Abstract
For tissue immunostaining, antibodies are currently the only clinically validated and commercially available probes. Aptamers, which belong to a class of small molecule ligands composed of short single-stranded oligonucleotides, have emerged as probes over the last several decades; however, their potential clinical value has not yet been fully explored. Using cultured cells and an RNA-based CD30 aptamer, we recently demonstrated that the synthetic aptamer is useful as a specific probe for flow cytometric detection of CD30-expressing lymphoma cells. In this study, we further validated the use of this aptamer probe for immunostaining of formalin-fixed and paraffin-embedded lymphoma tissues. Using CD30 antibody as a standard control, we demonstrated that the synthetic CD30 aptamer specifically recognized and immunostained tumor cells of classical Hodgkin lymphoma and anaplastic large cell lymphoma, but did not react with background cells within tumor sites. Notably, the CD30 aptamer probe optimally immunostained lymphoma cells with lower temperature antigen retrieval (37 vs 96°C for antibody) and shorter probing reaction times (20 vs 90 min for antibody) than typical antibody immunostaining protocols. In addition, the CD30 aptamer probe showed no nonspecific background staining of cell debris in necrotic tissue and exhibited no cross-reaction to tissues that do not express CD30, as confirmed by a standard CD30 antibody staining. Therefore, our findings indicate that the synthetic oligonucleotide CD30 aptamer can be used as a probe for immunostaining of fixed tissue sections for disease diagnosis.
Keywords: anaplastic large cell lymphoma, CD30 expression, classical Hodgkin lymphoma, immunohistochemistry, immunostaining, oligonucleotide aptamer probe, paraffin-embedded tissue staining
Although pathological diagnosis can be performed by morphological/histological evaluation of tissue sections, detecting specific cell biomarkers is almost indispensable for diagnosis confirmation in the majority of neoplasms. The most popular method for detection of biomarkers is immunostaining of fixed tissue sections by antibodies, which are currently the only commercially available and clinically validated probes for this purpose. Production of antibodies is a time-consuming and complicated process and requires animal care and cell culture facilities. Thus, researchers have searched for other ligand molecules that are easier and less costly to produce. One such new class of small molecule ligands is aptamers, which are composed of short single-stranded oligonucleotides (usually 30–50 bases).1–7 With the use of the Systematic Evolution of Ligands by EXponential (SELEX) amplification technique, researchers have discovered a variety of RNA- and DNA-based aptamers, which specifically bind to their target molecules with high affinity at pico- to nanomolar levels.8,9 As specific ligands, aptamers have been tested as a probe for a variety of purposes including dot plot assays,10 western blot assays,11 enzyme-linked immunosorbent assay,11,12 and flow cytometry analyses of cell lines or isolated cells.13,14
A recent study discovered an RNA-based aptamer that specifically binds to the tumor necrosis factor (TNF) receptor (TNFR) family proteins.15 Among its binding targets, this aptamer binds to CD30 proteins with an affinity more than 1000 times greater than other tested proteins of the TNFR family members in solution. To explore the potential use of aptamers for disease diagnosis, we previously validated this reported CD30 aptamer for immunophenotyping of human lymphoma cells using multi-color flow cytometry analysis.16 Our studies demonstrated that the CD30 aptamer probe specifically binds to intact cells of classical Hodgkin lymphoma (CHL) and anaplastic large cell lymphoma (ALCL) without any cross-reaction with other types of lymphoma or blood cells. In this study, we further demonstrated that the CD30 aptamer probe is suitable for immunostaining formalin-fixed and paraffin-embedded lymphoma tissues.
Materials and methods
CD30 Aptamer, CD30 Antibody, and Tissue Sections
As previously described, an oligonucleotide RNA aptamer probe with the following sequence was synthesized: 5′-GAUUCGUAUGGGUGGGAUCG GGAAGGGCUACGAACACCG-3′ (Integrated DNA Technologies, Coralville, IA, USA).15,16 For immunostaining, the synthetic aptamer probes were biotinylated at the 5′ end. A control probe of equal length (5′-GAU-random rN (n=33)-CCG-3′) and biotinylation at the 5′ end was also generated and used to rule out nonspecific tissue staining of the synthetic oligonucleotide probe.16 The monoclonal mouse anti-human CD30 antibody was purchased from Dako (Carpinteria, CA, USA). A secondary antibody staining kit, including horseradish peroxidase (HRP)-conjugated horse anti-mouse IgG antibody and DAB peroxidase substrate solution for color development, was purchased from Vector Laboratories (Burlingame, CA, USA).
