman HA. The identification of homocysteine in the urine. Biochem Biophys Res Commun. 1962;9:493–496. doi: 10.1016/0006-291x(62)90114-6. [DOI] [PubMed] [Google Scholar]
- 21.Gibbons GH. Dzau VJ. The emerging concept of vascular remodeling. N Engl J Med. 1994;330:1431–1438. doi: 10.1056/NEJM199405193302008. [DOI] [PubMed] [Google Scholar]
- 22.Green JM. Ballou DP. Matthews RG. Examinationof the role of methylenetetrahydrofolate reductase in incorporation of methyltetrahydrofolate into cellular metabolism. FASEB J. 1988;2:42–47. doi: 10.1096/fasebj.2.1.3335280. [DOI] [PubMed] [Google Scholar]
- 23.Griffiths R. Williams DC. O'Neill C. Dewhurst IC. Ekuwem CE. Sinclair CD. Synergistic inhibition of [3H]muscimol binding to calf-brain synaptic membranes in the presence of L-homocysteine and pyridoxal 5′-phosphate. A possible mechanism for homocysteine-induced seizures. Eur J Biochem. 1983;137:467–478. doi: 10.1111/j.1432-1033.1983.tb07850.x. [DOI] [PubMed] [Google Scholar]
- 24.Guttormsen AB. Ueland PM. Svarstad E. Refsum H. Kinetic basis of hyperhomocysteinemia in patients with chronic renal failure. Kidney Int. 1997;52:495–502. doi: 10.1038/ki.1997.359. [DOI] [PubMed] [Google Scholar]
- 25.Hart SR. Mangoni AA. Swift CG. Jackson SH. Effect of methionine loading on pulse wave analysis in elderly volunteers. Postgrad Med J. 2006;82:524–527. doi: 10.1136/pgmj.2005.044610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayden MR. Tyagi SC. Intimal redox stress: accelerated atherosclerosis in metabolic syndrome and type 2 diabetes mellitus. Atheroscleropathy Cardiovasc Diabetol. 2002;1:3. doi: 10.1186/1475-2840-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayden MR. Tyagi SC. Homocysteine and reactive oxygen species in metabolic syndrome, type 2 diabetes mellitus, and atheroscleropathy: the pleiotropic effects of folate supplementation. Nutr J. 2004;3:4. doi: 10.1186/1475-2891-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heinecke JS. Superoxide mediated oxidation of low-density lipoproteins by thiols. In: Cerutti PA, editor; Cerutti JM, editor; McCord I, editor; Fridovich I, editor. Oxy-radicals in Molecular Biology and Pathology. New York: Liss; 1988. pp. 433–457. [Google Scholar]
- 29.Hoffman DR. Cornatzer WE. Duerre JA. Relationship between tissue levels of S-adenosylmethionine, S-adenylhomocysteine, and transmethylation reactions. Can J Biochem. 1979;57:56–65. doi: 10.1139/o79-007. [DOI] [PubMed] [Google Scholar]
- 30.Hofmann MA. Lalla E. Lu Y. Gleason MR. Wolf BM. Tanji N. Ferran LJ. Kohl B. Rao V. Kisiel W. Stern DM. Schmidt AM. Hyperhomocysteinemia enhances vascular inflammation and accelerates atherosclerosis in a murine model. J Clin Invest. 2001;107:675–683. doi: 10.1172/JCI10588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hultberg B. Andersson A. Sterner G. Plasma homocysteine in renal failure. Clin Nephrol. 1993;40:230–234. [PubMed] [Google Scholar]
- 32.Jacobsen DW. Catanescu O. DiBello PM. Barbato JC. Molecular targeting by homocysteine: a mechanism for vascular pathogenesis. Clin Chem Lab Med. 2005;43:1076–1083. doi: 10.1515/CCLM.2005.188. [DOI] [PubMed] [Google Scholar]
