Abstract
Coronary vessel distensibility is reduced with atherosclerosis and normal aging but direct measurements have historically required invasive measurements at cardiac catheterization. Therefore, we sought to assess coronary artery distensibility non-invasively with 3.0T coronary magnetic resonance imaging (MRI) and to test the hypothesis that this non-invasive technique can detect differences in coronary distensibility between healthy and coronary artery disease (CAD) subjects. Thirty-eight healthy, adult subjects (23 men, mean age 31±10 years) and 21 patients with CAD defined on X-ray angiography (11 men, mean age 57±6 years) were studied on a commercial whole-body MR imaging system (Achieva 3.0 T; Philips, Best, The Netherlands). In each subject, the proximal segment of a coronary artery was imaged for cross-sectional area measurements using cine spiral MRI. Distensibility (mmHg−1*103) was determined as: (end-systolic lumen area–end-diastolic lumen area) / (pulse pressure multiplied by the end-diastolic lumen area). Pulse pressure was calculated as the difference between the systolic and diastolic brachial blood pressures. Thirty-four healthy subjects and nineteen patients had adequate image quality for coronary area measurements. Coronary artery distensibility was significantly higher in healthy subjects, than in the CAD patients (mean ± 1 SD: 2.4 ± 1.7 mmHg−1*103 vs. 1.1 ± 1.1 mmHg−1*103 respectively, p=0.007); (median: 2.2 vs. 0.9 mmHg−1*103). In a subgroup of 10 CAD patients we found a significant correlation between coronary artery distensibility measurements assessed with MRI and X-ray coronary angiography (R=0.65; p=0.003). In a group of 10 healthy subjects repeated distensibility measurements demonstrated a significant correlation (R=0.80; p=0.006). In conclusion, 3.0T MRI, a reproducible non-invasive means to assess human coronary artery vessel wall distensibility, is able to detect significant differences in distensibility between healthy subjects and CAD patients.
Keywords: coronary artery distensibility, non-invasive, cardiac magnetic resonance, 3.0 Tesla
Introduction
Magnetic resonance imaging (MRI) offers the opportunity to non-invasively measure local vessel wall abnormalities and is not limited to the assessment of superficial vessels. Moreover, MRI can provide extensive coverage of large arteries with high accuracy and reproducibility 1. It has been used to characterize atherosclerotic plaques in the aorta 2 and to measure the response to therapy at multiple vascular sites 3. In addition to structural information, MRI can acquire dynamic, physiological data within the same vessels 4,5. High-field (3.0 Tesla (T)) MRI offers the advantage of high temporal and/or spatial resolution that can be used to quantify functional and morphological alterations required to non-invasively measure in vivo coronary arterial distensibility. We sought to test the hypotheses that coronary artery distensibility can be quantified non-invasively using 3.0 T coronary MRI and that differences in distensibility can be detected between healthy subjects and coronary artery disease (CAD) subjects.
Methods
Thirty-eight healthy, adult subjects (23 male, mean 31±10 years) and 21 patients with X-ray angiography documented CAD (11 male, mean 57±6 years) were studied. Healthy subjects were defined as those without a history of CAD and the absence of traditional CAD risk factors other than male gender. CAD subjects were defined by the presence of coronary stenosis ≥ 50% on previous coronary X-ray angiography, documented history of myocardial infarction, or history of coronary revascularization including percutaneous coronary intervention or coronary artery bypass grafting. The study protocol was approved by the Institutional Review Board at Johns Hopkins University, Baltimore, USA and all participants provided written informed consent. The coronary MRI examination was performed in the morning while the subjects were in a fasting state and before administration of prescribed vasoactive medications in patients. No contrast agent was used. Imaging was performed with subjects in the prone position and distensibility measurements obtained after at least 20 minutes of rest in the magnet. The peripheral blood pressure was recorded. Total imaging time was approximately 25–30 minutes. A commercial human 3.0 T MRI scanner (Achieva, Philips, Best, The Netherlands) with a 6-element cardiac coil for signal reception was used. Scout scans were performed to determine the 3D course of the proximal coronary arteries. MR angiography of the right coronary artery (RCA), left anterior descending (LAD) and/or left circumflex artery (LCX) was performed with a navigator-gated free-breathing and