Abstract
Objectives
To compare patients' preference for transdermal fentanyl or sustained release oral morphine, their level of pain control, and their quality of life after treatment.
Design
Randomised, multicentre, international, open label, crossover trial.
Setting
35 centres in Belgium, Canada, Denmark, Finland, the United Kingdom, the Netherlands, and South Africa.
Participants
256 patients (aged 26-82 years) with chronic non-cancer pain who had been treated with opioids.
Main outcome measures
Patients' preference for transdermal fentanyl or sustained release oral morphine, pain control, quality of life, and safety assessments.
Results
Of 212 patients, 138 (65%) preferred transdermal fentanyl, whereas 59 (28%) preferred sustained release oral morphine and 15 (7%) expressed no preference. Better pain relief was the main reason for preference for fentanyl given by 35% of patients. More patients considered pain control as being “good” or “very good” with fentanyl than with morphine (35% v 23%, P=0.002). These results were reflected in both patients' and investigators' opinions on the global efficacy of transdermal fentanyl. Patients receiving fentanyl had on average higher quality of life scores than those receiving morphine. The incidence of adverse events was similar in both treatment groups; however, more patients experienced constipation with morphine than with fentanyl (48% v 29%, P<0.001). Overall, 41% of patients experienced mild or moderate cutaneous problems associated with wearing the transdermal fentanyl patch, and more patients withdrew because of adverse events during treatment with fentanyl than with morphine (10% v 5%). However, within the subgroup of patients naive to both fentanyl and morphine, similar numbers of patients withdrew owing to adverse effects (11% v 10%, respectively).
Conclusion
Transdermal fentanyl was preferred to sustained release oral morphine by patients with chronic non-cancer pain previously treated with opioids. The main reason for preference was better pain relief, achieved with less constipation and an enhanced quality of life.
What is already known on this topic
The clinical use of potent opioids in the treatment of chronic non-cancer pain is supported by retrospective, survey data and small randomised controlled trials showing efficacy and safety
Studies with transdermal fentanyl have shown efficacy and preference over sustained release oral morphine in the treatment of cancer pain
What this study adds
This is the first study to provide comparative data supporting treatment options with potent opioids for chronic non-cancer pain
Both transdermal fentanyl and sustained release oral morphine provided effective and well tolerated pain relief
During fentanyl treatment patients experienced superior pain relief, higher quality of life, and less constipation; fentanyl was preferred to morphine by 65% of patients
Introduction
In 1998 the World Health Organization survey of nearly 26 000 patients from five continents reported that 22% had had persistent pain sometime over the previous year.1 Pain is one of the commonest reasons for visiting a doctor and, when pain is chronic, the multimillion healthcare costs are only exceeded by the multibillion costs of work loss, disability, and social welfare benefits. Global socioeconomic costs at one end of the spectrum are matched by personal suffering at the other. Pain is often undertreated or mistreated, with patients going from doctor to doctor for relief and finally moving outside mainstream medicine in increasing numbers.2
Opioids are the mainstay of management of cancer pain, providing effective pain relief.3,4 Opioids are the most powerful analgesics, but politics, prejudice, and continuing ignorance still impede optimum prescribing.5 A review of retrospective and survey data confirms the efficacy of opioids in the treatment of chronic non-cancer pain and found that fears of addiction were not justified.6 Randomised controlled trials of intravenous opioids in chronic non-cancer pain show benefit over placebo for morphine and fentanyl, whereas oral placebo controlled trials show efficacy for codeine, morphine, and oxycodone.7–11 Worldwide, the value of opioids in this role has led to the development of management guidelines, with recommendations from national organisations.12–15
Morphine is the standard opioid against which others are judged and is usually prescribed in a sustained release oral formulation for the treatment of chronic pain.5,10 Severe constipation, a persistent complication of oral opioids, may affect some patients' quality of life more than their pain.16
Fentanyl, a lipid soluble synthetic opioid, can be delivered in a transdermal controlled release formulation, providing continuous, controlled systemic delivery of fentanyl for up to 72 hours.17 Studies with transdermal fentanyl have shown analgesic efficacy in cancer pain.18–22 Most patients preferred fentanyl, which was associated with less constipation than morphine.21,22 Also, fentanyl has been shown to relieve neuropathic pain that is relatively insensitive to opioids.8,23
Opioids are individually titrated to an effective dose. Therefore little difference would be expected between opioids in efficacy or improvements in quality of life, which is confirmed by studies in cancer pain.5,6,24,25 Recognising the increasing importance of patients' preference and choice, we investigated in a large, multicentre, two way crossover trial whether patients with chronic non-cancer pain accustomed to opioids would prefer transdermal fentanyl over sustained release oral morphine, has been found in patients with cancer pain.21 We also assessed pain control, quality of life, and adverse events.
Participants and methods
Protocol
The study was approved by local ethics committees, and patients giving written, informed consent were recruited from 35 specialist pain clinics in Belgium (15 patients), Canada (58), Denmark (67), Finland (16), the United Kingdom (65), the Netherlands (21), and South Africa (14).
