Abstract
The central nervous system (CNS) depressant and anticonvulsant activities of iso-6-spectaline (SPEC) were investigated in animal models. The SPEC from Senna spectabilis var. excelsa (Schrad) (0.1, 0.5 and 1.0 mg/ kg) injected by oral route (p.o.) in mice caused a significant decrease in the motor activity up to 30 days after the administration and in the dose of 1.0 mg/kg significantly reduced the remaining time on the Rota-rod apparatus. Additionally, SPEC (0.1, 0.5 and 1.0 mg/kg, p.o.) was also capable of promoting increase of latency for development of convulsions induced by pentylenetetrazole. This SPEC was also capable of promoting an increase of latency for development of convulsions induced by picrotoxin at highest dose. In the same way, the anticonvulsant effect of SPEC was affected by pretreatment with flumazenil, a selective antagonist of the benzodiazepine site of the GABAA receptor. These results suggest possible CNS depressant and anticonvulsant activities in mice that needs further investigation.
Keywords: Fabaceae, open field, pentylenetetrazole, picrotoxin, senna spectabilis
INTRODUCTION
Numerous herbal medicines are recognized as active in the central nervous system (CNS), and they have at least a hypothetical potential to affect chronic conditions such as anxiety, depression, headaches or epilepsy, that do not respond well to conventional treatments. Thus, iso-6-spectaline (SPEC) may possess a modulatory role in the treatment of neurodegenerative diseases, since their piperidine alkaloid compound can interrupt cellular oxidative processes in the CNS. The effects of SPEC on the CNS have not yet been determined, therefore, would be important to conduct these studies to clarify its brain action mechanism.
Piperidine alkaloids are abundant in nature and many of them are known to exhibit some biological activity. In our search for potential anxiolytic, antidepressant or anticonvulsant agents employing a mechanism-based yeast bioassay for CNS-modifying agents,[1] we have isolated iso-6-spectaline (SPEC; 14-[(2R,3R,6R)-3-hydroxy -2-methylpiperidine]-tetradecan-13-one), piperidine alkaloids, is a heterocyclic organic not aromatic compound [Figure 1] found in many plant species. It was isolated for the first time from Cassia sp., species previously known as Cassia excelsa, hence the term cassine.[2] Piperidine alkaloid derivatives with CNS effects include SPEC which exerts neuroprotective effects against depression model.[2]
Figure 1.
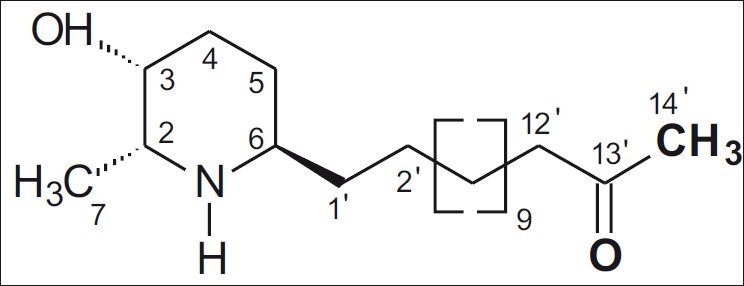
Chemical structure of iso-6-spectaline (SPEC; 14-[(2R,3R,6R)- 3-hydroxy-2-methylpiperidine]-tetradecan-13-one)
In Brazilian northeast folk medicine, Senna spectabilis is used as anti-inflammatory, analgesic, laxative, purgative, antimicrobial and antiulcerogenic.[3–5] Studies have shown that the extract of Senna sp. inhibits lipid peroxidation of bovine brain phospholipids, indicating its antioxidant activity.[6] Then, the excessive production of free radicals in the organism, and the imbalance between the concentrations of these and the antioxidant defenses may be related to the pathogenesis of neurodegenerative diseases.[7,8]
The genus Cassia possesses about 600 species distributed worldwide, being well known due to its diverse biological and pharmacological properties.[4] Senna spectabilis (DC) Irwin and Barneby var. spectabilis (Cassia spectabilis DC) is widely grown as an ornamental plant in tropical and subtropical areas, and has been commonly used in traditional medicine for many years. It has also been used in traditional Brazilian medicine for the treatment of flu and cold, as a laxative and purgative.[9,10]
Preliminary behavioral screening realized with the SPEC demonstrates that it reduces lipid peroxidation in pilocarpine-induced seizures, increasing survival rate and reducing number of seizures in rats. Additionally, there is no work demonstrating SPEC effects in neurodegenerative diseases in animal models of anxiety, depression or epilepsy. Therefore, we decided to assess the SPEC effects on the CNS of mice.
