Abstract
Objective:
To investigate the clinical features, prevalence, role of surgical intervention and the visual prognosis of macular holes (MH) in patients with Behcet's disease (BD).
Materials and Methods:
Retrospective study of patients with BD and MH from January 1998 to November 2008.
Results:
Out of 159 patients, 21 eyes of 17 patients were identified with MH. The mean age was 38.59 (range 23-61) years and the mean follow-up period was 5.1 years (range 13-164 months). The prevalence of MH was 7%. Visual acuity (VA) at the time of presentation ranged from 20/70 to hand-motion. Optical coherence tomography (OCT) findings revealed intraretinal cysts at the edge of the MH. The mean size of MH was 983.6 um; 52% had elevated edges, 43% had flat edges and only one eye (5%) was closed postoperatively. Fluorescein angiography (FA) was consistent with macular ischemia in 76% of the cases. Human leukocyte antigen (HLA) B51 association was found in 14 of the 15 patients investigated. Six patients (out of 17) underwent pars plana vitrectomy. The final VA on their last follow-up ranged from 20/70 to 2/200. Surgical intervention for MH did not result in any visual improvement as compared to non-operated eyes. One patient lost vision completely due to elevated intraocular pressure post vitrectomy and silicon oil tamponade.
Conclusions:
MH in patients with BD may lead to significant visual disability. Surgical intervention does not seem to have any potential beneficial effect on the VA, probably due to significant macular ischemia and sequelae from the ocular inflammation.
Keywords: Behcet's disease, HLA-B51, optical coherence tomography, macular hole
Behçet's disease (BD) is a systemic disease characterized by obliterative vasculitis, recurrent aphthous ulcers and intraocular inflammation.[1] The diagnostic criteria of The International Study Group for BD require evidence of recurrent oral ulceration and at least two additional criteria, including recurrent genital ulcers, ocular lesions, skin lesions, and a positive pathergy test.[2] The cause and the pathogenesis of BD remains elusive, but the tumor necrosis factor-alpha (TNF-alpha) pathway has been proposed in the pathogenesis of BD.[3–6] Co-expression of the TNF beta 2 (TNFB2) allele with human leukocyte antigen (HLA-B51) has been reported to increase the severity of eye involvement in BD.[7–9] Prevalence of BD has been reported to be 2-30 cases per 100,000 in the Asian continent and 0.1-7.5 per 100,000 in Europe and the United States.[10]
Uveitis in BD is nongranulomatous and can be anterior, intermediate, posterior or panuveitis, which is the most frequent type (60%) in both sexes.[11] The severity and number of repeated inflammatory attacks involving the posterior segment determine the extent of permanent anatomical changes and the degree of irreversible visual loss. The incidence of posterior segment involvement is reported to be 50-93%.[12]
Macular edema is the most frequent sign and complication from posterior uveitis which can evolve into cystoid degeneration and occasionally macular hole (MH). Macular lesions and macular ischemia in particular are often irreversible, making prognosis uncertain, despite the various treatments available.[13,14]
A full-thickness MH is an uncommon complication and reported in 3.4% of patients with macular involvement in BD.[15] It is thought that chronic cystoid macular edema (CME) and retinal vasculitis may be the risk factors for the development of MH in BD.[13,15] However, natural history, clinical and morphological characteristic features, as well as the outcome of surgical intervention for MH in patients with BD are not well studied due to its rare occurrence.
Materials and Methods
We performed a retrospective chart review of all patients with BD and MH seen at our eye specialist center from January 1998 to December 2008. Approval of the local ethics committee for this review was obtained. The collected data included the demographics of BD patients with MH, the clinical features of MH in these patients, including best corrected visual acuity (BCVA) at presentation and last follow-up. We also studied the morphological characteristics of MH in BD by the use of ancillary tests such as optical coherence tomography (OCT) and fluorescein angiography (FA), HLA association and the outcome of surgical intervention including complications of MH surgery.
