Abstract
Adipose-specific inactivation of both AP-1 and C/EBP families of B-ZIP transcription factors in transgenic mice causes severe lipoatrophy. To evaluate if inactivation of only C/EBP members was critical for lipoatrophy, A-C/EBP, a dominant-negative protein that specifically inhibits the DNA binding of the C/EBP members, was expressed in adipose tissue. For first 2 weeks after birth, aP2-A-C/EBP mice had no white adipose tissue(WAT), drastically reduced brown adipose tissue(BAT) and exhibited marked hepatic steatosis, hyperinsulinemia, and hyperlipidemia. However, WAT appeared during the third week, coinciding with significantly improved metabolic functioning. In adults, BAT remained reduced, causing cold intolerance. At 30 weeks, the aP2-A-C/EBP mice had only 35% reduced WAT, with clear morphological signs of lipodystrophy in subcutaneous fat. Circulating leptin and adiponectin levels were less than the wild type levels and these mice exhibited impaired triglyceride clearance. Insulin resistance, glucose intolerance, and reduced free fatty acid release in response to β3-adrenergic agonist suggest improper functioning of the residual WAT. Gene-expression analysis of inguinal WAT identified reduced mRNA levels of several enzymes involved in fatty acid synthesis and glucose metabolism that are known C/EBPα transcriptional targets. There were increased levels for genes involved in inflammation and muscle differentiation. However, when dermal-fibroblasts from aP2-A-C/EBP mice were differentiated into adipocytes in tissue culture, muscle markers were elevated more than the inflammatory markers. These results demonstrate that the C/EBP family is essential for adipose tissue development during the early postnatal period, contribute to glucose and lipid homeostasis in adults, and the suppression of the muscle lineage.
Keywords: C/EBP, dominant negative, adipose tissue, diabetes, lipodystrophy, transgenic mouse
INTRODUCTION
Studies in humans and mice demonstrate that obesity greatly increases the risk of insulin resistance, dyslipidemia, type 2 diabetes, and cardiovascular diseases (Kopelman 2000; Reitman 2004). Paradoxically, lipodystrophy, a paucity of adipose tissue, also leads to very similar metabolic conditions (Huang-Doran, et al. 2010), indicating the importance of adipose tissue in the regulation of glucose and lipid homeostasis (Garg and Misra 2004; Reitman, et al. 2000).
Lipodystrophy in humans comprises a heterogeneous group of rare metabolic disorders characterized by partial or complete loss of adipose tissue (Garg and Misra 2004; Reitman et al. 2000). Lipodystrophy are either inherited or acquired, although inherited lipodystrophic syndromes are exceedingly rare. Etiologically lipodystrophies are categorized as congenital or acquired and according to the pattern of adipose tissue loss, it can be classified as either generalized that affects the whole body or partial that affects specific body regions. Mutations in several genes have been identified in congenital generalized lipodystrophy (Fiorenza, et al. 2010) including AGPAT2 (1-acylglycerol-3-phosphate O-acyltransferase 2) (Agarwal, et al. 2002; Garg and Misra 2004), BSCL2 (Berardinelli-Seip congenital lipodystrophy 2) (Ebihara, et al. 2004; Garg and Misra 2004; Magre, et al. 2001), CAV1 (Kim, et al. 2008) and PTRF (Hayashi, et al. 2009). In case of rare familial partial lipodystrophy type 1 (FPLD1), although any genetic locus has not yet been identified, but numerous genetic mutations have been implicated in other types of inherited partial lipodystrophy (Fiorenza et al. 2010), including the LMNA (lamin A/C) (Garg and Misra 2004), PPARg (peroxisome proliferator-activated receptor-γ) (Agarwal and Garg 2002; Savage, et al. 2003), AKT2 (George, et al. 2004; Tan, et al. 2007), CIDEC (Rubio-Cabezas, et al. 2009) and ZMPSTE24 (Agarwal, et al. 2003).
Lipodystrophy is also common among human immunodeficiency virus (HIV) patients receiving highly active antiviral therapy (Gougeon, et al. 2004; Koutkia and Grinspoon 2004). The similarity of the metabolic manifestations observed in obesity and lipoatrophy suggests that common pathways might be involved in the pathogenesis of both diseases. The identification of several fat derived hormones, including leptin, adiponectin, and resistin, has begun to help clarify this issue, although the precise nature of such pathways remains largely unclear (Kadowaki and Yamauchi 2005; Kershaw and Flier 2004; Steppan and Lazar 2004).
Transgenic mice with fat ablation have been produced by inhibition of adipocyte differentiation and development (Laustsen, et al. 2002; Moitra, et al. 1998; Peterfy, et al. 2001; Shimomura, et al. 1998; Yamauchi, et al. 2001) or induction of apoptosis (Burant, et al. 1997; Pajvani, et al. 2005; Ross, et al. 1993). Independent of the actual cause of fat ablation, all these mutant mice demonstrate insulin resistance, hyperinsulinemia, and hyperlipidemia. In some cases, type 2 diabetes was exhibited, the phenotype characteristic of a patient with lipodystrophy. Similar to human lipodystrophy, the severity of metabolic syndrome in mice correlates with the degree of fat loss (Colombo, et al. 2003).
The A-ZIP/F-1 mouse has severe lipoatrophic diabetes (Moitra et al. 1998). In this model, fat ablation was achieved by adipose specific expression of a dominant negative (DN) protein that inhibits the function of both the C/EBP and JUN families of transcription factors (Vinson, et al. 1993) implicated in adipocyte growth and development (Herrera, et al. 1989; Linhart, et al. 2001). The resulting transgenic mice, A-ZIP/F-1, have virtually no white adipose tissue (WAT) and show many features of patients with severe congenital generalized lipodystrophy. The goal of this study was to determine if the inactivation of only C/EBP members in adipose tissue was critical for the A-ZIP/F-1 phenotype. Transgenic mice were generated that used the adipocyte specific aP2 enhancer/promoter to express A-C/EBP, a dominant negative (DN) protein that specifically inhibits the DNA binding of C/EBP family of B-ZIP transcription factors (Vinson, et al. 2002). These aP2-A-C/EBP transgenic mice have a unique biphasic lipodystrophy syndrome. They have severe lipodystrophy and insulin resistance during early postnatal development, but during weaning, adipose tissue grows along with partial recovery from the metabolic syndrome.
MATERIALS AND METHODS
Transgenic (TG) mice
The plasmid directing fat-specific expression using the 7,621 bp aP2 gene enhancer/promoter (Bernlohr, et al. 1985) was obtained from Dr. M. D. Lane (Moitra et al. 1998). The A-C/EBP DN is a 104-amino acid protein consisting of an N-terminal 9-amino acid FLAG epitope, a 13-amino acid linker, a 21-amino acid designed acidic amphipathic helix, and a 61-amino acid C/EBPα leucine zipper extending to the natural C-terminus (Krylov, et al. 1995). We cloned the A-C/EBP dominant-negative as a KpnI-SmaI fragment to produce the final construct "Bluescript aP2 A-C/EBP SV40 polyA."
For microinjection, an 11,410 bp DNA fragment containing the aP2 promoter, A-C/EBP open reading frame, SV40 splicing site, and polyA site (Figure 1A) was obtained by HindIII - NotI digestion and gel purification and microinjected into the male FVB/N mice (Taketo, et al. 1991) pronuclei and screened by PCR. The transgene specific primers were 934 (5'-TTCAGAGGCTCATAGCACCCTCCT-3') and 933 (5'-TGTCACACCACAGAAGTAAGGTTCC -3'), giving a 561-bp product. Zfy primer pairs (Koopman, et al. 1991) producing a 150-bp product were included in equimolar concentration in the standard multiplex reaction to determine sex. All reactions were performed for 10′ at 93°C using the AmpliTaq™ Gold system, followed by 30 cycles of 45″ at 94°C, 30″ at 65°C, and 45″ at 72° C and a final extension for 10′ at 72° C. Mice were fed a standard pellet diet (NIH-07, 11% calories from fat; Zeigler Brothers, Inc., Gardners, PA). For tissue collection mice were euthanized by cervical dislocation under ketamine (100 mg/kg) /xylazine (10 mg/kg) anesthesia delivered intraperitoneally. All mice were kept on a 12 hour light/dark cycle, with light beginning at 6 am and dark at 6 pm. The animal study protocol was approved by IACUC and the study was carried out following appropriate guidelines.
Figure 1. A-C/EBP transgene is expressed in adipose tissue.
A) Schematic representation of the transgene used for adipose specific expression of A-C/EBP. The open box represents the 7.6 kb aP2 promoter/enhancer, the solid box the 298 bp A-C/EBP DN open reading frame with a FLAG epitope, and the stippled box the ~1.0 kb SV40 small t-antigen splice site and polyadenylation sequences. The unique restriction sites used to form junctions between these three different elements are shown, as well as the 5' and 3' sites used for isolating DNA for microinjection from the plasmid. B) mRNA expression of A-C/EBP transgene. Total RNA was isolated from wild type and aP2-A-C/EBP BAT (B), WAT (W) and liver (L) at the indicated ages, and hybridized to a A-C/EBP specific probe (see Methods) (Top). All lanes contain 10 µg of RNA except for the indicated lanes. Ethidium bromide staining confirms RNA loading (Bottom).
Primary cultures of dermal fibroblasts
Dermal fibroblasts were cultured from newborn wild type or transgenic mice that express the A-C/EBP dominant negative under the control of the 422 adipocyte specific promoter (Moitra et al. 1998) according to Lichti et al. (Lichti, et al. 2008). Primary dermal fibroblasts were seeded at a density of one mouse dermis per 10 cm dish or equivalent in DMEM/F12: GlutaMAX medium (Invitrogen) with 10% FBS. Adipogenesis in the cultured cells was induced as described earlier (Qi, et al. 2003; Rishi, et al. 2010). Briefly, after cells grew to confluence, cultures were treated with 0.5 mM 3-isobutyl-1-methyl-xanthine (Sigma), 1 µM dexamethasone (Sigma), 5 µg/ml insulin (Sigma), and 0.5 µM rosiglitazone for 48 hours. Cells were then changed to medium containing only 5 µg/ml insulin (Sigma) and 0.5 µM rosiglitazone. The induction lasted for 8 days with the medium being replaced every 2 days. Cell with fat droplets, indicative of adipogenesis were revealed by Oil Red O (Sigma) staining.
Isolation and analysis of RNA
RNA was initially extracted using RNeasy kits (Qiagen), as per the manufacturer’s protocol. Nucleic acid elutes from the RNeasy spin-columns were treated with DNaseI in the presence of 1 mM MgCl2 for 30 min at 37°C, treated twice with acid phenol (Ambion) in heavy Phase Lock tubes (Eppendorf), and the aqueous supernatant precipitated successively with isopropanol and 95% ethanol. Northern blots (Maximum Strength Nytran Plus; Schleicher & Schuell) were hybridized using UltraHyb (Ambion) at 42°C overnight, washed for 30 min at medium stringency (2X SSC + 0.2% SDS at 68°C), and exposed to film or quantitated with a Phosphorimager. Probes used were specific for A-C/EBP (1100-bp HindIII 3'UTR fragment including only SV40 sequences).
Microarray analysis
Total RNA was isolated from inguinal fat of six-month old wild type and aP2-A-C/EBP male mice and from dermal fibroblasts primary cultures of wild type and aP2-A-C/EBP induced for adipogenic differentiation. cDNA was synthesized from 5 micrograms of total RNA using the SuperScript Double-Stranded cDNA Synthesis Kit (Invitrogen). This was amplified to cRNA and labeled with biotin using the BioArray RNA Transcript Labeling Kit (Enzo). The labeled cRNA samples were hybridized to Affymetrix GeneChip® Mouse Genome 430 2.0 Array allowing to analyze 39,000 transcripts. The arrays were washed and stained in the Affymetrix GeneChip® Fluidics Station 450 and scanned using Affymetrix GeneChip® Scanner 3000.
Biochemical assays
Blood was collected in non-fasted state mice from their tail or retro-orbital vein at 9 am. Glucose was measured from whole blood or serum using a Glucometer Elite (Bayer). Serum insulin, leptin, adiponectin, and resistin were measured by RIA (Linco, St. Charles, MO). Serum triglycerides (Thermo Electron, Louisville, CO,.# 2780-400H) and free fatty acids (Roche Diagnostics GmbH, Penzberg, Germany, #1 383 175) were measured using indicated colorimetric assay. Tissue triglycerides were measured as described (Colombo et al. 2003).