ECG-triggered, T2-prepared, 3D, segmented k-space, gradient-echo imaging sequence. In each subject, the proximal segment of the coronary artery best identified on scout images (RCA and/or LAD and/or LCX) was then imaged in cross-section using cine spiral MRI for area measurements (Figure 1). In CAD patients, the cross-sectional imaging plane was located in a region at least two cm proximal to the location of a significant stenosis. The imaging plane for cross-sectional area measurements was localized in a proximal arterial segment that was straight over a distance of at least 2 cm. Vein grafts were not included in the analysis. MRI parameters were: echo time (TE)=1.5ms, radiofrequency (RF) excitation angle=20° and spectral spatial excitation, breath-hold duration~14–24s, acquisition window=10ms, repetition time (TR)=14ms, 21 spiral interleaves, spatial resolution (acquired/reconstructed)= 0.89×0.89×8.00mm3/0.69×0.69×8.00 mm3. In a subgroup of 10 CAD patients, clinically indicated invasive X-ray coronary angiography was performed within 6 months of MRI imaging using a standard Judkins technique. Hemodynamics were monitored continuously throughout the procedure, and pulse pressure measured during coronary artery imaging. Coronary angiography was performed in multiple projections to obtain optimum images for quantitative analysis. Coronary angiographic images were recorded in the universal DICOM format and forwarded to the Angiographic Core Laboratory for blinded analysis. Cross-sectional areas of proximal coronary artery segments of 10 randomly selected subjects (7 healthy adults and 3 CAD patients) were measured by two independent observers (S.K. and A.H.) and by one observer (A. H.) at two separate times. These data were used in the assessment of inter-observer and intra-observer variability. Furthermore, 10 of our healthy subjects underwent two coronary MRI examinations to test for repeatability. After the first examination, the study subjects were removed from the scanner. After approximately 20 minutes outside of the scanner, the subjects were then repositioned and the complete examination was repeated. Coronary images were analyzed for absolute cross-sectional area changes using the full-width half-maximum criteria (Cine version 3.15.17, GE, Milwaukee, WI, USA). Images were magnified three-fold, and a circular region-of-interest (ROI) was manually traced around the coronary artery. The computer algorithm then automatically measured the cross sectional coronary area using full-width half-maximum criteria. Distensibility (mmHg−1) was determined as: (end-systolic lumen area–end-diastolic lumen area) / (pulse pressure multiplied by the end-diastolic lumen area) 6. Pulse pressure was calculated as the difference between the systolic and diastolic blood pressure 7. End-systole was defined as the period that began before the onset of left ventricular relaxation. End-diastole was defined as the period that began after the largest left ventricular volume was reached. For analysis of invasive coronary artery angiography, validated post-processing software for computer-assisted quantitative coronary angiography (QCA) was used (PIE Medical Imaging BV CAAS 2.0.1 research). The luminal diameter of the major epicardial coronary artery segment was quantified at end-systole and end-diastole in the cardiac cycle by quantitative angiographic methods by an experienced interventionalist (J.M. and J.T.) who was blinded to the results of MR imaging. Luminal diameter measurements were acquired at end-systole and end-diastole, defined by ECG. Following analysis, MRI and invasive angiography were matched by using distances from anatomic fiducial markers, such as ostia or side branches, to ensure corresponding segments were measured by both modalities. Statistical package for the social sciences (SPSS) 12.0 for Windows was used for all statistical analyses. Data are expressed as mean values ± one standard deviation (SD). Characteristics of the study population were compared using Wilcoxon-signed rank test. A non-parametric statistical test (Mann-Whitney-U-Test) was used to compare coronary cross-sectional area measurements and distensibility between the healthy and CAD subjects. Student’s paired t-test was used to compare invasive and MRI-measurements in those CAD patients in which both modalities were performed. To test for intra- and interobserver variability as well as for repeatability, data were analyzed using Pearson’s correlation coefficient and Bland-Altman agreement analysis. P-values for Bland-Altman were derived from Pitman´s test of difference in variance. Statistical significance was defined as a two-tailed p-value <0.05.
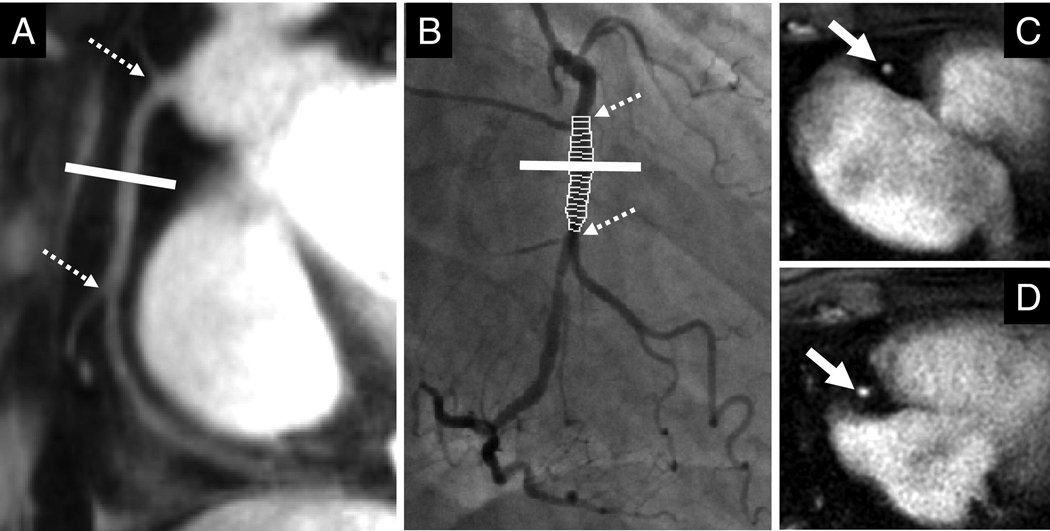
Figure 1.