- 33.Jacobsen DW. Gatautis VJ. Green R. Robinson K. Savon SR. Secic M. Ji J. Otto JM. Taylor LM., Jr Rapid HPLC determination of total homocysteine and other thiols in serum and plasma: sex differences and correlation with cobalamin and folate concentrations in healthy subjects. Clin Chem. 1994;40:873–881. [PubMed] [Google Scholar]
- 34.Jencks DA. Matthews RG. Allosteric inhibition of methylenetetrahydrofolate reductase by adenosylmethionine. Effects of adenosylmethionine and NADPH on the equilibrium between active and inactive forms of the enzyme and on the kinetics of approach to equilibrium. J Biol Chem. 1987;262:2485–2493. [PubMed] [Google Scholar]
- 35.Jin L. Caldwell RB. Li-Masters T. Caldwell RW. Homocysteine induces endothelial dysfunction via inhibition of arginine transport. J Physiol Pharmacol. 2007;58:191–206. [PubMed] [Google Scholar]
- 36.Kang SS. Wong PW. Malinow MR. Hyperhomocysteinemia as a risk factor for occlusive vascular disease. Ann Rev Nutr. 1992;12:279–298. doi: 10.1146/annurev.nu.12.070192.001431. [DOI] [PubMed] [Google Scholar]
- 37.Lominadze D. Roberts AM. Tyagi N. Moshal KS. Tyagi SC. Homocysteine causes cerebrovascular leakage in mice. Am J Physiol Heart Circ Physiol. 2006;290:1206–1213. doi: 10.1152/ajpheart.00376.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malinow MR. Kang SS. Taylor LM. Wong PW. Coull B. Inahara T. Mukerjee D. Sexton G. Upson B. Prevalence of hyperhomocysteimia in patients with peripheral aterial occlusive disease. Circulation. 1989;79:1180–1188. doi: 10.1161/01.cir.79.6.1180. [DOI] [PubMed] [Google Scholar]
- 39.McCully KS. Vascular pathology of homocysteinemia: implications for the pathogenesis of arteriosclerosis. Am J Pathol. 1969;56:111–128. [PMC free article] [PubMed] [Google Scholar]
- 40.McCully KS. Homocysteine and vascular disease. Nat Med. 1996;2:386–389. doi: 10.1038/nm0496-386. [DOI] [PubMed] [Google Scholar]
- 41.McCully KS. Wilson RB. Homocysteine theory of arteriosclerosis. Atherosclerosis. 1975;22:215–227. doi: 10.1016/0021-9150(75)90004-0. [DOI] [PubMed] [Google Scholar]
- 42.Moshal KS. Singh M. Sen U. Rosenberger DS. Henderson B. Tyagi N. Zhang H. Tyagi SC. Homocysteine-mediated activation and mitochondrial translocation of calpain regulates MMP-9 in MVEC. Am J Physiol Heart Circ Physiol. 2006;291:2825–2835. doi: 10.1152/ajpheart.00377.2006. [DOI] [PubMed] [Google Scholar]
- 43.Mudd SH. Finkelstein JD. Refsum H. Ueland PM. Malinow MR. Lentz SR. Jacobsen DW. Brattstrom L. Wilcken B. Wilcken DL. Blom HJ. Stabler SP. Allen RH. Selhub J. Rosenburg IH. Homocysteine and its disulfide derivatives: A suggested consensus terminology. Arterioscler Thromb Vasc Biol. 2000;20:1704–1706. doi: 10.1161/01.atv.20.7.1704. [DOI] [PubMed] [Google Scholar]
- 44.Mudd SH. Levy HL, et al. Disorders of transsulfuration. In: Scriver CR, editor; Beaudet AL, editor; Sly WS, editor; Valle D, editor. The Metabolic and Molecular Bases of Inherited Disease. NewYork: McGraw-Hill; 1995. pp. 1279–1327. [Google Scholar]
