Abstract
Background:
Over the past 30 years, pretransfusion tests have undergone considerable modification. In 1984, AABB recommended that the full cross match could be replaced by an abbreviated cross match in patients with negative antibody screen. However, before implementation of such a policy, issue regarding safety of T & S needs to be evaluated.
Objectives:
The aim of pretransfusion testing (PTT) is to ensure that enough red blood cells (RBCs) in the selected red cell components will survive when transfused.
Results and Conclusion:
We have, therefore in this study; evaluated safety of T & S procedure for PTT in comparison with conventional test tube cross match. The T & S procedure gave a safety of 91.6%. Also, the usefulness of the T & S was shown through the detection of unexpected antibodies in 0.75% (15 out of 2026) of cases.
Keywords: Cross matching, pre transfusion testing, red cell transfusion, type and screen
Introduction
The aim of pretransfusion testing (PTT) is to ensure that enough red blood cells (RBCs) in the selected red cell components will survive when transfused. PTT can assure ABO compatibility between donor and patient blood as well as detect most clinically significant RBC alloantibodies that react with antigens on donor RBCs. Over the past 30 years, PTT have undergone considerable modification. Most of the early methods for antibody screening and crossmatching involved testing at room temperature also. In 1978, the American Association of Blood Banks (AABB) deleted the room temperature requirement from its standards.[1] The main reason for abbreviating crossmatching is to save the cost of reagents and labor. More recently, it has been recognized that patients with a negative antibody screen and no history of red cell antibodies do not require a complete 20-30 minute crossmatch. The chances of a clinically significant red cell antibody being missed in a patient with a negative antibody screen (false negative) are 1-4/10,000.[2,3]
Approximately 95% of transfusions occur in patients with a negative antibody screen. The issue of whether to omit anti-human globulin crossmatch (AHG-XM) for patients screened as negative for RBC alloantibodies remains controversial. In 1984, AABB recommended that the full cross match could be replaced by an abbreviated cross match in patients with negative antibody screen.[4,5]
A T and S includes typing the patient's red cells for ABO, Rh blood group, and screening patient's serum for the presence of unexpected antibodies by using reagent red cells (screen cells) in an antihuman globulin (AHG) phase. These screen cells carry all common red cell antigens capable of inducing clinically significant red cell antibody reactions. If the antibody screen is negative and the patient has no past history of unexpected antibodies, it can be predicted that more than 99.99% of ABO compatible red blood cell units would be compatible in an AHG crossmatch.[6] ABO- and Rh-compatible blood can be selected from the inventory and issued within five to 10 minutes. If the antibody screen is positive, which is extremely rare in an un-transfused patient, the unexpected antibody must be identified before antigen negative compatible red blood cells can be issued.
In many hospitals in India, blood units are fully cross matched and reserved for patient even though many of them have low probability of requiring transfusion. A significant number of these reserved units are held until expiry and therefore wasted. A T and S policy for PTT would be the best alternative in such cases. However, before implementation of such a policy, issue regarding safety of T and S needs to be evaluated. We have, therefore; evaluated safety of T and S procedure for PTT in comparison with conventional test tube cross match.
Materials and Methods
The study was carried in the Department of Transfusion Medicine, SGPGIMS, Lucknow. A total of 2026 requisitions were received during the study period for PTT. Antibody screening was done in all the patients where a request was sent for pre-transfusion testing to the cross-match laboratory. Each patient was tested only once in case of multiple requests. Routine indirect antiglobulin cross-matching was done to check the compatibility between donor cells and patient serum. In all the samples, antibodies were screened using cell panel Asia [ID-DiaCell I-II-III Asia (Mia+)]. The actual crossmatch was carried out routinely and the screening was carried out blindly, without knowing the result of the compatibility test. The results of the two tests were later compared. Whenever antibody screening was positive, antibody was identified using 11 cell panel (DiaMed). In presence of clinically significant red cell antibody, respective antigen negative red cell units were issued after compatible indirect antiglobulin cross-match. The sensitivity (safety level), specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated.
Results
There were 2026 requests considered for antibody testing by indirect antiglobulin testing using three cell panel Asia (DiaMed) in gel cards. Antibody screening was positive in twenty-six cases (1.28%). The specificity of the detected antibody was anti E (10), anti N (2), anti M (3), anti Leb (2), anti Lea (2), anti JKb, anti Mia, anti D, anti K, anti Fya, anti s (one each) and a suspected antibody to a low incidence antigen.
Comparison of cross match vs type and screen
Out of 12 incompatible cross match samples, one patient showed negative results with antibody screening (false negatives 8.33%). There was no blood group error, cross match was repeated to confirm the incompatibility and the possibility of dosage was ruled out by the use of 11 cell panel. Since testing was performed on fresh samples there was no chance of deterioration of the antibody due to storage. The T and S procedure gave a safety of 91.6%. In the rest 2014 patients with compatible cross matches, antibodies were detected in 26 individuals. The details are given in the Table 1. The safety parameters for T and S are given in Table 2.
Table 1.