MATERIALS AND METHODS
Plant material and chemistry study
The plant was collected in September 2003, at Boa Viagem, State of Ceará, Brazil, and was identified by Prof. A.G. Fernandes, in the Department of Biology of the Federal University of Ceará. The voucher specimen is deposited at the Prisco Bezerra Herbarium under the voucher number 33013.
The botanical material, leaves (1.5 kg), stem (5.1 kg) and roots (3.8 kg), were triturated and exhaustingly extracted with ethanol and concentrated in rotative evaporator, producing 39 g, 27.5 g and 42 g respectively. The leaf extract (39 g) was then submitted to technical of selective extraction of alkaloids a fraction rich in alkaloids (FA) and a non alkaloids (FNA) were obtained. The alkaloid fraction was submitted to chromatography on SEPHADEX, with methanol as movable phase. The dichloromethane fraction (7.6 g) was submitted to the same chromatography process.
The analysis of the fractions was made in chromatography in thin layer (CCD), which revealed the purity of the rich fraction in SPEC (30 mg) which when subjected to the test with the reagent Dragendoff revealed an orange stain, and was thus positive for alkaloids.[11] Its spectra of NMR RMN1H, RMN13C, DEPT 135, COSY, HMBC e HSQC1 were obtained and compared with the data from the literature for identification. The iso-6-spectaline is an amorphous white solid with M.P. 130.8-132.3°C; the value of TLC in MeOH/EtOAc (1:1), Rf = 0.58; NMR spectra description of 1H is 1H NMR (MeOD, 500 MHz) δH 3.83 (1H, H-3); 3.30 (1H, H-6); 3.23 (1H, H-2); 2.47 (2H, H-12’); 2.15 (3H, H-14’); 1.44 (3H, H-7); 1.29-1.33 (12H, H-4’- H-9’); NMR spectra description of 13C is 13C NMR (MeOD, 125 MHz) δC – 212.4 (C, C-13’); 66.1 (CH, C-3); 58.8 (CH, C-2); 57.7 (CH, C-6); 44.4 (CH2, C-12’); 34.9 (CH2, C-1’); 31.2 (CH2, C-4); 29.9 (CH3, C-14’); 30.9 (CH2, C-3’); 30.5 (CH2, C-10’); 30.5-30.9 (CH2, C-4’-C-9’); 26.4 (CH2, C-2’); 23.8 (CH2, C-5); 23.8 (CH2, C-11’); 16.1 (CH3, C-7). In the present work, the iso-6-spectaline was suspended in 0.5% Tween 80 distilled in water, and sonicated before use. Agents were administrated orally (p.o.) and intraperitoneally (i.p.) at a dose volume of 0.1 ml/10 g.
Animals
Male Swiss mice (25-30 g), two months of age were used. The animals were randomly housed in appropriate cages at 23 ± 2° C on a 12-h light/dark cycle (lights on 08:00 a.m. – 18:00 p.m.) with free access to food (Purina®) and water. All experiments were carried out between 08:00 a.m. and 18:00 p.m. in a quiet room. Experimental protocols and procedures were approved by the Ethics Committee on Animal Experiments at the Federal University of Piaui (CEEA/UFPI # 44/09).
Behavioral effects, locomotor activity and motor coordination test (rota-rod test)
Behavioral screening (n = 7, per group) was performed following parameters described by Almeida et al.,[12] and animals were observed at 30 days after oral (p.o.) administration of SPEC (0.1, 0.5, and 1.0 mg/kg, p.o.).