All patients were quiet in their inflammation at the time of the surgery. In order to prevent an exacerbation of the inflammation due to the surgical intervention, all patients were treated with 1 mg prednisolone/kg one week before surgery. This treatment was slowly tapered during the first three postoperative months. Other therapeutic modalities that were rendered to the patients were continued during the follow-up period including topical steroids and systemic immunosuppressive medications as indicated.
The results were analyzed using the Statistical Package for Social Science for Windows Version 12 (SPSS Inc., Chicago, IL, USA). Categorical data were expressed as number and percentage. Ordinal and categorical data were compared using the Chi square test. P value of 0.05 was used as the level of significance.
Results
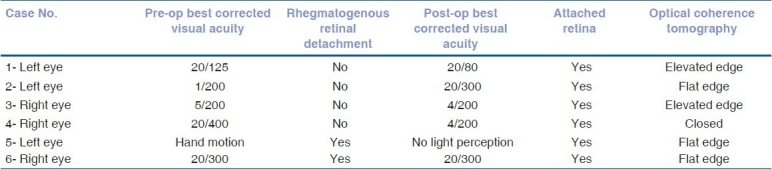
The medical records of 159 patients with BD were reviewed. A total of 17 patients (21 eyes) were identified with MH. The prevalence was 7%. The follow-up period ranged from 13 to 164 months with a mean of 61.18 +/-SD: 45.20. Fifteen of the patients were male and two were female. The age ranged from 23 to 61 years with a mean of 38.9 years (59% less than 40 years old). The right eye was involved in six patients, left eye in seven patients and bilateral involvement was found in four patients. The BCVA of the involved eyes at the presentation and at the last follow-up is described in Table 1. Fundus changes that were associated with MH included pigmentary maculopathy changes in 10 eyes (47%), vascular sheathing in nine (42%), pale disc in two (9%) and retinal detachment (RD) in two eyes (9%) [Figs. 1 and 2]. The minimal size of the MH measured by OCT was 494 um and the maximum 1666 um with a mean of 983.6 um. All patients showed intraretinal cysts with retinal thickening at the edges of the MH. At the last follow-up visit, OCT showed elevated edges of the MH in 52% whereas 43% showed flat edges and only one (5%) MH was closed after undergoing surgical repair [Fig. 3]. FA revealed macular ischemia in 16 eyes (76.2%). There was no significant correlation between macular ischemia and the status of the MH edges (P=0.051) [Table 2]. BCVA at presentation and last follow-up was not significantly associated with macular ischemia (P=0.055) [Tables 3a and 3b]. HLA association was investigated in 15 patients and the result showed positive HLA B51 in 14 (out of 15) and only one patient had HLA B55 (7%). Four eyes underwent pars plana vitrectomy (PPV), indocyanin green (ICG)-assisted internal limiting membrane peeling (ILMP), air-fluid (AF) exchange and gas (C3F8 or SF6) tamponade with postoperative face down position. Two patients who presented with MH-related rhegmatogenous RD underwent PPV, ICG-assisted ILMP, AF exchange and silicon oil tamponade with flat retina postoperatively [Table 4]. Postoperative visual acuity (VA) did not show significant improvement in comparison to preoperative VA except in one patient who did not have macular ischemia. MH closure was achieved in only one eye (out of six) which was confirmed by OCT examination and the remaining five eyes continued to have persistent open MH. One of the operated eyes suffered complete loss of vision secondary to elevated intraocular pressure (IOP) three months post vitrectomy with silicon oil.
Table 1.
Best corrected visual acuity of involved eye at presentation and last visit
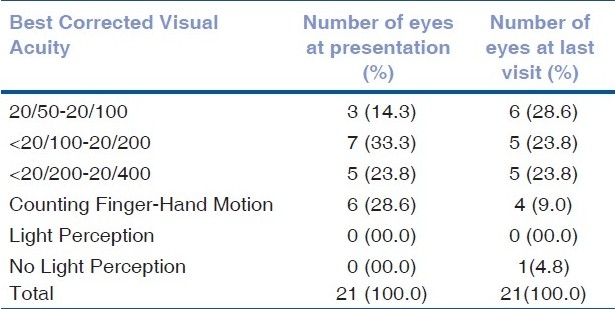
Figure 1.