The formalin-fixed and paraffin-embedded tissue sections from patient specimens were obtained from archived files in our department according to an Institutional Review Board-approved protocol. These samples included CHL (n=5), ALCL (n=5), and different normal tissues as indicated in Figure 4. Tissue sections were prepared, and hematoxylin and eosin staining was performed for morphological confirmation following routine laboratory protocol.
Figure 4.
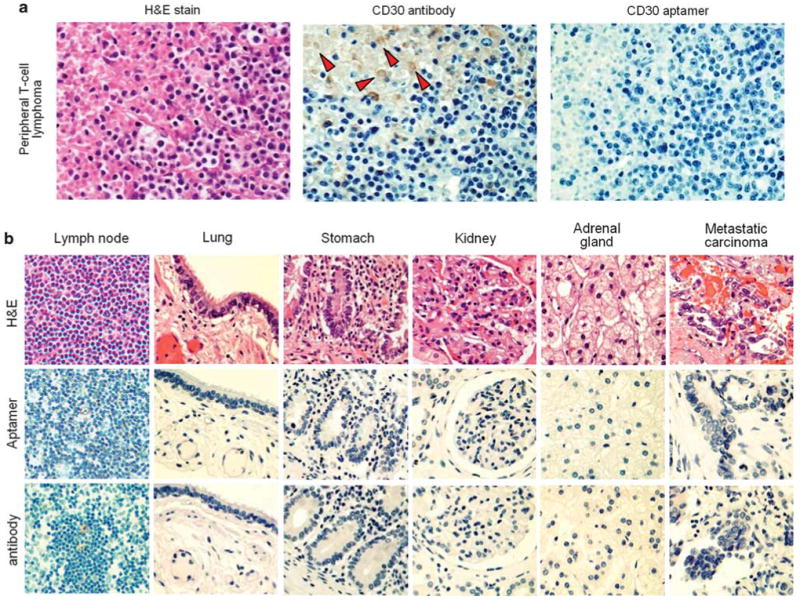
The CD30 aptamer-mediated immunostaining in different tissues. (a) Immunostaining of necrotic tissue. Hematoxylin and eosin (H and E) staining of a CD30-negative peripheral T-cell lymphoma revealed the presence of necrosis in the upper left portion and viable lymphoma cells in the lower right portion of the tissue section. Probing of the tissue with the standard CD30 antibody showed nonspecific staining on cell debris/‘ghost’ cells (indicated by arrows) within the necrotic area, although no reaction with adjacent viable lymphoma cells that do not express CD30 was noted. In contrast, immunostaining with the synthetic CD30 aptamer probe showed no nonspecific background staining in the necrotic areas or in the adjacent viable lymphoma cells. (b) Comparison of the immunostaining by the synthetic CD30 aptamer probe and the CD30 antibody on sections of different CD30-negative tissues. Formalin-fixed and paraffin-embedded tissue sections of normal lymph node, lung, stomach, kidney, adrenal gland, and a metastatic carcinoma of the lung were prepared with H and E stain (top row), immunostained with the CD30 aptamer probe (middle row), or immunostained with the CD30 antibody as a standard control (bottom row).
Immunostaining of Fixed Tissue Sections
For aptamer staining, the tissue sections were deparaffinized two times in xylene for 15 min each, washed with 100% ethanol followed by 95, 80, and 70% ethanol, and then rinsed in distilled water. For antigen retrieval, tissue sections were incubated in 10mM sodium citrate buffer (pH 6.0) at 95 or 37°C for 30 min. After two washes with phosphate-buffered saline solution (PBS) containing 0.05% Tween-20 (pH 7.4), tissue sections were incubated in 3% H2O2 for 20 min. To mask endogenous biotin binding, sections were treated with biotin-blocking solution (Vector Laboratories) following the manufacturer's instructions. Finally, tissue sections were probed with 200 μl of the biotinized CD30 aptamer (60 nmol final concentration in PBS) at room temperature for 20 min. After two washes with PBS Tween-20 solution, the tissue sections were incubated with 200 μl of the HRP-conjugated streptavidin solution (Dako) at room temperature for 20 min and subsequently treated with 200 μl of DAB peroxidase substrate solution (Dako) for color development at room temperature for 5 min. Counterstaining of cell nuclei in tissue sections was performed with the hematoxylin solution for 3 min following routine laboratory protocol. Stained tissue sections were examined under a light microscope.
