Abstract
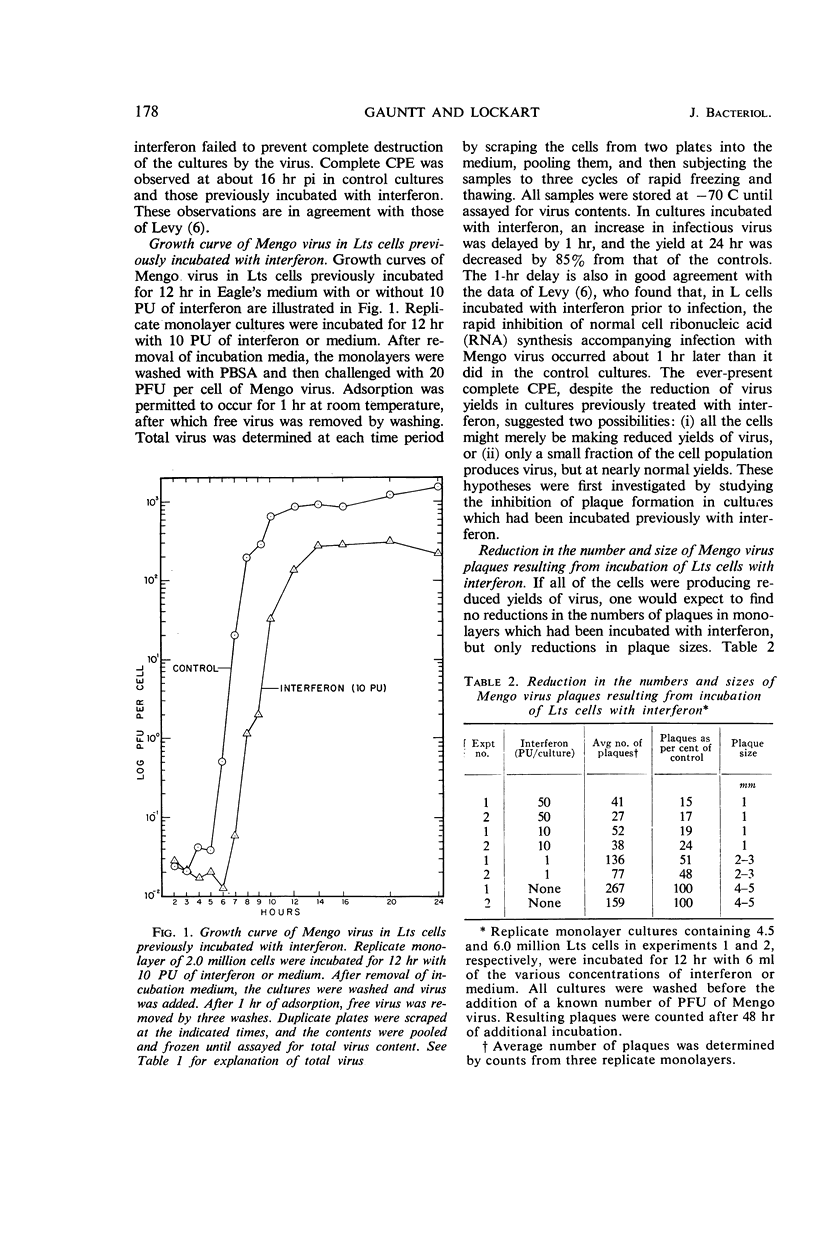
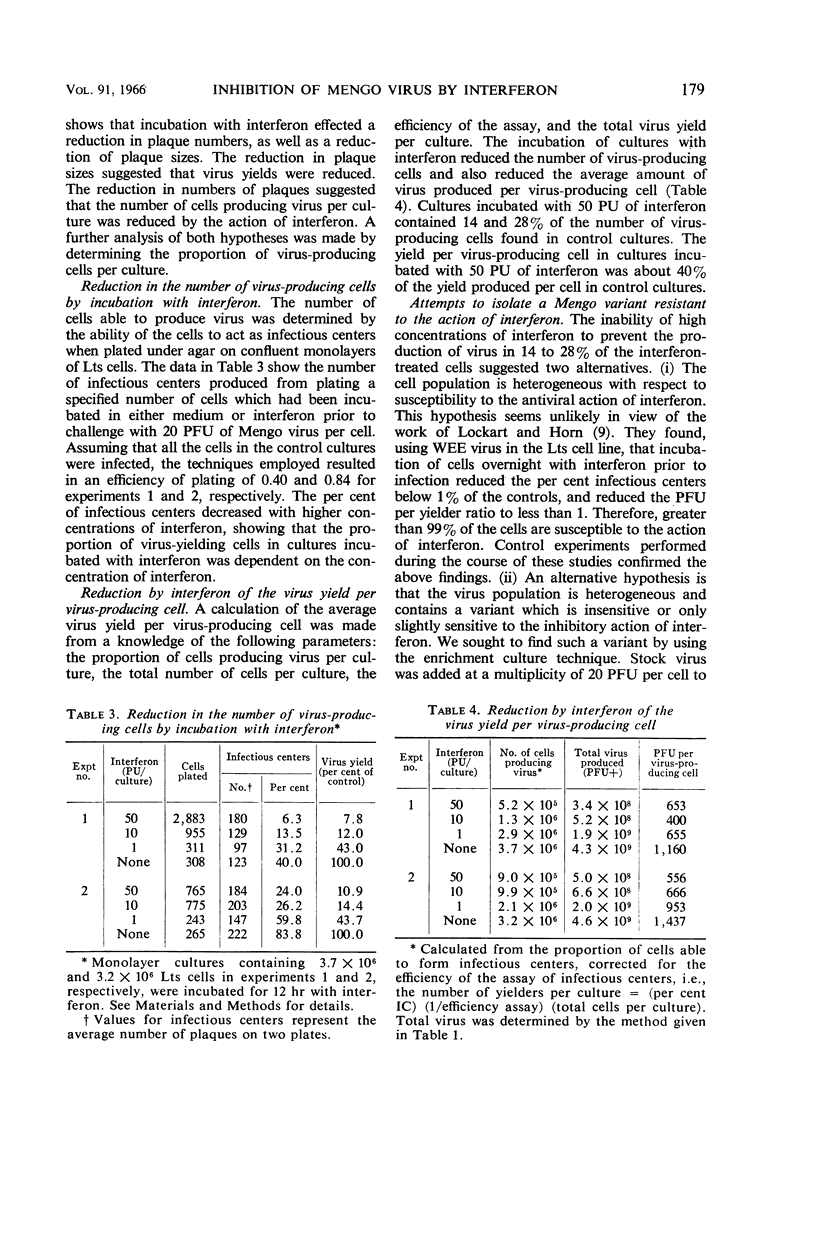
Gauntt, Charles J. (The University of Texas, Austin), and Royce Z. Lockart, Jr. Inhibition of Mengo virus by interferon. J. Bacteriol. 91:176–182. 1966.—The inhibition of Mengo virus replication in L cells resulting from interferon was studied quantitatively. Interferon was titrated on L cells with Western equine encephalomyelitis (WEE) virus as the challenge virus. One protective unit (PU) of interferon is the least amount of interferon which prevents cytopathic effects when a large multiplicity of WEE virus is added subsequent to overnight incubation with interferon. Ten PU of interferon reduced the yields of Mengo virus by about 90%. Larger doses of interferon, up to 220 PU, caused no further reduction in the amount of virus produced. Plaque formation by Mengo virus was also reduced in number by about 85 to 90%, but could not be further reduced. The plaques which formed on interferon-treated cells were reduced in size. We were unable to obtain a virus population with increased resistance to interferon action by use of five successive growth cycles in interferon-treated cultures. Analysis of the cell population for the proportion of cells able to act as infectious centers revealed that incubation of cells with 10 PU of interferon decreased the proportion of virus-yielding cells by 80%. The yield of virus per virus-producing cell was decreased by 40 to 60%. Despite the reduction in yields, plaques, and infectious centers resulting from interferon, all doses of interferon failed to prevent the complete destruction of the cells. Experiments with puromycin indicated that the cytopathic effects observed in L cells infected with Mengo virus required that a virus-directed protein be synthesized between 4 and 5 hr postinfection. The evidence suggested, therefore, that the Mengo virus genome was able to code for new protein synthesis in the absence of the production of infectious virus.