Patients were invited to participate if they were aged over 18 years and had chronic non-cancer pain requiring continuous treatment with potent opioids for six weeks preceding the trial. They had to have achieved moderate pain control with a stable dose of oral opioid for seven days before the trial. Exclusion criteria included pain not responding to opioids, a history of allergy or hypersensitivity to opioids, life threatening disease, skin disease precluding the transdermal system, reduced level of consciousness, social isolation, concomitant psychiatric disorders, history of substance misuse, clinically relevant cardiac, nervous system, or respiratory disease, participation in another clinical research project, and possible pregnancy or lactation.
Treatments
Baseline assessment included recording the patients' characteristics, medical history, physical abnormalities, and vital signs. Efficacy and safety data were collected at day 7, 16, and 28 of each treatment period. Patients' pain was classified according to the International Association for the Study of Pain as nociceptive (stimulation of intact nociceptors by noxious stimuli), neuropathic (disease or trauma of the nervous system), or combined nociceptive and neuropathic (table 1).
Table 1.
Characteristics at baseline of patients receiving transdermal fentanyl or sustained release oral morphine. Values are numbers (percentages) unless stated otherwise
| Characteristic | Randomised to fentanyl first (n=126) | Randomised to morphine first (n=130) |
|---|---|---|
| Female:male | 60:66 | 60:70 |
| Mean age (range) in years | 50.9 (28-82) | 51.9 (26-82) |
| Mean weight (range) in kg | 74.3 (38-138) | 77.9 (45-130) |
| Mean height (range) in cm | 170 (144-200) | 170.5 (151-196) |
| White | 125 (99) | 126 (97) |
| Other | 1 (1) | 4 (3) |
| Mean (SE) duration of chronic pain (range) in years | 9.5 (0.74) (0.2-50) | 9.1 (0.73) (0.3-46) |
| Pain type: | ||
| Neuropathic | 31 (25) | 35 (27) |
| Nociceptive | 64 (51) | 64 (49) |
| Combined neuropathic and nociceptive | 31 (25) | 31 (24) |
| Classification of (most common) pain*: | ||
| Axis I: region | ||
| Lower back | 52 (41) | 50 (39) |
| Lower limbs | 27 (21) | 32 (25) |
| Axis II: system | ||
| Musculoskeletal or connective tissue | 51 (41) | 64 (50) |
| Nervous system | 61 (48) | 47 (36) |
| Axis III: temporal characteristics | ||
| Continuous, fluctuating severity | 52 (41) | 62 (48) |
| Continuous, non-fluctuating severity | 38 (30) | 32 (25) |
| Axis IV: intensity; time since onset | ||
| Medium (>6 months) | 27 (21) | 34 (26) |
| Mild (>6 months) | 4 (3) | 2 (2) |
| Axis V: aetiology | ||
| Degenerative or mechanical | 40 (32) | 46 (36) |
| Trauma, operation, or burns | 37 (29) | 33 (26) |
| Opioid use before trial: | ||
| Morphine or morphine sulphate | 91 (72) | 103 (79) |
| Efficacy evaluation “bad” or “very bad” | 9 (10) | 15 (15) |
According to the International Association for the Study of Pain.
The requirement for opioid was determined individually over the 24 hours before the first dose of study drug was given. Analgesic doses of fentanyl equivalent to the patients' previous opioid dose were calculated from the manufacturer's recommendations.26 Patients were treated with fentanyl patches (Durogesic, Janssen-Cilag) releasing 25, 50, 75, or 100 μg fentanyl/hr and sustained release oral morphine as 10, 30, 60, 100, or 200 mg tablets (MS Contin, Napp Laboratories). Patients receiving morphine received half the equivalent analgesic dose every 12 hours. At crossover, patients received the same opioid dose as before the study. Patients randomised to receive fentanyl first were given the first dose of morphine when the last fentanyl patch was removed. Patients randomised to receive morphine first had the first fentanyl patch applied when the last dose of morphine was given.
Patients were prescribed immediate release morphine (initially 5 mg) every four hours as needed. Patients requiring more than 60 mg of this rescue drug over two days of a three day period with fentanyl could increase their fentanyl dose. Patients receiving morphine needing more than two doses of the rescue drug per day could titrate to a higher dose of morphine.
Subject preferences—The primary efficacy variable was the patient's preference for transdermal fentanyl or sustained release oral morphine and their main reason for preference. This evaluation was completed either at the end of the trial or at the end of treatment in patients who withdrew before completion.
Pain control and treatment assessment—At each visit patients were assessed for pain control compared with the previous visit. Both investigator and patient completed a global treatment assessment at the end of each treatment period.
Rescue drug—Patients recorded their use of rescue morphine for breakthrough or incident pain. The use of the drug in the first week of each study period was excluded from the analysis, which was considered the “titration period.”
Quality of life assessment—Quality of life (SF-36) and pain intensity (0 being low and 100 high) were assessed at baseline and at the end of each treatment period.27
Safety observations—The safety evaluation at the first visit, at crossover, and at the end of the trial included a physical examination, standardised measurement of vital signs, and overall assessment of disease progression. Details of all adverse events and presumed relation to the drugs were noted by the investigator. At each visit bowel function was assessed by using a questionnaire, and the application site of the fentanyl patch was evaluated.