Mice were divided into four groups (seven animals each). Vehicle (saline/Tween 80 0.5%; control group) and SPEC (0.1, 0.5, and 1.0 mg/kg, p.o.) were injected. The spontaneous locomotor activity of the animals was assessed in a cage activity (50 cm × 50 cm × 50 cm) after 30 days of treatment.[13]
A Rota-rod treadmill device (AVS®, Brazil) was used for the evaluation of motor coordination.[14] Initially, the mice able to remain on the Rota-rod apparatus longer than 180 s (9 rpm) were selected 24 h before the test. Thirty minutes after 30 days of administration of either SPEC (0.1, 0.5, and 1.0 mg/kg, p.o.), vehicle (saline/Tween 80 0.5%; control group) or diazepam (DZP, 2.0 mg/kg, i.p.), each animal was tested on the Rota-rod apparatus and the time(s) remained on the bar for up to 180 s was recorded after 30 days of treatment.
Pentylenetetrazole-induced convulsions
Pentylenetetrazole (PTZ) (60 mg/kg, i.p.) was used to induce clonic convulsions.[15] Mice were divided into five groups (n = 7 per group). First group received vehicle (two drops of Tween 80 0.5% in distilled water, the solvent for SPEC) while the second group was treated with diazepam (DZP, 2.0 mg/kg, i.p.). The remaining groups received an injection of SPEC (0.1, 0.5, and 1.0 mg/kg, p.o.). After 30 days of drug administration, the mice were treated with PTZ (i.p.) at a dose of 60 mg/kg. The latency and percentage of inhibition clonic convulsions were registered. The incidence of deaths was noted until 24 h after the injection of PTZ.
The effect of selective GABAA-BZD receptor antagonist flumazenil[16] on the anticonvulsant activity of SPEC was investigated. In the experimental groups, mice were given flumazenil (FLU) (10 mg/kg, i.p.) 30 min before the administration of SPEC (1.0 mg/kg, p.o.) (30 days before the injection of PTZ). In the standard group, the animals received FLU 30 min before the administration of diazepam (DZP, 2.0 mg/kg, i.p.) (30 days before the injection of PTZ). The anticonvulsant activity of SPEC and DZP in mice pretreated with FLU was assessed.
Picrotoxin-induced convulsion
The method has been described previously.[17,18] Animals were divided into five groups (n = 7 per group). Control group received vehicle and standard group was treated with diazepam (DZP, 2.0 mg/kg, i.p.). The remaining groups were treated with 0.1, 0.5 and 1.0 mg/kg (p.o.) of SPEC. After 30 days of drug administration, the mice were treated with PIC at a dose of 8 mg/kg (i.p.). Immediately after the injection of the convulsant, mice were individually placed in plastic boxes and observed for the time to onset of clonic convulsion (latency), percent clonic convulsion and deaths. The incidence of deaths was noted until 24 h after the injection of PIC.
Statistical analysis
The data obtained were evaluated by one-way analysis of variance (ANOVA) followed by Student-Neuman-Keuls t-test. The incidence (%) of clonic or tonic-clonic convulsions as well as the mortality were evaluated by Fisher's Exact Test. Differences were considered to be statistically significant when P < 0.05.
RESULTS AND DISCUSSION
Analysis of the 1H NMR and 13C NMR spectra showed that SPEC presented analytical and spectroscopic data in full agreement with its assigned structure. The chemical purity of the compound was determined by analysis of the 1H NMR and 13C NMR.
SPEC at doses of 0.1, 0.5 and 1.0 mg/kg (p.o.) showed behavioral changes in animals 30 days after of treatment: Decrease of spontaneous activity, palpebral ptosis, ataxia, analgesia, and sedation. Behavioral changes were more evident on the second day of treatment. The doses of 0.1, 0.5 and 1.0 mg/kg (p.o.) of SPEC caused significant decrease of 30%, 30% and 59% of ambulation (number of crossings) 30 days after administration, respectively [Figure 2]. In this test only the highest dose (1.0 mg/kg, p.o.) reduced (40%) the remaining time of animals on the Rota-rod apparatus [Figure 3].
Figure 2.