Body composition
Body composition was measured in non-anesthetized 30 week-old male and female mice using the Bruker minispec NMR analyzer mq10 (Bruker, TX, males) or Echo 3-in-1 NMR analyzer (Echo Medical systems, TX, females) under manufacture’s settings (Bruker Optics Inc., Woodlands, TX). Testing was conducted in randomly fed mice between 9 and 11 am.
Indirect calorimetry
Oxygen consumption and carbon dioxide production were measured in male and female 30 week old mice at using Oxymax Indirect Calorimetry System (Columbus Instruments, Columbus, OH), with 1 mouse per chamber, testing transgenic mice simultaneously with controls (Gavrilova, et al. 2000b). Mice had free access to food and water. Motor activity was determined by infrared beam interruption (Opto-Varimex mini; Columbus Instruments, Columbus, OH). Daily metabolic rate was measured at 24° C for 24 h after a 24-h acclimatization period. Resting oxygen consumption was calculated as the average of the points with less than 6 ambulating beam breaks per minute. The respiratory exchange ratio (RER; the ratio of carbon dioxide produced to oxygen consumed) was calculated from resting data points. Oxidation of carbohydrate produces RER of 1.00, whereas fatty acid oxidation results RER of approximately 0.70. The effect of the β3-adrenergic agonist, CL316,243 was measured in 30-week old male mice at 30° C with each mouse serving as its own control. Mice were allowed to acclimate to calorimetry chambers for 24 h. The baseline data were collected on the following day from 10 am until 1 pm. CL316, 243 was injected intraperitoneally (1 mg/kg in saline) at 1 pm and after a 1-hour delay, data was collected for 3 hours. Oxygen consumption data was normalized to body weight and expressed in ml/kg/min.
Food intake
Food intake was measured in male and female mice at 29 weeks. Mice were caged individually, and the amount of food in the feeding container was measured at day 0 and day 5. Food intake was expressed as g/mouse/day and was also normalized to body weight (mg/g body weight/day).
Cold tolerance
Female mice 30 weeks of age were individually caged with free access to food and water, but without bedding. Mice were placed in 4° C (cold room) at 9 am and body temperature was measured hourly for 8 hours using a rectal probe (Thermalert TH-5, Physitemp, Clifton, NJ).
In vivo assays of glucose homeostasis
Insulin tolerance test was performed at 9 am in non-fasted 32-week old male and female mice. Recombinant human insulin (Humulin R, Eli Lilly, Indianapolis, IN) was injected intraperitoneally (0.75 IU/kg). Blood glucose levels were measured 0, 15, 30, 45 and 60 min after the injection using glucometer. Glucose tolerance was tested in 30 week old male and female mice fasted for 6 hours. Glucose was injected intraperitoneally (2 g/kg) at 2 pm and its levels in blood were measured at 0, 15, 30, 60, and 120 minutes after the injection. In vivo glucose uptake into muscle and adipose tissue was measured in 36 week old male mice in a non-fasted state. At 9 am mice were injected intraperitoneally with (1–14C) 2-deoxyglucose (2-DG) (10 μCi; ICN Radiochemicals Inc., Irvine, CA) and insulin (0.75 IU/kg, Humulin R, Eli Lilly, Indianapolis, IN). After 45 min, tissues were removed and the (14C) 2-deoxyglucose 6-phosphate in muscle and fat was quantitated (Kim, et al. 1996).
Triglyceride clearance
Triglyceride clearance was measured in 24-week old male and female mice fasted for 4 hours (from 8 am until 12 pm) and then gavaged with 400 µl peanut oil (Colombo et al. 2003). Blood was taken hourly via tail vein for 6 hours, and plasma triglyceride were measured colorimetrically.
Western blotting
For protein analysis by Western blotting, tissue/cell lysates were prepared from inguinal fat or induced primary dermal fibroblasts. Inguinal fat tissues from 6 month old male mice was collected, snap frozen in liquid nitrogen and grounded by mortar and pestle. The tissue was lysed in modified RIPA buffer containing 50 mM Tris-Cl, 150 mM NaCl, 0.5% NP-40, 1% Triton-X, 1% sodium deoxycholate, 0.1% SDS, 1 mM EDTA, protease inhibitor (Complete Protease Inhibitor Cocktail Tablet, Roche), 10 mM NaF, 1 mM sodium vandate and 1 mM PMSF. The lysate was centrifuged for two-times at 15000 × g at 4°C for 30 mins and the infranatant was collected carefully without disturbing the upper layer of triglycerides and FFA. The whole cell lysates from primary cultures were prepared in RIPA buffer containing 50 mM Tris-Cl, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 1 mM EGTA, 5 mM EDTA, 10 mM NaF, 1 mM β-glycerophosphate, 1 mM sodium vandate and 1 mM PMSF. Protein concentrations were measured using a Bradford Protein Assay reagent (BioRad) and equal amounts were loaded onto the gel. Proteins were resolved on NuPAGE 4–12% Bis-Tris gels (Invitrogen) and blotted onto PVDF membranes (Hybond-P, Amersham Biosciences). Membranes were blocked in 5% skim milk for 1 hour at room temperature and incubated for another hour with the required primary antibodies followed by three washes, at 5 minutes each, of PBS with 0.1% Tween 20 (Sigma Chem Inc.). After washing, the blots were incubated for 1 hour with secondary antibodies against rabbit or mouse IgG (Amersham Biosciences, 1:5,000) and washed 3×5 minutes. Blots were developed using ECL plus Western Blotting detection system (Amersham Biosciences). The following primary antibodies were used: Polyclonal rabbit anti-myomesin-2 (sc-50435; Santa Cruz Biotechnology), Polyclonal goat anti-Steroyl-CoA desaturase 1 (SCD1) (sc-14719; Santa Cruz Biotechnology), Polyclonal rabbit anti-FLAG. All washes and dilutions were carried out using PBS with 0.1% Tween 20 (Sigma Chem Inc.).
Statistical analysis
The gene expression profile consists of RMA extracted log transformed expression ratios measured on the full set of genes represented on the microarray. Analyses were performed with the software package BRB Array Tools, developed by the Biometric Research Branch of the US National Cancer Institute. Genes were filtered based on T-test (One tailed, two-sampled equal variance) p-value ≤ 0.05. The filtered gene list was used to classify the misregulated genes in aP2-A-C/EBP mice compared to the wild type at a cut-off value greater than 1.5 fold change in expression (ratio between mean expression of 2 TG and 3 WT). The misregulated genes were used to identify the relatively enriched (p-value <0.01) Gene Ontology categories using Gene Ontology Tree Machine (GOTM) software developed and maintained by Vanderbilt University (available at: http://bioinfo.vanderbilt.edu/gotm). The significantly enriched GO categories were then manually grouped into functionally related classes. The statistical significance (P-value) of genes appearing in these functional classes was determined using Fisher’s exact test.
Values for other biochemical and physiological assays have been reported as mean ± SEM. Statistical significance was determined using ANOVA followed by t-tests, with differences considered as significant at p<0.05. Holm-Sidak test was performed for pairwise multiple comparisons.
RESULTS
Generation of the aP2-A-C/EBP-1 transgenic mouse line
A-C/EBP is a dominant negative (DN) protein that specifically heterodimerizes with C/EBP family members and is able to inhibit the DNA binding of C/EBP family members in an equimolar competition assay (Vinson et al. 2002). The A-C/EBP protein contains the C/EBPα leucine zipper and a designed acidic protein sequence that replaces the C/EBP basic region. The acidic protein sequence heterodimerizes with the basic region of endogenous C/EBP proteins by forming a coiled coil extension of the leucine zipper (Ahn, et al. 1998; Krylov et al. 1995; Olive, et al. 1997). We expressed the A-C/EBP protein selectively in adipose tissue by using the aP2 promoter/enhancer as described previously (Figure 1A) (Moitra et al. 1998). The founder with the most severe phenotype, termed aP2-A-C/EBP, is described here.
The expression of A-C/EBP dominant negative transcript was detectable in brown adipose tissue (BAT) from 3 days after birth expressed until adulthood but was undetectable in liver (Figure 1B). Transgenic WAT grew into a biochemically traceable sample only after three weeks of age and contained less A-C/EBP transcript than BAT.
Morphological characteristics of young aP2-A-C/EBP mice
The aP2-A-C/EBP pups were similar to the wild type littermates in either body weight or length at birth (Figure 2A–B) but showed slower growth during the subsequent 4 weeks of life. At birth, BAT weight was 50% less in aP2-A-C/EBP mice than in wild type mice and it progressively decreased with age (Figure 2C). WAT was undetectable in aP2-A-C/EBP mice at birth but started to appear in its normal anatomical locations at week 3. The lower body weight of aP2-A-C/EBP mice can be partially explained by the lack of WAT. Histological examination of BAT from aP2-A-C/EBP mice identified patches of cells containing large fat droplets that were not detected in the wild type BAT but resembled white adipocytes (Figure 2E). The BAT from aP2-A-C/EBP mice is similar to the BAT from aP2-A-ZIP/F mice (Moitra et al. 1998). Subdermal white adipocytes histologically observed in one day old wild type pups were almost completely absent in the aP2-A-C/EBP pups (Figure 2F).
Figure 2. Morphological, metabolic and histological characteristics of young aP2-A-C/EBP mice.
Figures A–D & I–L show data from time course experiments (Age of mice: 1, 3, 5, 10, 12, 15, 18, 21 and 28 days) on aP2-A-C/EBP (○) and wild type littermate control mice (●). Data are presented as mean ± SEM. Since there was no big difference between mice of different sex before one month of age, data from males and females were combined together. Asterisks indicate significant (t-test, p<0.05) difference between wild type and aP2-A-C/EBP pups. Number of mice used for time course experiments are shown in Table 1. A) Body weight (in gms): The aP2-A-C/EBP pups at birth are similar in weight to wild type littermates but, they start to lag significantly (24% lighter; t-test, p<0.001) by day 3. B) Body length (in mm). C) BAT (percent body weight): At birth, transgenic pups had 43 % (t-test, p< 0.001) lower BAT compared to the wild type, the difference reduced while they underwent a growth spurt until day 18, then aP2-A-C/EBP BAT again regressed in weight and is on the average, 65% smaller (t-test, p< 0.001) than in controls by weaning. D) Liver (percent body weight): At day 1, aP2 A-C/EBP pups had 67 % larger livers than wild types (t-test, p< 0.001) and became 135 % larger (t-test, p< 0.001) in a week. Then aP2 A-C/EBP liver regressed over the next 3 weeks to reach the same relative weight as wild type. Figures E–H show histology and gross appearance of selected organs in aP2-A-C/EBP pups (panels on the right) and wild type littermates (panels on the left). E) Hematoxylin and eosin (H & E) staining of BAT sections from day old pups (200X magnification). BAT in transgenic animals has a larger proportion of WAT-like cells but looks mostly normal. F) H & E staining of skin section (magnification X200) of 1-day old pups. Note almost complete absence of WAT like cells in the subdermal region of transgenic mice. G) Livers in the aP2-A-C/EBP mice at day 1 of life are paler in color suggesting lipid accumulation. H) H & E staining of liver sections. Hepatocytes of transgenic mice appeared filled with lipid droplets. Figures I–L) Serum biochemistry in aP2-A-C/EBP pups and wild type littermates from birth till weaning; I) serum glucose (in mg/dL); J) serum insulin (in ng/ml); K) serum triglyceride (in mg/dL); L) serum free fatty acids (in mM).
At birth, the aP2-A-C/EBP liver was similar to the wild type liver in terms of both weight and reddish color, however, by day 1, it was 75% heavier than the wild type littermates and was pale in color (Figure 2D,G). Histological examination of the aP2-A-C/EBP liver revealed massive accumulation of lipid in hepatocytes (Figure 1H). The aP2-A-C/EBP liver remained large and fatty until day 21 (weaning), however by day 28 the gross difference between the wild type and the aP2-A-C/EBP liver in weight and color disappeared (Figure 2D) coinciding with the appearance of WAT.
Insulin resistance and dyslipidemia in young aP2-A-C/EBP mice
During the first 4 weeks of development, serum was analyzed to determine the levels of glucose, insulin, triglyceride, and free fatty acids in wild type and aP2-A-C/EBP mice (Figure 2I–L). During the first week of life the aP2-A-C/EBP mice had significantly high levels of blood glucose (Figure 2I) while serum insulin levels (Figure 2J) were dramatically elevated, 20–60 times higher than in controls. During the second week, hyperinsulinemia gradually decreased to wild type levels by week 4. Serum triglyceride (Figure 2K) and free fatty acids (Figure 2L) were also elevated in young aP2-A-C/EBP mice and gradually normalized by weaning, coinciding with appearance of WAT.
