Abstract
Background:
Fine needle aspiration cytology (FNAC) of oral and maxillofacial region has not been widely utilized for diagnosis due to diversity of lesion types, heterogeneity of cell populations and difficulties in reaching and aspirating these lesions.
Aim:
Our aim was to demonstrate the effectiveness of this cheap and simple procedure for the diagnosis of tumor and tumor like lesions of oral and maxillofacial region. In addition, we sought to highlight probable causes of errors in the cases showing lack of correlation between cytological and histological diagnoses.
Materials and Methods:
The study was conducted on 50 patients of all age groups with various palpable lesions in the oromaxillofacial region. A comparison between cytological and histological diagnosis was done wherever biopsy material was available.
Results:
The rate of unsatisfactory FNA was 4%. There were six false negative cases but no false positive case. The sensitivity of our study ranged from 77.7 to 75% including and excluding the suspicious cases, respectively. Specificity and positive predictive value was 100%.
Conclusion:
FNAC is a minimally invasive, highly accurate and cost-effective procedure for the assessment of patients with oromaxillofacial lesions. When applied in a proper manner, FNAC can help avoid a surgical biopsy in many cases.
Keywords: Fine needle aspiration cytology, oral and maxillofacial lesions, pitfalls
Introduction
The role of fine needle aspiration cytology/biopsy (FNAC/FNAB) in the diagnostic evaluation of neoplastic and non-neoplastic lesions has increased dramatically. This safe, reliable, cost-effective and easy procedure can eliminate the need for open biopsy procedure, with its potential untoward effects.[1]
FNAC of head and neck region was pioneered by Martin in the early 1930s.[2] There is a relatively large volume of literature documenting the effectiveness of FNA for diagnosis of head and neck and salivary gland lesions. Few reports, however, explore the potential of FNA for the diagnosis of intraoral and lesions of maxillofacial region.[3] Diversity of lesion types, heterogeneity of cell population, difficulties in reaching and aspirating the lesion, and rarity of this type of lesion make the cytological diagnosis of oromaxillofacial lesions difficult. The need for an expert cytopathologist, who is able to evaluate samples, may also act as a limitation in this region.[1] FNAC is a technique that allows more rapid diagnosis, and if necessary, a re-aspiration can be done quickly at the time of initial testing.[4]
The fundamental indication for FNAC is a lesional mass that is palpable or visible by a radiological imaging method. This technique may also assist in establishing a specific diagnosis for radiolucent lesions of the jaw. The thinning or destruction of cortical bone permits the use of thin needles to aspirate such abnormalities.
The present study was conducted to find out the role of cytology in accurate diagnosis and pitfalls of cytology in lesions of oromaxillofacial region.
Materials and Methods
The study was conducted on 50 patients of both sexes and all age groups, with clinically diagnosed tumors and tumor like conditions of oral and maxillofacial region, in the Department of Pathology.
The FNAC was performed using 21–25 G needle and a 20-ml syringe without local anesthetic. The lesional site was cleaned using povidone-iodine solution and ethyl alcohol (spirit). The needle was inserted into the lesion and aspirate from different portions of the mass lesion was collected by altering the direction of the needle inside the mass and by giving multiple passes. The material so obtained was smeared onto glass slide, fixed in 95% ethyl alcohol for hematoxylin and Eosin (H and E) staining and air dried for Romanowsky (Leishman) staining. Other stains were used in accordance with the type of lesion and requirement. Cytopathological diagnosis was made and correlated with histopathological diagnosis wherever possible.
