Abstract
Background/Aims: The impact of aging on esophageal motility is not completely understood. This study aims at assessing 1) whether degeneration of esophageal body motility occurs with age and 2) whether this development is influenced by gastroesophageal reflux disease (GERD).
Methods: 326 consecutive patients with symptoms of GERD underwent a diagnostic work-up including a water-perfused esophageal manometry. Patients were divided by age: 17–39 years (group 1, n=75), 40–49 years (group 2, n=79), 50–59 years (group 3, n=64), 60–69 years (group 4, n=74), and >70 years (group 5, n=34). GERD was diagnosed if patients had erosive esophagitis at endoscopy, a positive pH-metry, or both. The amplitude of esophageal contraction waves 3 cm and 8 cm above the lower esophageal sphincter and the percentage of peristaltic contraction waves of the tubular esophagus were analyzed and correlated to GERD.
Results: A normal esophageal manometry was found in 86.7%, 73.4%, 67.2%, 58.1%, and 55.9% (p<0.01) in groups 1–5, respectively. Esophageal contraction wave amplitudes were affected by age in patients positive for GERD only (p<0.01). Esophageal body peristalsis was affected by age (p<0.01) independent of the diagnosis of GERD.
Conclusion: Aging is correlated to esophageal motor abnormalities. GERD has a significant impact on esophageal contraction wave amplitude, but not on peristalsis.
Keywords: esophagus, dysmotility, manometry, age, GERD
Abstract
Hintergrund: Schluckstörungen sind ein häufiges klinisches Problem älterer Patienten. Die Ätiologie der sogenannten Presbyphagie ist multifaktoriell. Unter anderem wird auch eine altersabhängige Verschlechterung der Speiseröhrenmotilität diskutiert. Manometrische Daten aus größeren Patientenkollektiven liegen zu dieser Fragestellung bisher nicht vor.
Patienten und Methoden: 326 Patienten mit Verdacht auf gastroösophageale Refluxkrankheit (GERD) wurden mittels Ösophagogastroskopie, pH-Metrie und Ösophagusmanometrie untersucht. Die Patienten wurden je nach Alter in 5 Gruppen unterteilt: 17–39 Jahre (Gruppe 1, n=75), 40–49 Jahre (Gruppe 2, n=79), 50–59 Jahre (Gruppe 3, n=64), 60–69 Jahre (Gruppe 4, n=74) und älter als 70 Jahre (Gruppe 5, n=34). Kriterien der GERD waren eine erosive Ösophagitis oder eine positive pH-Metrie. Der Prozentsatz peristaltischer Kontraktionswellen sowie die ösophagealen Kontraktionsamplituden 3 und 8cm oberhalb des unteren Ösophagussphinkters wurden analysiert und mit dem Alter und der Diagnose GERD korreliert.
Ergebnisse: Eine normale Ösophagusmanometrie fand sich in 86,7%, 73,4%, 67,2%, 58,1%, und 55,9% (p<0.01) der Patienten in den Gruppen 1–5. Der Prozentsatz peristaltischer Kontraktionswellen war bei allen Patienten - unabhängig von der Diagnose GERD – mit zunehmendem Alter reduziert (p<0.01). Die Amplituden der ösophagealen Kontraktionen waren nur bei Patienten mit nachgewiesener GERD altersabhängig reduziert (p<0.01).
Schlussfolgerung: Mit dem Alter der Patienten sinkt der Prozentsatz peristaltischer Kontraktionswellen signifikant. Das Vorliegen einer GERD führt zusätzlich zu einer altersabhängigen ösophagealen Dyskontraktilität der distalen Speiseröhre.
Introduction
Aging can be defined as a universal and irreversible degeneration of the human body, resulting in a general decline of physiologic functions. In the foregut, this process is associated to a progressive increase of typical complaints such as dysphagia [1], regurgitation, and heartburn [2], [3].