- 45.Mudd SH. Uhlendorf BW. Freeman JM. Finkelstein JD. Shih VE. Homocystinuria associated with decreased methylenetretrahydrofolate reductase activity. Biochem Biophys Res Commun. 1972;46:905–912. doi: 10.1016/s0006-291x(72)80227-4. [DOI] [PubMed] [Google Scholar]
- 46.Mujumdar VS. Aru GM. Tyagi SC. Induction of oxidative stress by homocyst(e)ine impairs endothelial function. J Cell Biochem. 2001;82:491–500. doi: 10.1002/jcb.1175. [DOI] [PubMed] [Google Scholar]
- 47.Nygard O. Refsum H. Ueland PM. Stensvold I. Nordrehaug JE. Kvale G. Vollset SE. Coffee consumption and total plasma homocysteine: The Hordaland Homocysteine Study. Am J Clin Nutr. 1997;65:136–143. doi: 10.1093/ajcn/65.1.136. [DOI] [PubMed] [Google Scholar]
- 48.Nygard O. Vollset SE. Refsum H. Brattstrom L. Ueland PM. Total homocyteine and cardiovascular disease. J Intern Med. 1999;246:425–454. doi: 10.1046/j.1365-2796.1999.00512.x. [DOI] [PubMed] [Google Scholar]
- 49.Nygard O. Vollset SE. Refsum H. Stensvold I. Tverdal A. Nordrehaug JE. Ueland M. Kvale G. Total plasma homocysteine and cardiovascular risk profile. The Hordaland Homocysteine Study. JAMA. 1995;274:1526–1533. doi: 10.1001/jama.1995.03530190040032. [DOI] [PubMed] [Google Scholar]
- 50.Ovechkin AV. Tyagi N. Sen U. Lominadze D. Steed MM. Moshal KS. Tyagi SC. 3-Deazaadenosine mitigates arterial remodeling and hypertension in hyperhomocysteinemic mice. Am J Physiol Lung Cell Mol Physiol. 2006;291:905–911. doi: 10.1152/ajplung.00543.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Radomski A. Sawicki G. Olson DM. Radomski MW. The role of nitric oxide and metalloproteinases in the pathogenesis of hyperoxia-induced lung injury in newborn rats. Br J Pharmacol. 1998;125:1455–1462. doi: 10.1038/sj.bjp.0702216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Racek J. Rusnakova H. Trefil L. Siala KK. The influence of folate and antioxidants on homocysteine levels and oxidative stress in patients with hyperlipidemia and hyperhomocysteinemia. Physiol Res. 2005;54:87–95. doi: 10.33549/physiolres.930520. [DOI] [PubMed] [Google Scholar]
- 53.Refsum H. Ueland PM. Nygard O. Vollset SE. Homocysteine and cardiovascular disease. Annu Rev Med. 1998;49:31–62. doi: 10.1146/annurev.med.49.1.31. [DOI] [PubMed] [Google Scholar]
- 54.Robinson K. Mayer EL. Miller DP. Green R. van Lente F. Gupta A. Kottke-Marchant K. Savon SR. Selhub J. Nissen SE. Kutner M. Topol EJ. Jacobsen DW. Hyperhomocysteinemia and low pyridoxal phosphate. Circulation. 1995;92:2825–2830. doi: 10.1161/01.cir.92.10.2825. [DOI] [PubMed] [Google Scholar]
- 55.Ross R. The pathogenesis of atherosclerosis: a perspective of the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 56.Selhub J. Homocysteine metabolism. Annu Rev Nutr. 1999;19:217–246. doi: 10.1146/annurev.nutr.19.1.217. [DOI] [PubMed] [Google Scholar]
- 57.Selwyn AP. Prothrombotic and antithrombotic pathways in acute coronary syndromes. Am J Cardiol. 2003;91:3–11. doi: 10.1016/s0002-9149(03)00428-4. [DOI] [PubMed] [Google Scholar]
