Abstract
Frailty is an important geriatric syndrome that predicts disability and mortality. Substantial evidence suggests inflammation marked by elevated IL-6 levels as a key pathophysiologic factor that contributes to frailty. CXCL-10, a potent pro-inflammatory chemokine, has increased levels with age and is implicated in several inflammatory conditions. To better understand molecular mechanisms of inflammation activation in frailty, we evaluated monocytic expression of CXCL-10 and other inflammatory pathway genes by pathway-specific gene array analysis and quantitative RT-PCR. Frailty status was determined by the validated criteria. Sixteen pairs of community-dwelling frail and age-, race-, and sex-matched non-frail participants (mean age 83 years, range 72–94) completed the study. Here we report that frail participants had higher CXCL-10 expression levels than matched non-frail controls (1.05 ± 0.88 versus 0.53 ± 0.39, p = 0.04). CXCL-10 expression correlated with IL-6 levels only in frail participants (Spearman correlation coefficient r = 0.52, p = 0.03). Furthermore, frailty-associated CXCL-10 upregulation was highly correlated with IL-6 elevation, both measured by frail-over-non-frail ratios (r = 0.93, p < 0.0001). These findings suggest upregulated monocytic expression of CXCL-10 as an important molecular mechanism that contributes to inflammation activation in frail older adults. Therapeutic implications include potential development of CXCL-10-based interventional strategies for the prevention and treatment of frailty in older adults.
Keywords: Frailty, CXCL-10, IL-6, Monocytic gene expression, Inflammation
1. Introduction
Frailty is increasingly recognized as an important geriatric syndrome characterized by decreased physiologic reserve and increased vulnerability to stressors, leading to serious adverse health outcomes including disability, dependency, and mortality [1–3]. The estimated prevalence of this syndrome is 7% among community-dwelling men and women age 65 and older, and up to 25–40% of those aged 80 years and older [1,4]. Substantial evidence suggests that chronic inflammation marked by elevated interleukin-6 (IL-6) levels as a key pathophysiologic factor that contributes to frailty [3,5–10]. For example, frailty is associated with elevated IL-6, C-reactive protein (CRP), and white blood cells (WBC) counts [5–8,10]. In addition, circulating IL-6 levels have inverse associations with insulin-like growth factor-1 (IGF-1) levels and hemoglobin concentrations in frail older adults; low IGF-1 levels and low hemoglobin concentrations (or anemia) are each individually associated with frailty, as well [5,11,12]. These studies and others suggest that frail older adults manifest low-grade, systemic inflammation that may contribute directly or, through other intermediary pathophysiological processes, to frailty [9,10,13,14]. However, molecular mechanisms that lead to inflammation activation in frail older adults have not been elucidated.
CXC chemokine ligand 10 (CXCL-10), also termed interferon (IFN)γ inducible protein 10 kD (IP-10), is the prototype of the CXC chemokine superfamily. Many types of cells produce CXCL-10, including monocytes, and monocytic expression of CXCL-10 is induced by lipopolysaccharide (LPS), IFNγ, and other stimulating factors [15]. CXCL-10 is a potent pro-inflammatory biomediator with elevated levels during aging [16–19]. Upregulated CXCL-10 production has also been implicated in the pathogenesis of several inflammatory conditions including multiple sclerosis (MS) and autoimmune thyroiditis [20,21]. In addition, our data demonstrated that frailty was associated with increase in LPS-induced expression of key inflammatory pathway molecules by CD14+ monocytes, among which CXCL-10 was the most consistently upregulated [22]. However, unstimulated monocytic expression of CXCL-10 and its relationship with IL-6 levels in frail older adults have not been evaluated.
The objective of this study was to investigate monocytic CXCL-10 expression and its relationship with serum IL-6 levels in the syndrome of frailty. We hypothesized that frail older adults would have upregulated CXCL-10 expression by CD14+ monocytes compared to the matched non-frail controls. We further hypothesized that frailty-associated upregulation in CXCL-10 expression would be associated with elevation in serum IL-6 levels. Addressing these hypotheses will provide initial evidence for CXCL-10 upregulation in frail older adults and help delineate its role in inflammation activation and pathogenesis of frailty with potential therapeutic implications. To test these hypotheses, we evaluated ex vivo, unstimulated monocytic CXCL-10 expression and its relationship with serum IL-6 levels by pathway-specific gene array and quantitative RT-PCR in a case control study of community-dwelling frail and age-, race-, and sex-paired non-frail older adults.
