Abstract
Recently, significant attention has been drawn to the biology of small leucine-rich repeat proteoglycans (SLRPs) due to their multiple functionalities in various cell types and tissues. Here, we characterize a novel SLRP member, “Podocan-like (Podnl) protein” identified by a bioinformatics approach. The Podnl protein has a signal peptide, a unique cysteine-rich N-terminal cluster, 21 leucine-rich repeat (LRR) motifs, and 1 putative N-glycosylation site. This protein is structurally similar to podocan in SLRPs. The gene was highly expressed in mineralized tissues and in osteoblastic cells and the high expression level was observed at and after matrix mineralization in vitro. Podnl was enriched in newly formed bones based on immunohistochemical analysis. When Podnl was transfected into osteoblastic cells, the protein with N-glycosylation was detected mainly in the cultured medium, indicating that Podnl is a secreted N-glycosylated protein. The endogenous Podnl protein was also present in bone matrix. These data provide a new insight into our understanding of the emerging SLRP functions in bone formation.
Keywords: Small leucine-rich repeat proteoglycans (SLRPs), Podocan-like, Bone, N-glycosylation
Introduction
Mineralized tissues of vertebrates, except tooth enamel, are essentially composed of two phases, minerals (mainly carbonate-rich hydroxyapatite) and fibrillar type I collagen, where the latter serves as a structural template regulating the spatial aspect of mineralization. Thus, the size and stability of collagen fibrils are an important determinant for mineralization. Recently, it has been shown that the pattern of post-translational modifications of collagen has significant impact on collagen fibrillogenesis and subsequent mineralization [1,2,3,4]. In addition, several small leucine-rich repeat proteoglycans (SLRPs) have been implicated in controlling collagen fibrillogenesis, matrix mineralization and osteoblast differentiation [5,6,7,8,9,10,11,12,13]. SLRPs are an evolutionally conserved family of secreted proteins that are synthesized as a relatively small core protein containing 6–20 leucine-rich repeat (LRR) motifs and post-translationally modified with glycosaminoglycan (GAG) chain(s), N-glycosylation, and/or tyrosine sulfation. SLRPs are divided into several subgroups based on the numbers of exons, interspaced amino acid residues within the N-terminal cysteine cluster, and LRR motifs [14,15,16,17]. At present, fifteen SLRP members have been cloned and most recently, we have identified a novel SLRP member, nephrocan that is highly expressed in kidney and may modulate the function of transforming growth factor-β [18]. We have established a bioinformatics approach to identify new SLRP members using various prediction programs in the following manner. We obtained the protein sequence of decorin (DCN) in fish as a query protein sequence and searched mouse sequences by a BLAST program. The candidate sequences obtained were then screened by the PSORT II program to predict the subcellular localization. The criteria of new SLRP members include the presence of signal peptide sequence, presence of typical N-terminal cysteine cluster (commonly observed in all SLRPs with some variations) and absence of transmembrane region(s). By using this computational screening, we have now identified another novel SLRP member, named “Podocan-like (Podnl)”, that is present in bone matrix. In this study, we report the initial characterization of this novel member including its gene structure, its tissue distribution/gene expression pattern, its localization both in vivo and in vitro, and its post-translational modification.
Materials and methods
Cell culture
The mouse calvaria-derived preosteoblastic cell line, MC3T3-E1 cells (subclone 4) (MC cells), were purchased from American Type Culture Collection (CRL-2593) and maintained as previously described [13]. Human embryonic kidney 293 cells were purchased from Clontech and maintained in Dulbecco’s modified Eagle’s medium (DMEM, Gibco) as described [18].