Comparison of T and S with conventional Coombs cross match
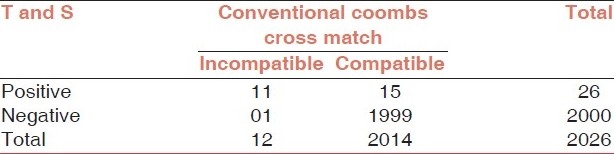
Table 2.
Safety parameters of T and S in Indian setting

Discussion
The main objective of this study was to demonstrate whether the T and S procedure is a safe method of PTT, when compared to the antiglobulin crossmatch currently in use. Although, this study did not show the T and S to reach the expected safety level of 99%, it has achieved a reasonable level of safety (91.6%). Also, the usefulness of the T and S was shown through the detection of unexpected antibodies in 0.75% (15 out of 2026) of cases, which would have been missed otherwise with conventional Coombs cross match.
In this study, only one sample gave a negative antibody screen while conventional crossmatch was incompatible. This may be due to a rare antibody in the patient sera against which the corresponding antigen was not present on the screening cells but present on the donor red cell. A transfusion reaction would have occurred in this case since it was reacting at 37°C in AHG. Since, the screening panel used in the present study was not from Indian subcontinent, therefore, it would be advisable to prepare screening cell panels that include RBC antigens which are prevalent within the local/regional population e.g, In, Mia etc.
By implementing the T and S test policy, the number of unnecessary crossmatch tests is reduced, as is the number of blood returns and extensions. Both the American and British standards stipulate that when an irregular antibody is detected, its specificity should be determined and its clinical significance assessed.[7,8] Furthermore, for transfusion purposes, antigen negative blood should be selected for crossmatching if the recipient has a clinically significant antibody. Based on these standards, it is clear that any hospital providing the T and S test should have in place means for investigating serological problems.
Although T and S is a safer alternative to conventional cross match, certain infrastructural and technical changes in the blood center must be included before its implementation. For instance, a reliable and validated, preferably by computer, checking procedure should be in place when the blood units are delivered. Secondly, sensitive techniques for the detection of erythrocyte antibodies should be employed.
One of the best means of evaluating transfusion practices is to determine the ratio of units cross matched to units transfused (cross match/transfusion or C/T ratio). The T and S procedure has proved effective in reducing unnecessary cross matches for effective surgery procedures.
The T and S offers several advantages over the older practice of crossmatching and reserving specific donor units for patients. It allows optimal use of donor blood, as it is not tied up by being crossmatched and held for patients who probably will not need it. It also has potential for a more economic transfusion service due to decreased blood inventory requirements,[9] decreased reagents, and more efficient use of technologist time. The estimated savings from eliminating the AHG phase of a crossmatch is approximately $1.00 in cost and 30% in technologist's time.[10] With approximately 8 million units of blood transfused annually in India, the cost and time savings would be substantial.
However, T and S should be used with caution in Indian blood bank setting as the essential infrastructure and technical expertise may not be available in all the centers. Moreover, the reagent cell panels are not indigenous and may therefore miss some of the clinically significant antibodies. Further studies on a large scale are necessary to assess the utility of T and S method before implementing it in routine practice.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Standards for Blood Banks and Transfusion Services. 9th ed. Washington, DC: American Association of Blood Banks; 1978. [Google Scholar]
- 2.Oberman HA, Barnes BA, Friedman BA. The risk of abbreviating the major crossmatch in urgent or massive transfusion. Transfusion. 1978;18:137–41. doi: 10.1046/j.1537-2995.1978.18278160574.x. [DOI] [PubMed] [Google Scholar]
- 3.Mintz PD, Haines A, Sullivan M. Incompatible crossmatch following negative antibody screen: Frequency and cause. Transfusion. 1980;20:651. doi: 10.1046/j.1537-2995.1982.22282177114.x. [DOI] [PubMed] [Google Scholar]
- 4.Standards for Blood Banks and Transfusion Services. Arlington: AABB; 1984. American Association of Blood Banks. [Google Scholar]
- 5.Equivalent methods for compatibility testing: Memorandum to establishment. Washington DC: FDA; 1984. Offi ce of Biologicals Research and Review. National Center for Drugs and Biochemicals. [Google Scholar]
- 6.Boral LI, Henry JB. The type and screen: A safe alternative and supplement in selected surgical procedures. Transfusion. 1977;17:163–8. doi: 10.1046/j.1537-2995.1977.17277151923.x. [DOI] [PubMed] [Google Scholar]
- 7.Walker RH. 11th ed. Bethesda (Md): American Association of Blood Banks; 1993. Technical manual. [Google Scholar]
- 8.Guidelines for pre-transfusion compatibility procedures in blood transfusion laboratories. BCSH Blood Transfusion Task Force. Transfus Med. 1996;6:273–83. [PubMed] [Google Scholar]
- 9.Pereira A. Blood inventory management in the type and screen era. Vox Sang. 2005;89:245–50. doi: 10.1111/j.1423-0410.2005.00700.x. [DOI] [PubMed] [Google Scholar]
- 10.Masouredis SP. Pretransfusion tests and compatibility: Question of safety and efficacy. Blood. 1982;59:873–5. [PubMed] [Google Scholar]