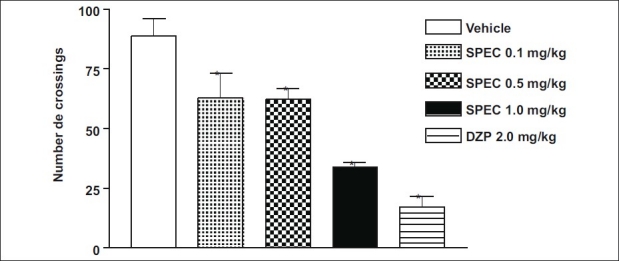
Effects of iso-6-spectaline (0.1, 0.5 and 1.0 mg/kg, p.o., SPEC) or diazepam (DZP, 2.0 mg/kg, i.p.) on locomotor activity of mice. The parameters evaluated were the total number of pulses of crossings in activity cage. Values are mean ± S.E.M. for seven mice per group. *P < 0.001 as compared to control (Vehicle), one-way ANOVA followed by Student-Neuman-Keuls t-test
Figure 3.
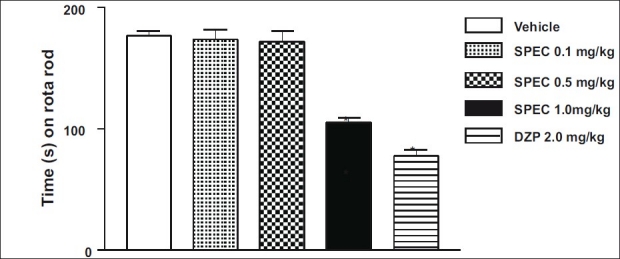
Time (s) on the Rota-rod observed in mice after oral route treatment with Vehicle (control), iso-6-spectaline (0.1, 0.5 and 1.0 mg/ kg, p.o., SPEC) or DZP (2.0 mg/kg, i.p.). The motor response was recorded for the following 180 s after drug treatment. Values are mean ± S.E.M. for seven mice per group. *P < 0.001 as compared to control (Vehicle), one-way ANOVA followed by Student-Neuman- Keuls t-test
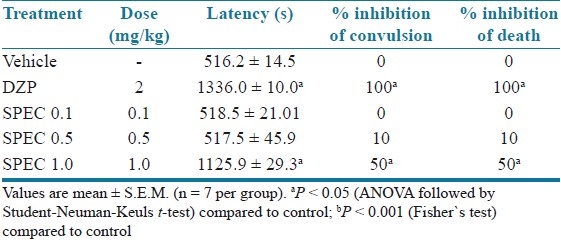
Table 1 shows that (PTZ) consistently induced clonic convulsions in 100% of mice. SPEC (0.1, 0.5 and 1.0 mg/kg, p.o.) delayed the onset of PTZ-induced tonic convulsion significantly and (1.0 mg/kg, p.o.) protected 80% (P < 0.001) of mice against the convulsion. Diazepam completely protected the animals against the tonic convulsion elicited by PTZ.
Table 1.
Effects of iso-6-spectaline (0.1, 0.5 and 1.0 mg/kg, p.o., SPEC) on PTZ-induced convulsion in mice
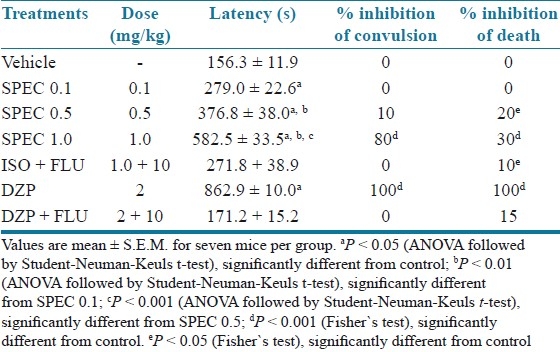
As described in Table 1, the administration of FLU (10 mg/kg, i.p.) antagonized the effect of SPEC (1.0 mg/kg, i.p.) and DZP (2 mg/kg, i.p.) in the prolongation of convulsion latency. When given i.p., only the highest dose of SPEC (1.0 mg/kg, i.p.) increased the latency for convulsions induced by PIC significantly when compared to the control (P < 0.001) [Table 2].
Table 2.