Morphological characteristics of 6-month old aP2-A-C/EBP mice
Body weights of adult aP2-A-C/EBP mice were slightly less than wild type mice, particularly in females (Figure 3A). There was a reduction in fat depots throughout the body and a severe reduction of BAT (Figure 3B). NMR analysis of the body composition showed a 35% decrease in the amount of fat in transgenic mice compared to the wild type controls, with no change in lean body mass (Table 2). To examine whether different fat depots were affected similarly, individual fat pad weights were determined (Figure 3C). BAT was the most affected, being 85% and 70% smaller in males and females, respectively, compared to wild type controls. WAT depots in aP2-A-C/EBP males, (inguinal, epididymal, retroperitoneal and mesenteric) were decreased in weight by 32–46%. In transgenic females, all other WAT depots were significantly reduced in size except for the parametrial fat pad.
Figure 3. Characteristics & gross appearance of aP2-A-C/EBP mice at 30 weeks.
A) Growth curves of male (top) and female (bottom) mice; Data are mean ± SD for n = 7–32. The A-C/EBP mice did not weigh any different from the wild type littermates at birth, but by weaning, were smaller on the average by 25% for females and 30% for males, respectively. This difference in weights persisted for female aP2-A-C/EBP mice throughout their lives, but for the males again becomes apparent only after 25 weeks. B) Gross appearance of male aP2-A-C/EBP mice (mouse to the right) compared to the male wild type littermate (mouse to the left) at 30 weeks of age. Arrows show decreased amount of subcutaneous fat in transgenic mouse. C) Weight of selected fat pads in aP2-A-C/EBP mice (open bar) and wild type littermates (filled bar); Abbreviations used are: Ing -inguinal fat, Epi – epididymal fat, Para – parametrial fat, Retro – retroperitoneal fat, Mes – mesenteric fat, BAT – brown fat. Data are mean ± SEM, n= 7–11/group (males 9 WT, 11 TG; females 7 WT, 10 TG). The amount of inguinal, retroperitoneal, mesenteric fat and BAT was decreased by 59%, 51%, 40% and 70%, respectively. D) H & E staining of inguinal fat from aP2-A-C/EBP (panel on the right) and wild type littermate (panel on the left).
Table 2.
Characteristics of aP2-A-C/EBP mice at 30 weeks of age.
| Characteristic | Male | Female | ||
|---|---|---|---|---|
| WT | A-C/EBP | WT | A-C/EBP | |
| Body weight (g) | 36.9 ± 1.4 (n=9) |
35.5 ± 1.1 (n=11) |
32.7 ± 2.5 (n=7) |
30.6 ± 1.3 (n=12) |
| FAT mass (g) |
4.7 ± 0.4 (n=6) |
2.78 ± 0.3* (n=6) |
8.4 ± 1.2 (n=7) |
5.1 ± 0.59* (n=9) |
| Lean mass (g) | 26.7 ± 1.1 (n=6) |
25.7 ± 0.9 (n=6) |
22.6 ± 0.6 (n=7) |
23.5 ± 0.85 (n=9) |
| Body length (mm) | 90.1 ± 1.4 (n=9) |
89 ± 1.0 (n=11) |
85.6 ± 1.5 (n=7) |
84.2 ± 1.1 (n=12) |
| Liver (% body weight) |
4.7 ± 0.1 (n=9) |
5.7 ± 0.2* (n=11) |
4.4 ± 0.2 (n=7) |
5.6 ± 0.2* (n=12) |
| Kidney (% body weight) |
1.4 ± 0.0 (n=9) |
1.6 ± 0.1* (n=11) |
1.1 ± 0.0 (n=7) |
1.2 ± 0.1 (n=12) |
| Spleen (% body weight) |
0.32 ± 0.1 (n=9) |
0.47 ± 0.0* (n=11) |
0.41 ± 0.0 (n=7) |
0.56 ± 0.0* (n=12) |
| Heart (% body weight) |
0.45 ± 0.0 (n=9) |
0.49 ± 0.0* (n=11) |
0.40 ± 0.0 (n=7) |
0.45 ± 0.0 (n=12) |
| Muscle mass (% body weight) |
76.6 ± 0.7 (n=6) |
81.4 ± 0.4* (n=6) |
71.8 ± 2.6 (n=7) |
80.9 ± 1.4* (n=9) |
| WAT mass (% body weight) |
13.4 ± 0.9 (n=6) |
8.7 ± 0.6* (n=6) |
26.2 ± 3.0 (n=7) |
17.1 ± 1.4* (n=9) |
| Resting oxygen consumption (ml/kg/h) |
2748 ± 93 (n=6) |
3130 ± 74* (n=6) |
3610 ± 120 (n=7) |
4202 ± 122* (n=9) |
| Total oxygen consumption (ml/kg/h) |
3281 ± 87 (n=6) |
3937 ± 74* (n=6) |
4404 ± 152 (n=7) |
5088 ± 108* (n=9) |
| Food intake (g/mouse/day) |
2.9 ± 0.2 (n=6) |
3.5 ± 0.2* (n=6) |
3.1 ± 0.1 (n=7) |
3.1 ± 0.1 (n=9) |
| Food intake (mg/g/day) |
84 ± 8 (n=6) |
114 ± 12* (n=6) |
95 ± 3 (n=7) |
106 ± 5* (n=9) |
| Serum glucose (mg/dL) | 183 ± 11 (n=10) |
186 ± 11 (n=12) |
172 ± 9 (n=7) |
173 ± 9 (n=12) |
| Serum insulin (ng/ml) |
1.7 ± 0.2 (n=10) |
3.0 ± 0.6* (n=12) |
1.0 ± 0.2 (n=7) |
1.4 ± 0.3 (n=12) |
| Serum triglyceride (mg/dL) | 216 ± 32 (n=10) |
269 ± 22 (n=12) |
164 ± 30 (n=7) |
211 ± 21 (n=12) |
| Serum free fatty acids (mM) | 0.37 ± 0.4 (n=9) |
0.32 ± 0.3 (n=12) |
0.39 ± 0.1 (n=6) |
0.48 ± 0.1 (n=12) |
| Serum leptin (ng/ml) |
12.3 ± 2.2 (n=9) |
4.1 ± 0.5* (n=9) |
15.7 ±4.1 (n=7) |
5.6 ± 0.7* (n=10) |
| Serum adiponectin (µg/ml) |
9.6 ± 1.2 (n=9) |
2.7 ± 0.3* (n=9) |
20.3 ± 2.1 (n=7) |
4.8 ± 0.7* (n=10) |
| Serum resistin (ng/ml) |
9.0 ± 0.5 (n=11) |
7.0 ± 1.1* (n=10) |
9.1 ± 0.6 (n=7) |
7.5 ± 0.5* (n=9) |
| Liver triglyceride (µmol/g) | 8.2 ± 0.6 (n=6) |
8.5 ± 0.8 (n=6) |
15.0 ± 0.6 (n=6) |
12.7 ± 1.7 (n=6) |
| Muscle triglyceride (µmol/g) | 12.0 ± 1.2 (n=6) |
12.5 ±1.0 (n=6) |
15.8 ±1.4 (n=6) |
12.9 ± 2.2 (n=6) |
Data are mean ± S.E. (n= 6–12).
Asterisk (*) indicates significant difference (p<0.05) between WT and A–C/EBP mice within each gender group.
Wild-type BAT consists of mitochondria-rich eosinophilic cells containing multiple lipid droplets. Wild-type WAT cells, in contrast, are larger containing a single large lipid droplet with the nucleus at the cell periphery. The aP2-A-C/EBP brown adipose tissue showed sparse eosinophilic staining, a single lipid droplet per cell, and peripheral nuclei, resembling BAT observed in the aP2-A-ZIP/F-1 mouse (Moitra et al. 1998). Histological examination of epididymal WAT was similar in the aP2-A-C/EBP and wild type mice. In contrast, the aP2-A-C/EBP inguinal fat revealed smaller adipocytes and presence of stretches of fibroblast-like cells unlike the wild type fat (Figure 3D). In addition, there was an increase in the number of lymphocytes.
In both male and female aP2-A-C/EBP mice spleen and livers were enlarged (Table 2). In addition, transgenic males had bigger kidneys and hearts, similar to the A-ZIP/F model of complete lipoatrophy (Moitra et al. 1998).
Cold sensitivity in aP2-A-C/EBP mice
BAT is a major site of adaptive thermogenesis in small mammals (Himms-Hagen, et al. 1994; Nicholls and Locke 1984). Since aP2-A-C/EBP mice had dramatically reduced and apparently inactive BAT, we analyzed metabolic rate and cold sensitivity in 6 month-old mice. The basal metabolic rate (per mouse or normalized to fat free mass) was comparable in the aP2-A-C/EBP and control mice (data not shown); however, when the data was normalized to total body weight, the metabolic rate of transgenic mice was nearly 15% higher than the wild type controls (Table 2). Relatively elevated metabolic rate was likely to be balanced with significantly increased food intake in the aP2-A-C/EBP mice (Table 2). To assess thermogenic capacity of the aP2-A-C/EBP male mice, we stimulated them with the β3-adrenergic agonist, CL316243, which induces lipolysis in WAT and thermogenesis in BAT (Himms-Hagen et al. 1994). In response to CL316243, the wild type mice doubled their metabolic rate, whereas the aP2-A-C/EBP mice increased their metabolic rate only by 50%, suggesting a defect in thermogenesis (Figure 4A). Both wild type and transgenic mice responded to β3-adrenergic stimulation by lowering respiratory exchange ratio (RER). However, the drop was significantly smaller in transgenic mice, indicating a relative decrease in fatty acid oxidation (Figure 4A). To assess WAT responsiveness to the β3-agonist, we measured in vivo FFA release in blood (Figure 4B). Within 20 minutes of acute stimulation with CL316243, wild type mice increased FFA 3 fold, however the response was significantly less (p= 0.003) in transgenic mice. Thus, the aP2-A-C/EBP mice have an impaired response to β3-adrenergic stimulation. This disparity can lead to cold intolerance as evident in the aP2-A-C/EBP female mice that were unable to maintain normal body temperature at 4° C (Figure 4C).
Figure 4. Characteristics of aP2-A-C/EBP mice at 30 weeks.
A) Oxygen consumption (panel on the left) and respiratory exchange ratio (panel on the right) were measured before (solid bars) and after CL316,243 (hatched bars) injection at 30°C. Note blunted response to β3-adrenergic agonist, CL316,243, in aP2-A-C/EBP mice. Data are mean + SEM, n=8/group. B) Free fatty acid release in response to β3 adrenergic agonist was significantly less (p=0.003) in aP2-A-C/EBP mice. The mice were injected with CL316,243 (1 mg/kg, intraperitoneally), and free fatty acids in serum were measured at 0, 20 and 120 min, n=6/group. C) aP2-A-C/EBP mice are cold intolerant (p= 0.033). The mice were placed at 4°C, and rectal temperature was measured hourly (0–8 hours). The graphs show body temperature of individual mice (4 TG and 5 WT). Significant (p<0.05) differences were observed starting from hour 5. D) Glucose tolerance test: Mice (littermates) were injected with glucose (2 g/kg) intraperitoneally. Data are mean ± SEM, n= 7–12/group (males 10 WT, 12 TG; females 7 WT, 10 TG) at 0, 15, 30, 60 and 120 minutes after injection. Male aP2-A-C/EBP mice showed significantly delayed clearance of glucose (p<0.05 at 60 and 120 minutes) E) Insulin tolerance test: Mice (littermates) were injected with insulin (0.75 IU/kg). Data are mean ± SEM, n= 7–13/group (males 10 WT, 13 TG; females 7 WT, 10 TG) at 0, 15, 30, 45 and 60 minutes after injection. Male aP2-A-C/EBP mice showed significantly less response to insulin (p<0.05 at 45 and 60 minutes) F) 2-deoxyglucose (2-DG) uptake into skeletal muscle and adipose tissue: aP2-A-C/EBP mice (open bar) and wild type littermates (filled bar) were injected with (1–14C) 2-deoxyglucose and insulin (0.75 IU/kg). SM –skeletal muscle, gastrocnemius; BAT – brown fat; Epi – epididymal fat; Ing inguinal fat. G) Triglyceride clearance after oral lipid load: mice (littermates) were gavaged with 400 ml peanut oil, and plasma triglyceride levels were measure hourly (0–6 hous). Data are mean ± SEM, n= 7–11/group (males 10 WT, 11 TG; females 7 WT, 10 TG). aP2-A-C/EBP mice showed delayed triglyceride clearance (p<0.05 at 3, 4 and 5 hours). The panels A, D, E, F, G and I show data from the aP2-A-C/EBP mice (○) and wild type littermates (●). Asterisks (*) indicate statistical significance at p< 0.05 from pairwise multiple comparison test (Holm-Sidak).