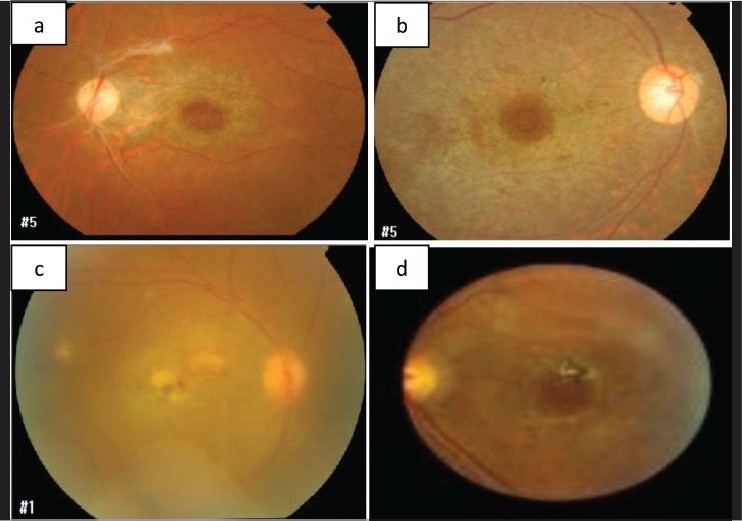
Fundus photos of patients with Behçet's disease and macular hole with the typical pigmentary changes in the macula as an inflammatory sequelae (a and b); active retinitis in the macula (c); and pigmented scar (d)
Figure 2.
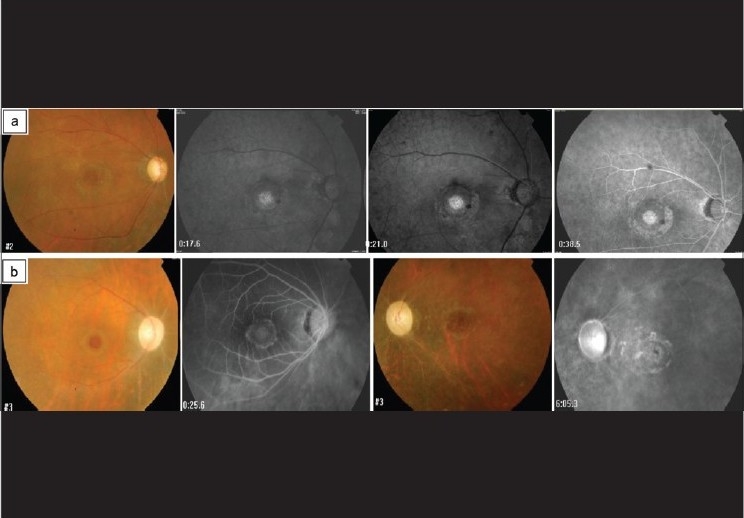
Fundus photos and fluorescein photographs of two patients with ischemic changes. The first patient had a delayed filling of fluorescein and almost no pericapillary perfusion (a). The other patient showed bilateral sclerosed vessels with compromised macular circulation and pale optic discs (b)
Figure 3.
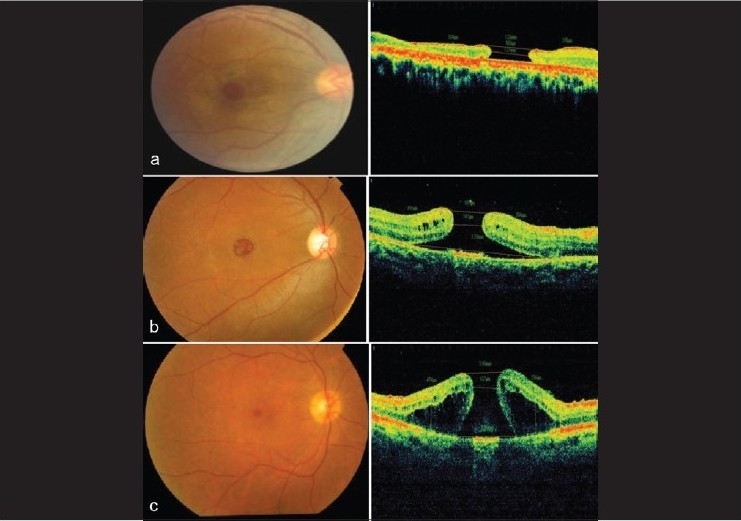
Fundus and optical coherence tomography (OCT) images of patients with Behçet's disease and macular hole. There were different OCT findings in that some patients had flat edges and no subretinal fluid (a); others had elevated edges with subretinal fluid (b) and some had elevated edges with intraretinal edema (c)
Table 2.