It is common knowledge that the causes of symptoms of the upper gastrointestinal tract in older patients are multifactorial. By nature, old patients have a higher incidence of obstructive pathologies like osteophytes, diverticula, or carcinoma [4]. Also, neurological disorders responsible for esophageal symptoms like Parkinson’s disease or apoplexia are far more frequent in this population [4]. On the other hand, the degenerating effect of aging on esophageal motor function and its influence on esophageal function is not clearly understood. Earlier literature suggested a continuous decrease of esophageal motor function with increasing age. Thus, in 1964, the term “presbyesophagus” was coined to describe an age related decrease in contractile amplitude, polyphasic waves in the esophageal body, incomplete sphincter relaxation, and esophageal dilation [5]. In this study, esophageal manometry revealed a high incidence of dysmotility in a cohort of nonagenarians. Later, this concept was challenged as others found only minor changes of esophageal motility in healthy elderly individuals [6], [7]. Moreover, it was stated that esophageal dysmotility in aged patients would rather be the effect of other underlying pathology such as gastroesophageal reflux disease (GERD) [8], than a simple consequence of aging. More recently however, it was found that esophageal function naturally deteriorates from the age of 40 years onwards [9]. This physiological loss of function presumably sets the scene for subsequent symptoms such as reflux or dysphagia, that are more prevalent in aged populations. In this context, it was challenging to review our department’s experience with patients of different age that underwent esophageal function testing for GERD in our upper-GI laboratory.
Material and methods
Patients
Between October 1999 and December 2009, 326 consecutive patients (127 women, 199 men) with a mean age of 51 years (range 17–88 years) underwent esophageal function testing for suspected GERD including an esophageal manometry in our laboratory. Inclusion criteria were typical (heartburn, regurgitation) or atypical (chronic cough) symptoms suggestive of GERD for a period longer than one year; exclusion criteria were a history of previous upper gastrointestinal surgery, actual medication interfering with GERD, and known specific esophageal motor disorder. Proton pump inhibitors were stopped for at least one week. Upper-GI endoscopy and esophageal 24h-pH-metry were performed in 96% and 92% of patients, respectively, and all patients underwent a standardized interview about the presence of symptoms.
For analysis, patients were subdivided into 5 groups according to age: 17–39 years (group 1, n=75), 40–49 years (group 2, n=79), 50–59 years (group 3, n=64), 6–69 years (group 4, n=74), and >70 years (group 5, n=34).
Esophageal manometry
Manometry was completed in all patients using a low-compliance, pneumohydraulic capillary assembly connected to a eight-channel water-perfused catheter (Medtronic Deutschland GmbH, Germany). Technical details of the procedure have been described elsewhere [10]. For analysis of esophageal body motility, the percentage of peristaltic contractions was noted. Esophageal body motility was evaluated according to Kharilas and coworkers [11]. Motility was considered normal if at least 60% of swallows were followed by peristaltic contractions, with a mean amplitude greater than 40 mmHg at the two distal measuring sites (3 and 8 cm above the lower esophageal sphincter (LES)).
Other clinical investigations (pH-monitoring, endoscopy)
Intraesophageal 24-hour pH monitoring was performed with a portable datalogger (Digitrapper Mark III, Medtronic Germany GmbH, Düsseldorf, Germany) connected to a multi-use antimony pH catheter (Medtronic) in 301 patients. Technical details of the procedure have been described elsewhere [10]. Cut-off values for distal esophageal pH-monitoring were adapted from [12]: acid reflux during total, upright and supine phases was defined pathologic if the percentage of time esophageal exposure to pH<4 exceeded 4.5%, 8.4%, and 3.5%, respectively.
314 patients underwent upper gastrointestinal endoscopy to assess mucosal injury (i.e. erythematous or erosive esophagitis, or metaplastic changes). Gastroscopy was performed with use of a PQ-20 or GIF-Q165 upper-GI-endoscope (Olympus Europa GmbH, Hamburg, Germany). Esophagitis was defined as macroscopically visible erosion within the distal esophagus during endoscopy. Grading of esophagitis was done according to Savary and Miller. Biopsies were taken and analyzed by pathology only if macroscopic lesions were present. BE was diagnosed only if intestinal metaplasia was confirmed by pathology.
GERD was diagnosed if patients had erosive esophagitis at endoscopy, a positive pH-metry, or both.
Assessment of data and statistical analysis
All data were collected from our archive and entered into an Excel Spreadsheet (Microsoft Office 2000®, Microsoft Corporation, USA). Statistical analysis was performed with Statistica® (StatSoft, USA). Kruskal-Wallis-, Mann-Whitney-U-, and Chi2-tests were used as appropriate. A p-value <0.05 was considered statistically significant.