Molecular cloning of mouse Podocan-like cDNA
The cDNAs containing the full length sequences of Podnl were isolated by reverse transcription-polymerase chain reaction (RT-PCR) using Hotstar Taq DNA polymerase (Qiagen). The sequences of the primers were as follows: forward primer, 5’-CTGTGCTGAGAGATGGAGGGCAATTG-3’ and reverse primer, 5’-CCTGTGAACATGAGAGGGCATCTGC-3’. The cDNA derived from MC cells was used as a template for RT-PCR. The PCR conditions were as follows: 15min at 95 °C; 30 s at 95 °C, 1min at 60 °C, 1min at 72 °C for 40 cycles. The PCR products were then ligated into the pcDNA3.1-V5/His-TOPO mammalian expression vector (Invitrogen) and the plasmids obtained were sequenced at the DNA Sequencing Facility (University of North Carolina, Chapel Hill, NC). During the course of molecular cloning of Podnl, we have obtained several splicing variants including the NCBI reference sequence (NM_001013384) and found that one particular splicing variant was predominantly expressed in MC3T3-E1 osteoblastic cells. Thus, this variant was cloned and further characterized (the Genbank™/EBI Data Bank accession number; DQ303463). The plasmid containing the coding sequence of Podnl was generated by RT-PCR with the primers designed as follows: forward primer, 5’-GCTATGAGGCCTCAAGAGCTGCTATTG-3’ and reverse primer, 5’-GACCAGGACCTGTTCCGGGTTTTCTG-3’, and successfully obtained (pcDNA3.1-Podnl-V5/His).
RNA extraction and quantitative real-time PCR
In order to investigate the Podnl gene expression in various tissues, real time PCR analysis was performed using cDNA derived from heart, brain, lung, kidney, calvaria and femur (C57BL/6, 5 weeks, male) and MC cells. MC cells were maintained and plated onto 35 mm dishes at a density of 2 × 105 cells/dish. After reaching confluence, the medium was replaced with the one supplemented with 50 µg/ml of ascorbic acid and 1mM of β-glycerophosphate (mineralization medium) (this time point will be referred to as day 0), and maintained for up to 35 days (fully mineralized stage). Cells were washed with phosphate buffered saline (PBS), and total RNA was extracted with TRIzol reagent solution (Invitrogen) at the end of every week. Mouse tissue samples were collected, and total RNA was extracted in the same manner. Two µg of the total RNA extract was used for RT using the Omniscript RT Kit (Qiagen). Real time PCR was performed in triplicate using the specific primers-probe for Podnl (Applied Biosystems, ABI assay number: Mm01247693_m1) or glyceraldehyde-3-phosphate dehydrogenase (GAPDH, ABI assay number: 4308313), and the expression levels were analyzed by the ABI Prism 7000 Sequence detection system (Applied Biosystems). The mean fold changes in the expression of Podnl relative to that of GAPDH were calculated using the values obtained from the heart or MC cells at day 0 cDNA as a calibrator by means of 2−ΔΔ CT method as described previously [12,18]. Three independent experiments were performed and results were essentially identical.
Overexpression of Podnl in osteoblasts
MC cells were maintained and plated onto 6 well culture plates at a density of 1 × 105 cells/dish. On the following day, cells were transfected with 2.5 µg each of an empty vector (EV, pcDNA3.1-V5/His A vector; Invitrogen) or pcDNA3.1-Podnl-V5/His vector using FuGENE6 transfection reagent (Roche Diagnostics). After 24 hours, cultured media were collected, and cell lysates were prepared using a lysis buffer in the same manner as described [18]. The cultured media and cell lysates were immunoprecipitated (IP) followed by Western blot (WB) analysis both with anti-V5 antibody (Invitrogen). The immunoreactivity was visualized in the same manner as previously described [18].
Glycosylation in Podnl
The state of N-glycosylation of Podnl was examined as previously described [18]. Briefly, MC cells were plated onto 6 well culture plates at a density of 1 × 105 cells/well, and transfected with EV or pcDNA3.1-Podnl-V5/His vector. After 24 hours, the cultured medium was immunoprecipitated with anti-V5 antibody and the immunocomplex was collected using protein A-sepharose beads. Aliquots of the samples were then treated with N-glycosidase-F enzyme (Prozyme Inc., San Leandro, CA) for 24 hours at 37 °C, subjected to WB analysis, and the susceptibility of Podnl to the treatment was compared with the untreated sample.