Statistical analyses—The primary efficacy variable was analysed with a binomial test determining whether the proportion of patients who either “preferred” or “very much preferred” a treatment was larger than 0.5. Differences in personal variables at baseline between treatment groups were analysed with the Van Elteren test for continuous variables and the Cochran-Mantel Haenszel test for categorical variables, both adjusted for country. The mean daily dose of rescue drug, assessment of global treatment, assessment of pain control, and quality of life scores were compared with the Koch non-parametric paired analysis for crossover designs and adjusted for country.28 Order effects were assessed by adding an interaction variable for treatment sequence and were excluded if P>0.10. All other P values less than 0.05 were considered significant.
Assignment—Patients were assigned to treatment groups by using the central randomisation minimisation technique.29 One group was randomised to four weeks of treatment with sustained release oral morphine followed by transdermal fentanyl for four weeks. The second group received the same treatments but in reverse order. A washout period was considered unethical.
Results
Participant flow
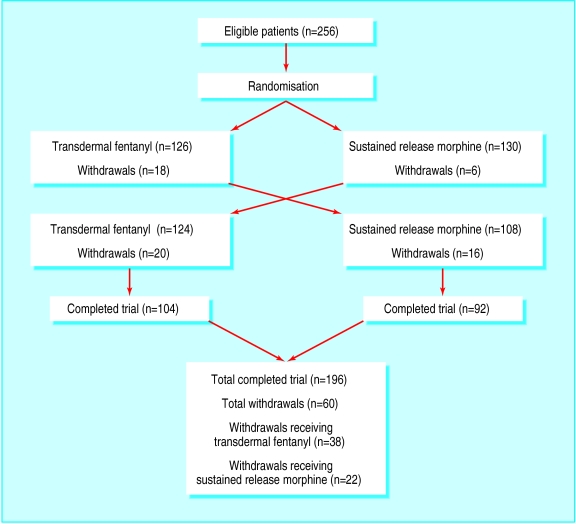
The figure shows the progress of patients through the study. Sixty patients withdrew; 37 because of adverse events, five because of insufficient efficacy, and 18 for other reasons. Five patients without baseline data were excluded from the efficacy analysis. All patients were included in the safety analysis.
Baseline characteristics, including type of pain and patients' previous use of opioids, did not differ between the two groups (tables 1 and 2). The mean starting dose of transdermal fentanyl was 39.7 μg/hr (range 25-200 μg/hr) and of sustained release oral morphine 123.0 mg/24 hr (range 10-700 mg/24 hr). The mean dose of fentanyl at the end of the study was 57.3 μg/hr (range 0-325 μg/hr) and of morphine 133.1 mg/24 hrs (range 0-800 mg/24 hrs).
Analysis
Patient preference
Preference could not be assessed in 39 of 251 patients, leaving a total of 212 patients for analysis. A higher proportion of patients preferred or very much preferred transdermal fentanyl to oral sustained release morphine (138 (65%) v 59 (28%); P<0.001) (table 3). Fifteen patients (7%) did not express a preference. After exclusion of 24 patients with a “bad” or “very bad” score while taking morphine before the study, 69% of patients expressed a “strong” or “very strong” preference for fentanyl.
Table 3.
Patients' preferences for transdermal fentanyl or sustained release oral morphine. Values are numbers (percentages) unless stated otherwise
| Preference | Randomised to fentanyl first (n=124) | Randomised to morphine first (n=127) | Overall (n=212) | Reasons for preference (%) |
|---|---|---|---|---|
| Strong preference for fentanyl | 41 (41) | 43 (38) | 138* (65) | Better pain relief (35), fewer adverse events (14), more convenient (15), other (1) |
| Preference for fentanyl | 13 (13) | 41 (36) | ||
| No preference | 12 (12) | 3 (3) | 15 (7) | |
| Preference for morphine | 20 (20) | 18 (16) | 59 (28) | Better pain relief (17), fewer adverse events (5), more convenient (5), other (0.9) |
| Strong preference for morphine | 13 (13) | 8 (7) | ||
| No preference assessed | 25 | 14 | — |
P<0.001 different from preference for morphine.
Patient preference for fentanyl was not significantly different in patients with nociceptive pain (75 of 108, 69%), neuropathic pain (31 of 53, 58%), or mixed neuropathic and nociceptive pain (32 of 51, 63%). The predominant reason given for preferring fentanyl was better pain relief, followed by greater convenience and fewer adverse events (table 3). In a subgroup of 66 patients who were neither accustomed to fentanyl nor morphine (they had taken other opioids before the study), 62% preferred fentanyl.
Pain control, treatment assessments, and rescue drug
Patients treated with transdermal fentanyl had on average lower pain intensity scores than those treated with sustained release oral morphine (mean 57.8, range 33.1-82.5 v mean 62.9, range 41.2-84.6; P<0.001), irrespective of the order of treatment. More patients receiving fentanyl considered their pain control to be good or very good than those receiving morphine (35% v 23%, P=0.002) (table 4). Similar satisfaction was found among patients receiving fentanyl with nociceptive pain (43 of 123, 35%), neuropathic pain (21 of 62, 34%), or combined neuropathic and nociceptive pain (23 of 63, 37%). The corresponding satisfaction rates with morphine were 15 or 59 (25%) patients, 27 of 116 (23%), and 12 of 59 (20%), respectively. Overall, about one quarter of patients considered their pain control poor or very poor with either treatment (table 4).