Correlation between macular hole status and macular ischemia
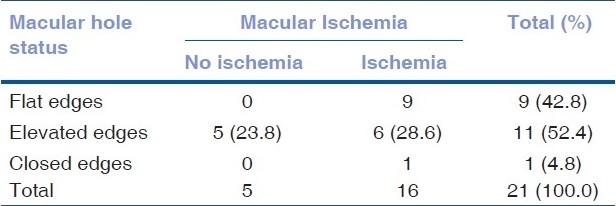
Table 3A.
Correlation between best corrected visual acuity at presentation and macular ischemia
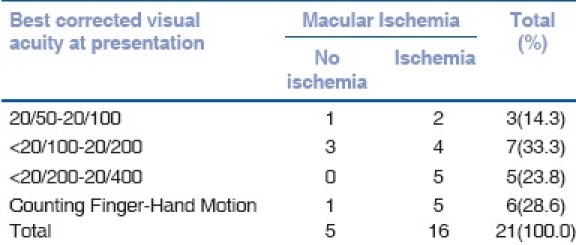
Table 3B.
Correlation between best corrected visual acuity at last follow-up and macular ischemia
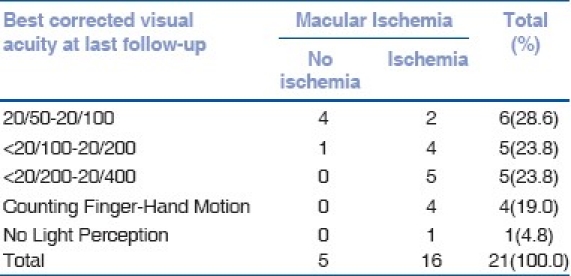
Table 4.
Surgical outcome
Discussion
Posterior segment involvement in BD often leads to irreversible and serious vision loss. Although most macular manifestations of the disease have been reported in the literature, MH is rarely discussed in detail except in anecdotal case reports.[13–15] Our study presents the largest case series of MH in BD with focus on clinical and morphological features, the natural history and the visual outcome, with or without surgical intervention, over a long period of follow-up.
MH in BD and probably in other uveitis is an inflammatory type of MH. Although the exact mechanisms of its development are not clear, it is thought to be related to induced posterior vitreous detachment (PVD) with minimal vitreous liquefaction by vitreous gel shrinkage secondary to vitritis, as discussed in previous papers.[16–19] Furthermore, the prognosis of MH in uveitis patients does not depend only on the closure of the MH, but probably more on other factors such as the perfusion status of the macula and disturbances of the retinal pigment epithelium (RPE) as a sequelae of the inflammation.
Our study showed that 88% of the patients were males with 59% of them less than 40 years of age. These findings are concordant with previous studies that have shown a male preponderance and it is generally associated with a more severe form of the disease in men, and a younger age of onset.[20] The prevalence of MH among our patients with BD was 6.6% which is higher than previously reported (3.4%) by Angioi-Duprez et al.[15] The possible explanation for this could be that our hospital is a tertiary eye hospital and we see late referrals of complicated cases. Either eye can be involved and bilateral involvement was found in 23.5% of the patients with MH.
The mean duration of the follow-up of BD patients with MH in this study was approximately five years which is a considerable time and should adequately address the natural course of the disease.