Generation of anti-Podnl antibody and purification of Podnl-V5/His protein
In order to characterize the Podnl protein, an affinity purified-polyclonal Podnl antibody was generated by immunizing rabbits using the synthetic peptide (Bethyl laboratories Inc.). To characterize the anti-Podnl antibody, recombinant Podnl protein was generated and purified as follows. The 293 cells were transfected with the pcDNA3.1-Podnl-V5/His vector. The cells were cultured in DMEM containing 10% FBS and 400 µg/ml of G418 (Gibco) for 3–4 weeks, the clones derived from G418-resistant cells were isolated by cloning rings and further grown under the same conditions. Equal number of cells in each clone were plated, cultured for 3 days, cultured media were collected, and immunoprecipitated with anti-V5 antibody. After addition of Protein A-sepharose 4B conjugate, the samples were subjected to WB analysis with anti-V5 antibody. The clone which synthesized the highest level of Podnl-V5/His protein was further cultured for 6 days and the cultured medium was collected. Podnl-V5/His protein was purified using a Ni-NTA agarose resin (Qiagen). Following the nickel column purification, the fraction was further purified by a Superdex-200 column (GE Healthcare) equilibrated and eluted with 0.025M NH4HCO3 using a Hitachi D-7000 HPLC system. The purity of the Podnl-V5/His protein was assessed by 4–12% SDS-PAGE stained with Coomassie Brilliant Blue R-250 (CBB, Bio-Rad) and by WB analyses with anti-V5 and anti-Podnl antibodies. The purified Podnl protein was pooled, dialyzed against distilled water, lyophilized and dissolved in distilled water. The protein concentration was measured using a DC protein assay kit (Bio-Rad) and it was kept at −20 °C until use.
Podnl protein in bone matrix
Fetal bovine femurs were obtained (Aries Scientific Inc.) and stored at −80°C until use. After both ends of the bones and periosteum were cut, the middle third was longitudinally cut, bone marrow was removed, washed with cold PBS, defatted, lyophilized and pulverized in liquid nitrogen by a freezer mill (Spex CertiPrep). Bone matrix was first fractionated into mineral-non-associated and -associated fractions. The latter (mineral-associated) was further fractionated into EDTA soluble and insoluble fractions by the method described by Termine et al. [19] with some modifications [20]. Ten grams of bone powder was first extracted with 100 ml of 6M guanidine-HCl, pH 7.4, for 5 days at 4°C, the supernatant was separated by centrifugation at 15,000 × g for 30 min, exhaustively dialyzed against cold distilled water and lyophilized (G1 representing the matrix molecules not associated with mineral). The residue (mineral-associated) was then demineralized with 0.5M EDTA, pH 7.4, for 2.5 weeks at 4°C with several changes of EDTA, the supernatant was separated by centrifugation as described above, dialyzed against cold distilled water and lyophilized (E representing soluble matrix molecules associated with mineral). The residue was further extracted with 100 ml of 6M guanidine-HCl, pH 7.4, for 5 days at 4°C, the extract was collected by centrifugation, dialyzed and lyophilized (G2 including mineral-associated, insoluble matrix). Fetal bovine skin sample (Aries Scientific Inc.) was also prepared for comparison. After the hair was shaved off, the dermis was cut into small pieces on ice by a razor blade, pulverized, washed with cold PBS and with distilled water and lyophilized. The powder was then extracted with 6M guanidine-HCl, pH 7.4, as described above, the supernatant was collected, dialyzed against distilled water and lyophilized. Aliquotes of the samples/fractions (skin, G1, E, and G2 fractions of bone matrix) were dissolved in the lysis buffer and centrifuged. The protein concentration in the supernatant was determined by a DC protein assay kit. The purified Podnl-V5/His protein was used as a positive control. Equal amounts of proteins measured were then subjected to WB analysis with anti-Podnl and anti-V5 antibodies. Preimmune serum was used to confirm the specificity of anti-Podnl antibody.