Metabolic characteristics of the adult aP2-A-C/EBP mice
6 month-old male and female aP2-A-C/EBP mice had normal glucose serum levels (Table 2). Insulin serum levels were significantly elevated only in aP2-A-C/EBP males (by 76%). Both male and female aP2-A-C/EBP mice had elevated serum triglyceride levels.
Glucose and insulin tolerance tests were used to characterize glucose metabolism in more detail (Figure 4D–E). The aP2-A-C/EBP males showed delayed clearance of glucose from the circulation after glucose load and a blunted response to insulin, the phenotype consistent with insulin resistance. Oppositely, the aP2-A-C/EBP females behaved similar to the wild type female littermate having normal glucose serum levels. To analyze whether glucose uptake was impaired in the aP2-A-C/EBP male mice, we measured 2-deoxyglucose uptake into skeletal muscle, BAT, epididymal and inguinal fat (Figure 4F). Glucose uptake into muscle and BAT was significantly reduced in transgenic male mice compared to controls (by 32% and 56%, respectively), whereas uptake into WAT was similar in both genotypes.
To determine whether impaired clearance of triglycerides might be causing the increase in serum triglyceride, we performed a triglyceride tolerance test (Figure 4G). After oral lipid load delivery, both male and female aP2-A-C/EBP mice demonstrated delayed triglyceride clearance, similar to mice with complete lipoatrophy (Colombo et al. 2003; Gavrilova, et al. 2003). Taken together, these data demonstrate that despite only a modest reduction of total fat, the aP2-A-C/EBP mice have abnormalities in glucose and lipid metabolism.
Circulating adipokine levels in aP2-A-C/EBP mice
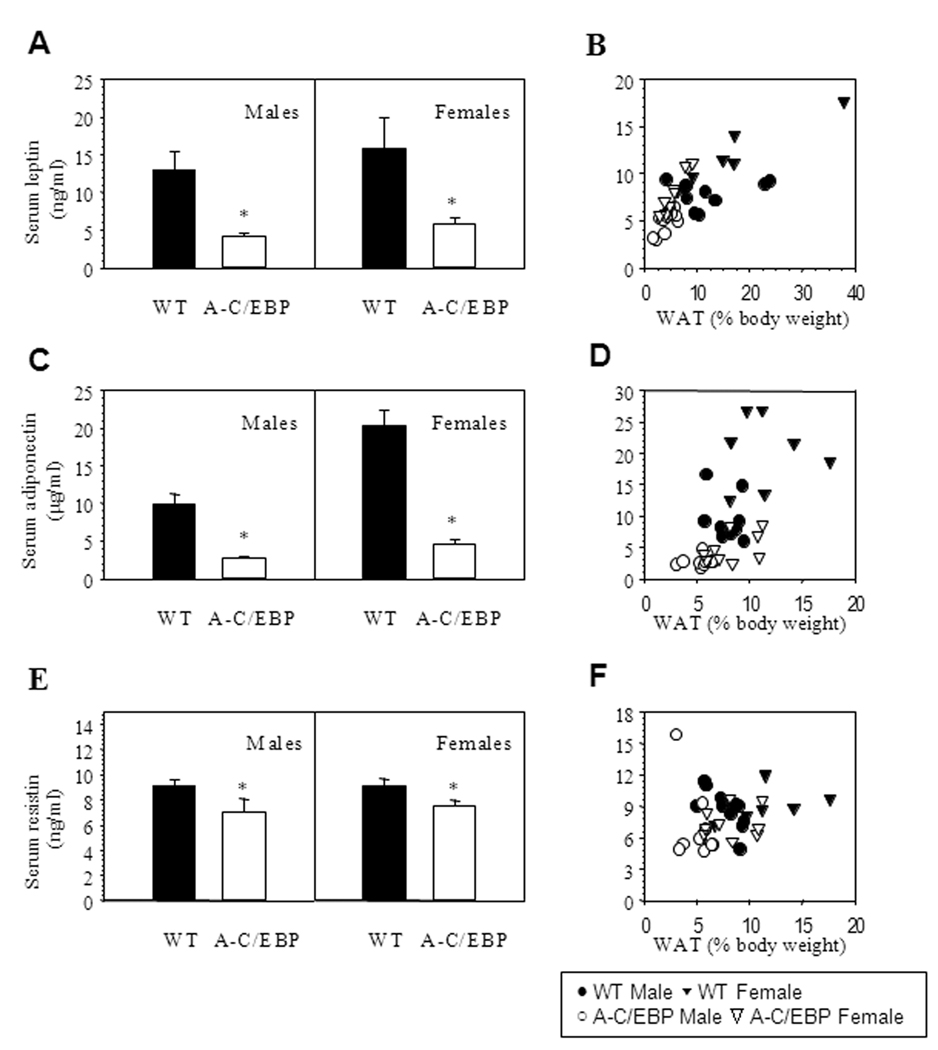
Three serum adipokines were measured to explore the mechanisms underlying the metabolic syndrome in adult aP2-A-C/EBP mice (Figure 5). Leptin levels were reduced in both males and females and positively correlated with % body fat (Figures 5A–B). Consistent with previously published data (Combs, et al. 2003), wild type females had higher adiponectin levels than males (Figure 5C). In both male and female aP2-A-C/EBP mice, adiponectin levels were significantly lower than in wild type controls (by 76% and 72%, respectively). Interestingly, transgenic mice had lower adiponectin levels even after normalization for percent of fat (Figure 5D). Resistin levels were also significantly reduced in transgenic mice (by 22% and 18%, males and females, respectively) and did not show a clear correlation with percent body fat (Figures 5E–F).
Figure 5. aP2-A-C/EBP mice have low circulating adipokines.
A–B) Serum leptin, and C–D) Serum adiponectin in 7–10 mice/ group (9 WT & 9 TG males; 7 WT & 10 TG females). E–F) Serum resistin in 7–11 mice/ group (11 WT & 10 TG males; 7 WT & 9 TG females). Serum was obtained from aP2-A-C/EBP mice (open bar) and wild type littermates (filled bar) at the age of 33 weeks in non-fasted state. In panels A, C and E data are mean ± SEM, n=7–11 mice per group. aP2-A-C/EBP mice had significantly (two way ANOVA test) less levels of leptin (p<0.001), adiponectin (p<0.001) and resistin (p=0.029). In panels B, D and F the data from the aP2-A-C/EBP mice (female, ∆; male, ○) and wild type littermates (female ▲; male ●) are plotted as a function of WAT mass, where WAT mass is a combined weight of inguinal, gonadal (epididymal in males or parametrial in females), retroperitoneal and mesenteric fat pads, expressed as percent body weight.
Gene expression changes in aP2-A-C/EBP inguinal fat
We determined the global mRNA expression profiles from inguinal fat of 6-month old wild type and aP2-A-C/EBP male mice. 542 transcripts were significantly (p< 0.05) misregulated by 1.5 fold, with similar numbers being down-regulated (246) and up-regulated (296) in the aP2-A-C/EBP mice (Figure 6A). Potential transcriptional targets of the C/EBP family members were amongst the down-regulated genes. Among the enriched GO terms for down-regulated genes, 37 genes were grouped in the GO category of metabolism of lipid, alcohol or carbohydrate (Table 3). These include the well-known adipose genes (Table 4) stearoyl-Coenzyme A desaturase 1 (Scd1), stearoyl-Coenzyme A desaturase 2 (Scd2), Agpat2, adiponectin receptor 2 (Adipor2) and glycerol-3-phosphate dehydrogenase 1 (soluble) (Gpd1). The four genes involved in fat cell differentiation include the adrenergic receptor, beta 1 (Adrb1), lipin 1(Lpin1) and PPARγ. The depressed mRNA expression of adipokine genes (Table 4) in the aP2-A-C/EBP inguinal depot was consistent with their lower circulating protein levels (Table 2).
Figure 6. Gene expression changes in male inguinal fat from wild type vs. aP2-A-C/EBP mice.
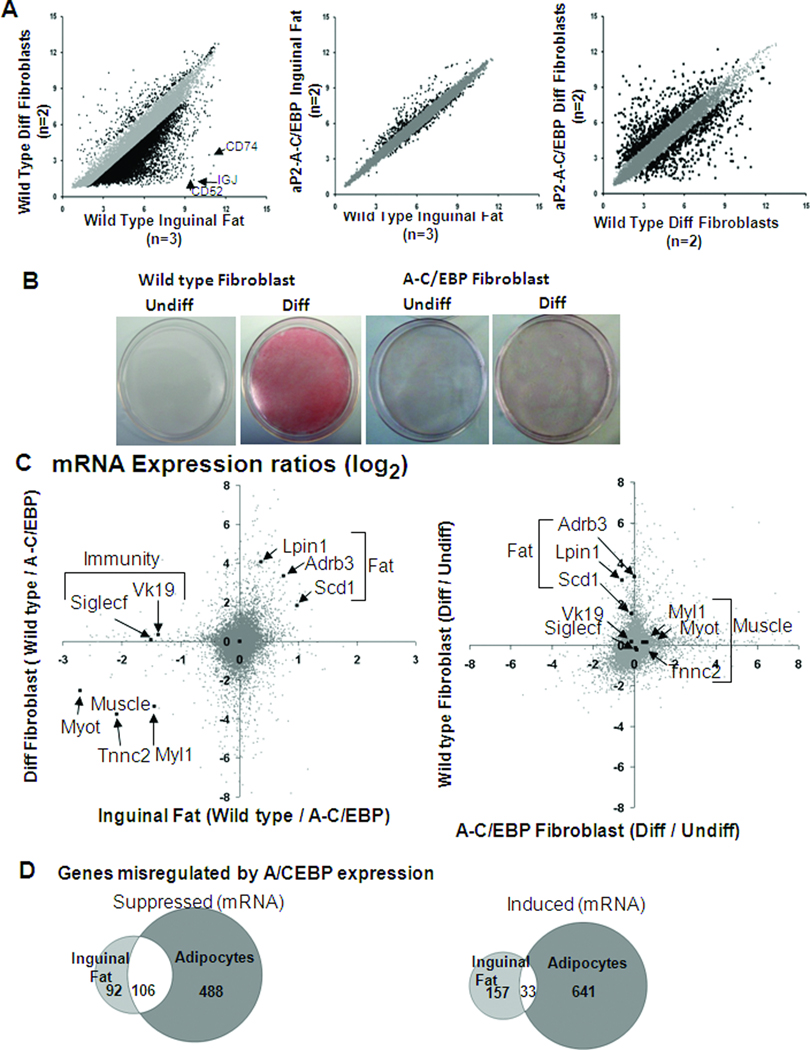
A) cRNA were made from inguinal fat tissue or primary dermal fibroblast cultures induced for adipogenic differentiation, hybridized to 45,000 feature Affymetrix arrays microarrays and data presented as a scatter graph. Mean fluorescence intensity (log2) values from mRNA of wild type inguinal fat (n= 2) vs. wild type (n= 2) primary dermal fibroblast cultures induced for adipogenic differentiation (left panel). Genes whose expression levels changes significantly (p≤0.05) over 2.0-fold are highlighted in black dots (left panel). Mean fluorescence intensities (log2) from mRNA of wild type (n= 3) vs. aP2-A-C/EBP (n= 2) mice inguinal fat. Genes whose expression levels changes significantly (p≤0.05) over 1.5-fold are highlighted in black dots (middle panel). Mean fluorescence intensity (log2) values from mRNA of wild type (n= 2) vs.aP2-A-C/EBP (n= 2) primary dermal fibroblast cultures induced for adipogenic differentiation (right panel). Genes whose expression levels changes significantly (p≤0.05) over 3.5-fold are highlighted. B) Oil-Red-O stained dermal fibroblast cultures from new-born wild type and aP2-A-C/EBP mice with (Diff) or without (Undiff) induction for adipogenesis. Oil-Red-O stains neutral lipids that accumulate in adipocytes. Wild type or aP2-A-C/EBP fibroblast cultures that were not induced for adipogenesis (Undiff) did not show any lipid accumulation. Wild type cultures showed almost complete differentiation to adipocytes 8 days after induction for adipogenesis. Induction of adipogenesis is inhibited in cultures expressing A-C/EBP. C) cRNA was made from mRNA of wild type (n= 3) and aP2-A-C/EBP (n= 2) mice inguinal fat tissue, and from wild type undifferentiated (n= 2), wild type differentiated (n= 2) and aP2-A-C/EBP undifferentiated (n= 2) and aP2-A-C/EBP differentiated (n= 2) primary dermal fibroblasts. Each of these was hybridized to 45,000 feature Affymetrix microarrays. Left panel: The fold change in mRNA abundance (mean fluorescence intensity (log2) values) in inguinal fat, wild type / aP2-A-C/EBP, were plotted against the fold change of mRNA abundance (mean fluorescence intensity (log2) values) in primary dermal fibroblasts differentiated for adipogenesis, wild type / aP2-A-C/EBP. Arrows indicate some fat-specific genes suppressed by A-C/EBP both in tissues and primary cultures (top right quadrant), muscle-specific genes induced by A-C/EBP both in tissue and primary cultures (bottom left quadrant) and some genes from immune system that are induced by A-C/EBP in tissue but not in the culture (top left quadrant). Right panel: The fold change in mRNA abundance (mean fluorescence intensity (log2) values) in wild type primary dermal fibroblasts upon induction for adipogenic differentiation (ratio of Diff to Undiff) were plotted against the fold change of mRNA abundance (mean fluorescence intensity (log2) values) in aP2-A-C/EBP primary dermal fibroblasts upon induction for adipogenic differentiation. Arrows indicate the genes marked in left panel. D) Genes (mRNA) suppressed (left panel) or induced (right panel) by A-C/EBP in inguinal fat tissue and / or primary dermal fibroblasts induced for adipogenic differentiation were plotted as Venn diagram.