2. Materials and methods
2.1. Human subjects
Community-dwelling adults aged 72 and older from Baltimore, Maryland, were recruited from outpatient medical clinics, senior centers, and residential retirement communities and screened by a trained clinical coordinator. The validated and widely utilized screening criteria for frailty included low grip strength, slow walking speed, subjective exhaustion, low levels of physical activity, and unintentional weight loss [1]. Those meeting three or more of the above five items were categorized as frail and those who had none as non-frail. Exclusion criteria included Parkinson's disease, cerebrovascular accident with residual hemiparesis, symptomatic congestive heart failure, malignancy, uncompensated endocrine disorders, rheumatoid arthritis or any other inflammatory conditions, or significant cognitive deficit (Folstein mini-mental status exam score below 18/30). Individuals on steroids or other immune modulating agents were also excluded. These individuals were excluded to minimize the impact of one single disease in mimicking or initiating the presence of frailty or potential modulating effects on monocytes from medications. Those who qualified came to the General Clinical Research Center at Johns Hopkins Bayview Medical Center for a medical history and physical examination by a physician investigator to ensure that they met the eligibility and exclusion criteria and did not have exacerbation of chronic conditions or an acute infection. To minimize potential influences from major demographic variables, a frailty case was matched with a non-frail control by age (within 1 year), race, and sex. The Johns Hopkins Institutional Review Board approved the study protocol. Written informed consent was obtained from all participants.
2.2. CD14+ monocyte preparation and RNA isolation
Peripheral blood mononuclear cells (PBMCs) were isolated from fresh peripheral blood samples by centrifugation over Ficoll-Hypaque density gradient (specific density, 1.077 g/ml) for 10 min at 600g at room temperature. PBMCs were washed three times with PBS containing 2 mM EDTA and 0.5% bovine albumin (pH 7.4) and CD14+ monocytes were purified using a MACS monocyte isolation kit (Miltenyi Biotec, Auburn, CA), according to the manufacturer's instructions. The cells obtained from MACS were further purified by additional 1 h-incubation in RPMI 1640 medium containing 10% heat-inactivated fetal bovine serum (FBS) (Gibco, Gaithersberg, MD) in plastic culture dishes at 37 °C in a humidified 5% CO2 incubator, after which non-adherent cells were removed by repeated rinsing with serum—free RPMI 1640.
Total RNA was extracted from purified CD14+ monocytes using Trizol (Invitrogen/Life Technologies, Carlsbad, CA) following the manufacturer's instructions. The concentration and quality of the RNA preparations were determined by spectrophotometer and electrophoresis.
To minimize potential laboratory variability, blood samples from frail and matched non-frail participants in pairs were collected in the same morning and subsequent experiments were performed in parallel at the same time by the same investigator using the same reagents and equipment. All the reagents and plastic wares were endotoxin-free.
2.3. CXCL-10 expression by pathway-specific gene array
Commercially available pathway-specific gene array (GEArray, SuperArray Bioscience Corp., Frederick, MD) were utilized. The GEArray membrane contains specific oligonucleotide sequences for 367 inflammatory pathway genes including CXCL-10, along with the control sequences [PUC18 as negative control; β-actin and glyceraldehydes 3 phosphate dehydrogenase (GAPDH) for loading] spotted in triplets. GEArray analyses were performed according to the manufacturer's protocols. Briefly, 3 μg of purified total RNA were applied for the synthesis of cDNA probes using GEArray TrueLabeling-Reverse Transcription kit (TL-RT, SuperArray Bioscience Corp) and labeled with [α-32P]-dCTP (Amersham Pharmacia BioTech, Piscataway, NJ). The 32P-labeled cDNA probes were hybridized under precisely specified conditions to the GEArray membrane. After washing, the CXCL-10 expression signal on the GEArray membrane was quantified by exposure to PhosphorImager screens for 1–3 h and recorded on a Molecular Dynamics PhosphoImager (Amersham Biosciences Corp, Piscataway, NJ) using the manufacturer's ImageQuant program. The recorded data were then analyzed using GEArray Analyzer software (SuperArray Bioscience Corp) with appropriate background subtraction and data normalization following manufacturer's instructions. The corrected and normalized signals were used to determine the relative abundance of CXCL-10 transcripts or expression level.