Table 4.
Assessment of pain control at end point of each four week intervention period after treatment with transdermal fentanyl or sustained release oral morphine. Values are numbers (percentages)
| Pain control | Treatments
|
Crossover
|
|||
|---|---|---|---|---|---|
| Fentanyl (n=124) | Morphine (n=127) | Morphine (n=107) | Fentanyl (n=123) | ||
| Good or very good | 50 (40) | 24 (19) | 30 (28) | 37 (30) | |
| Moderate | 45 (36) | 71 (56) | 43 (40) | 50 (41) | |
| Poor or very poor | 29 (24) | 32 (25) | 34 (32) | 36 (29) | |
In the investigators' opinion, global efficacy of fentanyl was good or very good in 131 of 225 (58%) patients compared with 75 of 224 (33%) patients receiving morphine (P<0.001). The corresponding percentages from the patient assessments were 60% for fentanyl and 36% for morphine (P<0.001).
Analysis of the consumption of rescue drug during the last three weeks of each treatment period showed that the mean (standard deviation) consumption was significantly higher with fentanyl (29.4 (33.0) mg) than with morphine (23.6 (32.0) mg; P<0.001). A significant (P<0.05) period effect was also observed: the higher consumption during fentanyl treatment was more apparent in the second trial period (mean 32.4 (SD 38.5) mg) than the first (26.3 (26.0) mg), where the consumption of the rescue drug remained essentially the same over the two treatment periods in the morphine group (23.7 (35.3) mg v 23.6 (27.3) mg).
Quality of life—Patients had, on average, quality of life scores below the median on a scale ranging from 0 to 100 (lowest to highest wellbeing) (table 5). Patients receiving transdermal fentanyl had higher overall quality of life scores than patients receiving sustained release oral morphine in each of eight categories measured by the SF-36. Differences were significant in the categories for bodily pain, vitality, social functioning, and mental health. No period effect was noted.
Table 5.
Quality of life scores* based on SF-36 of both intervention periods after treatment with transdermal fentanyl or sustained release oral morphine. Values are means (95% confidence intervals)
| Category | Fentanyl | Morphine | P value |
|---|---|---|---|
| Physical functioning | |||
| Physical functioning | 35.4 (32.2 to 38.7) | 34.9 (31.7 to 38.2) | 0.712 |
| Role physical | 20.3 (15.8 to 24.8) | 15.1 (11.3 to 18.9) | 0.100 |
| Bodily pain | 32.5 (30.1 to 34.9) | 27.8 (25.5 to 30.1) | <0.001 |
| General health | 42.5 (39.6 to 54.4) | 40.7 (37.8 to 43.5) | 0.246 |
| Summary measurement | 28.6 (27.5 to 29.7) | 27.4 (26.3 to 28.5) | 0.004 |
| Mental health | |||
| Vitality | 38.0 (35.4 to 40.6) | 34.6 (31.7 to 37.3) | <0.001 |
| Social functioning | 47.1 (43.6 to 50.5) | 44.0 (40.5 to 47.5) | 0.002 |
| Role emotional | 48.6 (43.6 to 50.5) | 44.8 (38.8 to 50.8) | 0.458 |
| Mental health | 61.5 (58.8 to 64.2) | 59.1 (56.3 to 61.8) | 0.020 |
| Summary measurement | 44.4 (42.8 to 46.0) | 43.1 (41.5 to 44.8) | 0.030 |
From 0 (worst possible) to 100 (best possible).
Adverse effects—The overall incidence of treatment related adverse events was similar in both groups (table 6), as was the proportion of patients with adverse events (74% v 70%). Transdermal fentanyl was associated with a higher incidence of nausea (26% v 18%) than was sustained release oral morphine, whereas constipation was less common with fentanyl than with morphine (16% v 22%). Reduced constipation was confirmed by the bowel function questionnaire (29% fentanyl v 48% morphine; P<0.001) (table 6). Erythema and itching at application sites commonly occurred in patients receiving fentanyl (101 of 250, 41%) but were of mild to moderate intensity. Few patients had serious adverse events (2.8% v 3.8% for fentanyl and morphine, respectively), and only one patient, in the morphine group, hypoventilated. No deaths occurred, and no clinically important changes of vital signs were observed.
Table 6.