Table 3. Genes significantly (p≤ 0.05) misregulated in aP2-A-C/EBP mice compared to Wild type mice.
Relatively enriched (p <0.01) GO_BP (Gene Ontology Biological Process) category has been presented singly or several such categories has been grouped together in the Gene Function column. Total number of genes within each gene function group has been given in parenthesis and they include genes that may be in more than one group (overlaps). The six topmost genes (with respect to fold mRNA change) have been enlisted for groups with more than 6 genes. The ratio of mean hybridization intensities from wild type (n=3) over aP2-A-C/EBP (n= 2) male inguinal fat mRNA (n=3) male inguinal fat mRNA is presented as mRNA fold change.
| Gene Function | Gene Symbol |
Gene Name | mRNA fold change (WT/A-CEBP) |
|---|---|---|---|
| Metabolism: Lipid, Alcohol, Carbohydrate (n=37) | Scd1 | stearoyl-Coenzyme A desaturase 2 | 3.5 |
| Cidea | cell death-inducing DNA fragmentation factor, alpha subunit-like effector A | 3.4 | |
| Gpd1 | glycerol-3-phosphate dehydrogenase 1 (soluble) | 3.4 | |
| Acaca | acetyl-Coenzyme A carboxylase alpha | 2.9 | |
| Mbtps1 | membrane-bound transcription factor peptidase, site 1 | 2.8 | |
| Pcx | pyruvate carboxylase | 2.7 | |
| Transport, Signaling and Cytoskeleton Related (n=15) | Sorbs1 | sorbin and SH3 domain containing 1 | 2.3 |
| Acss2 | acyl-CoA synthetase short-chain family member 2 | 2.1 | |
| Akt2 | thymoma viral proto-oncogene 2 | 2.1 | |
| Slc2a4 | solute carrier family 2 (facilitated glucose transporter), member 4 | 2.0 | |
| Slc36a2 | solute carrier family 36 (proton/amino acid symporter), member 2 | 2.0 | |
| Acly | ATP citrate lyase | 2.0 | |
| Fat cell differentiation (n=4) | BC054059 | cDNA sequence BC054059 | 1.9 |
| Adrb1 | adrenergic receptor, beta 1 | 1.7 | |
| Lpin1 | lipin 1 | 1.7 | |
| PPARg | peroxisome proliferator activated receptor, gamma | 1.7 | |
| Muscle related (n= 30) | Myom2 | myomesin 2 | 0.19 |
| Trim63 | tripartite motif-containing 63 | 0.21 | |
| Sgcg | sarcoglycan, gamma (dystrophin-associated glycoprotein) | 0.22 | |
| Mybpc2 | myosin binding protein C, fast-type | 0.24 | |
| Ryr1 | ryanodine receptor 1, skeletal muscle | 0.25 | |
| Tpm1 | tropomyosin 1, alpha | 0.26 | |
| Metabolism (n= 23) | Pgam2 | phosphoglycerate mutase 2 | 0.23 |
| Pygm | muscle glycogen phosphorylase | 0.29 | |
| Ampd1 | adenosine monophosphate deaminase 1 (isoform M) | 0.31 | |
| Adssl1 | adenylosuccinate synthetase like 1 | 0.32 | |
| Eno3 | enolase 3, beta muscle | 0.33 | |
| Pfkm | phosphofructokinase, muscle | 0.34 | |
| Neuron differentiation (n=4) | Bex1 | brain expressed gene 1 | 0.19 |
| Cd24a | CD24a antigen | 0.56 | |
| Eya1 | eyes absent 1 homolog (Drosophila) | 0.63 | |
| Sema4d | sema domain, immunoglobulin domain (Ig), transmembrane domain (TM) and short cytoplasmic domain, (semaphorin) 4D | 0.67 | |
| Immune response related (n= 8) | PTX3 | pentraxin related gene | 0.29 |
| IGH-6 | immunoglobulin heavy chain 6 (heavy chain of IgM) | 0.33 | |
| Fcer1a | Fc receptor, IgE, high affinity I, alpha polypeptide | 0.42 | |
| Fcgr2b | Fc receptor, IgG, low affinity IIb | 0.56 | |
| Ion homeostasis and transport (n=16) | Cacna1s | calcium channel, voltage-dependent, L type, alpha 1S subunit | 0.30 |
| Hrc | histidine rich calcium binding protein | 0.37 | |
| Atp2a1 | ATPase, Ca++ transporting, cardiac muscle, fast twitch 1 | 0.37 | |
| Cacng1 | calcium channel, voltage-dependent, gamma subunit 1 | 0.4 | |
| Sypl2 | synaptophysin-like 2 | 0.45 | |
| Cacnb1 | calcium channel, voltage-dependent, beta 1 subunit | 0.48 |
Table 4.
mRNA levels of known adipose tissue expressed genes in inguinal fat of aP2-C/EBP (TG) mice
| Gene Name | TG* (n=2) |
WT* (n=3) |
TG/WT† |
|---|---|---|---|
| Leptin (Lep) | 11.0 | 11.5 | 0.7 |
| Resistin (Retn) | 12.9 | 13.4 | 0.7 |
| insulin receptor (Insr) | 6.5 | 6.7 | 0.8 |
| insulin receptor substrate 1 (Irs1) | 11.5 | 11.6 | 1.0 |
| solute carrier family 2 (facilitated glucose transporter), member 4 (Slc2a4) | 10.5 | 11.5 | 0.5 |
| CCAAT/enhancer binding protein, beta (C/EBPβ) | 9.2 | 9.6 | 0.8 |
| peroxisome proliferator activated receptor, gamma (Pparg) | 10.8 | 11.5 | 0.6 |
| fatty acid binding protein 4, adipocytes (Fabp4) | 12.7 | 12.9 | 0.9 |
| stearoyl-Coenzyme A desaturase 1 (Scd1) | 12.6 | 13.4 | 0.6 |
| stearoyl-Coenzyme A desaturase 2 (Scd2) | 6.9 | 8.7 | 0.3 |
| 1-acylglycerol-3-phosphate O-acyltransferase 2 (lysophosphatidic acid acyltransferase, beta) (Agpat2) | 11.9 | 12.8 | 0.6 |
| adiponectin receptor 2 (Adipor2) | 8.7 | 9.4 | 0.6 |
indicates average of hybridization intensities;
indicates ratio of hybridization intensities for aP2-A-C/EBP over wild type male inguinal fat.
Among the up-regulated genes, sixty-two GO categories were enriched (p<0.01), and the most prominent enriched GO terms are muscle related (18 categories) or immune-related (16 categories). Thirty of the up-regulated genes identified in the enriched GO categories were involved in the muscle-related biological processes like muscle contraction (15 genes), muscle development (9 genes), and cytoskeletal organogenesis and biogenesis (18 genes) (Table 3).
Adipocytes from dermal fibroblast cultures do not express immuno-related genes
We differentiated new born primary dermal fibroblast cultures from wild type and aP2-A-C/EBP mice (Lichti et al. 2008; Rishi et al. 2010) into adipocytes using a 3-isobutyl-1-methyl-xanthine (IBMX), dexamethasone, insulin, and rosiglitazone (Qi et al. 2003; Rishi et al. 2010). Lipid droplets, a hallmark of adipogenic differentiation, appeared in differentiated wild type cells but, were almost completely absent in A-C/EBP expressing primary cultures (Figure 6B). Comparison of mRNA expression from inguinal fat and differentiated wild type dermal fibroblasts reveals that both the cells express similar adipocyte specific genes, whereas immune response genes are more prevalently expressed in inguinal fat (Figure 6A). It is well established that the adipose tissue has several important immune functions (Schaffler and Scholmerich). The absence of immune genes in the primary culture suggests that adipose tissue might be infiltrated by immune cells or macrophages. The immune cell molecular signature is more severe in inguinal fat tissue expressing A-C/EBP (Figure 6C).
Dermal fibroblast expressing A-C/EBP express muscle markers
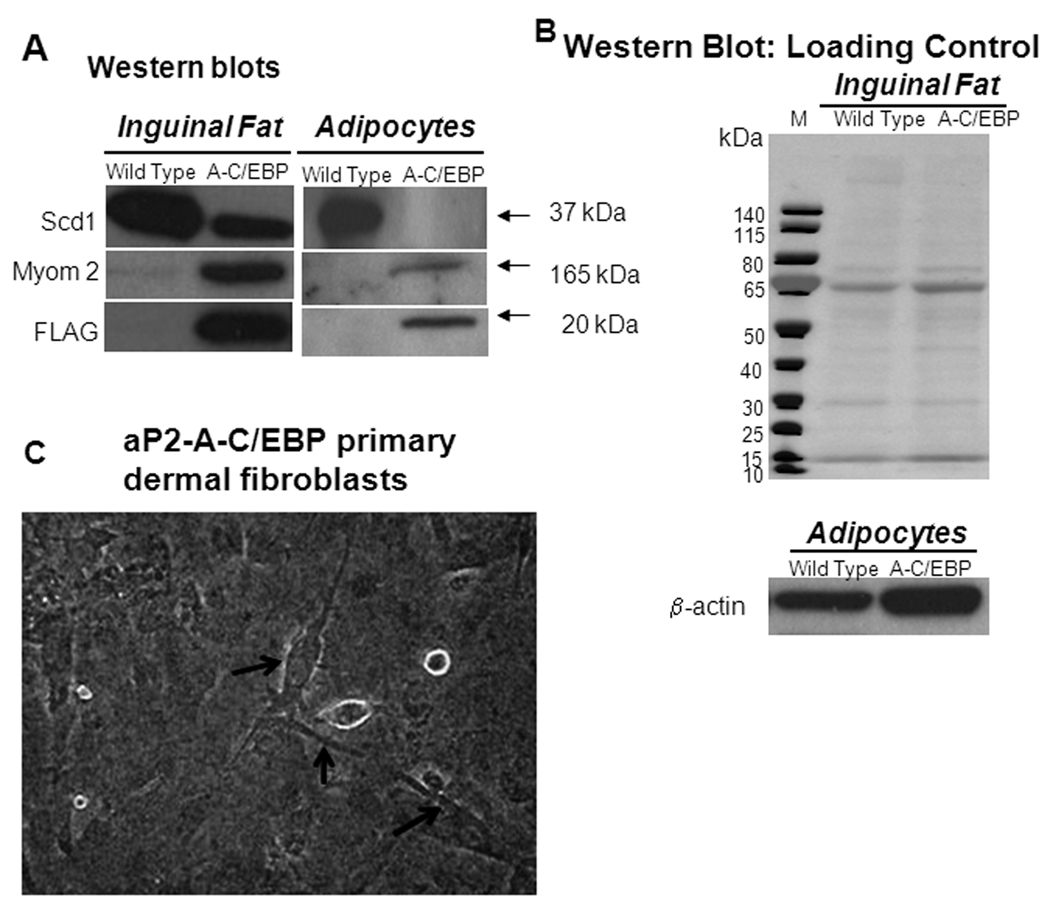
The genes down-regulated in both inguinal fat and primary cultures expressing A-C/EBP are involved in fat metabolism (Figure 6C–D). Over 50% of the genes (106 genes (53.5%)) down-regulated in inguinal fat were also down-regulated in the primary cells induced to differentiate. Genes related to fat cell differentiation were predominant among the suppressed genes, while genes related to muscle were over represented among the induced genes in primary cultures from aP2-A-C/EBP mice, as also observed in inguinal fat tissue. We have marked a few of these misregulated fat-specific (Lpin1, Adrb3 and Scd1) and muscle specific (Tnnc2 and Myl1) genes in Figure 6C. Protein expression data that confirmed the expression of A-C/EBP transgene in inguinal fat and primary cultures from aP2-A-C/EBP mice also showed a decreased level of SCD1, a classical adipocyte marker and an increased level of Myom2, a muscle-specific gene compared to wild type (Figure 7A–B).