For CD30 antibody staining, tissue section preparation, antigen retrieval, and H2O2 treatment were carried out under the same conditions as described above. In addition, tissue sections were blocked with horse serum for 30 min at room temperature and then probed with CD30 antibody (1:50 dilution) for 90 min. After two washes with PBS Tween-20 solution, the tissue sections were incubated with HRP-conjugated anti-mouse IgG antibody for 30 min and subsequently treated with DAB peroxidase substrate solution for color development.
Results
Specific Immunostaining of Lymphoma Cells by the Synthetic CD30 Aptamer Probe
For immunostaining, the formalin-fixed and paraffin-embedded tissue sections of CHL, in which the tumor cells express CD30, were prepared and probed with the synthetic CD30 aptamer or the clinically validated CD30 antibody as a standard control (Figure 1, for overview). First, tissue antigen retrieval was performed at 96°C in citrate buffer, and tissue sections were probed with the CD30 antibody for 90 min. Tumor cells in lymphoma tissues were visualized by reaction with HRP-conjugated secondary antibody and subsequent color development as described under ‘Materials and methods’ section. In place of antibody, lymphoma tissues were also probed with the synthetic CD30 aptamer for 20 min and subsequently incubated with HRP-conjugated streptavidin for visualization. Under these conditions, both CD30 antibody and CD30 aptamer specifically recognized the CHL tumor cells, but did not react with background cells within tumor sites (Figure 2a–c). Subsequently, we tested antigen retrieval at 37°C, a lower temperature that is easier to perform in a clinical laboratory. Tissue immunostaining was performed as described above. Notably, under this condition, the CD30 aptamer probe specifically stained CHL tumor cells, whereas the CD30 antibody did not (Figure 2d and e).
Figure 1.
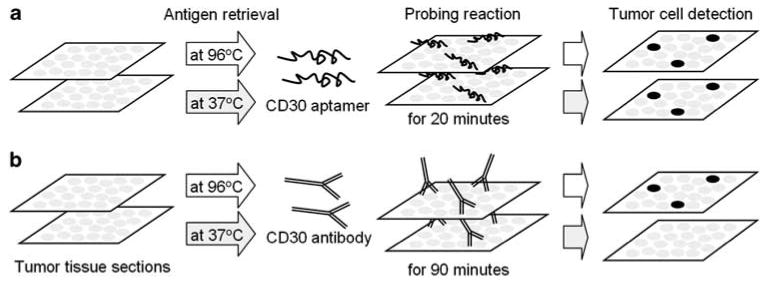
Overview of tissue immunostaining with the CD30 aptamer probe and the CD30 antibody. The formalin-fixed and paraffin-embed tissue sections were prepared, and antigen retrieval was performed at 96 or 37°C. Tissues were then probed with the synthetic CD30 aptamer probe for 20 min (a) or the CD30 antibody for 90 min as a standard control (b). CD30-expressing lymphoma cells were visualized by horseradish peroxidase-induced color development as described in the ‘Materials and methods’ section.
Figure 2.