(A) Magnetic resonance coronary angiography (MRCA) of the right coronary artery (RCA) is used to select a cross-sectional perpendicular plane (indicated by white line), (B) corresponding X-ray coronary angiography image. Dotted arrows indicate side-branches used as markers. Images of the proximal RCA (white arrow) at end-systole (C) and in end-diastole (D) in a CAD patient.
Results
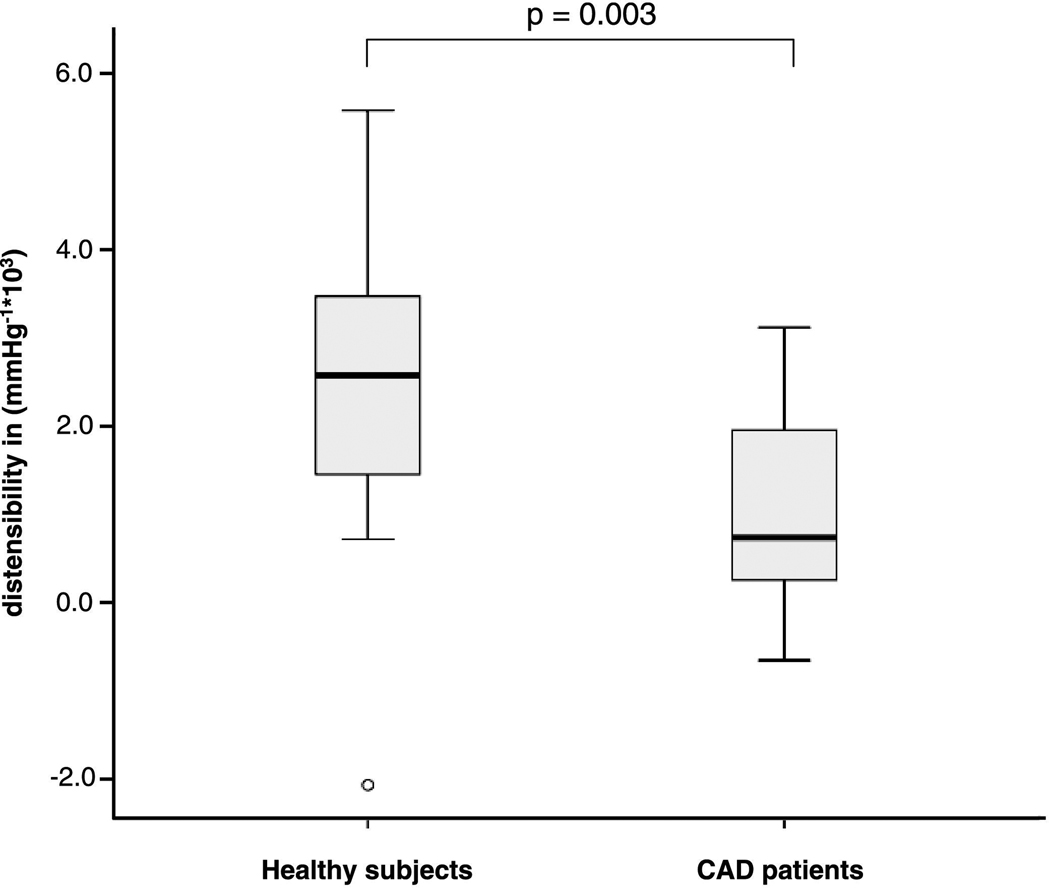
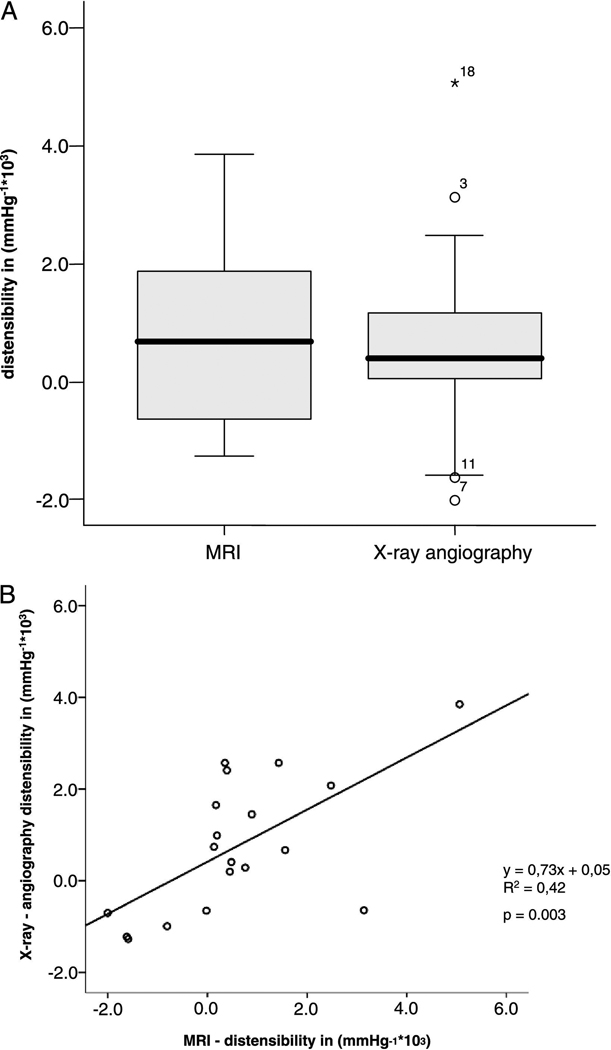
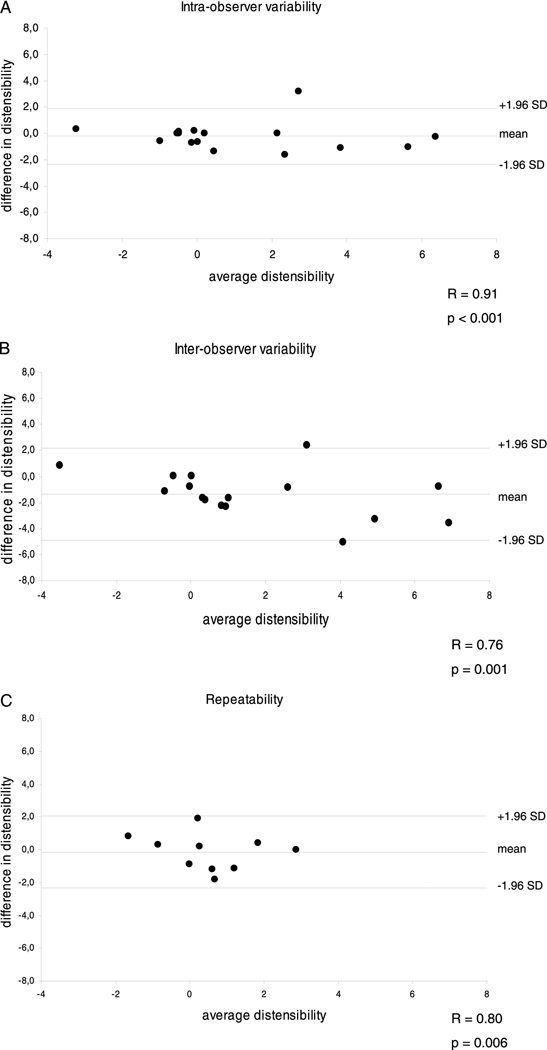
Image quality was adequate for coronary artery measurements in 34 of 38 healthy subjects (90%) and in 19 of 21 CAD patients (90%). Four healthy subjects were excluded due to a broken coil (N=1), non-diagnostic image quality because of excess subject motion (N=1), shoulder pain (N=1) and breath-holding problems (N=1). Two CAD patients were excluded because of claustrophobia (N=1) and non-diagnostic image quality because of body habitus (N=1). In healthy subjects, 54 coronary artery segments (32 RCA and 22 LAD) and in CAD patients, 31 coronary artery segments (20 RCA and 10 LAD and 1 LCX) were evaluable for analysis. Baseline characteristics of the study population are presented in Table 1. The mean systolic and diastolic blood pressures in the healthy subjects were significantly lower than those in the CAD patients (121 ± 15 mmHg vs. 141 ± 14 mmHg for systolic pressures, respectively, p<0.001) and (59 ± 10 mmHg vs. 73 ±13 mmHg for diastolic pressures, respectively, p<0.001). The mean pulse pressure in healthy adults (62 ± 10 mmHg) was also significantly lower than that in CAD patients (68 ± 10 mmHg), (p<0.05). At end-systole, the luminal area was 11.6 ± 3.2 mm2 in healthy subjects and 14.3 ± 4.2 mm2 in CAD patients (p<0.01). The luminal area during end-diastole was 10.3 ± 2.5 mm2 for healthy subjects and 13.5 ± 3.9 mm2 for CAD patients (p<0.001). Coronary vessel area change between end-systole and end-diastole was significantly higher in the healthy subjects than in the CAD patients, 13.5 ± 15.8 % vs. 5.9 ± 8.9 % respectively (p<0.01). Coronary artery distensibility was significantly higher in healthy subjects, than in the CAD patients 2.4 ± 1.7 mmHg−1*103 vs. 1.1 ± 1.1 mmHg−1*103 respectively (p=0.007); (median: 2.2 vs. 0.8 mmHg−1*103) (Figure 2). The distensibility measurements as well as the correlations between corresponding coronary MRI and invasive X-ray coronary angiography distensibilty measures in a subgroup of 10 CAD patients, including 19 coronary artery segments are presented in Table 2. At end-systole, the luminal area was 13.8 ± 4.1 mm2 at MRI and 10.0 ± 4.9 mm2 at X-ray (p=0.002). The luminal area during end-diastole was 13.0 ± 3.4 mm2 at MRI and 9.7 ± 4.8 mm2 at invasive coronary angiography for the CAD patients (p=0.005). The mean pulse pressure measured at the time of the MRI in CAD patients (71 ± 10 mmHg) was significantly higher than that at X-ray angiography (51 ± 11 mmHg, p<0.001), likely due to sedation and nitroglycerine administered prior to invasive angiography. The average coronary artery luminal diameter stenosis assessed by X-ray coronary angiography was 18 ± 13%. We found no significant difference in distensibility measurements between both modalities (p=0.62) (Figure 3A). There was a significant correlation between coronary artery distensibility measurements assessed with MRI and invasive X-ray coronary angiography (y = 0.73x + 0.05; R2 = 0.42; p=0.003) (Figure 3B). Intra-observer and inter-observer analyses for measurement of coronary distensibility were performed in 10 subjects (7 healthy subjects and 3 CAD patients) including 16 proximal coronary artery segments. We found a high correlation for distensibility measurements performed by one observer (R=0.91; p<0.001) or between two observers (R=0.76; p=0.001) (Figure 4A–B). In 10 healthy subjects, including 10 proximal coronary artery segments, repeatability measurements for distensibility between two different MRI exams performed on the same day demonstrated a significant correlation (R=0.80; p=0.006) (Figure 4C).
Table 1.
Baseline characteristics of study population.