Figure 7.
A) Western blots using a gradient gel (4% to 12%) with 20 µg of protein loaded in each lane. Immunoblotting to detect adipocyte specific marker SCD1 (37 kDa), muscle-specific marker Myom2 (165 kDa) and transgenic A-C/EBP (20 kDa) protein from wild type or aP2-A-C/EBP inguinal fat tissue or primary dermal fibroblasts induced for adipogenesis were done using antibodies against SCD1, Myom2 and FLAG. B) Western blots using a gradient gel (4% to 12%) with 20 µg of protein loaded in each lane. Blot stained with Ponceau S solution to show equal loading of protein from wild type and A-C/EBP inguinal fat tissue (left panel). Immunoblotting to detect β-actin protein from wild type or aP2-A-C/EBP primary dermal fibroblasts induced for adipogenesis (right panel). C) Primary dermal fibroblasts cultures from aP2-A-C/EBP transgenic mice. Beating cardiac muscle cells are marked by arrows.
DISCUSSION
Adipocyte growth and differentiation is regulated by sequence-specific DNA binding transcription factors that include AP-1 and C/EBP B-ZIP families (Morrison and Farmer 2000; Rosen, et al. 2002). AP-1 promotes precursor cell proliferation (Stephens, et al. 1992) while C/EBP family members mediate adipocyte differentiation via a sequential pattern of expression beginning with C/EBPβ and C/EBPα followed by C/EBPα (Mandrup and Lane 1997; Rosen, et al. 2000). Adipose specific expression of a promiscuous dominant negative protein termed A-ZIP/F that inactivates both AP-1 and C/EBP families caused severe lipoatrophy in the transgenic mouse (Moitra et al. 1998). However, which of these B-ZIP families is critical for the severe lipoatrophy phenotype is not clear. In this study, A-C/EBP, a dominant negative protein that inhibits only the DNA binding of C/EBP family members (Vinson et al. 1993) was expressed selectively in adipose tissue of transgenic mice named aP2-A-C/EBP. These mice have impaired adipocytes function.
During the first three weeks of life, the aP2-A-C/EBP transgenic mice have virtually no WAT, reduced BAT, hyperinsulinemia, hyperlipidemia and hepatic steatosis that is more severe than the A-ZIP/F-1 mice (Moitra et al. 1998). These data suggest that inactivation of only the C/EBP family of proteins is sufficient for induction of lipoatrophy in young transgenic mice. C/EBPα null mice, which died soon after birth due to hypoglycemia, had no visible WAT (Wang, et al. 1995). C/EBPα deficient mice rescued from early death by transgenic expression of C/EBPα in liver had almost no WAT, but showed minimal changes in BAT (Linhart et al. 2001). In contrast, double knockout of C/EBPβ and C/EBPα caused dramatic reduction of BAT, but only modest decrease in the amount of WAT (Tanaka, et al. 1997). Taken together, these data suggest that in vivo C/EBPα is essential for differentiation of WAT and C/EBPβ and C/EBPα play more important role in differentiation of BAT. Lack of WAT and severe reduction of BAT in the aP2-A-C/EBP mouse is suggested to be caused by combined inhibition of C/EBPα, C/EBPβ and C/EBPδ function.
The mRNA down-regulated in adult aP2-A-C/EBP inguinal fat include GO terms for fat cell differentiation (n=3) including the nuclear receptor, Pparγ, a known to be a key regulator of adipogenesis (Rosen, et al. 1999), lipid metabolism and transport (n=20), and insulin receptor signaling (n=3) (Table 3). We observed lower mRNA levels for Pparγ responsive adipocyte genes, including Scd1, Scd2, Agpat2 and Adipor2 (Yao-Borengasser, et al. 2008). This data suggests that C/EBP family members are critical for the regulation of adipogenic signals.
The enriched GO terms for up-regulated genes in aP2-A-C/EBP inguinal fat tissue (Table 3) were related to muscle, metabolism and energy generation, and immune response. Several skeletal muscle specific mRNAs including regulatory factors MyoD and MRF4 and differentiation markers myosin light chain, myosin heavy chain, and α-actin 1 (Acta1) and GO terms muscle development (n= 9), contraction (n= 15), and cytoskeleton organization and biogenesis (n= 18) were increased in the transgenic fat tissue. The increase in muscle genes is consistent with the increased connective tissue observed histologically. These findings possibly indicate the existence of a switch between white fat cells and muscle that is controlled directly or indirectly by C/EBP family members. A closer look to the differentiated dermal fibroblasts from A-C/EBP mice revealed a few cardiac beating cells in the culture (Figure 7C). As reported earlier, that dermal cell preparation may contain some precursors to other cells (Lichti et al. 2008). Therefore, another possibility might be that when adipocytes differentiation is inhibited in A-C/EBP cells, precursors to muscle cells are prominent in the culture and may contribute to the mRNA expression.
In contrast to the aP2-A-ZIP/F-1 mice, which did not have WAT, the aP2-A-C/EBP mice developed WAT during weaning. A difference between these two dominant negatives is that the A-ZIP/F but not the A-C/EBP dominant negative inhibits the DNA binding of the AP-1 heterodimer complex composed of a FOS and a JUN family member. Thus, one possible explanation for the delayed appearance of WAT is that the absence of AP-1 DN activity in aP2-A-C/EBP mice allows preadipocytes to proliferate at weaning. Clonal expansion of preadipocytes is a prerequisite for WAT-type adipogenesis in vitro, and requires AP-1 as well as c-myc (Rosen et al. 2000). Previously, we reported that although A-C/EBP blocked cell proliferation associated with mitotic clonal expansion, it did not inhibit normal proliferation of 3T3-L1 preadipocytes (Zhang, et al. 2004). Thus, once the developmental bottleneck is overcome, WAT-type adipocytes may differentiate depending upon networks that can bypass C/EBP family members. The reappearance of WAT to near normal amounts and in normal anatomical locations suggests that although C/EBP’s role as a transcription factor is essential for normal adipogenesis, it’s inhibition by A-C/EBP expression has no major effect on the number of adipocyte precursors and their ability to differentiate.
Reappearance of WAT in the aP2-A-C/EBP mice coincided with recovery from insulin resistance, hyperlipidemia and hepatic steatosis, suggesting that the lack of fat was primarily caused by metabolic abnormalities associated with lipodystrophy. This is in agreement with our previous data demonstrating that surgical implantation of adipose tissue reversed lipoatrophic diabetes in the AZIP/F-1 mouse (Gavrilova, et al. 2000a). Similarly, in the FAT-ATTAC mouse, a novel model of inducible and reversible fat ablation caused by regulated apoptosis, cessation of pro-apoptotic treatment reversed many of the metabolic effects observed in the lipoatrophic state (Pajvani et al. 2005). Taken together, the data demonstrates the beneficial effects of a minimal amount of functional WAT on glucose and lipid metabolism.
At 6 months of age, aP2-A-C/EBP mice exhibited signs of partial lipoatrophy, including reduced fat mass and serum leptin and adiponectin, increased food intake, and mild insulin resistance observed in males. It is not clear whether this is a long-term complication of metabolic stress the mice experience early in life or a direct effect of the dominant negative protein. As expression of C/EBPα and C/EBPα in mice declines with age (Karagiannides, et al. 2001), it is possible that we may see stronger effects of the dominant negative protein in older mice due to the relative stoichiometric increase in expression of the DN protein in WAT. Both reduced fat mass and defective WAT could be responsible for the adult aP2-A-C/EBP phenotype. The likelihood of A-C/EBP expression in macrophages might also have some effect on the phenotype.
Dysfunction of adipose tissue can lead to the whole body insulin resistance. For example, inactivation of glucose transporter 4 (GLUT4) selectively in adipose tissue impairs insulin sensitivity in muscle and liver (Abel, et al. 2001). Several studies showed that while PPARγ is sufficient to trigger the adipogenic program, C/EBPα is required for establishment of insulin-sensitive glucose transport in mature adipocytes (El-Jack, et al. 1999; Rosen et al. 2002; Wu, et al. 1999). Insulin did not stimulate glucose uptake into adipocytes lacking C/EBPα, which were shown to have decreased levels of messenger RNA for insulin receptor, insulin receptor substrate 1 (IRS1) and GLUT4 (SLC2A4) (Rosen et al. 2002). C/EBPα is also required for the intracellular retention of GLUT4 and may control the expression of the proteins that determine the basal, slow exocytosis of GLUT4 (Wertheim, et al. 2004). Adipocytes derived from C/EBPβ and C/EBPα-deficient mouse embryonic fibroblasts also show reduced GLUT4 and IRS2 expression and reduced glucose uptake in response to insulin (Yamamoto, et al. 2002). IR and GLUT4 mRNA levels in the aP2-A-C/EBP inguinal fat were reduced by 20% and 50%, respectively. Yet, insulin stimulation of 2-DG uptake into epididymal and inguinal fat was similar in aP2-A-C/EBP and control mice, suggesting normal insulin sensitivity in WAT. Interestingly, aP2-A-C/EBP mice also showed significantly reduced insulin stimulated glucose uptake into skeletal muscle, which does not express a transgene, suggesting that circulating factor(s) might be involved.
The aP2-A-C/EBP mice had low levels of circulating adipokines and less mRNA in inguinal fat suggesting that they may be transcriptional targets of C/EBP family members (Table 2). A large body of evidence suggests that leptin deficiency contributes to insulin resistance. The decrease in plasma leptin was proportional to the percent body fat and thus can simply result from reduction of fat mass. However, C/EBPα had been shown to activate transcription of the leptin gene (Hollenberg, et al. 1997; Mason, et al. 1998; Miller, et al. 1996), and gene expression analysis showed a 34% reduction in leptin mRNA levels in the aP2-A-C/EBP mice. Thus, direct inhibition of leptin mRNA expression and reduction of fat mass may contribute to low leptin plasma levels in the aP2-A-C/EBP mice. C/EBPα activates both the adiponectin (Gustafson, et al. 2003; Park, et al. 2004; Saito, et al. 1999) and resistin genes (Hartman, et al. 2002; Seo, et al. 2003) that are both are reduced in the aP2-A-C/EBP mice (Table 2). Reduction of resistin should not contribute to the aP2-A-C/EBP phenotype (Hartman et al. 2002), if anything, low resistin can mask insulin resistance caused by a leptin or adiponectin deficiency.
The partial lipodystrophic phenotype of the aP2-A-C/EBP mouse makes it a valuable mouse model of the human disease of partial lipodystrophy and more comparable than complete fat ablation observed in the A-ZIP/F-1 mouse (Moitra et al. 1998). Demthylation by 5-azacytidine treatment or by DNMT1 depletion is aslo reported to inhibit the adipocyte differentiation (Rishi et al. 2010). So, these mice will be of interest to examine any possible epigenetic consequences of inhibiting C/EBP family function in adipose tissue.
Table 1. Number of mice used for time-course experiments (Figure 2A–D, I–L).