Results
Demographic data
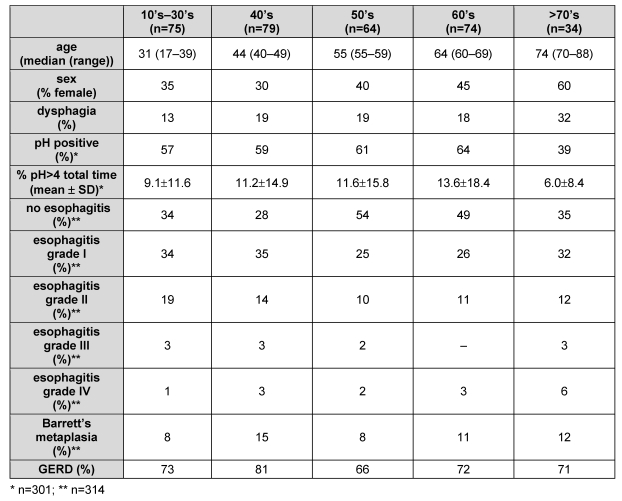
There was no significant difference between the 5 groups for the distribution of males and females, the prevalence of dysphagia, of a positive pH-metry, or of esophagitis at endoscopy (Table 1 (Tab. 1)). Also GERD (i.e. patients with a positive pH-metry, macroscopic esophagitis at endoscopy, or both) was equally distributed among the different age brackets.
Table 1. Demographic data, prevalence of dysphagia, and diagnostic work-up.
Amplitude of distal esophageal body contractions and esophageal body peristalsis
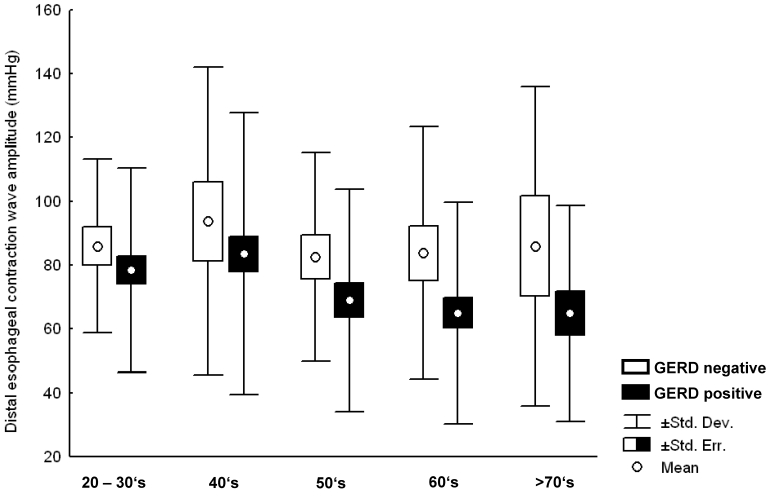
There was a slight but significant decrease of distal esophageal wave amplitudes (mean ± standard deviation) with increasing age for the whole population (81.2±33.9, 85.5±47.1, 73.8±36.6, 70.3±40.8, 70.9±41.3 (p<0.01) in groups 1–5, respectively). Further analysis showed that wave amplitudes were significantly lower in GERD compared with non-GERD patients (p<0.01), so that mean distal esophageal wave amplitudes dropped significantly only in the subgroup positive for GERD (p<0.01), whereas this was not the case in patients tested negative for reflux disease (Figure 1 (Fig. 1)).
Figure 1. Distal esophageal wave amplitude in patients tested negative for GERD (white columns), and patients tested positive for GERD (black columns). A significant decrease of mean esophageal wave amplitude with increasing age was found for patients tested positive for GERD (p<0.01), but not for patients tested negative for GERD (p=n.s.).