Effects of iso-6-spectaline (0.1, 0.5 and 1.0 mg/k g, p.o., SPEC) on PIC-induced convulsion in mice
The antidepressant and anticonvulsants effects of SPEC isolated from S. spectabilis were emphasized in the present work since the management of neurodegenerative diseases faces a number of problems including limited number of effective antidepressant agents and toxicity of the available anticonvulsant agents. In folk medicine of the Brazilian Northeast, the Senna spectabilis is used as an anti-inflammatory, analgesic, laxative, purgative, antimicrobial and antiulcerogenic.[3,4] In pharmacological behavioral screening, the animals treated with SPEC showed decrease of response to the touch, palpebral ptosis, ataxia, analgesia, sedation and reduction of motor activity. These data are indicative of the depressive activity of the CNS.,[2] indicating effects on the CNS similar to drugs that reduce the CNS activity.[5,10,19–23]
The reduction of the locomotor activity observed after treatement with SPEC an be due to either an inhibitory effect of the SPEC in the CNS or by muscular relaxant activity in the periphery. Our results suggest that SEPC could show a neuro-sedative activity or a profile for a hypnotic drug.
Our results suggest that the higher dose of SPEC produces loss of motor coordination in mice. Thus, the lack of motor coordination is characteristic of a drug that reduces the CNS activity such as anxiolytics, sedatives and hypnotics.[12,24,25]
Pentylenetetrazole, picrotoxin and strychnine are all convulsant agents.[26,27] Data from this study show that the onset of tonic-clonic convulsion produced by PTZ was significantly delayed by SPEC [Table 2] and mortality was significantly reduced (P < 0.05). According to De Sarro et al.,[28] PTZ may be exerting its convulsing effect by inhibiting the activity of gamma aminobutyric acid (GABA) at GABAA receptors. Gamma aminobutyric acid is the major inhibitory neurotransmitter implicated in epilepsy. The enhancement and inhibition of GABA neurotransmission will attenuate and enhance convulsion, respectively.[29] Since the SPEC delayed the occurrence of PTZ convulsion, it is probable that it may interfere in GABAergic mechanisms to exert its anticonvulsant effect.
In order to determine the role of BZD receptors in the SPEC-induced anticonvulsant effects, flumazenil (FLU), a specific antagonist of the benzodiazepine site in the GABA-benzodiazepine receptor complex, was used.[16] The results obtained from PTZ-induced convulsion model in mice pretreated with FLU suggest that SPEC could facilitate the inhibitory activity of the GABAergic system probably through a competitive agonist action in the BZD site of the GABA receptors. The significant effect on the motor coordination, in high doses, might support this theory, as GABAergic drugs usually are sedative.[30]
According to previous studies, picrotoxin, a GABAA-receptor antagonist, produces seizures by blocking the chloride-ion channels linked to GABAA-receptors, preventing the entry of chloride ions into the brain and, consequently, inhibitory transmission in the brain.[31] Therefore, the findings of the present study suggest that SPEC (1.0 mg/kg, p.o.) might have inhibited and/or attenuated the PIC-induced convulsions by interfering with GABAergic neurotransmission.[32]
Summarizing our data, the results suggest depressant CNS and anticonvulsant effects of iso-6-spectaline from Senna spectabilis. The precise mechanisms of the possible behavioral effects of iso-6-spectaline are not clear, however, the GABAergic neurotransmitter system might be involved. So, more studies will be required for elucidation of this effect in the CNS.
ACKNOWLEDGMENT
We would like to thank the National Council of Technological and Scientific Development (CNPq/Brazil), the Research Supporting Foundation of State of Piaui (FAPEPI/Brazil) for the financial support and Dr. Paulo Michel Pinheiro Ferreira (UFPI, Picos) for his help with English editing of the manuscript.
Footnotes
Source of Support: National Council of Technological and Scientific Development (CNPq/Brazil), the Research Supporting Foundation of State of Piaui (FAPEPI/Brazil)
Conflict of Interest: None declared.