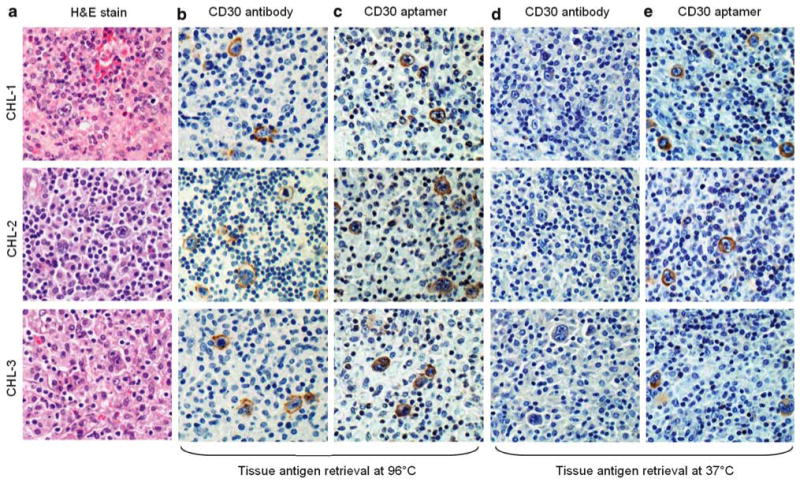
Tissue immunostaining of classical Hodgkin lymphoma (CHL) by the CD30 aptamer probe and the CD30 antibody. CHL tumor cases were selected with hematoxylin and eosin stain for morphological confirmation (a). Tissue antigen retrieval was performed at 96°C, and lymphoma cells were probed with CD30 antibody for 90 min or CD30 aptamer probe for 20 min (b and c). The same sets of tissue immunostains were also performed with antigen retrieval at 37°C (d and e). All images were taken under a light microscope with ×200 magnification.
For further confirmation, tumor tissues of CD30-expressing ALCL were also prepared, and antigen retrieval was carried out at 37°C as described above. The synthetic CD30 aptamer probe specifically immunostained the ALCL cells but not the background cells within tumor sites (Figure 3a and b); however, under the same antigen retrieval conditions, the CD30 antibody did not immunostain the ALCL tumor cells (Figure 3c), although the antibody reacted with the ALCL cells when antigen retrieval was performed at 96°C (Figure 3d). Moreover, tumor tissues were prepared with antigen retrieval at both temperatures and probed with the synthetic control RNA probe, which has the same sequence length as the CD30 aptamer and contains random rN within its central portion.16 No tissue staining of CHL or ALCL by the control RNA probe was observed (data not shown).
Figure 3.
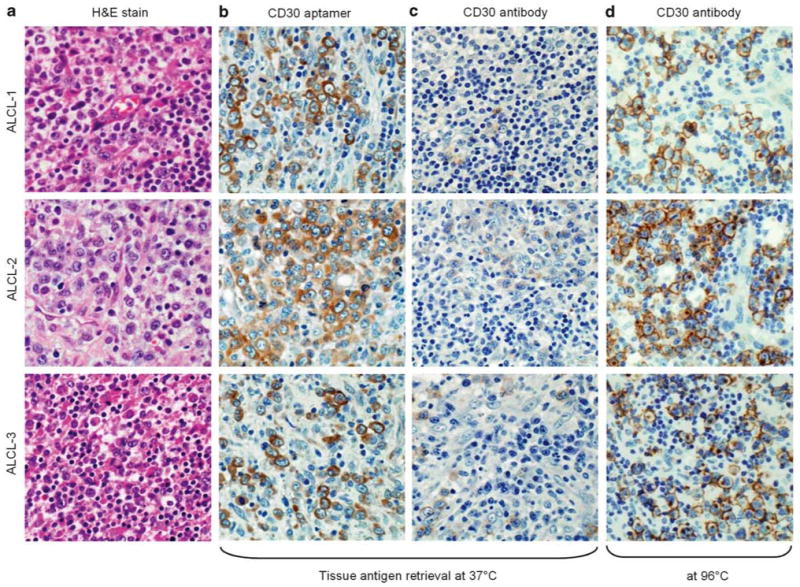
Tissue immunostaining of anaplastic large cell lymphoma (ALCL) by the CD30 aptamer probe and the CD30 antibody. ALCL tumor cases were selected with hematoxylin and eosin stain for morphological confirmation (a). Tissue antigen retrieval was performed at 37°C, and lymphoma cells were probed with the CD30 aptamer probe for 20 min or CD30 antibody for 90 min (b and c). Tissue immunostaining with the CD30 antibody was performed following antigen retrieval at 96°C (d) as a positive control. All pictures were taken under a light microscope with ×200 magnification.