Results
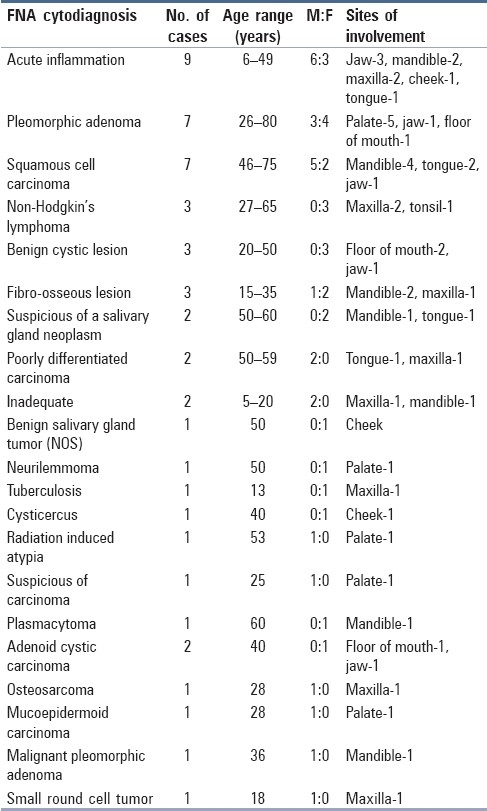
FNA was performed on 50 patients ranging in age from 5 to 80 years, with maximum number of cases in the age group 21–30 years. The male:female ratio was 1.1:1. Out of 50 cases, a definite positive diagnosis of malignancy or benignancy was given in 45 cases (90%). Of these 45 cases, 21 were proved malignant and 19 were benign on biopsy. For five cases, biopsy was not available. On cytology, three cases were signed out as suspicious for malignancy which turned out to be malignant on biopsy. Two cases were unsatisfactory because of inadequate material.
The diagnosis of acute inflammatory lesion/inflammatory change in lesion was the commonest (18.7%). Another common diagnosis was pleomorphic adenoma (PA) (14.5%) with palate being the commonest site (71%).
Squamous cell carcinoma was the most common malignancy (7 cases, 14.5%), with M:F ratio of 5:2. The sites involved were mandible (57%), tongue (28%) and jaw (14%) [Table 1].
Table 1.
Fine needle aspiration cytological diagnosis with respect to site of involvement and age, sex distribution of cases
Tables 2 and 3 show a comparison between benign and malignant cases given on FNA with their respective histological diagnoses. No false positivity was reported in this study. All 14 cases diagnosed as malignant on FNA were proved malignant on biopsy. Three cases which were reported suspicious of malignancy were also confirmed on histopathology as malignant, giving an overall diagnostic accuracy of 100% for positive malignant FNA. Of the 27 benign diagnoses on FNA, six were false negative (22.2%) and had a subsequent diagnosis of malignancy on histopathology. The specific histopathological diagnosis rendered in these cases was adenoid cystic carcinoma (ACC) (3), polymorphous low grade adenocarcinoma (PLGA) (1), malignant peripheral nerve sheath tumor (1) and metastatic breast carcinoma (1). ACC was misdiagnosed as PA due to the presence of little nuclear pleomorphism and scanty number of hyaline globules. The cytology smears of PLGA were richly cellular with moderate to abundant cytoplasm, round nuclei, fine chromatin and inconspicuous nucleoli.
Table 2.
Benign diagnoses based on fine needle aspiration report and comparison with histological diagnosis
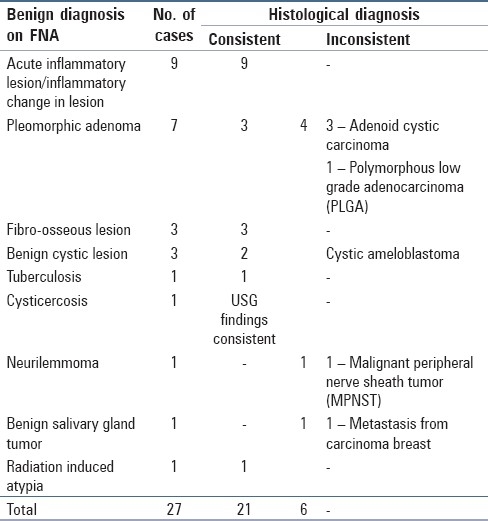
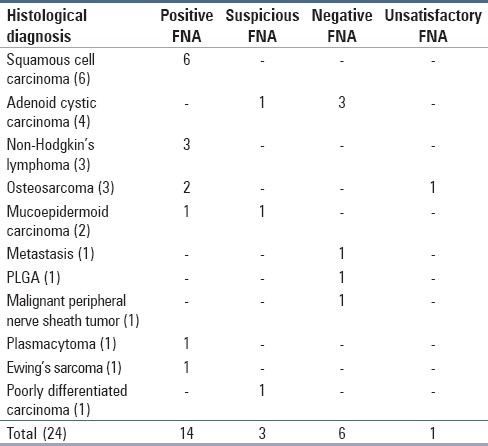
Table 3.