REFERENCES
- 1.Silva FO, Silva MG, Feng D, de Freitas RM. Evaluation of central nervous system effects of iso-6-cassine isolated from Senna spectabilis var.excelsa (Schrad) in mice. Fitoterapia. 2010;82:255–9. doi: 10.1016/j.fitote.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Junior CV, Rezende A, Silva DH, Castro-Gambôa I, Bolzani VS, Barreiro EJ, et al. Ethnopharmacological, biological and chemical aspects of the Cassia genus. Quim Nova. 2006;29:1279–86. [Google Scholar]
- 3.Sansores-Peraza P, Rosado-Valladi M, Brito-Loeza W, Mena-Rejon GJ, Quijano L. Cassine, an antimicrobial alkaloid from Senna racemosa. Fitoterapia. 2000;71:690–2. doi: 10.1016/s0367-326x(00)00152-0. [DOI] [PubMed] [Google Scholar]
- 4.Viegas C, Jr, da S Bolzani V, Furlan M, Barreiro EJ, Young MC, Tomazela D, et al. Further bioactive piperidine alkaloids from the flowers and green fruits of cassia spectabilis. J Nat Prod. 2004;67:908–10. doi: 10.1021/np0303963. [DOI] [PubMed] [Google Scholar]
- 5.Freitas RM, Souza FC, Viana GS, Fonteles MM. Acetylcholinesterase activities in hippocampus, frontal cortex and striatum of Wistar rats after pilocarpine-induced status epilepticus. Neurosci Lett. 2006;399:76–8. doi: 10.1016/j.neulet.2006.01.028. [DOI] [PubMed] [Google Scholar]
- 6.Sunil KK, Müller K. Inhibition of leukotriene biosynthesis and lipid peroxidation in biological models by the extract of Cassia fistula. Phytother Res. 1998;12:465–531. [Google Scholar]
- 7.Simonié A, Laginja J, Varljen J, Zupan G, Erakovié V. Lithium plus pilocarpine induced status epilepticus-biochemical changes. Neurosci Res. 2000;36:157–66. doi: 10.1016/s0168-0102(99)00120-0. [DOI] [PubMed] [Google Scholar]
- 8.Freitas RM, Souza FC, Vasconcelos SM, Viana GS, Fonteles MM. Oxidative stress in the hippocampus after status epilepticus in rats. FEBS J. 2005;272:1307–12. doi: 10.1111/j.1742-4658.2004.04537.x. [DOI] [PubMed] [Google Scholar]
- 9.Lorenzi H, Matos FJ. Instituto Plantarum, Nova Odessa; 2002. Plantas Medicinais do Brasil Nativas e Exóticas; p. 291. [Google Scholar]
- 10.Carlini EA. Plants and the central nervous system. Pharmacol Biochem Behav. 2003;75:501–12. doi: 10.1016/s0091-3057(03)00112-6. [DOI] [PubMed] [Google Scholar]
- 11.Mahato SB, Sen S. Advances in triterpenoid research 1990-1994. Phytochemistry. 1997;44:1185–1236. doi: 10.1016/s0031-9422(96)00639-5. [DOI] [PubMed] [Google Scholar]
- 12.Almeida RN, Falcão AC, Diniz RS, Quintans-Júnior LJ, Polari RM, Barbosa-Filho JM, et al. Metodologia para avaliação de plantas com atividade no sistema nervoso central e alguns dados experimentais. Rev Bras Farm. 1999;80:72–6. [Google Scholar]
- 13.Asakura W, Matsumoto K, Ohta H, Watanbe H. Effects of alpha 2-adrenergic drugs on REM sleep deprivation-induced increase in swimming activity. Pharmacol Biochem Behav. 1993;46:111–5. doi: 10.1016/0091-3057(93)90325-n. [DOI] [PubMed] [Google Scholar]
- 14.Perez RM, Perez JA, Garcia LM, Sossa H. Neuropharmacological activity of Solanum nigrum fruit. J Ethnopharmacol. 1998;62:43–8. doi: 10.1016/s0378-8741(98)00059-2. [DOI] [PubMed] [Google Scholar]
- 15.Smith M, Wilcox KS, White HS. Discovery of antiepileptic drugs. Neurotherapeutics. 2007;4:12–7. doi: 10.1016/j.nurt.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.File SE, Pellow S. Intrinsic actions of the benzodiazepine receptor antagonist Ro 15-1788. Psychopharmacology (Berl) 1986;88:1–11. doi: 10.1007/BF00310505. [DOI] [PubMed] [Google Scholar]
- 17.Lehmann J, Hutchison A, McPherson SE, Mondadori C, Schmutz M, Sinton CM, et al. CGS 19755 a selective and competitive N-Metil-D-aspartate-type excitatory amino acid receptor antagonist. J Pharmacol Exp Ther. 1988;246:65–75. [PubMed] [Google Scholar]