No Cross-Reaction of the Synthetic CD30 Aptamer Probe to Necrotic and/or Non-CD30-Expressing Tissues
Not uncommonly, necrotic tumor tissues produce a nonspecific stain of cell debris by an antibody probe, and this nonspecificity may be problematic for disease diagnosis. The CD30 antibody reacted nonspecifically to cell debris/‘ghost’ cells within the necrotic area of a peripheral T-cell lymphoma tissue, although no CD30 expression was detected in viable lymphoma tumor cells within these same tissue sites by the CD30 antibody (Figure 4a). In contrast, under the same conditions, the synthetic CD30 aptamer probe did not exhibit any cross-reactivity to either necrotic or viable lymphoma cells, confirming nonspecific staining of CD30 antibody at necrotic tumor sites.
Finally, sections of different tissues, including normal lymph node, lung, stomach, kidney, adrenal gland, and metastatic carcinoma, were prepared and immunostained with the CD30 aptamer probe as described above. The CD30 antibody was used as a standard immunostain control in parallel (Figure 4b). No nonspecific or background staining by the CD30 aptamer probe was observed in any of these known CD30-negative tissues.
Discussion
Although the CD30 aptamer and the CD30 antibody exhibited nearly identical specificity to CD30-expressing lymphoma cells, the cell staining patterns were slightly different. The CD30 antibody staining was concentrated at the cell membrane and Golgi zone in CHL and ALCL cells (Figures 2 and 3). In contrast, the CD30 aptamer probe stained the cell membranes and, more diffusely, the cytoplasm of lymphoma cells. This slightly different staining pattern may be due to (1) the better penetration and more efficient reaction of aptamer probes within the fixed tissues and/or (2) because the aptamer probe and antibody recognize different epitopes/portions of cellular CD30 proteins. Our previous studies showed that the CD30 aptamer probe and CD30 antibody can simultaneously bind to the same lymphoma cells without any disruption of binding by either molecule.16 Notably, in vitro protein-binding studies showed that the CD30 aptamer has a more than 1000 times higher affinity to CD30 molecules than to other proteins of the TNFR family, and this data, thus, ruled out the possibility of a nonspecific cross-reaction.15 In addition, findings from both immunophenotyping studies of cultured lymphoma cells by flow cytometry16 and immuno-staining of fixed lymphoma tumor tissues demonstrated that the CD30 aptamer probe and the CD30 antibody have very similar sensitivities and specificities for tumor cells in CHL and ALCL. Furthermore, immunostaining of normal lymph nodes revealed that reactive immunoblasts react with CD30 aptamer probes similarly to CD30 antibody (Figure 4b, far left column). Occasional plasma cells also exhibited weak cytoplasmic staining by the CD30 aptamer probe and by the CD30 antibody (data not shown), although flow cytometry analysis revealed that cultured plasma cells did not react to the CD30 aptamer probe or antibody.16
The concept of the aptamer has emerged over the past few decades;1–3 however, its clinical application has not yet been significantly advanced. This study provides the first demonstration that the synthetic RNA oligonucleotide aptamer probe is useful for immunostaining of formalin-fixed and paraffin-embedded tissues. The CD30 aptamer specifically detected CD30-expressing lymphoma cells. In comparison with antibody, the aptamer probes are synthetic oligonucleotides and thus cost much less to produce. In addition, for tissue immunostaining, the aptamer probes provide optimal staining with shorter reaction times and less restricted antigen retrieval conditions. Furthermore, when probing tumor tissue with necrosis, the aptamer probes shows no or much less background staining of cell debris. These advantages strongly encourage the use of the aptamer probe in tissue immunostaining for disease diagnosis. Because of the discovery of more clinically relevant aptamer probes, advances in the chemical technology required to generate more stable RNA-based aptamer probes,17–19 and because of more validation studies conducted in clinical specimens, one can expect that synthetic aptamer probes will become another powerful tool for pathologists in addition to currently used antibodies.
Acknowledgments
This study was supported in part by a clinical translation study award from the Methodist Hospital Research Institute (CTSA) and grants from the National Cancer Institutes (K22 CA113493, R43 CA148735, and developmental research project award of P50 CA126752).
Footnotes
Disclosure/conflict of interest
The authors declare no conflict of interest.