| Characteristics | Healthy subjects (N = 38) |
CAD patients (N = 21) |
p-value |
|---|---|---|---|
| Age* (years) | 31 ± 10 | 57 ± 6 | < 0.001 |
| Men | 23 (61%) | 11 (52%) | 0.002 |
| Family history of CAD | 0 | 6 (29%) | |
| Previous MI | 0 | 4 (19%) | |
| PCI/Stent | 0 | 10 (48%) | |
| CABG | 0 | 3 (14%) | |
| Smoking | 0 | 8 (38%) | 0.005 |
| Dyslipidemia | 0 | 18 (86%) | < 0.001 |
| Diabetes mellitus | 0 | 8 (38%) | 0.005 |
| Arterial hypertension | 0 | 15 (71%) | < 0.001 |
| Target coronary artery | |||
| left | 22 (41%) | 11 (35%) | |
| right | 32 (59%) | 20 (65%) |
Data are expressed as number (%) of subjects.
Mean ± one standard deviation. CAD=coronary artery disease; MI=myocardial infarction; PCI=percutaneous coronary intervention; CABG=coronary artery bypass grafting. The target coronary artery is defined by left, including LAD (left anterior descending artery) and LCX (left circumflex artery) and right, including RCA (right coronary artery). Dyslipidemia was defined as total cholesterol > 200 mg/dl or treatment with lipid-lowering therapy. Arterial hypertension was defined as blood pressure ≥ 140/90 mmHg or treatment with antihypertensive medication.
Figure 2.
Boxplot comparing measurements of coronary artery distensibility in healthy subjects and CAD patients.
Table 2.
Comparison of magnetic resonance imaging (MRI) vs. X-ray angiography.
| MRI | X-ray | p-value (MRI vs. X-ray) |
|
|---|---|---|---|
| Cross-sectional area change (%) | 5.3 ± 10.3 | 2.9 ± 8.7 | 0.27 |
| Distensibility (mmHG−1*103) | 0.8 ± 1.5 | 0.6 ± 1.7 | 0.62 |
Values are given as mean ± one standard deviation; ES = end-systole; ED = end-diastole.
Figure 3.
A–B A) Boxplot comparing measurements of coronary artery distensibility in CAD patients with MRI and X-ray-angiography. B) Correlation of coronary artery distensibility: MRI vs. X-ray coronary angiography.
Figure 4.
A–C Bland-Altman plots for intra-observer variability (A); inter-observer variability (B) and inter-study repeatability (C) of coronary artery distensibilty measurements.