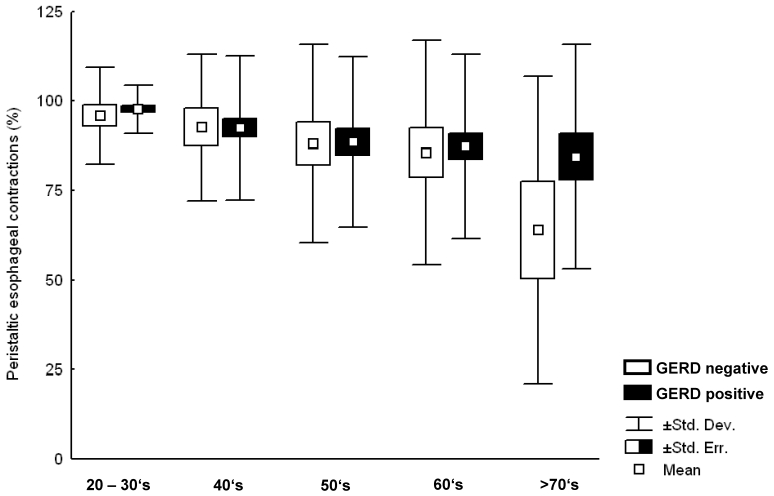
Analysis of esophageal body motility in the total population revealed a significant decrease (96.8±9.0, 92.5±20.1, 88.5±25.0, 86.9±27.2, 78.5±35.8 (p=0.03) (mean ± standard deviation) in groups 1–5, respectively) of peristaltic esophageal contractions with increasing age. This decrease was similar but not statistically significant for both GERD and non-GERD patients (Figure 2 (Fig. 2)).
Figure 2. Percentage of peristaltic response to wet swallows in patients tested negative for GERD (white columns), and patients tested positive for GERD (black columns). A decrease of the percentage of peristaltic swallows with increasing age was found for patients tested positive for GERD and for patients tested negative for GERD.
The prevalence of a normal esophageal manometry (i.e. a mean distal wave pressure >40 mmHg and ≥60% peristaltic contractions) was 86.7%, 73.4%, 67.2%, 58.1%, and 55.9% (p<0.01) in groups 1–5, respectively. The tendency to a drop with increasing age was found in both GERD and non-GERD patients.
Lower esophageal sphincter
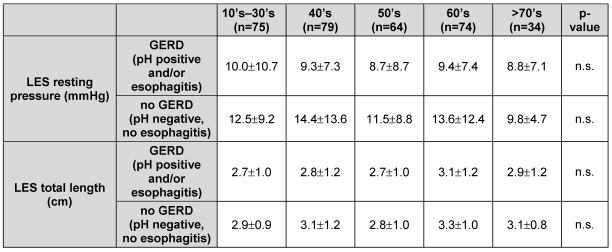
No difference was noted between the groups for both the resting pressure and the length of the LES (Table 2 (Tab. 2)). Moreover, there was no correlation with age for both parameters among GERD and non-GERD patients. However, GERD patients had a significantly lower LES resting pressure than non-GERD patients; this difference was not found for the length of the LES.
Table 2. Lower esophageal sphincter: Resting pressure distal to the respiratory inversion point and total length of the LES in patients with GERD and in patients with no evidence of GERD.
Discussion
This study on a large group of patients with reflux symptoms shows that both aging and GERD have an impact on esophageal motility. Our first result was that patients tested positive for GERD had significantly lower peristaltic wave amplitudes compared with those without GERD; and that this effect was more relevant in older individuals than in younger patients. In contrast, patients without GERD had similar distal esophageal contractile amplitudes in all age brackets. Similarly, Dantas and coworkers have shown a progressive reduction of esophageal wave amplitudes with higher degrees of esophagitis in a clinical study on GERD patients; however, the authors did not find significant differences between patients younger or older than 50 years [13]. All in all, conflicting results have been published in the literature on esophageal contractile amplitudes in younger and older patients. In the first study addressing this issue, a normal amplitude of esophageal waves was reported in nonagenarians; however, inadequate manometric equipment (uninfused catheters) was used [5]. It is known that such a system gives inadequate estimates of esophageal contractile pressure [14]. Using state of the art waterperfused manometry, Hollis et al. found a marked decline of peristaltic amplitude in healthy elderly men (70 to 87 years) without reflux symptoms compared to a group of younger subjects (19 to 27 years) [7]. On the other hand, Richter et al. reported increased mean distal contractile amplitudes with age peaking in the 5th decade in a cohort of 95 asymptomatic volunteers [15]. In another study, two groups (≥75 years; n=66 and ≤50 years; n=122) from a cohort of unselected patients with various symptoms referred for esophageal function testing were found to have similar peristaltic pressure and duration [16]. In the only other study focusing specifically on GERD and age, younger (≤40 years; n=48) and older (≥65 years; n=133) subjects with reflux symptoms were stratified according to acid exposure at pH-metry (<5%, 5–10%, >10%). The authors reported a trend toward higher distal esophageal pressure in the older group for acid exposure <5% and 5–10%; however, this was not found in patients with the highest acid exposure [8].