References
- 1.Ellington AD, Szostak JW. In vitro selection of RNA molecules that bind specific ligands. Nature. 1990;346:818–822. doi: 10.1038/346818a0. [DOI] [PubMed] [Google Scholar]
- 2.Tuerk C, Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science. 1990;249:505–510. doi: 10.1126/science.2200121. [DOI] [PubMed] [Google Scholar]
- 3.Jayasena SD. Aptamers: an emerging class of molecules that rival antibodies in diagnostics. Clin Chem. 1999;45:1628–1650. [PubMed] [Google Scholar]
- 4.Brody EN, Gold L. Aptamers as therapeutic and diagnostic agents. J Biotechnol. 2000;74:5–13. doi: 10.1016/s1389-0352(99)00004-5. [DOI] [PubMed] [Google Scholar]
- 5.Hermann T, Patel DJ. Adaptive recognition by nucleic acid aptamers. Science. 2000;287:820–825. doi: 10.1126/science.287.5454.820. [DOI] [PubMed] [Google Scholar]
- 6.Blank M, Blind M. Aptamers as tools for target validation. Curr Opin Chem Biol. 2005;9:336–342. doi: 10.1016/j.cbpa.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 7.Mairal T, Ozalp VC, Lozano Sánchez P, et al. Aptamers: molecular tools for analytical applications. Anal Bioanal Chem. 2008;390:989–1007. doi: 10.1007/s00216-007-1346-4. [DOI] [PubMed] [Google Scholar]
- 8.Bunka DH, Stockley PG. Aptamers come of age—at last. Nat Rev. 2006;4:588–596. doi: 10.1038/nrmicro1458. [DOI] [PubMed] [Google Scholar]
- 9.Cerchia L, Hamm J, Libri D, et al. Nucleic acid aptamers in cancer medicine. FEBS Lett. 2002;528:12–16. doi: 10.1016/s0014-5793(02)03275-1. [DOI] [PubMed] [Google Scholar]
- 10.Lin Y, Nieuwlandt D, Magallanez A, et al. High-affinity and specific recognition of human thyroid stimulating hormone (hTSH) by in vitro-selected 2′-amino-modified RNA. Nucleic Acids Res. 1996;24:3407–3414. doi: 10.1093/nar/24.17.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drolet DW, Moon-McDermott L, Romig TS. An enzyme-linked oligonucleotide assay. Nat Biotechnol. 1996;14:1021–1025. doi: 10.1038/nbt0896-1021. [DOI] [PubMed] [Google Scholar]
- 12.Ulrich H, Wrenger C. Disease-specific biomarker discovery by aptamers. Cytometry A. 2009;75:727–733. doi: 10.1002/cyto.a.20766. [DOI] [PubMed] [Google Scholar]
- 13.Davis KA, Abrams B, Lin Y, et al. Use of a high affinity DNA ligand in flow cytometry. Nucleic Acids Res. 1996;24:702–706. doi: 10.1093/nar/24.4.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis KA, Lin Y, Abrams B, et al. Staining of cell surface human CD4 with 2′-F-pyrimidine-containing RNA aptamers for flow cytometry. Nucleic Acids Res. 1998;26:3915–3924. doi: 10.1093/nar/26.17.3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mori T, Oguro A, Ohtsu T, et al. RNA aptamers selected against the receptor activator of NF-kappaB acquire general affinity to proteins of the tumor necrosis factor receptor family. Nucleic Acids Res. 2004;32:6120–6128. doi: 10.1093/nar/gkh949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang P, Zhao N, Zeng Z, et al. Using an RNA aptamer probe for flow cytometry detection of CD30-expressing lymphoma cells. Lab Invest. 2009;89:1423–1432. doi: 10.1038/labinvest.2009.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faria M, Ulrich H. Sugar boost: when ribose modifications improve oligonucleotide performance. Curr Opin Mol Ther. 2008;10:168–175. [PubMed] [Google Scholar]
- 18.Kainz S, Czaja R, Greiner-Stoffele T, et al. Selection of RNase-resistant RNAs. Handb Exp Pharmacol. 2006;173:447–455. doi: 10.1007/3-540-27262-3_23. [DOI] [PubMed] [Google Scholar]
- 19.Andreola ML, Calmels C, Michel J, et al. Towards the selection of phosphorothioate aptamers optimizing in vitro selection steps with phosphorothioate nucleotides. Eur J Biochem. 2000;267:5032–5040. doi: 10.1046/j.1432-1327.2000.01557.x. [DOI] [PubMed] [Google Scholar]