Most common adverse events related to treatment with transdermal fentanyl or sustained release oral morphine and bowel function, skin assessments, and disease progression. Values are numbers (percentages) of patients
| Fentanyl (n=250) | Morphine (n=238) | |
|---|---|---|
| Whole body | ||
| Fatigue | 14 (6) | 8 (3) |
| Central and peripheral nervous system | ||
| Dizziness | 28 (11) | 9 (4) |
| Headache | 22 (9) | 15 (6) |
| Gastrointestinal system | ||
| Abdominal pain | 11 (4) | 8 (3) |
| Constipation | 41 (16) | 52 (22) |
| Diarrhoea | 13 (5) | 7 (3) |
| Dry mouth | 21 (8) | 22 (9) |
| Nausea | 64 (26) | 44 (18) |
| Vomiting | 25 (10) | 24 (10) |
| Psychiatric disorders | ||
| Anorexia | 11 (4) | 3 (1) |
| Somnolence | 45 (18) | 34 (14) |
| Skin and appendages | ||
| Pruritus | 26 (10) | 20 (8) |
| Increased sweating | 22 (9) | 11 (5) |
| Bowel function (assessed by questionnaire) | ||
| Constipation* | 71 (29) | 112 (48) |
| Diarrhoea | 16 (7) | 16 (7) |
| Skin assessments | ||
| Erythema | 101 (41) | — |
| Oedema | 14 (6) | — |
| Itching | 80 (33) | — |
| Papules or pustules | 29 (12) | — |
| Disease progression | (n=225) | (n=224) |
| Improved | 15 (7) | 3 (1) |
| Deteriorated | 6 (3) | 14 (6) |
| Stable | 204 (91) | 207 (92) |
P<0.001.
Patient withdrawals—Within the total patient population the number of withdrawals in the fentanyl group was almost double (16%) that in the morphine group (9%) (table 7). More patients withdrew because of adverse events during treatment with transdermal fentanyl (11%) than with sustained release oral morphine (4%). However, subgroup analysis of 66 patients who had taken neither fentanyl nor morphine before the study showed that both the total number of withdrawals and withdrawals for adverse events were similar between treatment groups (table 7). Ten patients (16%) in the fentanyl group and 11 patients (18%) in the morphine group withdrew from the trial; seven patients receiving fentanyl (11%) and six receiving morphine (9.8%) withdrew because of an adverse event.
Table 7.
Number of patient withdrawals per treatment group for whole population and subpopulation of patients who had taken neither morphine nor fentanyl before study (naive patients). Values are numbers (percentages) of patients unless stated otherwise
| Fentanyl (n=250) | Morphine (n=238) | Ratio of fentanyl:morphine | |
|---|---|---|---|
| Total population | |||
| Total No of withdrawals | 39 (16) | 21 (9) | 1.9 |
| Withdrawals owing to adverse events | 27 (11) | 10 (4) | 2.7 |
| Naive population | n=63 | n=61 | |
| Total No of withdrawals | 10 (16) | 11 (18) | 0.9 |
| Withdrawals owing to adverse events | 7 (11) | 6 (10) | 1.2 |
Discussion
Patients with chronic non-cancer pain generally preferred treatment with transdermal fentanyl (65%) than with sustained release oral morphine (28%). A similar result was observed in patients with cancer pain.21,22 Furthermore, our findings confirm that potent opioids can provide satisfactory pain relief for the difficult clinical problem of chronic non-cancer pain. Although recruitment bias cannot be excluded, it cannot entirely explain the observed difference in treatment outcome.
Despite preference and better pain relief, more patients withdrew because of adverse events in the first fentanyl period than in the first morphine period. The phenomenon of preference for an opioid despite higher reporting of adverse events is well recognised in blinded controlled trials.7,9–11 Most patients (76%) had taken morphine for six weeks before entry to the study and would be accustomed to its side effects, making it unlikely that they would report additional adverse events when randomised to sustained release oral morphine. This may represent “incomplete cross tolerance” leading to a greater than anticipated potency.3,30 It is supported by analysis of a subgroup of patients who had taken neither morphine nor fentanyl before the trial. Here, withdrawals in total and in relation to adverse events were similar in both groups.
Comparisons of opioid action must be made at equianalgesic doses. It would be possible to explain the observed improvement in pain control and constipation with fentanyl if the initial dose ratios were wrong. The dosage of fentanyl increased consistently during each four week period, and these patients consumed more rescue drug than those receiving morphine. These findings confirm that a higher ratio of starting dose may be required compared with the conservative equianalgesic dose table used in this study.19 However, individual dose titration is vital and allows for the variability in patients' response to different opioids and the reported need to reduce the dose during “opioid rotation” in patients showing toxicity.3,21,31
The tables for equianalgesic dose derive from studies of single doses in selected populations and should be regarded as tentative for incomplete cross tolerance.3,30 Our patients were conditioned to opioids, mainly morphine, and switching to fentanyl may partly explain the improved pain control. The switch may have raised expectation of increased pain relief, partly attributable to a placebo analgesic effect.32 Most patients, however, preferred fentanyl regardless of the order of treatment. Exclusion of patients dissatisfied with morphine did not affect the percentage of patients preferring fentanyl.
The higher consumption of rescue drug during treatment with fentanyl was small (5.8 mg/24 hr overall), and probably not clinically important, but may reflect a less flexible dose titration with fentanyl. Furthermore, the difference in consumption of rescue drug was not significantly different between treatments in the first period. Differences in pain relief may also be explained by selectivity of opioid receptors. Indeed, recent research indicates a genetic basis for differences in pain sensitivity and response to analgesics.33
A significantly lower incidence of constipation was detected in the formal assessment of bowel function by patients receiving fentanyl (29% fentanyl v 48% morphine; P<0.001), confirming previous reports.18–22 In rats, fentanyl has a more favourable dose-analgesia to dose-constipation ratio than morphine, probably because the higher lipid solubility of fentanyl enables it to pass through the blood-brain barrier more easily than morphine.34 Giving fentanyl transdermally limits gastrointestinal concentration compared with oral morphine and consequently has less effect on opioid receptors in the gut.