The second result of our study was that the percentage of peristaltic contractions after wet swallows decreased with age independent of GERD. This observation is consistent with the results of studies looking at unselected patient cohorts [16], [17], and with a previous study that reported increasing prevalence of ineffective peristalsis with age in a large group of patients referred for investigations of reflux symptoms [18]. In contrast, Achem and coworkers found a significant decrease of esophageal peristalsis with age only in patients with highly elevated (>10% of pH<4), but not in patients with normal or mildly increased (<5% or 5–10% of pH<4) esophageal acid exposure [8].
As in other research, we could not detect significant changes of LES parameters (total length and resting pressure) among the five age brackets. This is consistent with the results of other retrospective studies that have evaluated esophageal manometry in GERD [8], [18], in unselected patients [16], or in healthy adults [15].
Our observations support the assumption of others, that the esophagus is subject to a general decline of motor function with age that is aggravated by GERD [4]. It is one of the few papers in the literature that clearly illustrates an interaction between the effect of age itself and that of caustic esophageal injury. Although the thickness of the esophageal wall does not change with age [19], it has been shown that the esophageal wall becomes less flexible in older people [9], and therefore it is probably less able to compensate additional caustic injury. The loss of peristaltic function in the older patients of our series may be explained by the fact that the number of esophageal myenteric neurones decreases with age [19], a phenomenon also known from spastic esophageal motility disorders such as achalasia [20], [21].
The pathophysiology of acid-induced impairment of esophageal motility is complex and incompletely understood. The smooth muscle of the thoracic esophagus is innervated by intramural inhibitory (nitric oxide releasing) and excitatory (acetylcholine releasing) neurons [22]. In animal studies, it has been shown that peristalsis is mainly dependent on inhibitory neurons that cause a latency gradient from the proximal to the distal esophagus [23]. On the other hand, the strength of esophageal squeeze in response to swallowing is largely modulated by a well-balanced mechanism involving both acetylcholine- (stimulating) and nitric oxide- (inhibiting) mediated neurons [24]. In a recent paper, Cheng and coworkers [25] have shown that GERD may have a particular impact on acetylcholine releasing neurons, resulting in reduced peristaltic contractile strength: Exposure to acid induced the release of platelet-activating factor (PAF) in the esophageal mucosa that in turn provoked a sequential production of proinflammatory cytokines (IL-6, IL-1β) and H2O2 in the esophageal muscle layer. This process initiated a spiral of mutual stimulation of cytokines, H2O2, and PAF; all of which are known to depress neurogenic muscle contraction by inhibiting release of acetylcholine [24], [26]. Thus, it can be presumed that acid reflux induces a complex mechanism leading to an overbalance of inhibitory (not acetylcholine-mediated) neurons. It would be obvious to assume that this predominance of inhibitory neurons could be responsible for a reduced peristaltic squeeze on the one hand and a relatively unaffected peristaltic function of the thoracic esophagus on the other.
Conclusions
The physiology of esophageal motility is still a subject of speculation in many details. Our clinical observations suggest an influence of GERD and age on esophageal contractile strength and an influence of age, largely independent of GERD, on esophageal peristaltic function. The literature on this subject is limited and inconsistent, especially regarding clinical studies. Experimental data suggests that acid reflux induces a vicious circle of proinflammatory cytokines and H2O2 within the esophageal wall that influences muscle contraction. By and large, our results emphasize the importance of esophageal manometry in patients with dysphagia and/or GERD. The information provided may be of specific value for gastroenterologists, for colleages working in geriatric medicine, and for esophageal surgeons specializing in benign pathologies like GERD or spastic esophageal motor disorders.