Discussion
We used 3.0 T MRI to non-invasively assess human coronary artery vessel wall distensibility. We compared MRI-measured distensibility to that measured at cardiac catheterization in a subset of CAD patients and found no significant difference between the two techniques. We observed a significant ~50% reduction in coronary artery distensibility measured by MRI in CAD patients as compared to that of healthy subjects. Coronary arterial distensibility is considered to be an important determinant of cardiovascular risk at an early stage of the disease 8. Although other vascular beds like the aorta have been studied and are useful 9–12, the presence and extent of any relationship between aortic and coronary distensibilities are not known. As an example of the heterogeneity of the vascular beds, in elderly patients with hypertension or diabetes, the carotid artery may become stiffer than either the common femoral or radial arteries, which stiffen little with age or hypertension 13,14. No two vascular territories have identical vasoelastic properties, and thus it is difficult to extrapolate segmental arterial properties from one vascular bed to other parts of the arterial tree 15. Moreover, local differences in coronary artery distensibility may be important for local atherosclerotic progression and local coronary plaque rupture, and such heterogeneity in distensibility throughout the coronary artery tree, if present, could only be detected with direct, local measures of the coronaries. Healthy subjects demonstrated significantly smaller cross-sectional areas compared to CAD patients, both in systole and diastole. Our measurements of absolute cross-sectional areas in healthy subjects and CAD patients are in agreement with invasive studies 7,16–18. Compensatory arterial enlargement in response to the development of atherosclerosis has been reported to be an important mechanism for luminal preservation in the face of increasing plaque accumulation 19. In a histopathological study, coronary arteries were larger in older than in younger patients 20. In a recent publication, cross-sectional areas were assessed with CTA were larger in patients with mild and moderate CAD than in a control group 21. A possible explanation might be higher pulse-pressure in CAD patients – leading to larger coronary diameters at rest 22. Findings in our study and in the study by Nakatani et al. 7 suggest that there is a relation between pulse pressure and lumen area. In our study, the mean pulse pressure in healthy adults (62 ± 10 mmHg) was significantly lower than that in CAD patients (68 ± 10 mmHg), (p<0.05). A previous study demonstrated a significant correlation between coronary area measurements obtained with X-ray angiography and those by MRI 23, as did our measures of distensibility. Our values of arterial distensibility assessed with MRI were slightly higher than the values obtained invasively 7,17,18 and the standard deviation was larger, potentially related to the lower spatial resolution of MRI. In a recently published study, CTA overestimated LAD diameter compared with QCA (3.7% at early-diastole and 3.5% at mid-diastole, respectively) 21. Also at cath, area measurements demonstrate a high variability dependent on reconstruction (2D or 3D) 24. Comparing invasive angiography and IVUS, invasive angiography systematically underestimated the luminal cross-sectional areas by 0.45 mm2 25. In normal coronary arteries, luminal dimensions may vary from 10% to 15% during the cardiac cycle 26. Our MRI-measurement in normal subjects of a mean 13.5% area change throughout the cardiac cycle is within that range. The systolic to diastolic differences are reported to range from 2.0% – 5.7% in CAD patients 27,28. These data are similar to our data with a mean area-change of 2.9% in CAD patients measured by X-ray angiography. The corresponding area-change measured with MRI was somewhat higher (5.3%); however, this difference was not statistically significant. Although direct measurements of intra-coronary pressures were not possible with our non-invasive protocol, we did monitor peripheral pressure and the non-invasive coronary distensibility measures agreed closely with the invasive measures. This totally non-invasive approach has greater clinical applicability than one dependent on invasive coronary measurements. Accurate and reproducible assessment of the vessel dimensions is necessary for detection of atherosclerosis progression or regression. In our study, the observed intra-observer and inter-observer variability was low and studies were repeatable. These findings are in agreement with earlier MRI studies testing observer-variability and repeatability for vessel wall imaging in the aorta 1 and coronary arteries 29. Heterogeneity in distensibility throughout the coronary artery tree can only be detected with direct, local measures of the coronaries along their path and not only in a single position. Future studies will be needed to address this issue. Due to the limited spatial resolution of MRI cross-sectional area measurements, the standard deviation for distensibility measures appears high. Other factors that might influence the distensibility also should be considered. These factors include diabetes mellitus 30 and the presence and extent of calcifications. Further studies in larger patient groups are necessary to evaluate the role of these variables in determining coronary artery distensibility.
Acknowledgments
Grant support Dr. Kelle is supported by a scholarship from the German Cardiac Society. This work is supported by NIH grant R01-HL084186, HL61912 and by the Donald W. Reynolds Foundation.
Footnotes
Disclosures There is no conflict of interests to disclose.