The fentanyl patch formulation affords a convenient system of delivery over 72 hours. It may prevent “clock watching” and breakthrough pain associated with shorter acting formulations, thus improving compliance.4 In a “double dummy” design, preference for one delivery system would have been difficult to assess if patients were receiving both drugs together, particularly considering the difficulties and risks associated with simultaneously titrating morphine and fentanyl, as they have different dose schedules. Placebo effects can explain analgesia but not poor analgesia.35 Therefore, although a placebo effect is a possible explanation for our findings, given an overtly different administration, it is a less plausible explanation for those receiving fentanyl or morphine who had poor pain control. These findings are consistent with other reports that opioids do not provide adequate pain control to all patients with chronic non-cancer pain.5,9,10
Finally, we believe that using a pragmatic, clinical practice based approach, particularly in a large sample size, is justified, especially in the light of recent problems applying quality designs to clinical trials.36,37 The “explanatory” (evidence based) approach requires a placebo for comparison, whereas the “pragmatic” approach generally compares a new treatment with the best in clinical use for the particular clinical circumstances of patients.38 The existence of a gold standard treatment allows direct comparison rather than a placebo control, so that transdermal fentanyl can be directly compared with sustained release oral morphine.39
Strong treatment preferences can present difficulties but may be avoided by the crossover design.40,41 Patients' preference, although important for all clinical decisions, deserves special emphasis when diseases or treatments affect quality of life, the treatment involves risks or side effects, or the choice between treatments is a “close call.”42 The patient may be the best judge of the delicate balance between analgesic efficacy, side effects, and the overall experience of pain. This reflects our choice of patients' preference as the primary efficacy variable. Furthermore, pragmatic outcome measures such as quality of life and patients' preference may, ultimately, form a more accurate evaluation of treatment effects than pain measures alone.
Figure.
Flow of patients through trial
Table 2.
Use of opioids in month before entry to study to receive transdermal fentanyl and sustained release oral morphine
| Opioid | No of patients taking drug | No (%) of patients randomised to treatment
|
|
|---|---|---|---|
| Morphine then fentanyl (n=130) | Fentanyl then morphine (n=126) | ||
| Morphine | 194 | 103 (79) | 91 (72) |
| Codeine | 19 | 8 (6) | 11 (9) |
| Buprenorphine | 18 | 10 (8) | 8 (6) |
| Ketobemidone | 18 | 8 (6) | 10 (8) |
| Oxycodone | 15 | 8 (6) | 7 (6) |
| Methadone | 7 | 5 (4) | 2 (2) |
| Dextropropoxyphene | 7 | 3 (2) | 4 (3) |
| Anileridine | 5 | 2 (2) | 3 (2) |
| Dipipanone | 5 | 1 (1) | 4 (3) |
| Fentanyl | 5 | 1 (1) | 4 (3) |
| Dihydrocodeine | 5 | 2 (2) | 3 (2) |
| Pethidine | 4 | 1 (1) | 3 (2) |
| Dextromoramide | 4 | 3 (2) | 1 (1) |
| Nicomorphine | 2 | 1 (1) | 1 (1) |
| Pentazocine | 2 | 0 | 2 (2) |
| Tilidine | 2 | 1 (1) | 1 (1) |
| Hydromorphone | 2 | 1 (1) | 1 (1) |
| Tramadol | 1 | 1 (1) | 0 |
Acknowledgments
We thank all the investigators who participated in the trial: J Maeyaert, L Plaghki (Belgium); J Clark, A Mailis, D Moulin, M Ong-Lam, D Reid, P Watson (Canada); S Andersen, C Christiansen, S Clemensen, K Glahn, T Jonsson, S Larsen, F Molke Borgbjerg, A Schou Olesen, J Mølgaard (Denmark); V Järvimäki, T Heiskanen (Finland); R Atkinson, P Brown, F Campbell, R Gautam, M Hanna, D Hughes, C Knight, W Notcutt (United Kingdom); G Braak, J Helmers, G Van Oss, W Zuurmond (Netherlands); D Lines (South Africa).
Footnotes
Funding: The study was supported by a grant from Janssen Research Foundation, Belgium.
Competing interests: LA receives support from both Janssen-Cilag, the manufacturer of transdermal fentanyl (Durogesic) and Napp Laboratories, the manufacturer of sustained release morphine. EK has been reimbursed by Janssen-Cilag for participation at a meeting sponsored by Janssen-Cilag.