Histological diagnoses of malignancies and fine needle aspiration results
Sensitivity in our study ranged from 77.7 to 75% including and excluding the suspicious cases, respectively. Specificity and accuracy/positive predictive value were 100% [Table 4].
Table 4.
Efficacy of fine needle aspiration cytology in lesions of oral and maxillofacial region

Discussion
FNA of 50 patients with various palpable lesions in the oral cavity and jaw bones was done. Forty-eight (96%) aspirations were considered satisfactory, one was considered suboptimal due to hypocellularity and one was unsatisfactory consisting of only blood. Biopsy findings were available in 45 cases. In three cases, no biopsy was available but clinicoradiological findings correlated with FNA diagnosis.
FNA findings in 42 out of 48 cases correlated with histological/clinicoradiological findings (87.5%). Of the 27 cases diagnosed as benign lesion on FNA, 6 were finally diagnosed malignant, giving a false negativity of 22.2% [Table 2]. Nineteen of 26 histologically/clinicoradiologically malignant lesions were cytologically diagnosed as positive for malignancy [Table 3]. These findings are in accordance with the findings of Castelli et al.[5]
The most commonly aspirated malignancy in our study was squamous cell carcinoma (seven cases). Gunhan et al.[1] and Cramer et al.[3] also encountered maximum cases of squamous cell carcinoma in their studies. This finding is explainable as squamous cell carcinoma accounts for the vast majority of malignancies of head and neck region.
Other commonly diagnosed malignancy in our study was non-Hodgkin's lymphoma (three cases) and was consistent on histology. Lilliemark et al.[6] has also reported that for some cases, FNA is superior to routine biopsy for the diagnosis of non-Hodgkin's lymphoma of the oral cavity.
In the present study, seven cases were diagnosed as PA cytologically; three were histologically consistent whereas three cases were diagnosed as ACC and one case as PLGA. [Table 2]. The presence of little pleomorphism in the nuclei and scanty number of hyaline globules in the cytological smears led to the misdiagnosis in our cases. The differential diagnosis between PA and ACC cannot be based solely on the stromal component. Cytological detail must be closely studied. A well-defined cytoplasm, absent or few stripped nuclei and a bland finely granular nuclear chromatin favor PA; scanty cytoplasm, a high N:C ratio, naked nuclei, nuclear molding, nuclear hyperchromasia and coarseness favor ACC.[7]
Lee et al.[8] noted that plasmacytoid appearance of individual tumor cells with abundant cytoplasm is a reliable finding in PA for differentiating it from ACC. Ustundag et al.[9] found that distinction of ACC from PA is not always easy as myxoid acellular material may occur in both and globules of basement membrane material so characteristic of ACC can sometimes be seen in PA.
A case of palatal mass in a 30-year-old man was diagnosed as PA on FNAC. However, on histology, diagnosis of PLGA was made. Sahai et al.,[10] in their study on cytology of tumors of minor salivary glands, mentioned that cytodiagnosis and differentiation of PLGA from various other tumors remains a challenge as their cytological findings are not well characterised. On retrospective reviewing of cytological findings in our case, few important findings were noted. The smears are richly cellular containing a large number of cell clusters arranged in sheets or in a branching papillary pattern. Tumor cells had moderate to abundant cytoplasm, round nuclei, fine chromatin and inconspicuous nucleoli. Nuclear pleomorphism was minimal [Figures 1 and 2].
Figure 1.
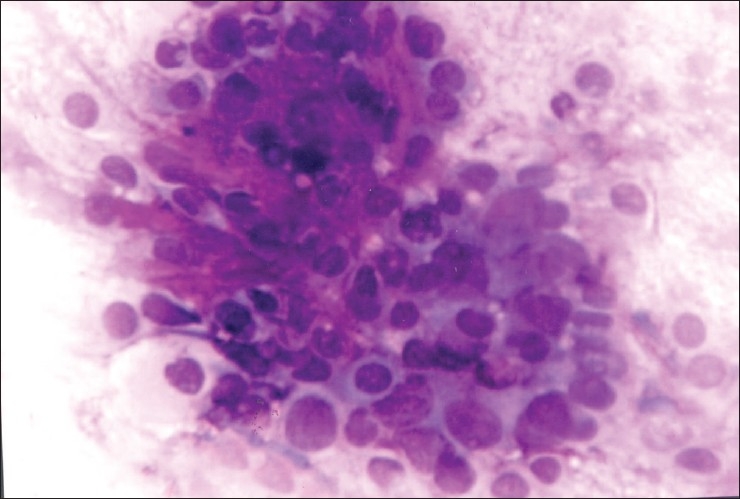
FNA smear of polymorphous low grade adenocarcinoma showing cluster of small round to oval cells in the background of fibromyxoid stroma which was misdiagnosed as pleomorphic adenoma (Leishman stain, ×400)
Figure 2.