- 58.Shastry S. Moning L. Tyagi N. Steed M. Tyagi SC. GABA receptors and nitric oxide ameliorate constrictive collagen remodeling in hyperhomocysteinemia. J Cell Physiol. 2005;205:422–427. doi: 10.1002/jcp.20416. [DOI] [PubMed] [Google Scholar]
- 59.Siwik DA. Pagano PJ. Colucci WS. Oxidative stress regulates collagen synthesis and matrix metalloproteinase activity in cardiac fibroblasts. Am J Physiol Cell Physiol. 2001;280:53–60. doi: 10.1152/ajpcell.2001.280.1.C53. [DOI] [PubMed] [Google Scholar]
- 60.Stamler JS. Redox signaling: nitrosylation and related target interactions of nitric oxide. Cell. 1994;78:931–936. doi: 10.1016/0092-8674(94)90269-0. [DOI] [PubMed] [Google Scholar]
- 61.Stipanuk MH. Sulfur amino acid metabolism: pathways for production and removal of homocysteine and cysteine. Annu Rev Nutr. 2004;24:539–577. doi: 10.1146/annurev.nutr.24.012003.132418. [DOI] [PubMed] [Google Scholar]
- 62.Stuhlinger MC. Tsao PS. Her J. Kimotot M. Balint RF. Cooke JP. Homocysteine impairs the nitric oxide synthase pathway, role of asymmetric dimethylarginine. Circulation. 2001;104:2569–2575. doi: 10.1161/hc4601.098514. [DOI] [PubMed] [Google Scholar]
- 63.Sydow K. Schwedhelm E. Arakawa N. Bode-Boger SM. Tsikas D. Hornig B. Frolich JC. Boger RH. ADMA and oxidative stress are responsible for endothelial dysfunction in hyperhomocyst(e)inemia: effects of L-arginine and B vitamins. Cardiovasc Res. 2003;57:244–252. doi: 10.1016/s0008-6363(02)00617-x. [DOI] [PubMed] [Google Scholar]
- 64.Syme SL. Rethinking disease: where do we go from here? Ann Epidemiol. 1996;6:463–468. doi: 10.1016/s1047-2797(96)00097-x. [DOI] [PubMed] [Google Scholar]
- 65.Tawakol A. Omland T. Gerhard M. Wu JT. Creager MA. Hyperhomocystinemia is associated with impaired endothelium-dependent vasodilation in humans. Circulation. 1997;95:1119–1121. doi: 10.1161/01.cir.95.5.1119. [DOI] [PubMed] [Google Scholar]
- 66.Tsai J. Perrella MA. Yoshizumi M. Hsieh C. Haber E. Schlegel R. Lee M. Promotion of vascular smooth muscle cell growth by homocysteine: a link to atherosclerosis. Proc Natl Acad Sci. 1994;91:6369–6373. doi: 10.1073/pnas.91.14.6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Turko IV. Murad F. Protein nitration in cardiovascular diseases. Pharmacol Rev. 2002;54:619–634. doi: 10.1124/pr.54.4.619. [DOI] [PubMed] [Google Scholar]
- 68.Tyagi N. Ovechkin AV. Lominadze D. Moshal KS. Tyagi SC. Mitochondrial mechanism of microvascular endothelial cells apoptosis in hyperhomocysteinemia. J Cell Biochem. 2006;98:1150–1162. doi: 10.1002/jcb.20837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tyagi N. Sedoris KC. Steed M. Ovechkin AV. Moshal KS. Tyagi SC. Mechanisms of homocysteine-induced oxidative stress. Am J Physiol Heart Circ Physiol. 2005;289:2649–2656. doi: 10.1152/ajpheart.00548.2005. [DOI] [PubMed] [Google Scholar]
- 70.Tyagi SC. Homocysteine redox receptor and regulation of extracellular matrix components in vascular cells. Am J Physiol. 1998;274:396–405. doi: 10.1152/ajpcell.1998.274.2.C396. [DOI] [PubMed] [Google Scholar]