References
- 1.Roes SD, Westenberg JJ, Doornbos J, van der Geest RJ, Angelie E, de Roos A, Stuber M. Aortic vessel wall magnetic resonance imaging at 3.0 Tesla: a reproducibility study of respiratory navigator gated free-breathing 3D black blood magnetic resonance imaging. Magn Reson Med. 2009;61:35–44. doi: 10.1002/mrm.21798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kramer CM, Cerilli LA, Hagspiel K, DiMaria JM, Epstein FH, Kern JA. Magnetic resonance imaging identifies the fibrous cap in atherosclerotic abdominal aortic aneurysm. Circulation. 2004;109:1016–1021. doi: 10.1161/01.CIR.0000116767.95046.C2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corti R, Fayad ZA, Fuster V, Worthley SG, Helft G, Chesebro J, Mercuri M, Badimon JJ. Effects of lipid-lowering by simvastatin on human atherosclerotic lesions: a longitudinal study by high-resolution, noninvasive magnetic resonance imaging. Circulation. 2001;104:249–252. doi: 10.1161/01.cir.104.3.249. [DOI] [PubMed] [Google Scholar]
- 4.Lee JM, Shirodaria C, Jackson CE, Robson MD, Antoniades C, Francis JM, Wiesmann F, Channon KM, Neubauer S, Choudhury RP. Multi-modal magnetic resonance imaging quantifies atherosclerosis and vascular dysfunction in patients with type 2 diabetes mellitus. Diab Vasc Dis Res. 2007;4:44–48. doi: 10.3132/dvdr.2007.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hays AG, Hirsch GA, Kelle S, Gerstenblith G, Weiss RG, Stuber M. Noninvasive visualization of coronary artery endothelial function in healthy subjects and in patients with coronary artery disease. J Am Coll Cardiol. 2010;56:1657–1665. doi: 10.1016/j.jacc.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 6.Oliver JJ, Webb DJ. Noninvasive assessment of arterial stiffness and risk of atherosclerotic events. Arterioscler Thromb Vasc Biol. 2003;23:554–566. doi: 10.1161/01.ATV.0000060460.52916.D6. [DOI] [PubMed] [Google Scholar]
- 7.Nakatani S, Yamagishi M, Tamai J, Goto Y, Umeno T, Kawaguchi A, Yutani C, Miyatake K. Assessment of coronary artery distensibility by intravascular ultrasound. Application of simultaneous measurements of luminal area and pressure. Circulation. 1995;91:2904–2910. doi: 10.1161/01.cir.91.12.2904. [DOI] [PubMed] [Google Scholar]
- 8.Cohn JN, Duprez DA, Grandits GA. Arterial Elasticity as Part of a Comprehensive Assessment of Cardiovascular Risk and Drug Treatment. Hypertension. 2005;46:217–220. doi: 10.1161/01.HYP.0000165686.50890.c3. [DOI] [PubMed] [Google Scholar]
- 9.Dart AM, Lacombe F, Yeoh JK, Cameron JD, Jennings GL, Laufer E, Esmore DS. Aortic distensibility in patients with isolated hypercholesterolaemia, coronary artery disease, or cardiac transplant. Lancet. 1991;338:270–273. doi: 10.1016/0140-6736(91)90415-l. [DOI] [PubMed] [Google Scholar]
- 10.Lee JM, Wiesmann F, Shirodaria C, Leeson P, Petersen SE, Francis JM, Jackson CE, Robson MD, Neubauer S, Channon KM, Choudhury RP. Early changes in arterial structure and function following statin initiation: quantification by magnetic resonance imaging. Atherosclerosis. 2008;197:951–958. doi: 10.1016/j.atherosclerosis.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cruickshank K, Riste L, Anderson SG, Wright JS, Dunn G, Gosling RG. Aortic Pulse-Wave Velocity and Its Relationship to Mortality in Diabetes and Glucose Intolerance: An Integrated Index of Vascular Function? Circulation. 2002;106:2085–2090. doi: 10.1161/01.cir.0000033824.02722.f7. [DOI] [PubMed] [Google Scholar]
- 12.Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Benetos A. Aortic Stiffness Is an Independent Predictor of All-Cause and Cardiovascular Mortality in Hypertensive Patients. Hypertension. 2001;37:1236–1241. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- 13.Boutouyrie P, Laurent S, Benetos A, Girerd XJ, Hoeks AP, Safar ME. Opposing effects of ageing on distal and proximal large arteries in hypertensives. J Hypertens Suppl. 1992;10:S87–S91. [PubMed] [Google Scholar]
- 14.Benetos A, Laurent S, Hoeks AP, Boutouyrie PH, Safar ME. Arterial alterations with aging and high blood pressure. A noninvasive study of carotid and femoral arteries. Arterioscler Thromb. 1993;13:90–97. doi: 10.1161/01.atv.13.1.90. [DOI] [PubMed] [Google Scholar]
- 15.Paini A, Boutouyrie P, Calvet D, Tropeano AI, Laloux B, Laurent S. Carotid and aortic stiffness: determinants of discrepancies. Hypertension. 2006;47:371–376. doi: 10.1161/01.HYP.0000202052.25238.68. [DOI] [PubMed] [Google Scholar]