Immunohistochemistry
To determine the distribution of Podnl in vivo, immunohistochemical staining was performed using VECTASTAIN Elite ABC Kit (Vector Laboratories Inc., Burlingame, CA). Formalin fixed paraffin embedded sections of normal mouse heads were deparaffinized with xylene and graded ethanol. The sections were then incubated with 0.3% H2O2 in ethanol for 30 min, followed by incubation with anti-Podnl antibody (diluted in 1:600) overnight at 4 °C. After washing with PBS several times, sections were further incubated with biotinylated IgG for 30 min and subsequently with avidin and biotinylated horseradish peroxidase for 30 min. Following several washes with PBS, 3, 3’-diaminobenzidine substrate (DAB; Vector Laboratories Inc.) was applied and the sections were also counterstained with hematoxylin (Sigma-Aldrich, St. Louis, MO). To verify the specificity of the anti-Podnl antibody, the effect of the synthetic peptide used for immunization on the immunoreactivity was evaluated. The anti-Podnl antibody with the synthetic peptide was incubated overnight at 4 °C and subjected to immunostaining in the same manner as describe above. The immunoreactivities of specimens visualized were photographed under a light microscope.
Results and discussion
Podnl is a novel member of SLRP family
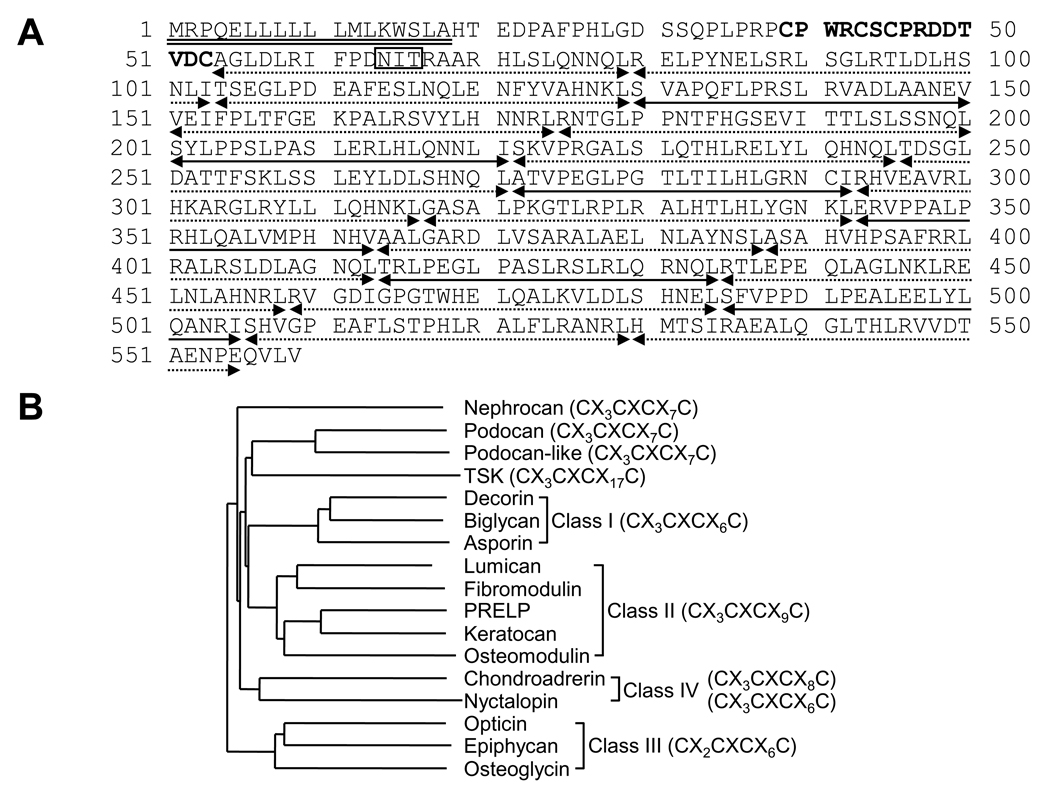
Through the computational screening, a SLRP-like extracellular molecule containing a signal peptide sequence lacking a transmembrane domain was identified. As shown in Fig. 1, Podnl possesses the structural features characteristic of SLRP family, i.e. a 18 amino acid long signal peptide sequence, a cysteine rich cluster (CX3CXCX7C, C; cysteine, X; any amino acids) at the N-terminus, a potential N-glycosylation site and 21 LRR motifs (Fig.1 A). There are two types of LRR motifs, type-T (zzxxaxxxxFxxaxxLxxLxLxxNxL) and type-S (xxaPzxLPxxLxxLxLxxNxI), where “z” is frequently a gap, “x” variable residues, and “a” alanine, valine, leucine, or isoleucine. In Podnl, of 21 LRR motifs, there are 15 type-T and 6 type-S (Fig. 1A). It has been reported that class I and II