2.4. Quantitative real time RT-PCR
cDNAs were synthesized from purified total RNA (2 μg) samples in 20 μl reactions using random primers (Promega, Madison, WI) and Omniscript® RT kit (Qiagen, Valencia, CA) following the manufacturer's instructions. Quantitative real time PCR (QPCR) experiments were performed using the M×3000P Real-Time PCR System (Stratagene, La Jolla, CA). Forward and reverse primers for CXCL-10 were purchased from SuperArray Bioscience Corp (Frederick, MD) and QPCR was performed using a Brilliant SYBR Green QPCR Master Mix buffer (Stratagene) containing SYBR Green I dye, SureStart Taq DNA polymerase, and a reference dye with the thermal cycling program of enzyme activation for 10 min at 95 °C and 40 cycles of 30 s denaturation at 95 °C, 45 s annealing at 60 °C, and 30 s extension at 72 °C. Data analyses were performed using the data software (Version 2.0) equipped with M×3000P Real-Time PCR System (Stratagene, La Jolla, CA) according to the manufacturer's instructions.
2.5. Measurement of serum IL-6 levels
IL-6 was measured in duplicate by enzyme-linked immunosorbent assay (ELISA) from frozen serum with a commercial kit (High-Sensitivity Quantikine Kit, R&D Systems, Minneapolis, MN) as previous reported [5]. The average of the two measures was used in the analysis.
2.6. Statistical analysis
Demographic and clinical characteristics of the study participants are presented as mean ± standard deviation (SD) or percentages of the total participants in the study group, where two-sided t tests were employed to determine the statistical significance of a difference between two means (p values), as appropriate. Relative CXCL-10 expression levels are also presented as mean ± SD (log-transformed) and Wilcoxon signed-rank sum test was utilized to evaluate difference between frail and non-frail participants using a two-sided α-value of 0.05 to determine statistical significance. Frail-over-non-frail ratios of CXCL-10 expression and serum IL-6 levels were calculated for individual frail and matched non-frail pairs. Spearman correlation coefficient (r) was used for assessing association between CXCL-10 expression levels and serum IL-6 concentrations in each of the frail and non-frail study groups as well as between the frail-over-non-frail ratios of CXCL-10 expression and serum IL-6 levels. All statistical analyses were performed using Stata 9 (StataCorp, College Station, TX).
3. Results
3.1. Characteristics of the study participants
A total of 32, or 16 pairs, of community-dwelling frail and age-, race-, and sex-matched non-frail participants, completed the study. Table 1 summarizes basic demographic and clinical characteristics of the study participants. The mean age was 83 years (range 72–94) and the majority subjects were white and females. The two study groups had comparable body mass index. None of the participants was current or recent (within past 10 years) smoker. Both groups had comparable total number of medical diagnoses and similar disease profiles. In addition, both groups were comparable in their total and specific medication use. None of the participants reported illicit drug use or heavy alcohol consumption.
Table 1.
Demographic and clinical characteristics of the study participants.
| Characteristics | Frail (N = 16) | Non-frail (N = 16) | P |
|---|---|---|---|
| Age (years)* | 83 ± 5 (range 72–94) | ||
| Race (% white) | 93.70% | ||
| Sex (% female) | 87.50% | ||
| Body mass index (kg/m2)* | 26.5 ± 5.5 | 27.1 ± 4.6 | 0.73 |
| Comorbid chronic conditions: | |||
| Total number of diagnosis* | 4.1 ± 1.7 | 4.0 ± 1.7 | 0.92 |
| Specific diseases: | |||
| Hypertension | 50.00% | 81.30% | |
| Osteoporosis | 43.80% | 37.50% | |
| Osteoarthritis | 43.80% | 31.30% | |
| Hypercholesterolemia | 25.00% | 18.80% | |
| Congestive heart failure (controlled) | 37.50% | 25.00% | |
| Hypothyroidism (compensated) | 31.30% | 31.30% | |
| Coronary heart disease (asymptomatic) | 18.80% | 25.00% | |
| Malignancy (reported cured) | 31.30% | 37.50% | |
| Medication usage: | |||
| Total number of medications* | 4.3 ± 1.9 | 3.8 ± 1.7 | 0.39 |
| Specific medications: | |||
| Aspirin | 37.50% | 31.30% | |
| b-Blockers | 43.80% | 31.30% | |
| Diuretics | 37.50% | 43.80% | |
| Calcium-channel blockers | 25.00% | 50.00% | |
| ACE inhibitors | 31.30% | 31.30% | |
| HMG-CoA reductase inhibitors | 31.30% | 25.00% | |
| Thyroid (T4) supplement | 31.30% | 31.30% |
Mean ± SD.