Notes
Acknowledgements
We thank Mrs. Haddaoui and Mrs. Marlies Janson for her helpful assistance in data acquisition and Mr. David Peters for proof reading of the manuscript.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Steele CM, Greenwood C, Ens I, Robertson C, Seidman-Carlson R. Mealtime difficulties in a home for the aged: not just dysphagia. Dysphagia. 1997;12(1):43–51. doi: 10.1007/pl00009517. [DOI] [PubMed] [Google Scholar]
- 2.Bollschweiler E, Knoppe K, Wolfgarten E, Hölscher AH. Prävalenz von Refluxbeschwerden in der Kölner Normalbevölkerung. [Prevalence of reflux symptoms in the general population of Cologne]. Z Gastroenterol. 2007;45(2):177–181. doi: 10.1055/s-2006-927402. (Ger). Available from: http://dx.doi.org/10.1055/s-2006-927402. [DOI] [PubMed] [Google Scholar]
- 3.Tack J, Vantrappen G. The aging oesophagus. Gut. 1997;41(4):422–424. doi: 10.1136/gut.41.4.422. Available from: http://dx.doi.org/10.1136/gut.41.4.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Achem SR, Devault KR. Dysphagia in aging. J Clin Gastroenterol. 2005;39(5):357–371. doi: 10.1097/01.mcg.0000159272.88974.54. Available from: http://dx.doi.org/.org/10.1097/01.mcg.0000159272.88974.54. [DOI] [PubMed] [Google Scholar]
- 5.Soergel KH, Zboralske FF, Amberg JR. Presbyesophagus: esophageal motility in nonagenarians. J Clin Invest. 1964;43:1472–1479. doi: 10.1172/JCI105023. Available from: http://dx.doi.org/10.1172/JCI105023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adamek RJ, Wegener M, Wienbeck M, Gielen B. Long-term esophageal manometry in healthy subjects. Evaluation of normal values and influence of age. Dig Dis Sci. 1994;39(10):2069–2073. doi: 10.1007/BF02090352. Available from: http://dx.doi.org/10.1007/BF02090352. [DOI] [PubMed] [Google Scholar]
- 7.Hollis JB, Castell DO. Esophageal function in elderly man. A new look at "presbyesophagus". Ann Intern Med. 1974;80(3):371–374. doi: 10.7326/0003-4819-80-3-371. [DOI] [PubMed] [Google Scholar]
- 8.Achem AC, Achem SR, Stark ME, DeVault KR. Failure of esophageal peristalsis in older patients: association with esophageal acid exposure. Am J Gastroenterol. 2003;98(1):35–39. doi: 10.1111/j.1572-0241.2003.07188.x. Available from: http://dx.doi.org/10.1111/j.1572-0241.2003.07188.x. [DOI] [PubMed] [Google Scholar]
- 9.Gregersen H, Pedersen J, Drewes AM. Deterioration of muscle function in the human esophagus with age. Dig Dis Sci. 2008;53(12):3065–3070. doi: 10.1007/s10620-008-0278-y. Available from: http://dx.doi.org/10.1007/s10620-008-0278-y. [DOI] [PubMed] [Google Scholar]
- 10.Gutschow CA, Bludau M, Vallböhmer D, Schröder W, Bollschweiler E, Hölscher AH. NERD, GERD, and Barrett's esophagus: role of acid and non-acid reflux revisited with combined pH-impedance monitoring. Dig Dis Sci. 2008;53(12):3076–3081. doi: 10.1007/s10620-008-0270-6. Available from: http://dx.doi.org/10.1007/s10620-008-0270-6. [DOI] [PubMed] [Google Scholar]
- 11.Kahrilas PJ, Dodds WJ, Hogan WJ, Kern M, Arndorfer RC, Reece A. Esophageal peristaltic dysfunction in peptic esophagitis. Gastroenterology. 1986;91(4):897–904. doi: 10.1016/0016-5085(86)90692-x. [DOI] [PubMed] [Google Scholar]
- 12.Jamieson JR, Stein HJ, DeMeester TR, Bonavina L, Schwizer W, Hinder RA, Albertucci M. Ambulatory 24-h esophageal pH monitoring: normal values, optimal thresholds, specificity, sensitivity, and reproducibility. Am J Gastroenterol. 1992;87(9):1102–1111. [PubMed] [Google Scholar]
- 13.Dantas RO, Aprile LR. Relacao entre idade e motilidade do esofago em pacientes com doenca do refluxo gastroesofagico. [Aging and esophageal motility in patients with gastroesophageal reflux disease]. Arq Gastroenterol. 2006;43(2):107–111. doi: 10.1590/S0004-28032006000200009. Available from: http://dx.doi.org/10.1590/S0004-28032006000200009. [DOI] [PubMed] [Google Scholar]