Data on various parameters were collected on days 1, 3, 5, 7, 10, 12, 15, 18, 21 and 28 days after birth from wild type and aP2-A-C/EBP litter mates and presented in Figure 1. Numbers of mice used in each group are presented in this table.
| Parameter | Genotype | Age (days) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 5 | 7 | 10 | 12 | 15 | 18 | 21 | 28 | ||
|
Body weight Fig. 2A |
WT | 14 | 27 | 16 | 14 | 10 | 11 | 11 | 6 | 13 | 17 |
| A-C/EBP | 15 | 23 | 17 | 11 | 10 | 9 | 12 | 7 | 9 | 22 | |
|
Body length Fig. 2B |
WT | 14 | 19 | 13 | 14 | 8 | 11 | 8 | 6 | 13 | 8 |
| A-C/EBP | 20 | 10 | 14 | 11 | 8 | 9 | 7 | 7 | 9 | 6 | |
|
BAT weight Fig. 2C |
WT | 14 | 19 | 9 | 8 | 8 | 9 | 8 | 6 | 10 | 6 |
| A-C/EBP | 20 | 10 | 7 | 8 | 8 | 9 | 7 | 7 | 5 | 8 | |
|
Liver weight Fig. 2D |
WT | 14 | 10 | 12 | 11 | 10 | 9 | 11 | 6 | 11 | 6 |
| A-C/EBP | 9 | 10 | 10 | 11 | 10 | 9 | 12 | 7 | 8 | 8 | |
|
Blood Glucose Fig. 2I |
WT | 14 | 15 | 16 | 14 | 10 | 11 | 11 | 6 | 11 | 6 |
| A-C/EBP | 15 | 20 | 17 | 11 | 10 | 9 | 12 | 7 | 8 | 8 | |
|
Serum Insulin Fig. 2J |
WT | 4 | 6 | 6 | 6 | 5 | 6 | nd | 6 | 5 | nd |
| A-C/EBP | 6 | 6 | 6 | 5 | 5 | 6 | nd | 6 | 5 | nd | |
|
Serum Triglyceride Fig. 2K |
WT | 5 | 6 | 6 | 6 | 5 | 6 | 6 | 6 | 6 | 6 |
| A-C/EBP | 4 | 5 | 5 | 5 | 4 | 6 | 4 | 6 | 5 | 5 | |
|
Serum FFA Fig. 2L |
WT | 4 | 6 | 12 | 6 | 5 | 6 | 6 | 6 | 6 | 6 |
| A-C/EBP | 6 | 5 | 12 | 6 | 3 | 5 | 4 | 6 | 5 | 4 | |
ACKNOWLEDGEMENTS
Microarray analyses were performed using BRB-Array Tools Version 3.7.0 Patch_1 developed by Dr. Richard Simon and Amy Peng Lam (available at: http://linus.nci.nih.gov/BRB-ArrayTools.html). Gene Ontology analyses was carried out using Gene Ontology Tree Machine (GOTM) developed and maintained by Bing Zhang, Stefan Kirov, Denise Schmoyer and Jay Snoddy of Vanderbilt University (available at: http://bioinfo.vanderbilt.edu/gotm). We thank Eric Rios-Doria for his editing and valuable comments to the manuscript.
FUNDING
This research was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health (NIH) and NIDDK, NIH. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
Footnotes
DECLARATION OF INTEREST
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
REFERENCES
- Abel ED, Peroni O, Kim JK, Kim YB, Boss O, Hadro E, Minnemann T, Shulman GI, Kahn BB. Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature. 2001;409:729–733. doi: 10.1038/35055575. [DOI] [PubMed] [Google Scholar]
- Agarwal AK, Arioglu E, De Almeida S, Akkoc N, Taylor SI, Bowcock AM, Barnes RI, Garg A. AGPAT2 is mutated in congenital generalized lipodystrophy linked to chromosome 9q34. Nat Genet. 2002;31:21–23. doi: 10.1038/ng880. [DOI] [PubMed] [Google Scholar]
- Agarwal AK, Fryns JP, Auchus RJ, Garg A. Zinc metalloproteinase, ZMPSTE24, is mutated in mandibuloacral dysplasia. Hum Mol Genet. 2003;12:1995–2001. doi: 10.1093/hmg/ddg213. [DOI] [PubMed] [Google Scholar]
- Agarwal AK, Garg A. A novel heterozygous mutation in peroxisome proliferator-activated receptor-gamma gene in a patient with familial partial lipodystrophy. J Clin Endocrinol Metab. 2002;87:408–411. doi: 10.1210/jcem.87.1.8290. [DOI] [PubMed] [Google Scholar]
- Ahn S, Olive M, Aggarwal S, Krylov D, Ginty DD, Vinson C. A dominant-negative inhibitor of CREB reveals that it is a general mediator of stimulus-dependent transcription of c-fos. Mol Cell Biol. 1998;18:967–977. doi: 10.1128/mcb.18.2.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernlohr DA, Doering TL, Kelly TJ, Jr, Lane MD. Tissue specific expression of p422 protein, a putative lipid carrier, in mouse adipocytes. Biochem Biophys Res Commun. 1985;132:850–855. doi: 10.1016/0006-291x(85)91209-4. [DOI] [PubMed] [Google Scholar]
- Burant CF, Sreenan S, Hirano K, Tai TA, Lohmiller J, Lukens J, Davidson NO, Ross S, Graves RA. Troglitazone action is independent of adipose tissue. J Clin Invest. 1997;100:2900–2908. doi: 10.1172/JCI119839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo C, Haluzik M, Cutson JJ, Dietz KR, Marcus-Samuels B, Vinson C, Gavrilova O, Reitman ML. Opposite effects of background genotype on muscle and liver insulin sensitivity of lipoatrophic mice. Role of triglyceride clearance. J Biol Chem. 2003;278:3992–3999. doi: 10.1074/jbc.M207665200. [DOI] [PubMed] [Google Scholar]
- Combs TP, Berg AH, Rajala MW, Klebanov S, Iyengar P, Jimenez-Chillaron JC, Patti ME, Klein SL, Weinstein RS, Scherer PE. Sexual differentiation, pregnancy, calorie restriction, and aging affect the adipocyte-specific secretory protein adiponectin. Diabetes. 2003;52:268–276. doi: 10.2337/diabetes.52.2.268. [DOI] [PubMed] [Google Scholar]
- Ebihara K, Kusakabe T, Masuzaki H, Kobayashi N, Tanaka T, Chusho H, Miyanaga F, Miyazawa T, Hayashi T, Hosoda K, et al. Gene and phenotype analysis of congenital generalized lipodystrophy in Japanese: a novel homozygous nonsense mutation in seipin gene. Clin Endocrinol Metab. 2004;89:2360–2364. doi: 10.1210/jc.2003-031211. [DOI] [PubMed] [Google Scholar]
- El-Jack AK, Hamm JK, Pilch PF, Farmer SR. Reconstitution of insulin-sensitive glucose transport in fibroblasts requires expression of both PPARgamma and C/EBPalpha. J Biol Chem. 1999;274:7946–7951. doi: 10.1074/jbc.274.12.7946. [DOI] [PubMed] [Google Scholar]
- Fiorenza CG, Chou SH, Mantzoros CS. Lipodystrophy: pathophysiology and advances treatment. Nat Rev Endocrinol. 2010 doi: 10.1038/nrendo.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg A, Misra A. Lipodystrophies: rare disorders causing metabolic syndrome. Endocrinol Metab Clin North Am. 2004;33:305–331. doi: 10.1016/j.ecl.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Gavrilova O, Haluzik M, Matsusue K, Cutson JJ, Johnson L, Dietz KR, Nicol CJ, Vinson C, Gonzalez FJ, Reitman ML. Liver peroxisome proliferator-activated receptor gamma contributes to hepatic steatosis, triglyceride clearance, and regulation of body fat mass. J Biol Chem. 2003;278:34268–34276. doi: 10.1074/jbc.M300043200. [DOI] [PubMed] [Google Scholar]
- Gavrilova O, Marcus-Samuels B, Graham D, Kim JK, Shulman GI, Castle AL, Vinson C, Eckhaus M, Reitman ML. Surgical implantation of adipose tissue reverses diabetes in lipoatrophic mice. J Clin Invest. 2000a;105:271–278. doi: 10.1172/JCI7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilova O, Marcus-Samuels B, Reitman ML. Lack of responses to a beta3-adrenergic agonist in lipoatrophic A-ZIP/F-1 mice. Diabetes. 2000b;49:1910–1916. doi: 10.2337/diabetes.49.11.1910. [DOI] [PubMed] [Google Scholar]
- George S, Rochford JJ, Wolfrum C, Gray SL, Schinner S, Wilson JC, Soos MA, Murgatroyd PR, Williams RM, Acerini CL, et al. A family with severe insulin resistance and diabetes due to a mutation in AKT2. Science. 2004;304:1325–1328. doi: 10.1126/science.1096706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gougeon ML, Penicaud L, Fromenty B, Leclercq P, Viard JP, Capeau J. Adipocytes targets and actors in the pathogenesis of HIV-associated lipodystrophy and metabolic alterations. Antivir Ther. 2004;9:161–177. [PubMed] [Google Scholar]
- Gustafson B, Jack MM, Cushman SW, Smith U. Adiponectin gene activation by thiazolidinediones requires PPAR gamma 2, but not C/EBP alpha-evidence for differential regulation of the aP2 and adiponectin genes. Biochem Biophys Res Commun. 2003;308:933–939. doi: 10.1016/s0006-291x(03)01518-3. [DOI] [PubMed] [Google Scholar]
- Hartman HB, Hu X, Tyler KX, Dalal CK, Lazar MA. Mechanisms regulating adipocyte expression of resistin. J Biol Chem. 2002;277:19754–19761. doi: 10.1074/jbc.M201451200. [DOI] [PubMed] [Google Scholar]
- Hayashi YK, Matsuda C, Ogawa M, Goto K, Tominaga K, Mitsuhashi S, Park YE, Nonaka I, Hino-Fukuyo N, Haginoya K, et al. Human PTRF mutations cause secondary deficiency of caveolins resulting in muscular dystrophy with generalized lipodystrophy. J Clin Invest. 2009;119:2623–2633. doi: 10.1172/JCI38660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera R, Ro HS, Robinson GS, Xanthopoulos KG, Spiegelman BM. A direct role for C/EBP and the AP-I-binding site in gene expression linked to adipocyte differentiation. Mol Cell Biol. 1989;9:5331–5339. doi: 10.1128/mcb.9.12.5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himms-Hagen J, Cui J, Danforth E, Jr, Taatjes DJ, Lang SS, Waters BL, Claus TH. Effect of CL-316,243, a thermogenic beta 3-agonist, on energy balance and brown and white adipose tissues in rats. Am J Physiol. 1994;266:R1371–R1382. doi: 10.1152/ajpregu.1994.266.4.R1371. [DOI] [PubMed] [Google Scholar]
- Hollenberg AN, Susulic VS, Madura JP, Zhang B, Moller DE, Tontonoz P, Sarraf P, Spiegelman BM, Lowell BB. Functional antagonism between CCAAT/Enhancer binding protein-alpha and peroxisome proliferator-activated receptor-gamma on the leptin promoter. J Biol Chem. 1997;272:5283–5290. doi: 10.1074/jbc.272.8.5283. [DOI] [PubMed] [Google Scholar]
- Huang-Doran I, Sleigh A, Rochford JJ, O'Rahilly S, Savage DB. Lipodystrophy: metabolic insights from a rare disorder. J Endocrinol. 2010;207:245–255. doi: 10.1677/JOE-10-0272. [DOI] [PubMed] [Google Scholar]
- Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26:439–451. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- Karagiannides I, Tchkonia T, Dobson DE, Steppan CM, Cummins P, Chan G, Salvatori K, Hadzopoulou-Cladaras M, Kirkland JL. Altered expression of C/EBP family members results in decreased adipogenesis with aging. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1772–R1780. doi: 10.1152/ajpregu.2001.280.6.R1772. [DOI] [PubMed] [Google Scholar]
- Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- Kim CA, Delepine M, Boutet E, El Mourabit H, Le Lay S, Meier M, Nemani M, Bridel E, Leite CC, Bertola DR, et al. Association of a homozygous nonsense caveolin-1 mutation with Berardinelli-Seip congenital lipodystrophy. J Clin Endocrinol Metab. 2008;93:1129–1134. doi: 10.1210/jc.2007-1328. [DOI] [PubMed] [Google Scholar]
- Kim JK, Wi JK, Youn JH. Plasma free fatty acids decrease insulin-stimulated skeletal muscle glucose uptake by suppressing glycolysis in conscious rats. Diabetes. 1996;45:446–453. doi: 10.2337/diab.45.4.446. [DOI] [PubMed] [Google Scholar]
- Koopman P, Ashworth A, Lovell-Badge R. The ZFY gene family in humans and mice. Trends Genet. 1991;7:132–136. doi: 10.1016/0168-9525(91)90458-3. [DOI] [PubMed] [Google Scholar]
- Kopelman PG. Obesity as a medical problem. Nature. 2000;404:635–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- Koutkia P, Grinspoon S. HIV-associated lipodystrophy: pathogenesis, prognosis, treatment, and controversies. Annu Rev Med. 2004;55:303–317. doi: 10.1146/annurev.med.55.091902.104412. [DOI] [PubMed] [Google Scholar]