3.2. Ex Vivo monocytic expression of CXCL-10 in frail and non-frail older adults
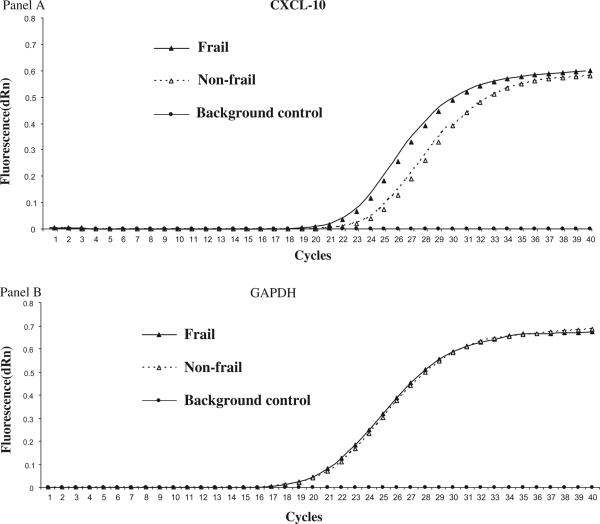
The purity of the CD14+ monocyte preparations was 98.8 ± 0.5% as assessed by routine flow cytometric analysis with phycoerythrin (PE)-conjugated anti-CD14 monoclonal antibody (Becton Dickinson, San Jose, CA). Monocytic CXCL-10 expression was first evaluated by GEArray analysis. As shown in Table 2, frail participants had significantly higher monocytic CXCL-10 expression levels compared to the matched non-frail controls (1.05 ± 0.88 versus 0.53 ± 0.39, p = 0.04). These results were confirmed by QPCR (1.46 + 0.91 versus 0.62 + 0.41, p = 0.02). Fig. 1 shows a representative QPCR experiment from a pair of frail and matched non-frail participants where the frail participant has marked upregulated CXCL-10 expression in the entire linear range of the QPCR reaction, compared with the non-frail participant.
Table 2.
Monocytic expression of CXCL-10, IL-1β, and TNF-α and serum IL-6 levels between frail and matched non-frail study groups.
| Frail group | Non-frail group | P | |
|---|---|---|---|
| Ex vivo monocytic expression levels * : | |||
| CXCL-10 | 1.05 ± 0.88 | 0.53 ± 0.39 | 0.04 |
| IL-1β | 0.57 ± 0.32 | 0.51 ± 0.22 | 0.38 |
| TNF-α | 0.15 ± 0.11 | 0.14 ± 0.09 | 0.72 |
| Serum IL-6 levels (pg/ml): | 3.0 ± 1.6 | 1.6 ± 1.3 | 0.01 |
Values were log-transformed.
Fig. 1.
Representative quantitative real time RT-PCR experiments demonstrating differential expression of CXCL-10 by unstimulated CD14+ monocytes from frail and matched non-frail older adults (Panel A). Expression of GAPDH (a house-keeping gene) in these experiments is shown in Panel B as the control.
To investigate the possibility that the observed upregulation of CXCL-10 expression was due to differential activation of the CD14+ monocyte preparations, we first performed QPCR evaluation of unstimulated expression of CXCL-10 and IFN-γ by monocytes purified by method used in this study and monocytes purified through elutriation from three regular blood donors. No significant activation of our monocyte preparations was apparent as their CXCL-10 and IFN-γ expression was similar to that by monocytes purified through elutriation from the same blood donors (data not shown). In addition, unstimulated expression of IL-1β and tumor necrosis factor (TNF)-α was assessed at the mRNA level by GEArray analysis. As shown in Table 2, frail and matched non-frail participants had comparable monocytic expression levels of IL-1β and TNF-α, indicating no apparent differential activation of these monocyte preparations. Results of the 364 other genes examined in GEArray analysis are provided in the supplementary data file.