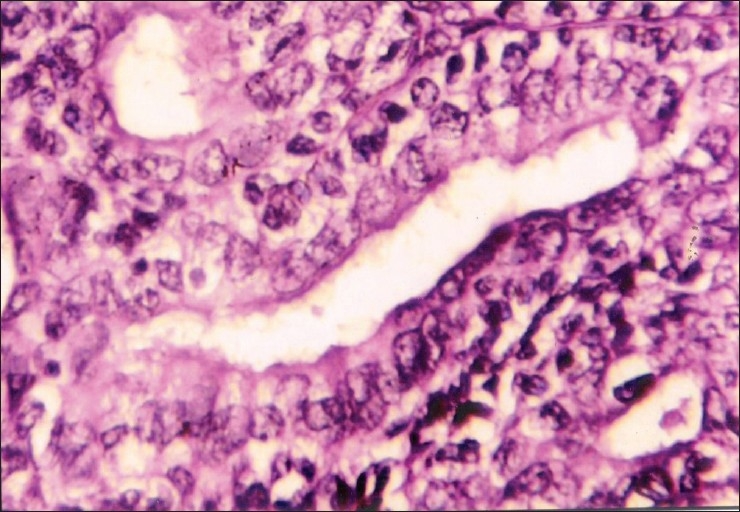
Corresponding histopathology section of PLGA (H and E, ×400)
Wantanabe et al.,[11] in addition to the above findings, also found that palisading of tumor cells around myxoid stroma situated in the centre of cell clusters is useful in diagnosis of PLGA.
FNAC can play an important role in the diagnosis of oral lesions especially for those present as a mass or growth. Tongue swellings and growths are easily accessible to FNA.[12]
There were two cases of metastasis in the oral cavity and maxillofacial region. The first case was large cell carcinoma of lung metastatic to tongue. In another case, a 50-year-old female with previous history of surgery for carcinoma breast presented with a swelling in the mandible and cheek. With no radiological information available and low cellularity on FNAC, a diagnosis of salivary gland neoplasm was made. Subsequent biopsy of the case showed metastasis from carcinoma breast involving mandible and extending into soft tissues.
Tumors metastatic to the oral region usually involve jaw bones rather than oral soft tissue. While breast is the most common primary site of tumors metastasising to the jaw bones, lung is the most common source of metastasis to oral soft tissues.[3]
A case of 60-year-old female with mandibular swelling was diagnosed as plasmacytoma on FNAC. Jaw lesions, though not uncommon, rarely present as the first sign in multiple myeloma and involve the mandible more frequently than maxilla.[13]
The presence of inflammatory cells, histiocytes, cellular debris characterized the inflammatory lesion in nine aspirates. Histologically, four of them proved to be cystic lesions, rest were non-specific inflammatory lesions. All these four cystic lesions were odontogenic cysts: Two were benign radicular cysts and the other two were dentigerous cyst and keratocyst each.
Ramzy et al.[14] reported that cysts of the jaw bones may pose a problem in differential diagnosis. The exact locations, history and radiological appearances must be considered before a definitive cytological diagnosis is made. They noted the presence of anucleated and superficial squamous cells in aspirates of odontogenic keratocysts and less so in follicular cyst. When infected, it is difficult to diagnose odontogenic cysts specifically.
In one case, aspiration showed few clusters of benign epithelial cells and macrophages against a background of mucoid fluid. A cytological diagnosis of benign cystic lesion was given. It histologically proved to be ameloblastoma. Review of the cytology smears showed clusters of basaloid cells revealing peripheral palisading [Figures 3 and 4].
Figure 3.