References
- 1.Gureje O, Von Korff M, Simon G, Gater R. Persistent pain and well-being: A World Health Organization study in primary care. JAMA. 1998;280:147–151. doi: 10.1001/jama.280.2.147. [DOI] [PubMed] [Google Scholar]
- 2.Ashburn MA, Staats PS. Management of chronic pain. Lancet. 1999;353:1865–1869. doi: 10.1016/S0140-6736(99)04088-X. [DOI] [PubMed] [Google Scholar]
- 3.Portenoy RK. Opioid and adjuvant analgesics. In: Mitchell M, editor. Pain 1999—an updated review. Seattle: IASP Press; 1999. pp. 3–18. [Google Scholar]
- 4.World Health Organization. Cancer pain relief, 2nd ed. Geneva: WHO; 1996. [Google Scholar]
- 5.McQuay H. Opioids in pain management. Lancet. 1999;353:2229–2232. doi: 10.1016/S0140-6736(99)03528-X. [DOI] [PubMed] [Google Scholar]
- 6.Portenoy RK. Opioid therapy for chronic non-malignant pain: a review of critical issues. J Pain Symptom Manage. 1996;11:203–217. doi: 10.1016/0885-3924(95)00187-5. [DOI] [PubMed] [Google Scholar]
- 7.Rowbotham MC, Reisner-Keller LA, Fields HL. Both intravenous lidocaine and morphine reduce the pain of postherpetic neuralgia. Neurology. 1991;41:1024–1028. doi: 10.1212/wnl.41.7.1024. [DOI] [PubMed] [Google Scholar]
- 8.Dellemijn PLI, Vanneste JAL. Randomised double-blind active-placebo-controlled crossover trial of intravenous fentanyl in neuropathic pain. Lancet. 1997;349:753–758. doi: 10.1016/S0140-6736(96)09024-1. [DOI] [PubMed] [Google Scholar]
- 9.Arkinstall W, Sandler A, Goughnour B, Babul N, Harsanyi Z, Drake A. Efficacy of controlled-release codeine in chronic non-malignant pain: a randomized, placebo-controlled clinical trial. Pain. 1995;62:169–178. doi: 10.1016/0304-3959(94)00262-D. [DOI] [PubMed] [Google Scholar]
- 10.Moulin DE, Iezzi A, Amireh R, Sharpe WKJ, Boyd D, Merskey H. Randomised trial of oral morphine for chronic non-cancer pain. Lancet. 1996;347:143–147. doi: 10.1016/s0140-6736(96)90339-6. [DOI] [PubMed] [Google Scholar]
- 11.Watson CPN, Babul N. Efficacy of oxycodone in neuropathic pain: a randomized trial in postherpetic neuralgia. Neurology. 1998;50:1837–1841. doi: 10.1212/wnl.50.6.1837. [DOI] [PubMed] [Google Scholar]
- 12.Schug SA, Merry AF, Acland RH. Treatment principles for the use of opioids in pain of nonmalignant origin. Drugs. 1991;42:228–239. doi: 10.2165/00003495-199142020-00005. [DOI] [PubMed] [Google Scholar]
- 13.Brown RL, Fleming MF, Patterson JJ. Chronic opioid analgesic therapy for chronic low back pain. J Am Board Fam Pract. 1996;9:191–204. [PubMed] [Google Scholar]
- 14.Graziotti PJ, Goucke CR. The use of oral opioids in patients with chronic non-cancer pain. Management strategies. Med J Austr. 1997;167:30–34. [PubMed] [Google Scholar]
- 15.Anon. The use of opioids for the treatment of chronic pain: a consensus statement from the American Academy of Pain Medicine and the American Pain Society. Pain Forum. 1997;6:77–79. [PubMed] [Google Scholar]
- 16.Cummings-Ajemian I. Treatment of related symptoms. In: Patt RB, editor. Cancer pain, section III, non-pharmacological treatment and novel approaches to management. Philadelphia: JB Lippincott; 1993. [Google Scholar]
- 17.Jeal W, Benfield P. Transdermal fentanyl—a review of its pharmacological properties and therapeutic efficacy in pain control. Drugs. 1997;53:109–138. doi: 10.2165/00003495-199753010-00011. [DOI] [PubMed] [Google Scholar]
- 18.Grond S, Zech D, Lehman KA, Radbruch L, Breintenbach H, Hertel D. Transdermal fentanyl in the long-term treatment of cancer pain: a prospective study of 50 patients with advanced cancer of the gastrointestinal tract or the head and neck origin. Pain. 1997;69:191–198. doi: 10.1016/s0304-3959(96)03254-x. [DOI] [PubMed] [Google Scholar]
- 19.Donner B, Zenz M, Tryba M, Strumpf M. Direct conversion from oral morphine to transdermal fentanyl: a multicentre study in patients with cancer pain. Pain. 1996;64:527–534. doi: 10.1016/0304-3959(95)00180-8. [DOI] [PubMed] [Google Scholar]
- 20.Donner B, Zenz M, Strumpf M, Raber M. Long-term treatment of cancer pain with transdermal fentanyl. J Pain Symptom Manage. 1998;15:168–175. doi: 10.1016/s0885-3924(97)00361-8. [DOI] [PubMed] [Google Scholar]
- 21.Ahmedzai S, Brooks D. Transdermal fentanyl versus sustained release oral morphine in cancer pain: preference, efficacy, and quality of life. J Pain Symptom Manage. 1997;13:254–261. doi: 10.1016/s0885-3924(97)00082-1. [DOI] [PubMed] [Google Scholar]