3.3. Associations of ex vivo monocytic CXCL-10 expression and serum IL-6 levels
As expected, frail participants had elevated serum IL-6 levels compared with matched non-frail controls (3.0 ± 1.6 versus 1.6 ± 1.3 pg/ml, p = 0.01, Table 2). Among frail participants, ex vivo monocytic CXCL-10 expression was correlated with serum IL-6 levels (r = 0.52, p = 0.03). No such correlation was observed among non-frail participants (r = 0.17, p = 0.53).
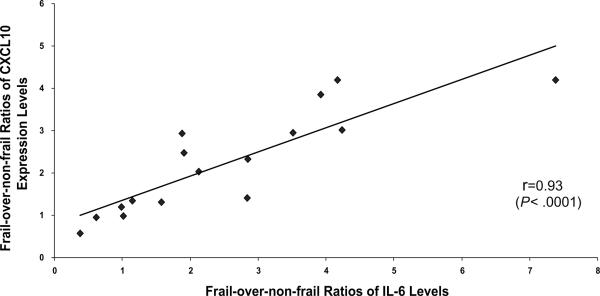
To further evaluate the relationship between upregulation of monocytic CXCL-10 expression and elevation of serum IL-6 levels in frailty, frail-over-non-frail ratios of monocytic CXCL-10 expression and serum IL-6 levels were determined for each individual pairs of frail and matched non-frail participants. As shown in Fig. 2, the frail-over-non-frail ratios of monocytic CXCL-10 expression were highly correlated with those of serum IL-6 levels (r = 0.93, p < 0.0001).
Fig. 2.
Correlation between CXCL-10 upregulation and IL-6 elevation as measured by frail-over-non-frail ratios of monocytic CXCL-10 expression or serum IL-6 levels (calculated for individual frail and matched non-frail pairs. Spearman correlation analysis demonstrated significant correlation between CXCL-10 upregulation and IL-6 elevation in frailty (r = 0.93, p < 0.0001).
4. Discussion
The present study reports, for the first time, that community-dwelling frail older adults had significant upregulation in monocytic CXCL-10 expression, compared to their matched non-frail controls. In addition, upregulation of monocytic CXCL-10 expression was highly correlated with elevation of serum IL-6 levels in the syndrome of frailty.
Monocytes and macrophages express numerous cytokine and chemokine mediators and play a pivotal role in the activation and regulation of local and systemic inflammation. Studies have suggested that monocytes become increasingly activated during aging, which likely contributes to the age-related increased levels of inflammation in older adults [23,24]. In this study, ex vivo CXCL-10 expression by freshly isolated CD14+ monocytes was evaluated without any stimulation or prolonged in vitro culture to best reflect in vivo CXCL-10 expression. To adjust the effect of age, frail and non-frail participants were matched by age and evaluated in pairs. It is interesting to note that in patients with MS, CXCL-10 is expressed by CD14+ monocytes after stimulation, but not by unstimulated monocytes [25], suggesting a potentially more robust CXCL-10 upregulation in frailty than in MS. In our frailty cohort studies, frail older adults had further upregulated CXCL-10 expression by CD14+ monocytes upon LPS stimulation [22]. In addition, none of the other 366 genes screened by pathway-specific GEArray analysis had consistent frailty-associated upregulated expression by unstimulated CD14+ monocytes (Table 2 and Supplement data), suggesting that this upregulated ex vivo expression in frail older adults appears to be CXCL-10 specific.
CXCL-10 is a strong chemoattractant for Th1 lymphocytes and NK cells. In addition to MS and autoimmune thyroiditis, CXCL-10 is implicated in other Th1-mediated inflammatory conditions including type-1 diabetes mellitus and allograft rejection [20,21,26,27]. CXCL-10 upregulation observed in this study suggests the possibility that inflammation activation in the frailty is likely Th1-mediated. This is consistent with our recent findings that frail older adults had increased frequencies of T lymphocytes expressing CC chemokine receptor-5 (CCR5), a pro-inflammatory Th1 phenotype [28].