- 18.Bum EN, Schmutz M, Meyer C, Rakotonirina A, Bopelet M, Portet C, et al. Anticonvulsant properties of the methanolic extract of Cyperus articulatus (Cyperaceae) J Ethnopharmacol. 2001;76:145–50. doi: 10.1016/s0378-8741(01)00192-1. [DOI] [PubMed] [Google Scholar]
- 19.Morais LC, Quintans-Júnior LJ, Franco CI, Almeida JR, Almeida RN. Antiparkinsonian-like effects of Plumbago scandens on tremorine-induced tremors methodology. Pharmacol Biochem Behav. 2004;79:745–9. doi: 10.1016/j.pbb.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Netto SM, Warela RW, Fechine MF, Queiroga MN, Quintans-Júnior LJ. Anxiolytic-like effect of Rauvolfia ligustrina willd. Ex Roem. and Schult. Apocynaceae, in the elevated plus-maze and hole-board tests. Braz J Pharmacogn. 2009;19:888–92. [Google Scholar]
- 21.Freire CM, Marques MO, Costa M. Effects of seasonal variation on the central nervous system activity of Ocimum gratissimum L. essential oil. J Ethnopharmacol. 2006;105:161–6. doi: 10.1016/j.jep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 22.Leite MP, Fassin JJ, Baziloni EM, Almeida RN, Mattei R, Leite JR. Behavioral effects of essential oil of Citrus aurantium L.inhalation in rats. Braz J Pharmacogn. 2008;18:661–6. [Google Scholar]
- 23.Quintans-Júnior LJ, Souza TT, Leite BS, Lessa NM, Bonjardim LR, Santos MR, et al. Phythochemical screening and anticonvulsant activity of Cymbopogon winterianus jowitt (Poaceae) leaf essential oil in rodents. Phytomedicine. 2008;15:619–24. doi: 10.1016/j.phymed.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 24.Dallmeier K, Carlini EA. Anesthetic, hypotermic, myorelaxant and anticonvulsant effects of synthetic eugenol derivatives and natural analogues. Pharmacology. 1981;22:113–27. doi: 10.1159/000137479. [DOI] [PubMed] [Google Scholar]
- 25.Olayiwola G, Obafemi CA, Taiwo FO. Synthesis and neuropharmacological activity of some quinoxalinone deriv African. J Biotechnol. 2007;6:777–86. [Google Scholar]
- 26.Nicoll RA. Introduction to the pharmacology of the central nervous system (CNS) In: Katzung BG, editor. Basic and Clinical Pharmacology. 8th ed. New York: McGraw-Hill; 2001. p. 351. [Google Scholar]
- 27.Rang HP, Dale MM, Ritter JM, Moore PK. 5th ed. Edinburgh: Churchill Livingstone; 2003. Pharmacology; p. 585. [Google Scholar]
- 28.De Sarro A, Cecchetti V, Fravolini V, Naccari F, Tabarrini O, De Sarro G. Effects of novel 6-desfluoroquinolones and classic quinolones on pentylenetetrazole-induced seizures in mice. Antimicrob Agents Chemother. 1999;43:1729–36. doi: 10.1128/aac.43.7.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Westmoreland BF, Benarroch EE, Dube JR, Regan TJ, Sandok BA. Rochester: Mayo Foundation; 1994. Medical Neurosciences; p. 307. [Google Scholar]
- 30.Pedersen ME, Vestergaard HT, Hansen SL, Bah S, Diallo D, Jäger AK. Pharmacological screening of Malian medicinal plants used against epilepsy and convulsions. J Ethnopharmacol. 2009;121:472–5. doi: 10.1016/j.jep.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 31.Löscher W, Schmidt D. New horizons in the development of antiepileptic drugs: Innovative strategies. Epilepsy Res. 2006;69:183–272. doi: 10.1016/j.eplepsyres.2006.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oliveira FA, de Almeida RN, Sousa MF, Barbosa-Filho JM, Diniz SA, Medeiros IA. Anticonvulsant properties of N-salicyloyltryptamine in mice. Pharmacol Biochem Behav. 2001;68:199–202. doi: 10.1016/s0091-3057(00)00484-6. [DOI] [PubMed] [Google Scholar]