- 16.Leung WH, Stadius ML, Alderman EL. Determinants of normal coronary artery dimensions in humans. Circulation. 1991;84:2294–2306. doi: 10.1161/01.cir.84.6.2294. [DOI] [PubMed] [Google Scholar]
- 17.Jeremias A, Spies C, Herity NA, Ward MR, Pomerantsev E, Yock PG, Fitzgerald PJ, Yeung AC. Coronary artery distensibility and compensatory vessel enlargement--a novel parameter influencing vascular remodeling? Basic Res Cardiol. 2001;96:506–512. doi: 10.1007/s003950170033. [DOI] [PubMed] [Google Scholar]
- 18.Shaw JA, Kingwell BA, Walton AS, Cameron JD, Pillay P, Gatzka CD, Dart AM. Determinants of coronary artery compliance in subjects with and without angiographic coronary artery disease. J Am Coll Cardiol. 2002;39:1637–1643. doi: 10.1016/s0735-1097(02)01842-9. [DOI] [PubMed] [Google Scholar]
- 19.Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med. 1987;316:1371–1375. doi: 10.1056/NEJM198705283162204. [DOI] [PubMed] [Google Scholar]
- 20.Roberts CS, Roberts WC. Cross-sectional area of the proximal portions of the three major epicardial coronary arteries in 98 necropsy patients with different coronary events. Relationship to heart weight, age and sex. Circulation. 1980;62:953–959. doi: 10.1161/01.cir.62.5.953. [DOI] [PubMed] [Google Scholar]
- 21.Ahmadi N, Shavelle D, Nabavi V, Hajsadeghi F, Moshrefi S, Flores F, Azmoon S, Mao SS, Ebrahimi R, Budoff M. Coronary distensibility index measured by computed tomography is associated with the severity of coronary artery disease. J Cardiovasc Comput Tomogr. 2010;4:119–126. doi: 10.1016/j.jcct.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 22.Frobert O, Schionning J, Gregersen H, Baandrup U, Petersen JA, Bagger JP. Impaired human coronary artery distensibility by atherosclerotic lesions: a mechanical and histological investigation. Int J Exp Pathol. 1997;78:421–428. doi: 10.1046/j.1365-2613.1997.470374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scheidegger MB, Stuber M, Boesiger P, Hess OM. Coronary artery imaging by magnetic resonance. Herz. 1996;21:90–96. [PubMed] [Google Scholar]
- 24.Wellnhofer E, Wahle A, Mugaragu I, Gross J, Oswald H, Fleck E. Validation of an accurate method for three-dimensional reconstruction and quantitative assessment of volumes, lengths and diameters of coronary vascular branches and segments from biplane angiographic projections. Int J Card Imaging. 1999;15:339–353. doi: 10.1023/a:1006322609072. discussion 355-356. [DOI] [PubMed] [Google Scholar]
- 25.Schuurbiers JC, Lopez NG, Ligthart J, Gijsen FJ, Dijkstra J, Serruys PW, Van der Steen AF, Wentzel JJ. In vivo validation of CAAS QCA-3D coronary reconstruction using fusion of angiography and intravascular ultrasound (ANGUS) Catheter Cardiovasc Interv. 2009;73:620–626. doi: 10.1002/ccd.21872. [DOI] [PubMed] [Google Scholar]
- 26.Ge J, Erbel R, Gerber T, Gorge G, Koch L, Haude M, Meyer J. Intravascular ultrasound imaging of angiographically normal coronary arteries: a prospective study in vivo. Br Heart J. 1994;71:572–578. doi: 10.1136/hrt.71.6.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weissman NJ, Palacios IF, Weyman AE. Dynamic expansion of the coronary arteries: implications for intravascular ultrasound measurements. Am Heart J. 1995;130:46–51. doi: 10.1016/0002-8703(95)90234-1. [DOI] [PubMed] [Google Scholar]
- 28.Peters RJ, Kok WE, Rijsterborgh H, van Dijk M, Koch KT, Piek JJ, David GK, Visser CA. Reproducibility of quantitative measurements from intracoronary ultrasound images. Beat-to-beat variability and influence of the cardiac cycle. Eur Heart J. 1996;17:1593–1599. doi: 10.1093/oxfordjournals.eurheartj.a014726. [DOI] [PubMed] [Google Scholar]
- 29.Desai MY, Lai S, Barmet C, Weiss RG, Stuber M. Reproducibility of 3D free-breathing magnetic resonance coronary vessel wall imaging. Eur Heart J. 2005;26:2320–2324. doi: 10.1093/eurheartj/ehi357. [DOI] [PubMed] [Google Scholar]
- 30.Vavuranakis M, Stefanadis C, Triandaphyllidi E, Toutouzas K, Toutouzas P. Coronary artery distensibility in diabetic patients with simultaneous measurements of luminal area and intracoronary pressure: evidence of impaired reactivity to nitroglycerin. J Am Coll Cardiol. 1999;34:1075–1081. doi: 10.1016/s0735-1097(99)00331-9. [DOI] [PubMed] [Google Scholar]