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BABLANIAN R., EGGERS H. J., TAMM I. STUDIES ON THE MECHANISM OF POLIOVIRUS-INDUCED CELL DAMAGE. II. THE RELATION BETWEEN POLIOVIRUS GROWTH AND VIRUS-INDUCED MORPHOLOGICAL CHANGES IN CELLS. Virology. 1965 May;26:114–121. doi: 10.1016/0042-6822(65)90031-0. [DOI] [PubMed] [Google Scholar]
- Balandin I. G., Franklin R. M. The effect of mengovirus infection on the activity of the DNA-dependent RNA polymerase of L-cells. II. Preliminary data on the inhibitory factor. Biochem Biophys Res Commun. 1964 Feb 18;15(1):27–32. doi: 10.1016/0006-291x(64)90097-x. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. One-step growth curve of Western equine encephalomyelitis virus on chicken embryo cells grown in vitro and analysis of virus yields from single cells. J Exp Med. 1954 Feb;99(2):183–199. doi: 10.1084/jem.99.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKLIN R. M., BALTIMORE D. Patterns of macromolecular synthesis in normal and virus-infected mammalian cells. Cold Spring Harb Symp Quant Biol. 1962;27:175–198. doi: 10.1101/sqb.1962.027.001.019. [DOI] [PubMed] [Google Scholar]
- LEVY H. B. STUDIES ON THE MECHANISM OF INTERFERON ACTION. II. THE EFFECT OF INTERFERON ON SOME EARLY EVENTS IN MENGO VIRUS INFECTION IN L CELLS. Virology. 1964 Apr;22:575–579. doi: 10.1016/0042-6822(64)90079-0. [DOI] [PubMed] [Google Scholar]
- LOCKART R. Z., Jr, HORN B. INTERACTION OF AN INTERFERON WITH L CELLS. J Bacteriol. 1963 May;85:996–1002. doi: 10.1128/jb.85.5.996-1002.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOCKART R. Z., Jr PRODUCTION OF AN INTERFERON BY L CELLS INFECTED WITH WESTERN EQUINE ENCEPHALOMYELITIS VIRUS. J Bacteriol. 1963 Mar;85:556–566. doi: 10.1128/jb.85.3.556-566.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAGNER R. R., LEVEY A. H., SNYDER R. M., RATCLIFF G. A., Jr, HYATT D. F. BIOLOGIC PROPERTIES OF TWO PLAQUE VARIANTS OF VESICULAR STOMATITIS VIRUS (INDIANA SEROTYPE). J Immunol. 1963 Jul;91:112–122. [PubMed] [Google Scholar]