SLRPs have 12 LRR motifs composed of 4 tandem STT ‘super-motifs’, i.e. (STT)4, and that class III 7 LRRs composed of (ST)T(ST)2. Chondroadherin, a representative class IV member, contains 10 type-T LRRs, i.e. T10 [21]. Although the conserved leucine residues of LRR 21 in Podnl are substituted with threonine (position 21) and glutamic acid (position 26), the organization of LRRs in Podnl is composing of T3(STT)6, indicating that this protein is a novel, unique member of SLRP family.
Fig. 1.
The amino acid sequence and phylogenetic analysis of Podnl. (A) The deduced amino acid sequence of Podnl. The putative signal peptide sequence and cleavage site (indicated by a double underline) were predicted using the PSORT II program. The N-terminal cysteine cluster (CX3CXCX7C; C: cysteine, X: any amino acid) is indicated in bold letters. A potential N-glycosylation sites (NIT) is shown by an open box. Twenty-one LRR motifs are categorized by type-S or -T of LRR motif. Type-S LRR motif is indicated by a dotted line with arrows, while type-T is by a line with arrows. (B) Phylogenetic tree analysis of Podnl together with other mouse SLRP members. PRELP; proline/arginine-rich end leucine-rich repeat protein, TSK; tsukushi.
Podnl is highly expressed in mineralized tissues
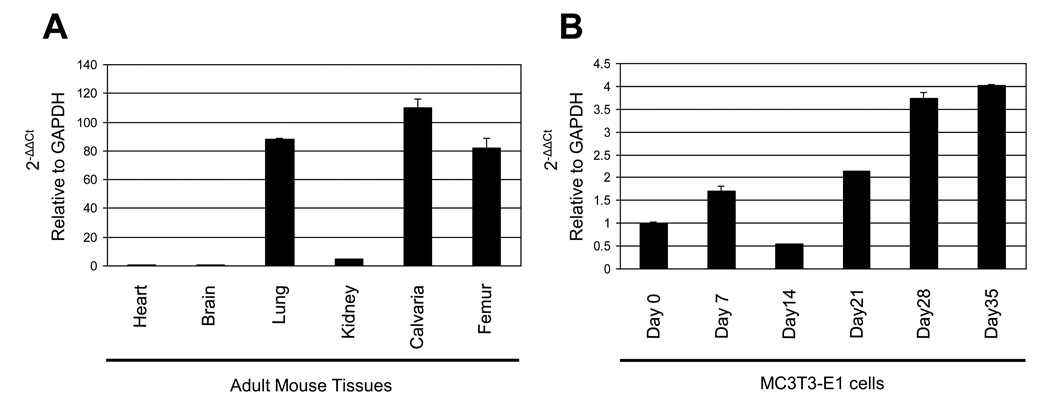
The expression levels of Podnl mRNA in various tissues are shown in Fig. 2A demonstrating that the expression in calvaria was the highest (~110 fold of that in heart) among the samples tested. The expression levels in lung and femur were also higher than those in other tissues (Fig. 2A). The Podnl mRNA expression pattern in MC cells during the course of cell differentiation and matrix mineralization is shown in Fig. 2B. Under the conditions used, by staining with Alizarin red, mineralized nodules were well formed at day 21 and further expanded at day 28 [13]. The expression level of Podnl was markedly increased at day 28 and 35 (i.e. ~3.9 fold at day 28 and ~4 fold at day 35 compared to that of day 0, Fig. 2B), suggesting that Podnl is potentially associated with matrix mineralization.