In contrast to the matched non-frail controls, frail participants had significant correlation between monocytic CXCL-10 expression and serum IL-6 levels. This association became even more striking when CXCL-10 upregulation and IL-6 elevation were measured by frail-over-non-frail ratios (Fig. 2). As previously suggested, circulating IL-6 could theoretically induce monocytic CXCL-10 expression through upregulating transcription factors NF-IL-6 and NF-kB [18,29]. However, this notion was not supported by the observations in our laboratory that recombinant IL-6 did not affect CXCL-10 expression by human or mouse monocytic cell lines (Leng, unpublished data). Considering IL-6 as an overall marker of inflammation in frail older adults, findings from this and other studies lead to our hypothesis, to be tested in future studies, that CXCL-10 plays an important role in Th1-mediated inflammation activation and pathogenesis of frailty (Fig. 3) [16–19,22]. Addressing the above hypothesis will have significant therapeutic implications in the management of the frailty syndrome as CXCL-10 is considered as a promising therapeutic target for several other Th1-mediated chronic inflammatory conditions [30,31]. In experimental allergic encephalomyelitis (EAE), an animal model for MS, administration of anti-CXCL-10 antibody or antisense oligonucleotides against CXCL-10 to the animal led to significant clinical and histological improvement of the disease [32,33]. DNA immunization, administration of plasmid DNA encoding CXCL-10, also suppressed EAE with production of anti-CXCL-10 antibodies and alteration of myelin antigen specific lymphoproliferation from Th1 and Th2 [34].
Fig. 3.
Hypothetical CXCL-10-mediated Th1 inflammation activation model pathway to the syndrome of frailty in older adults. The hypothetical causal directionality between CXCL-10 upregulation and IL-6 elevation remains to be determined in future longitudinal and/or interventional studies.
This study has several limitations. First, the sample size is relatively small. To address this limitation, this study included clinically well characterized group of frail and non-frail community-dwelling older adults who were age-, race-, and sex-matched in pairs. However, it is not possible to match all characteristics or differences between the frail and non-frail participants. In addition, this study is cross-sectional and the results were from one time-point measurement. Thus, causal directionality of the association between CXCL-10 upregulation and IL-6 elevation cannot be established in this study. Further investigations including longitudinal evaluation are needed. Despite these limitations, the results do support our original hypothesis. With careful control of major demographic variables and comparable clinical profiles between the frail and matched non-frail participants, the observed upregulated CXC10 expression by CD14+ monocytes and its association with IL-6 levels appear to be specific in frailty, above and beyond aging itself. These findings provide initial evidence supporting the role of CXCL-10 in contributing to inflammation activation and pathogenesis of frailty. They also provide a basis for potential development of CXCL-10-based interventional strategies for the prevention and treatment of this important geriatric syndrome.
Supplementary Material
Acknowledgments
Grant support This work was supported in part by the National Institutes of Health, National Institute on Aging, R21 AG024235 (Dr. Leng). Dr. Leng is a current recipient of the Paul Beeson Career Development Award in Aging Research, K23 AG028963.
Sponsor's role None.
Footnotes
Financial disclosure: There is no conflict of financial interest, relationships or affiliations other than those listed on the title page.
References
- [1].Fried LP, Tangen C, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56A(3):M1–M11. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- [2].Fried LP, Frailty Walston J. In: Hazzard's principles of geriatric medicine and gerontology. Halter JB, editor. Anne Arbor; McGraw-Hill: 2007. [Google Scholar]