VA assessment [Table 1] at presentation and last follow-up, including the operated eyes, demonstrated that 57.1% presented with BCVA of less than 20/200 and 28.6% had BCVA of counting finger (CF) to hand motion (HM) and only 14.3% presented with BCVA of 20/50-20/100. BCVA of 20/50-20/100 increased to 28.6% in the last visit while 47.6% continued to have BCVA of less than 20/100-20/400 and only 9% remained with BCVA of CF to HM. The improvement in the VA is mainly related to adequate control of the severe intraocular inflammation at presentation by systemic steroid and immunosuppressive treatments. Although the presence of macular ischemia on FA in BD is a predictor of poor visual outcome, macular ischemia may show partial recovery with the treatment of the disease as reported by Yilmaz et al.[14]
Evaluation of MH by OCT including the size, intraretinal cysts and edges showed an average size of 983.6 um which is large and associated with intraretinal cysts. The edge of MH was elevated in 52.4% and flat opened MH in 42.8% and only one MH closed postoperatively. Since the rate of closed MH is much lower than in idiopathic MH, it is reasonable to presume that the etiology differs between these and the MH in BD. The presence of inflammation and an already injured retina makes it less likely to achieve success in closing the MH with PPV and gas, as compared to idiopathic MH.
Macular ischemia was found in 76.2% of the eyes and despite non-significant correlation with poor vision at presentation and last follow-up it seems to be a limiting predictor factor to recover good VA.
The study showed that 93.3% patients have positive HLA B51 and this association is correlated with data that has been reported to increase the severity of eye involvement in BD.[7]
Post surgical intervention VA did not show significant improvement in comparison to preoperative VA except one patient who did not have macular ischemia, and 83% of operated eyes revealed persistent open MH [Table 4].
The major weaknesses and limitations of this study are that it is retrospective and that the number of patients operated for MH is small, in addition, two of the six operated patients presented with a rhegmatogenous RD caused by the MH.
In conclusion, surgical intervention for MH in BD does not seem to have a potential beneficial effect on the VA, probably due to significant macular ischemia. Prevention of CME, vasculitis and chronic inflammation by intensive and aggressive medical therapy, locally and systemically, might be the best effective treatment, rather than surgery in patients with BD and MH.
Acknowledgments
The authors would like to thank Dr Manal Bouhaimed from Kuwait University for her constructive review of the manuscript.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Yazici H, Yurdakul S, Hamuryudan V. Behçet's syndrome. Curr Opin Rheumatol. 1999;11:53–7. doi: 10.1097/00002281-199901000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Criteria for diagnosis of Behçet′s disease. International Study Group for Behçet's Disease. Lancet. 1990;335:1078–80. [PubMed] [Google Scholar]
- 3.Ahmad T, Wallace GR, James T, Neville M, Bunce M, Mulcahy-Hawes K, et al. Mapping the HLA association in Behçet's disease: A role for tumor necrosis factor polymorphisms? Arthritis Rheum. 2003;48:807–13. doi: 10.1002/art.10815. [DOI] [PubMed] [Google Scholar]
- 4.Akman A, Sallakci N, Coskun M, Bacanli A, Yavuzer U, Alpsoy E, et al. TNF-alpha gene 1031 T/C polymorphism in Turkish patients with Behçet's disease. Br J Dermatol. 2006;155:350–6. doi: 10.1111/j.1365-2133.2006.07348.x. [DOI] [PubMed] [Google Scholar]