Fig. 2.
Gene expression of Podnl. Quantitative real time PCR analysis of Podnl mRNA expression was performed using adult mouse tissues (A) and cultured osteoblasts (B). The mean fold changes in the expression of Podnl relative to that of GAPDH were calculated using the value of the expression of either heart (A) or MC3T3-E1 cells at Day 0 in culture (B) as a calibrator. The values are shown as mean ± S.D. based on triplicate assays.
Podnl is a secreted glycoprotein
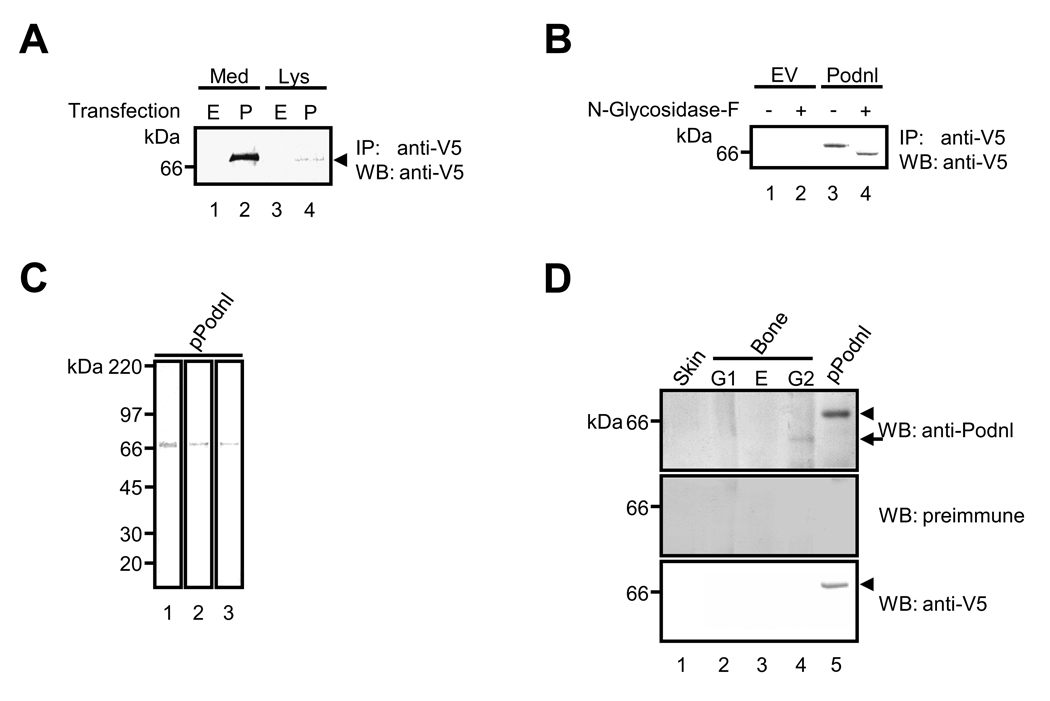
Since many, but not all, SLRP family members were secreted as proteoglycans carrying various types of GAG chains, we next determined whether Podnl is indeed secreted in the extracellular space and post-translationally modified with sugar moieties. After MC cells were transfected with Podnl expression vector, the presence of Podnl protein was examined in extracellular (medium) and intracellular (cell lysate) fractions. As shown in Fig. 3A, Podnl was mainly detected in the cultured medium fraction and to a much lesser extent in the cell lysate, demonstrating that Podnl is a secreted protein (Fig. 3A, lanes 2 and 4). The Podnl-V5/His protein produced by MC cells described above was subjected to various glycosidase digestion to examine the state of glycosylation. The treatment with endo-β-galactosidase, keratanase, or chondroitinase ABC did not change the migration position of the Podnl on the gel (data not shown), indicating that chondroitin/dermatan sulfate or keratin sulfate GAG chains are not attached to Podnl. However, when treated with N-glycosidase-F, the Podnl band migrated slightly faster than that of the untreated (Fig. 3B, lanes 3 and 4) confirming the presence of N-glycosylation in Podnl as the amino acid sequence suggested (Position 64 in Fig. 1A).