- Krylov D, Olive M, Vinson C. Extending dimerization interfaces: the bZIP basic region can form a coiled coil. EMBO J. 1995;14:5329–5337. doi: 10.1002/j.1460-2075.1995.tb00217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laustsen PG, Michael MD, Crute BE, Cohen SE, Ueki K, Kulkarni RN, Keller SR, Lienhard GE, Kahn CR. Lipoatrophic diabetes in Irs1(−/−)/Irs3(−/−) double knockout mice. Genes Dev. 2002;16:3213–3222. doi: 10.1101/gad.1034802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichti U, Anders J, Yuspa SH. Isolation and short-term culture of primary keratinocytes, hair follicle populations and dermal cells from newborn mice and keratinocytes from adult mice for in vitro analysis and for grafting to immunodeficient mice. Nat Protoc. 2008;3:799–810. doi: 10.1038/nprot.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linhart HG, Ishimura-Oka K, DeMayo F, Kibe T, Repka D, Poindexter B, Bick RJ, Darlington GJ. C/EBPalpha is required for differentiation of white, but not brown, adipose tissue. Proc Natl Acad Sci U S A. 2001;98:12532–12537. doi: 10.1073/pnas.211416898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magre J, Delepine M, Khallouf E, Gedde-Dahl T, Jr, Van Maldergem L, Sobel E, Papp J, Meier M, Megarbane A, Bachy A, et al. Identification of the gene altered in Berardinelli-Seip congenital lipodystrophy on chromosome 11q13. Nat Genet. 2001;28:365–370. doi: 10.1038/ng585. [DOI] [PubMed] [Google Scholar]
- Mandrup S, Lane MD. Regulating adipogenesis. J Biol Chem. 1997;272:5367–5370. doi: 10.1074/jbc.272.9.5367. [DOI] [PubMed] [Google Scholar]
- Mason MM, He Y, Chen H, Quon MJ, Reitman M. Regulation of leptin promoter function by Sp1, C/EBP, and a novel factor. Endocrinology. 1998;139:1013–1022. doi: 10.1210/endo.139.3.5792. [DOI] [PubMed] [Google Scholar]
- Miller SG, De Vos P, Guerre-Millo M, Wong K, Hermann T, Staels B, Briggs MR, Auwerx J. The adipocyte specific transcription factor C/EBPalpha modulates human ob gene expression. Proc Natl Acad Sci U S A. 1996;93:5507–5511. doi: 10.1073/pnas.93.11.5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moitra J, Mason MM, Olive M, Krylov D, Gavrilova O, Marcus-Samuels B, Feigenbaum L, Lee E, Aoyama T, Eckhaus M, et al. Life without white fat: a transgenic mouse. Genes Dev. 1998;12:3168–3181. doi: 10.1101/gad.12.20.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison RF, Farmer SR. Hormonal signaling and transcriptional control of adipocyte differentiation. J Nutr. 2000;130:3116S–3121S. doi: 10.1093/jn/130.12.3116S. [DOI] [PubMed] [Google Scholar]
- Nicholls DG, Locke RM. Thermogenic mechanisms in brown fat. Physiol Rev. 1984;64:1–64. doi: 10.1152/physrev.1984.64.1.1. [DOI] [PubMed] [Google Scholar]
- Olive M, Krylov D, Echlin DR, Gardner K, Taparowsky E, Vinson C. A dominant negative to activation protein-1 (AP1) that abolishes DNA binding and inhibits oncogenesis. J Biol Chem. 1997;272:18586–18594. doi: 10.1074/jbc.272.30.18586. [DOI] [PubMed] [Google Scholar]
- Pajvani UB, Trujillo ME, Combs TP, Iyengar P, Jelicks L, Roth KA, Kitsis RN, Scherer PE. Fat apoptosis through targeted activation of caspase 8: a new mouse model of inducible and reversible lipoatrophy. Nat Med. 2005;11:797–803. doi: 10.1038/nm1262. [DOI] [PubMed] [Google Scholar]
- Park BH, Qiang L, Farmer SR. Phosphorylation of C/EBPbeta at a consensus extracellular signal-regulated kinase/glycogen synthase kinase 3 site is required for the induction of adiponectin gene expression during the differentiation of mouse fibroblasts into adipocytes. Mol Cell Biol. 2004;24:8671–8680. doi: 10.1128/MCB.24.19.8671-8680.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterfy M, Phan J, Xu P, Reue K. Lipodystrophy in the fld mouse results from mutation of a new gene encoding a nuclear protein, lipin. Nat Genet. 2001;27:121–124. doi: 10.1038/83685. [DOI] [PubMed] [Google Scholar]
- Qi C, Surapureddi S, Zhu YJ, Yu S, Kashireddy P, Rao MS, Reddy JK. Transcriptional coactivator PRIP, the peroxisome proliferator-activated receptor gamma (PPARgamma)-interacting protein, is required for PPARgamma-mediated adipogenesis. J Biol Chem. 2003;278:25281–25284. doi: 10.1074/jbc.C300175200. [DOI] [PubMed] [Google Scholar]
- Reitman ML. Magic bullets melt fat. Nat Med. 2004;10:581–582. doi: 10.1038/nm0604-581. [DOI] [PubMed] [Google Scholar]
- Reitman ML, Arioglu E, Gavrilova O, Taylor SI. Lipoatrophy revisited. Trends Endocrinol Metab. 2000;11:410–416. doi: 10.1016/s1043-2760(00)00309-x. [DOI] [PubMed] [Google Scholar]
- Rishi V, Bhattacharya P, Chatterjee R, Rozenberg J, Zhao J, Glass K, Fitzgerald P, Vinson C. CpG methylation of half-CRE sequences creates C/EBPalpha binding sites that activate some tissue-specific genes. Proc Natl Acad Sci U S A. 2010;107:20311–20316. doi: 10.1073/pnas.1008688107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen ED, Hsu CH, Wang X, Sakai S, Freeman MW, Gonzalez FJ, Spiegelman BM. C/EBPalpha induces adipogenesis through PPARgamma: a unified pathway. Genes Dev. 2002;16:22–26. doi: 10.1101/gad.948702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, Spiegelman BM, Mortensen RM. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell. 1999;4:611–617. doi: 10.1016/s1097-2765(00)80211-7. [DOI] [PubMed] [Google Scholar]
- Rosen ED, Walkey CJ, Puigserver P, Spiegelman BM. Transcriptional regulation of adipogenesis. Genes Dev. 2000;14:1293–1307. [PubMed] [Google Scholar]
- Ross SR, Graves RA, Spiegelman BM. Targeted expression of a toxin gene to adipose tissue: transgenic mice resistant to obesity. Genes Dev. 1993;7:1318–1324. doi: 10.1101/gad.7.7b.1318. [DOI] [PubMed] [Google Scholar]
- Rubio-Cabezas O, Puri V, Murano I, Saudek V, Semple RK, Dash S, Hyden CS, Bottomley W, Vigouroux C, Magre J, et al. Partial lipodystrophy and insulin resistant diabetes in a patient with a homozygous nonsense mutation in CIDEC. EMBO Mol Med. 2009;1:280–287. doi: 10.1002/emmm.200900037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K, Tobe T, Yoda M, Nakano Y, Choi-Miura NH, Tomita M. Regulation of gelatin-binding protein 28 (GBP28) gene expression by C/EBP. Biol Pharm Bull. 1999;22:1158–1162. doi: 10.1248/bpb.22.1158. [DOI] [PubMed] [Google Scholar]
- Savage DB, Tan GD, Acerini CL, Jebb SA, Agostini M, Gurnell M, Williams RL, Umpleby AM, Thomas EL, Bell JD, et al. Human metabolic syndrome resulting from dominant-negative mutations in the nuclear receptor peroxisome proliferator-activated receptor-gamma. Diabetes. 2003;52:910–917. doi: 10.2337/diabetes.52.4.910. [DOI] [PubMed] [Google Scholar]
- Schaffler A, Scholmerich J. Innate immunity and adipose tissue biology. Trends Immunol. 31:228–235. doi: 10.1016/j.it.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Seo JB, Noh MJ, Yoo EJ, Park SY, Park J, Lee IK, Park SD, Kim JB. Functional characterization of the human resistin promoter with adipocyte determination- and differentiation-dependent factor 1/sterol regulatory element binding protein 1c and CCAAT enhancer binding protein-alpha. Mol Endocrinol. 2003;17:1522–1533. doi: 10.1210/me.2003-0028. [DOI] [PubMed] [Google Scholar]
- Shimomura I, Hammer RE, Richardson JA, Ikemoto S, Bashmakov Y, Goldstein JL, Brown MS. Insulin resistance and diabetes mellitus in transgenic mice expressing nuclear SREBP-1c in adipose tissue: model for congenital generalized lipodystrophy. Genes Dev. 1998;12:3182–3194. doi: 10.1101/gad.12.20.3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens JM, Butts MD, Pekala PH. Regulation of transcription factor mRNA accumulation during 3T3-L1 preadipocyte differentiation by tumour necrosis factor-alpha. J Mol Endocrinol. 1992;9:61–72. doi: 10.1677/jme.0.0090061. [DOI] [PubMed] [Google Scholar]
- Steppan CM, Lazar MA. The current biology of resistin. J Intern Med. 2004;255:439–447. doi: 10.1111/j.1365-2796.2004.01306.x. [DOI] [PubMed] [Google Scholar]
- Taketo M, Schroeder AC, Mobraaten LE, Gunning KB, Hanten G, Fox RR, Roderick TH, Stewart CL, Lilly F, Hansen CT, et al. FVB/N: an inbred mouse strain preferable for transgenic analyses. Proc Natl Acad Sci U S A. 1991;88:2065–2069. doi: 10.1073/pnas.88.6.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan K, Kimber WA, Luan J, Soos MA, Semple RK, Wareham NJ, O'Rahilly S, Barroso I. Analysis of genetic variation in Akt2/PKB-beta in severe insulin resistance, lipodystrophy, type 2 diabetes, and related metabolic phenotypes. Diabetes. 2007;56:714–719. doi: 10.2337/db06-0921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Yoshida N, Kishimoto T, Akira S. Defective adipocyte differentiation in mice lacking the C/EBPbeta and/or C/EBPdelta gene. EMBO J. 1997;16:7432–7443. doi: 10.1093/emboj/16.24.7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinson C, Myakishev M, Acharya A, Mir AA, Moll JR, Bonovich M. Classification of human B-ZIP proteins based on dimerization properties. Mol Cell Biol. 2002;22:6321–6335. doi: 10.1128/MCB.22.18.6321-6335.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinson CR, Hai T, Boyd SM. Dimerization specificity of the leucine zipper-containing bZIP motif on DNA binding: prediction and rational design. Genes Dev. 1993;7:1047–1058. doi: 10.1101/gad.7.6.1047. [DOI] [PubMed] [Google Scholar]
- Wang ND, Finegold MJ, Bradley A, Ou CN, Abdelsayed SV, Wilde MD, Taylor LR, Wilson DR, Darlington GJ. Impaired energy homeostasis in C/EBP alpha knockout mice. Science. 1995;269:1108–1112. doi: 10.1126/science.7652557. [DOI] [PubMed] [Google Scholar]
- Wertheim N, Cai Z, McGraw TE. The transcription factor CCAAT/enhancer-binding protein alpha is required for the intracellular retention of GLUT4. J Biol Chem. 2004;279:41468–41476. doi: 10.1074/jbc.M405088200. [DOI] [PubMed] [Google Scholar]
- Wu Z, Rosen ED, Brun R, Hauser S, Adelmant G, Troy AE, McKeon C, Darlington GJ, Spiegelman BM. Cross-regulation of C/EBP alpha and PPAR gamma controls the transcriptional pathway of adipogenesis and insulin sensitivity. Mol Cell. 1999;3:151–158. doi: 10.1016/s1097-2765(00)80306-8. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Kurebayashi S, Hirose T, Kouhara H, Kasayama S. Reduced IRS-2 and GLUT4 expression in PPARgamma2-induced adipocytes derived from C/EBPbeta and C/EBPdelta-deficient mouse embryonic fibroblasts. J Cell Sci. 2002;115:3601–3607. doi: 10.1242/jcs.00044. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- Yao-Borengasser A, Rassouli N, Varma V, Bodles AM, Rasouli N, Unal R, Phanavanh B, Ranganathan G, McGehee RE, Jr, Kern PA. Stearoyl-coenzyme A desaturase 1 gene expression increases after pioglitazone treatment and is associated with peroxisomal proliferator-activated receptor-gamma responsiveness. J Clin Endocrinol Metab. 2008;93:4431–4439. doi: 10.1210/jc.2008-0782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JW, Tang QQ, Vinson C, Lane MD. Dominant-negative C/EBP disrupts mitotic clonal expansion and differentiation of 3T3-L1 preadipocytes. Proc Natl Acad Sci U S A. 2004;101:43–47. doi: 10.1073/pnas.0307229101. [DOI] [PMC free article] [PubMed] [Google Scholar]