- 5.Duymaz-Tozkir J, Gül A, Uyar FA, Ozbek U, Saruhan-Direskeneli G. Tumour necrosis factor-alpha gene promoter region -308 and -376 G -- A polymorphisms in Behçet's disease. Clin Exp Rheumatol. 2003;21:S15–8. [PubMed] [Google Scholar]
- 6.Lee EB, Kim JY, Lee YJ, Park MH, Song YW. TNF and TNF receptor polymorphisms in Korean Behcet's disease patients. Hum Immunol. 2003;64:614–20. doi: 10.1016/s0198-8859(03)00057-0. [DOI] [PubMed] [Google Scholar]
- 7.Verity DH, Wallace GR, Vaughan RW, Kondeatis E, Madanat W, Zureikat H, et al. HLA and tumour necrosis factor (TNF) polymorphisms in ocular Behçet's disease. Tissue Antigens. 1999;54:264–72. doi: 10.1034/j.1399-0039.1999.540307.x. [DOI] [PubMed] [Google Scholar]
- 8.González-Escribano MF, Rodríguez MR, Walter K, Sanchez-Roman J, García-Lozano JR, Núñez-Roldán A. Association of HLA-B51 subtypes and Behçet's disease in Spain. Tissue Antigens. 1998;52:78–80. doi: 10.1111/j.1399-0039.1998.tb03027.x. [DOI] [PubMed] [Google Scholar]
- 9.Ohno S, Ohguchi M, Hirose S, Matsuda H, Wakisaka A, Aizawa M. Close association of HLA-Bw51 with Behçet's disease. Arch Ophthalmol. 1982;100:1455–8. doi: 10.1001/archopht.1982.01030040433013. [DOI] [PubMed] [Google Scholar]
- 10.Zouboulis CC. Epidemiology of Adamantiades-Behçet's disease. Ann Med Interne (Paris) 1999;150:488–98. [PubMed] [Google Scholar]
- 11.Tugal-Tutkun I, Onal S, Altan-Yaycioglu R, Huseyin Altunbas H, Urgancioglu M. Uveitis in Behçet disease: an analysis of 880 patients. Am J Ophthalmol. 2004;138:373–80. doi: 10.1016/j.ajo.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 12.Atmaca LS. Fundus changes associated with Behçet's disease. Graefes Arch Clin Exp Ophthalmol. 1989;227:340–4. doi: 10.1007/BF02169409. [DOI] [PubMed] [Google Scholar]
- 13.Benchekroun O, Lahbil D, Lamari H, Rachid R, El Belhadji M, Laouissi N, et al. Macular damage in Behcet's disease. J Fr Ophtalmol. 2004;27:154–9. doi: 10.1016/s0181-5512(04)96110-4. [DOI] [PubMed] [Google Scholar]
- 14.Yilmaz G, Akova Y, Aydin P. Macular ischaemia in Behçet's disease. Eye. 2000;14:717–20. doi: 10.1038/eye.2000.190. [DOI] [PubMed] [Google Scholar]
- 15.Angioi-Duprez K, Maalouf T, Gérin M, George JL. A full thickness macular hole as an uncommon complication of Behçet disease. J Fr Ophtalmol. 2001;24:172–4. [PubMed] [Google Scholar]
- 16.Hirokawa H, Takahashi M, Trempe CL. Vitreous changes in peripheral uveitis. Arch Ophthalmol. 1985;103:1704–7. doi: 10.1001/archopht.1985.01050110098035. [DOI] [PubMed] [Google Scholar]
- 17.Sheu SJ, Yang CA. Macular hole in Behcet's disease. Kaohsiung J Med Sci. 2004;20:558–62. doi: 10.1016/S1607-551X(09)70258-X. [DOI] [PubMed] [Google Scholar]
- 18.Wuu TT, Hong MC. Pars plana vitrectomy with internal limiting membrane removal for a macular hole associated with Behçet's disease. Eye. 2009;23:1606–7. doi: 10.1038/eye.2008.243. [DOI] [PubMed] [Google Scholar]
- 19.Georgalas I, Markomichelakis N, Lades I. Retinal detachment due to a macular hole in a patient with Behcet's disease treated with vitrectomy and silicone oil tamponade. Eur J Ophthalmol. 2008;18:1023–24. doi: 10.1177/112067210801800630. [DOI] [PubMed] [Google Scholar]
- 20.Tursen U, Gurler A, Boyvat A. Evaluation of clinical findings according to sex in 2313 Turkish patients with Behçet's disease. Int J Dermatol. 2003;42:346–51. doi: 10.1046/j.1365-4362.2003.01741.x. [DOI] [PubMed] [Google Scholar]