- 14.Hollis JB, Castell DO. Amplitude of esophageal peristalsis as determined by rapid infusion. Gastroenterology. 1972;63(3):417–422. [PubMed] [Google Scholar]
- 15.Richter JE, Wu WC, Johns DN, Blackwell JN, Nelson JL, 3rd, Castell JA, Castell DO. Esophageal manometry in 95 healthy adult volunteers. Variability of pressures with age and frequency of "abnormal" contractions. Dig Dis Sci. 1987;32(6):583–592. doi: 10.1007/BF01296157. Available from: http://dx.doi.org/10.1007/BF01296157. [DOI] [PubMed] [Google Scholar]
- 16.Ribeiro AC, Klingler PJ, Hinder RA, DeVault K. Esophageal manometry: a comparison of findings in younger and older patients. Am J Gastroenterol. 1998;93(5):706–710. doi: 10.1111/j.1572-0241.1998.210_a.x. Available from: http://dx.doi.org/10.1111/j.1572-0241.1998.210_a.x. [DOI] [PubMed] [Google Scholar]
- 17.Andrews JM, Heddle R, Hebbard GS, Checklin H, Besanko L, Fraser RJ. Age and gender affect likely manometric diagnosis: Audit of a tertiary referral hospital clinical esophageal manometry service. J Gastroenterol Hepatol. 2009;24(1):125–128. doi: 10.1111/j.1440-1746.2008.05561.x. Available from: http://dx.doi.org/10.1111/j.1440-1746.2008.05561.x. [DOI] [PubMed] [Google Scholar]
- 18.Lee J, Anggiansah A, Anggiansah R, Young A, Wong T, Fox M. Effects of age on the gastroesophageal junction, esophageal motility, and reflux disease. Clin Gastroenterol Hepatol. 2007;5(12):1392–1398. doi: 10.1016/j.cgh.2007.08.011. Available from: http://dx.doi.org/10.1016/j.cgh.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 19.Eckardt VF, LeCompte PM. Esophageal ganglia and smooth muscle in the elderly. Am J Dig Dis. 1978;23(5):443–448. doi: 10.1007/BF01072928. Available from: http://dx.doi.org/10.1007/BF01072928. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen NQ, Holloway RH. Recent developments in esophageal motor disorders. Curr Opin Gastroenterol. 2005;21(4):478–484. [PubMed] [Google Scholar]
- 21.Champion JK, Delise N, Hunt T. Myenteric plexus in spastic motility disorders. J Gastrointest Surg. 2001;5(5):514–516. doi: 10.1016/S1091-255X(01)80089-5. Available from: http://dx.doi.org/10.1016/S1091-255X(01)80089-5. [DOI] [PubMed] [Google Scholar]
- 22.Goyal RK, Chaudhury A. Physiology of normal esophageal motility. J Clin Gastroenterol. 2008;42(5):610–619. doi: 10.1097/MCG.0b013e31816b444d. Available from: http://dx.doi.org/10.1097/MCG.0b013e31816b444d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weisbrodt NW, Christensen J. Gradients of contractions in the opossum esophagus. Gastroenterology. 1972;62(6):1159–1166. [PubMed] [Google Scholar]
- 24.Rieder F, Biancani P, Harnett K, Yerian L, Falk GW. Inflammatory mediators in gastroesophageal reflux disease: impact on esophageal motility, fibrosis, and carcinogenesis. Am J Physiol Gastrointest Liver Physiol. 2010;298(5):G571–G581. doi: 10.1152/ajpgi.00454.2009. Available from: http://dx.doi.org/10.1152/ajpgi.00454.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng L, Cao W, Behar J, Fiocchi C, Biancani P, Harnett KM. Acid-induced release of platelet-activating factor by human esophageal mucosa induces inflammatory mediators in circular smooth muscle. J Pharmacol Exp Ther. 2006;319(1):117–126. doi: 10.1124/jpet.106.106104. Available from: http://dx.doi.org/10.1124/jpet.106.106104. [DOI] [PubMed] [Google Scholar]
- 26.Cao W, Cheng L, Behar J, Fiocchi C, Biancani P, Harnett KM. Proinflammatory cytokines alter/reduce esophageal circular muscle contraction in experimental cat esophagitis. Am J Physiol Gastrointest Liver Physiol. 2004;287(6):G1131–G1139. doi: 10.1152/ajpgi.00216.2004. Available from: http://dx.doi.org/10.1152/ajpgi.00216.2004. [DOI] [PubMed] [Google Scholar]