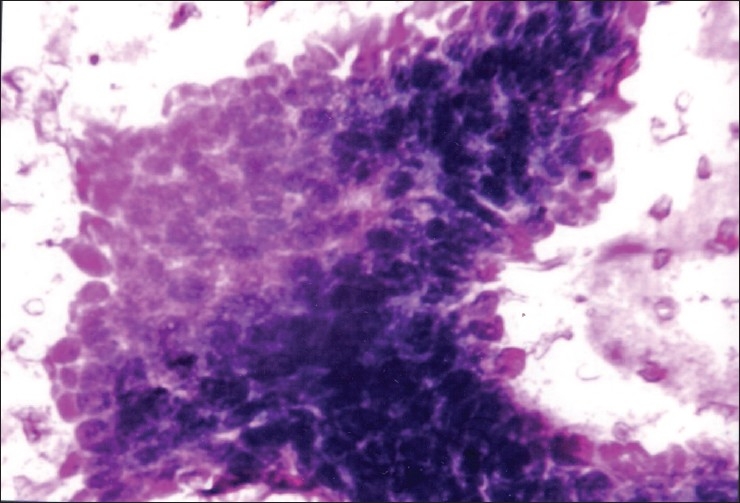
FNA smear of cystic ameloblastoma showing cluster of basaloid cells with peripheral palisading of nuclei (Leishman, ×400)
Figure 4.
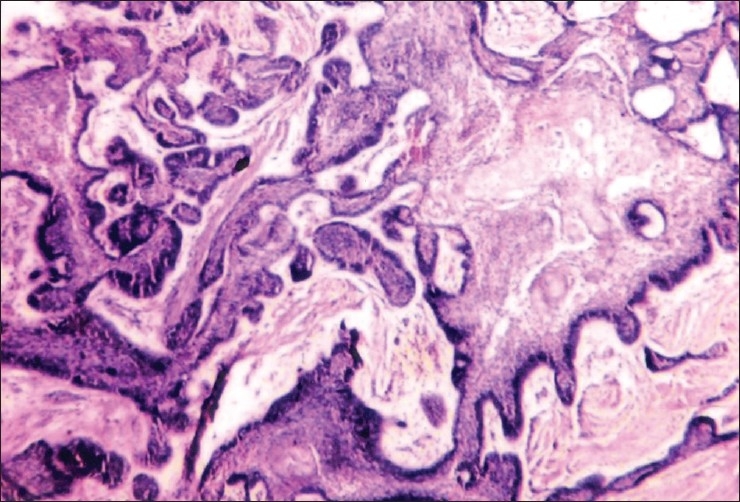
Histopathology from the same case (H and E, ×100)
Deshpande et al.[15] stated that in benign cystic jaw lesions and cystic ameloblastoma, a background of neutrophils, foamy macrophages and amorphous proteinaceous material may be seen and poses diagnostic problems. The characteristic combination of basaloid, stellate and polygonal and squamous cells helps to differentiate benign cystic jaw lesions from ameloblastoma.
The rate of unsatisfactory FNA in our study was 4% (two cases). Maxilla and mandible were the sites of aspiration in these cases. Gunhan et al.[1] also observed that even though thinning or destruction of cortical bone permits the use of fine needles, inadequate sampling is frequent in such lesions. The high number of insufficient aspirations which decreased with experience may be related to the peculiar anatomic structure of oral and maxillofacial tissues.
There were six false negative cases but no false positive case in our study. The sensitivity of our study ranged from 77.7 to 75% including and excluding the suspicious cases, respectively. Specificity and positive predictive value was 100% [Table 4].
Mondal et al.[16] studied 56 parapharyngeal masses over a period of 3.5 years. The overall diagnostic accuracy of FNAC was 98.8%, with sensitivity and specificity being 96 and 99%, respectively.
The sensitivity and specificity of the study conducted by Castelli et al.[5] was 80.6 and 96.9%, respectively. They suggested that inadequate sampling might be the most common source of false negative FNABs.
Conclusion
FNAC is a highly accurate procedure for differentiating benign and malignant lesions. However, specific cytological diagnosis may be difficult to make in the absence of characteristic architectural patterns. Diagnosis of aspirates from cystic lesions may be less specific than the solid lesions due to paucity of specific lesional cells in the former and also due to superimposed infection.