- 71.Tyagi SC. Homocysteine and heart disease: pathophysiology of extracellular matrix. Clin Exp Hypertens. 1999;21:181–198. doi: 10.3109/10641969909068660. [DOI] [PubMed] [Google Scholar]
- 72.Tyagi SC. Physiology and homeostasis of extracellular matrix: cardiovascular adaptation and remodeling. Pathophysiology. 2000;7:177–182. doi: 10.1016/s0928-4680(00)00046-8. [DOI] [PubMed] [Google Scholar]
- 73.Tyagi SC. Smiley LM. Mujumdar VS. Clonts B. Parker JL. Reductionoxidation (redox) and vascular tissue level of homocysteine in human coronary atherosclerotic lesions and role in vascular ECM remodeling and vascular tone. Mol Cell Biochem. 1998;181:107–116. doi: 10.1023/a:1006882014593. [DOI] [PubMed] [Google Scholar]
- 74.Ueland PM. Homocysteine species as components of plasma redox thiol status. Clin Chem. 1995;41:340–342. [PubMed] [Google Scholar]
- 75.Uppink JB. Vermaak WJ. van der Merwe A. Becker PJ. Vitamin B-12, vitamin B-6, and folate nutritional status in men with hyperhomocyst(e)inemia. Am J Clin Nutr. 1993;57:47–53. doi: 10.1093/ajcn/57.1.47. [DOI] [PubMed] [Google Scholar]
- 76.Welch GN. Upchurch GF., Jr. Loscalzo J. Homocysteine, oxidative stress, and vascular disease. Hosp Pract. 1997;32:81–92. doi: 10.1080/21548331.1997.11443510. [DOI] [PubMed] [Google Scholar]
- 77.Widner B. Enzinger C. Laich A. Wirleitner B. Fuchs D. Hyperhomocysteinemia, pteridines and oxidative stress. Curr Drug Metab. 2002;3:225–232. doi: 10.2174/1389200024605091. [DOI] [PubMed] [Google Scholar]
- 78.Wiklund O. Fager G. Andersson A. Lundstam U. Masson P. Hultberg B. N-acetylcysteine treatment lowers plasma homocysteine but not serum lipoprotein(a) levels. Atherosclerosis. 1996;119:99–106. doi: 10.1016/0021-9150(95)05635-1. [DOI] [PubMed] [Google Scholar]
- 79.Wilcken DEL. Wilcken B. The pathogenesis of coronary artery disease. A possible role for methionine metabolism. J Clin Invest. 1976;57:1079–1082. doi: 10.1172/JCI108350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wilson PW. D'Agostino RB. Levy D. Belanger AM. Silbershatz H. Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 81.Wouters MG. Moorrees MT. van der Mooren MJ. Blom HJ. Boers GH. Schellekens LA. Thomase CM. Eskes TK. Plasma homocysteine and menopausal status. Eur J Clin Invest. 1995;25:801–805. doi: 10.1111/j.1365-2362.1995.tb01687.x. [DOI] [PubMed] [Google Scholar]
- 82.Yusuf S. Reddy S. Ounpuu S. Anand S. Global burden of cardiovascular diseases: Part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation. 2001;104:2746–2753. doi: 10.1161/hc4601.099487. [DOI] [PubMed] [Google Scholar]
- 83.Yusuf S. Reddy S. Ounpuu S. Anand S. Global burden of cardiovascular diseases: Part II: variations in cardiovascular disease by specific ethnic groups and geographic regions and prevention strategies. Circulation. 2001;104:2855–2864. doi: 10.1161/hc4701.099488. [DOI] [PubMed] [Google Scholar]
- 84.Zhang X. Li H. Jin H. Ebin Z. Brodsky S. Goligorsky MS. Effects of homocysteine on endothelial nitric oxide production. Am J Physiol Renal Physiol. 2000;279:671–678. doi: 10.1152/ajprenal.2000.279.4.F671. [DOI] [PubMed] [Google Scholar]