- [3].Fried LP, Hadley EC, Walston JD, et al. From bedside to bench: research agenda for frailty. Sci Aging Knowledge Environ. 2005;2005(31):e24. doi: 10.1126/sageke.2005.31.pe24. [DOI] [PubMed] [Google Scholar]
- [4].Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59(3):255–63. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- [5].Leng S, Chaves P, Koenig K, Walston J. Serum interleukin-6 and hemoglobin as physiological correlates in the geriatric syndrome of frailty: a pilot study. J Am Geriatr Soc. 2002;50(7):1268–71. doi: 10.1046/j.1532-5415.2002.50315.x. [DOI] [PubMed] [Google Scholar]
- [6].Schmaltz HN, Fried LP, Xue QL, Walston J, Leng SX, Semba RD. Chronic cytomegalovirus infection and inflammation are associated with prevalent frailty in community-dwelling older women. J Am Geriatr Soc. 2005;53(5):747–54. doi: 10.1111/j.1532-5415.2005.53250.x. [DOI] [PubMed] [Google Scholar]
- [7].Walston J, McBurnie MA, Newman A, et al. Frailty and activation of the inflammation and coagulation systems with and without clinical morbidities: results from the Cardiovascular Health Study. Arch Intern Med. 2002;162:2333–41. doi: 10.1001/archinte.162.20.2333. [DOI] [PubMed] [Google Scholar]
- [8].Leng SX, Xue QL, Tian J, Walston JD, Fried LP. Inflammation and frailty in older women. J Am Geriatr Soc. 2007;55(6):864–71. doi: 10.1111/j.1532-5415.2007.01186.x. [DOI] [PubMed] [Google Scholar]
- [9].De MM, Franceschi C, Monti D, Ginaldi L. Inflammation markers predicting frailty and mortality in the elderly. Exp Mol Pathol. 2006;80(3):219–27. doi: 10.1016/j.yexmp.2005.11.004. [DOI] [PubMed] [Google Scholar]
- [10].Maggio M, Guralnik JM, Longo DL, Ferrucci L. Interleukin-6 in aging and chronic disease: a magnificent pathway. J Gerontol A Biol Sci Med Sci. 2006;61(6):575–84. doi: 10.1093/gerona/61.6.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Leng SX, Cappola AR, Andersen RE, et al. Serum levels of insulin-like growth factor-I (IGF-I) and dehydroepiandrosterone sulfate (DHEA-S), and their relationships with serum interleukin-6, in the geriatric syndrome of frailty. Aging Clin Exp Res. 2004;16(2):153–7. doi: 10.1007/BF03324545. [DOI] [PubMed] [Google Scholar]
- [12].Chaves PH, Semba RD, Leng SX, et al. Impact of anemia and cardiovascular disease on frailty status of community-dwelling older women: the Women's Health and Aging Studies I and II. J Gerontol A Biol Sci Med Sci. 2005;60(6):729–35. doi: 10.1093/gerona/60.6.729. [DOI] [PubMed] [Google Scholar]
- [13].Ferrucci L, Penninx BW, Volpato S, et al. Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. J Am Geriatr Soc. 2002;50(12):1947–54. doi: 10.1046/j.1532-5415.2002.50605.x. [DOI] [PubMed] [Google Scholar]
- [14].Paganelli R, Di IA, Cherubini A, et al. Frailty of older age: the role of the endocrine–immune interaction. Curr Pharm Des. 2006;12(24):3147–59. doi: 10.2174/138161206777947533. [DOI] [PubMed] [Google Scholar]
- [15].Neville LF, Mathiak G, Bagasra O. The immunobiology of interferon-gamma inducible protein 10 kD (IP-10): a novel, pleiotropic member of the C-X-C chemokine superfamily. Cytokine Growth Factor Rev. 1997;8(3):207–19. doi: 10.1016/s1359-6101(97)00015-4. [DOI] [PubMed] [Google Scholar]
- [16].Miles EA, Rees D, Banerjee T, et al. Age-related increases in circulating inflammatory markers in men are independent of BMI, blood pressure and blood lipid concentrations. Atherosclerosis. 2008;196(1):298–305. doi: 10.1016/j.atherosclerosis.2006.11.002. [DOI] [PubMed] [Google Scholar]
- [17].Antonelli A, Rotondi M, Fallahi P, et al. Increase of CXC chemokine CXCL10 and CC chemokine CCL2 serum levels in normal ageing. Cytokine. 2006;34(1–2):32–8. doi: 10.1016/j.cyto.2006.03.012. [DOI] [PubMed] [Google Scholar]
- [18].Antonelli A, Rotondi M, Fallahi P, et al. Age-dependent changes in CXC chemokine ligand 10 serum levels in euthyroid subjects. J Interferon Cytokine Res. 2005;25(9):547–52. doi: 10.1089/jir.2005.25.547. [DOI] [PubMed] [Google Scholar]
- [19].Shurin GV, Yurkovetsky ZR, Chatta GS, Tourkova IL, Shurin MR, Lokshin AE. Dynamic alteration of soluble serum biomarkers in healthy aging. Cytokine. 2007;39(2):123–9. doi: 10.1016/j.cyto.2007.06.006. [DOI] [PubMed] [Google Scholar]