Based on overall results and rarity of false positive FNA results in our study, we support the use of FNA in diagnosing lesions of oral and maxillofacial region.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Gunhan O, Dogan N, Celasun B, Sengun O, Onder T, Finci R. Aspiration cytology of oral cavity and jaw bone lesions.A report of 102 cases. Acta Cytol. 1993;37:135–41. [PubMed] [Google Scholar]
- 2.Martin HE, Ellis EB. Biopsy by needle puncture and aspiration. Ann Surg. 1930;92:169–81. doi: 10.1097/00000658-193008000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cramer H, Lampe H, Downing P. Intraoral and transoral fine needle aspiration.A review of 25 cases. Acta Cytol. 1995;39:683–8. [PubMed] [Google Scholar]
- 4.Scher RL, Oostingh PE, Levine PA, Cantrell RW, Feldman PS. Role of fine needle aspiration in the diagnosis of lesions of the oral cavity, oropharynx, and nasopharynx. Cancer. 1988;62:2602–6. doi: 10.1002/1097-0142(19881215)62:12<2602::aid-cncr2820621225>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 5.Castelli M, Gattuso P, Reyes C, Solans EP. Fine needle aspiration biopsy of intraoral and pharyngeal lesions. Acta Cytol. 1993;37:448–50. [PubMed] [Google Scholar]
- 6.Lilliemark J, Tani E, Mellstedt H, Skoog L. Fine-needle aspiration cytology and immunocytochemistry of malignant non-Hodgkin's lymphoma in the oral cavity. Oral Surg Oral Med Oral Pathol. 1989;68:599–603. doi: 10.1016/0030-4220(89)90247-8. [DOI] [PubMed] [Google Scholar]
- 7.Orell SR, Sterret GF, Whitaker D, Klijanienko J. Head and Neck; salivary glands. In: Orell SR, Sterett GF, Whitaker D, editors. Fine needle aspiration cytology. 4th ed. USA: Churchill Livingstone; 2005. pp. 41–77. [Google Scholar]
- 8.Lee SS, Cho KJ, Jang JJ, Ham EK. Differential diagnosis of adenoid cystic carcinoma from pleomorphic adenoma of the salivary gland on fine needle aspiration cytology. Acta Cytol. 1996;40:1246–52. doi: 10.1159/000333988. [DOI] [PubMed] [Google Scholar]
- 9.Ustundag E, Iseri M, Aydin O, Dal H, Almac A, Paksoy N. A denoid cystic carcinoma of tongue. J Laryngol Otol. 2000;114:477–80. doi: 10.1258/0022215001905922. [DOI] [PubMed] [Google Scholar]
- 10.Sahai K, Kapila K, Dahiya S, Verma K. Fine needle aspiration cytology of minor salivary gland tumours of the palate. Cytopathology. 2002;13:309–16. doi: 10.1046/j.1365-2303.2002.00429.x. [DOI] [PubMed] [Google Scholar]
- 11.Wantanabe K, Ono N, Saito K, Saito A, Suzuki T. Fine-needle aspiration cytology of polymorphous low-grade adenocarcinoma of the tongue. Diagn Cytopathol. 1999;20:167–9. doi: 10.1002/(sici)1097-0339(199903)20:3<167::aid-dc11>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 12.Patil R, Nayak S, Munshi M, Bobhate S. Scrape cytology in rare case of hairy tongue. J Cytol. 2009;26:91–3. doi: 10.4103/0970-9371.55232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehdi G, Ansari HA, Haider N. Cytological diagnosis of multiple myeloma presenting as a jaw swelling. J Cytol. 2009;26:80–2. doi: 10.4103/0970-9371.55228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramzy I, Aufdemorte TB, Duncan DL. Diagnosis of radiolucent lesions of jaw by fine needle aspiration biopsy. Acta Cytol. 1985;29:419–24. [PubMed] [Google Scholar]
- 15.Deshpande A, Umap P, Munshi M. Granular cell ameloblastoma of the jaw.A report of two cases with fine needle aspiration cytology. Acta Cytol. 2000;44:81–5. doi: 10.1159/000326231. [DOI] [PubMed] [Google Scholar]
- 16.Mondal P, Basu N, Gupta SS, Bhattacharya N, Mallick MG. Fine needle aspiration cytology of parapharyngeal tumors. J Cytol. 2009;26:102–4. doi: 10.4103/0970-9371.59395. [DOI] [PMC free article] [PubMed] [Google Scholar]