- 22.Payne R, Mathias SD, Pasta DJ, Wanke LA, Williams R, Mahmoud R. Quality of life and cancer pain: satisfaction and side effects with transdermal fentanyl versus oral morphine. J Clin Oncol. 1998;16:1588–1593. doi: 10.1200/JCO.1998.16.4.1588. [DOI] [PubMed] [Google Scholar]
- 23.Dellemijn PLI, van Duijn H, Vanneste JAL. Prolonged treatment with transdermal fentanyl in neuropathic pain. J Pain Symptom Manage. 1998;16:220–229. doi: 10.1016/s0885-3924(98)00070-0. [DOI] [PubMed] [Google Scholar]
- 24.TTS-Fentanyl Multicentre Study Group. Transdermal fentanyl in cancer pain. J Drug Dev. 1994;6:93–97. [Google Scholar]
- 25.Zech DFJ, Grond SAU, Dauer HG, Stollenwerk B, Lehmann KA. Transdermal fentanyl and initial dose-finding with patient-controlled analgesia in cancer pain. A pilot study with 20 terminally ill cancer patients. Pain. 1992;50:293–301. doi: 10.1016/0304-3959(92)90034-9. [DOI] [PubMed] [Google Scholar]
- 26.Southam MA. Transdermal fentanyl therapy: system design, pharmacokinetics and efficacy. Anticancer Drugs. 1995;6(suppl 3):29–34. doi: 10.1097/00001813-199504003-00005. [DOI] [PubMed] [Google Scholar]
- 27.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptional framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 28.Koch CG. The use of non-parametric methods in the statistical analysis of the two period change-over design. Biometrics. 1972;28:577–584. [PubMed] [Google Scholar]
- 29.Watson HR, Pearce AC. Treatment allocation in clinical trials: randomisation and minimisation compared in three test cases. Pharmaceut Med. 1990;4:207–212. [Google Scholar]
- 30.Collett B-J. Opioid tolerance: the clinical perspective. Br J Anaesth. 1998;81:58–68. doi: 10.1093/bja/81.1.58. [DOI] [PubMed] [Google Scholar]
- 31.Bruera E, Sloan P, Mount B, Scott J, Suarez-Almazar M. A randomized, double-blind, double-dummy, crossover trial comparing the safety and efficacy of oral sustained-release hydromorphine with immediate-release hydromorphine in patients with cancer pain. J Clin Oncol. 1996;14:1713–1717. doi: 10.1200/JCO.1996.14.5.1713. [DOI] [PubMed] [Google Scholar]
- 32.Amanzio M, Benedetti F. Neuropharmacological dissection of placebo analgesia: expectation-activated opioid systems versus conditioning-activated specific subsystems. J Neurosci. 1999;19:484–494. doi: 10.1523/JNEUROSCI.19-01-00484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uhl GR, Sora I, Wang Z. The mu opiate receptor as a candidate gene for pain: polymorphisms, variations in expression, nociception, and opiate responses. Proc NatlAcad Sci. 1999;96:7752–7755. doi: 10.1073/pnas.96.14.7752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Megens AAHP, Artois K, Vermeire J, Meert T, Awouters FHL. Comparison of the analgesic and intestinal effects of fentanyl and morphine in rats. J Pain Symptom Manage. 1998;15:253–258. doi: 10.1016/s0885-3924(97)00371-0. [DOI] [PubMed] [Google Scholar]
- 35.McQuay HJ, Jadad AR, Carroll D, Faura C, Glynn CJ, Moore RA, et al. Opioid sensitivity of chronic pain: a patient-controlled analgesia method. Anaesthesia. 1992;47:757–767. doi: 10.1111/j.1365-2044.1992.tb03253.x. [DOI] [PubMed] [Google Scholar]
- 36.Moore RA, Gavaghan D, Tramer MR, Collins SL, McQuay HJ. Size is everything—large amounts of information are needed to overcome random effects in estimating direction and magnitude of treatment effects. Pain. 1998;78:209–216. doi: 10.1016/S0304-3959(98)00140-7. [DOI] [PubMed] [Google Scholar]
- 37.Haynes B. Can it work? Does it work? Is it worth it? BMJ. 1999;319:652–653. doi: 10.1136/bmj.319.7211.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Max M. Methodological issues in the design of analgesic clinical trials. In: Mitchell M, editor. Pain 1999—an update review. Seattle: IASP Press; 1999. [Google Scholar]
- 39.Tramer MR. When placebo controlled trials are essential and equivalence trials are inadequate. BMJ. 1998;317:875–880. doi: 10.1136/bmj.317.7162.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Torgerson DJ, Sibbald B. Understanding controlled trials: what is a patient preference trial? BMJ. 1998;316:360. doi: 10.1136/bmj.316.7128.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Senn S. Within-patient studies: cross-over trials and n-of-1 studies. In: Mitchell M, editor. Pain 1999—an update review. Seattle: IASP Press; 1999. [Google Scholar]
- 42.Goodare H, Lockwood S. Involving patients in clinical research. BMJ. 1999;319:724–725. doi: 10.1136/bmj.319.7212.724. [DOI] [PMC free article] [PubMed] [Google Scholar]