- [20].Franciotta D, Martino G, Zardini E, et al. Serum and CSF levels of MCP-1 and IP-10 in multiple sclerosis patients with acute and stable disease and undergoing immunomodulatory therapies. J Neuroimmunol. 2001;115(1–2):192–8. doi: 10.1016/s0165-5728(01)00261-2. [DOI] [PubMed] [Google Scholar]
- [21].Antonelli A, Rotondi M, Fallahi P, et al. High levels of circulating CXC chemokine ligand 10 are associated with chronic autoimmune thyroiditis and hypothyroidism. J Clin Endocrinol Metab. 2004;89(11):5496–9. doi: 10.1210/jc.2004-0977. [DOI] [PubMed] [Google Scholar]
- [22].Qu T, Yang H, Fedarko N, et al. Upregulation of stress-responsive inflammatory pathway genes by LPS-induced CD14+ monocytes in frail older adults. Mech Ageing Dev. 2009;(130):161–6. doi: 10.1016/j.mad.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].De MM, Modesti M, Ginaldi L. Phenotypic and functional changes of circulating monocytes and polymorphonuclear leucocytes from elderly persons. Immunol Cell Biol. 2004;82(4):415–20. doi: 10.1111/j.0818-9641.2004.01242.x. [DOI] [PubMed] [Google Scholar]
- [24].Sadeghi HM, Schnelle JF, Thoma JK, Nishanian P, Fahey JL. Phenotypic and functional characteristics of circulating monocytes of elderly persons. Exp Gerontol. 1999;34(8):959–70. doi: 10.1016/s0531-5565(99)00065-0. [DOI] [PubMed] [Google Scholar]
- [25].Comabella M, Imitola J, Weiner HL, Khoury SJ. Interferon-beta treatment alters peripheral blood monocytes chemokine production in MS patients. J Neuroimmunol. 2002;126(1–2):205–12. doi: 10.1016/s0165-5728(02)00064-4. [DOI] [PubMed] [Google Scholar]
- [26].Nicoletti F, Conget I, Di MM, et al. Serum concentrations of the interferon-gamma-inducible chemokine IP-10/CXCL10 are augmented in both newly diagnosed Type I diabetes mellitus patients and subjects at risk of developing the disease. Diabetologia. 2002;45(8):1107–10. doi: 10.1007/s00125-002-0879-5. [DOI] [PubMed] [Google Scholar]
- [27].Rotondi M, Rosati A, Buonamano A, et al. High pretransplant serum levels of CXCL10/IP-10 are related to increased risk of renal allograft failure. Am J Transplant. 2004;4(9):1466–74. doi: 10.1111/j.1600-6143.2004.00525.x. [DOI] [PubMed] [Google Scholar]
- [28].De FU, Wang GC, Fedarko NS, Walston JD, Casolaro V, Leng SX. T-lymphocytes expressing CC chemokine receptor-5 are increased in frail older adults. J Am Geriatr Soc. 2008;56(5):904–8. doi: 10.1111/j.1532-5415.2008.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Matsusaka T, Fujikawa K, Nishio Y, et al. Transcription factors NF-IL6 and NF-kappa B synergistically activate transcription of the inflammatory cytokines, interleukin 6 and interleukin 8. Proc Natl Acad Sci USA. 1993;90(21):10193–7. doi: 10.1073/pnas.90.21.10193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lazzeri E, Romagnani P. CXCR3-binding chemokines: novel multifunctional therapeutic targets. Curr Drug Targets Immune Endocr Metabol Disord. 2005;5(1):109–18. doi: 10.2174/1568008053174723. [DOI] [PubMed] [Google Scholar]
- [31].Romagnani P. From basic science to clinical practice. use of cytokines and chemokines as therapeutic targets in renal diseases. J Nephrol. 2005;18(3):229–33. [PubMed] [Google Scholar]
- [32].Fife BT, Kennedy KJ, Paniagua MC, et al. CXCL10 (IFN-gamma-inducible protein-10) control of encephalitogenic CD4+ T cell accumulation in the central nervous system during experimental autoimmune encephalomyelitis. J Immunol. 2001;166(12):7617–24. doi: 10.4049/jimmunol.166.12.7617. [DOI] [PubMed] [Google Scholar]
- [33].Wojcik WJ, Swoveland P, Zhang X, Vanguri P. Chronic intrathecal infusion of phosphorothioate or phosphodiester antisense oligonucleotides against cytokine responsive gene-2/IP-10 in experimental allergic encephalomyelitis of lewis rat. J Pharmacol Exp Ther. 1996;278(1):404–10. [PubMed] [Google Scholar]
- [34].Wildbaum G, Netzer N, Karin N. Plasmid DNA encoding IFN-gamma-inducible protein 10 redirects antigen-specific T cell polarization and suppresses experimental autoimmune encephalomyelitis. J Immunol. 2002;168(11):5885–92. doi: 10.4049/jimmunol.168.11.5885. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.