Fig. 3.
Presence of Podnl protein in bone. (A) Podnl is an extracellular protein. The immunoprecipitation (IP) - Western blot (WB) analysis after the transfection into MC3T3-E1 osteoblastic cells demonstrated that Podnl is mainly detected in the cultured medium fraction (Med) (indicated by an arrow head), and to much lesser extent, in the cell lysate fraction (Lys). Markers of molecular mass are shown on the left. E; transfected with an empty vector, P; transfected with Podnl-V5/His vector. (B) Podnl is a glycoprotein with N-glycosylation. Podnl-V5/His vector (Podnl) or an empty vector (EV) was transfected into MC3T3-E1 cells and cultured media were incubated with N-glycosidase-F. Note that the molecular mass of Podnl was lowered upon the treatment (lane 3 vs lane 4) determined by WB analysis with anti-V5 antibody. (C) The purity of Podnl-V5/His fusion protein (pPodnl) produced by 293 cells was verified by Coomassie brilliant blue-R250 staining (lane 1). The pPodnl was subjected to WB analysis with anti-Podnl antibody (lane 3) showing its immunoreactivity to pPodnl. The presence of V5 tag was also confirmed by WB analysis with anti-V5 antibody (lane 2). (D) WB analyses with anti-Podnl antibody (upper panel), preimmune serum (middle panel), or anti-V5 antibody (lower panel) in bovine fetal bone and skin tissues. Podnl-V5/His fusion protein (pPodnl) produced by 293 cells was used as a positive control (lane 5). G1; matrix molecules not associated with mineral, E; soluble matrix molecules associated with mineral, G2; mineral-associated, insoluble matrix.
Presence of Podnl protein in mineralized tissues
Since mRNA expression of Podnl was high in mineralized tissues, i.e. calvaria, femur and osteoblasts, we further investigate the presence of Podnl protein in vivo, i.e. bone. Firstly, in order to confirm the purity of Podnl-V5/His protein produced by 293 cells, the protein was stained by CBB (Fig. 3C, lane 1) and a single band was observed at the expected molecular weight size at ~67 kDa, i.e. 62kDa Podnl + 5kDa tag. The same protein was further analyzed by WB analysis using anti-Podnl and anti-V5 antibodies (Fig. 3C, lanes 2 and 3), and it was immunoreactive to both antibodies. These results demonstrate that the full-length Podnl-V5/His protein was successfully purified and that anti-Podnl antibody recognizes Podnl. Secondly, the tissue distribution of Podnl was examined by WB analysis with this antibody using the protein extracts derived from bone. As shown in Fig. 3D, the immunoreactive band of Podnl was clearly observed only in G2 (mineral-associated, insoluble matrix) fraction of bone, but not in G1 or E fraction of bone nor in skin, as an immunoreactive protein band at ~62 kDa (indicated by an arrow, upper panel, lane 4) with anti-Podnl antibody was detected. This molecular weight is consistent with the expected molecular size for endogenous Podnl protein. With the same antibody, an immunoreactive band for Podnl-V5/His protein (a positive control) was detected at ~67 kDa due to the presence of a V5/His tag (~5 kDa) (indicated by an arrow head, upper panel, lane 5). The immunoreactivity of Podnl-V5/His fusion protein was also verified with anti-V5 antibody (indicated by an arrow head, lower panel, lane 5). No immunoreactivity was observed when treated with preimmune serum (middle panel), thus confirming the specificity of anti-Podnl antibody. The data demonstrate that Podnl protein is present in bone matrix that is associated with mineralized insoluble collagen (Fig. 3D). This protein appears to be absent (or, if present, in a small quantity) in skin, a non-mineralized tissue.
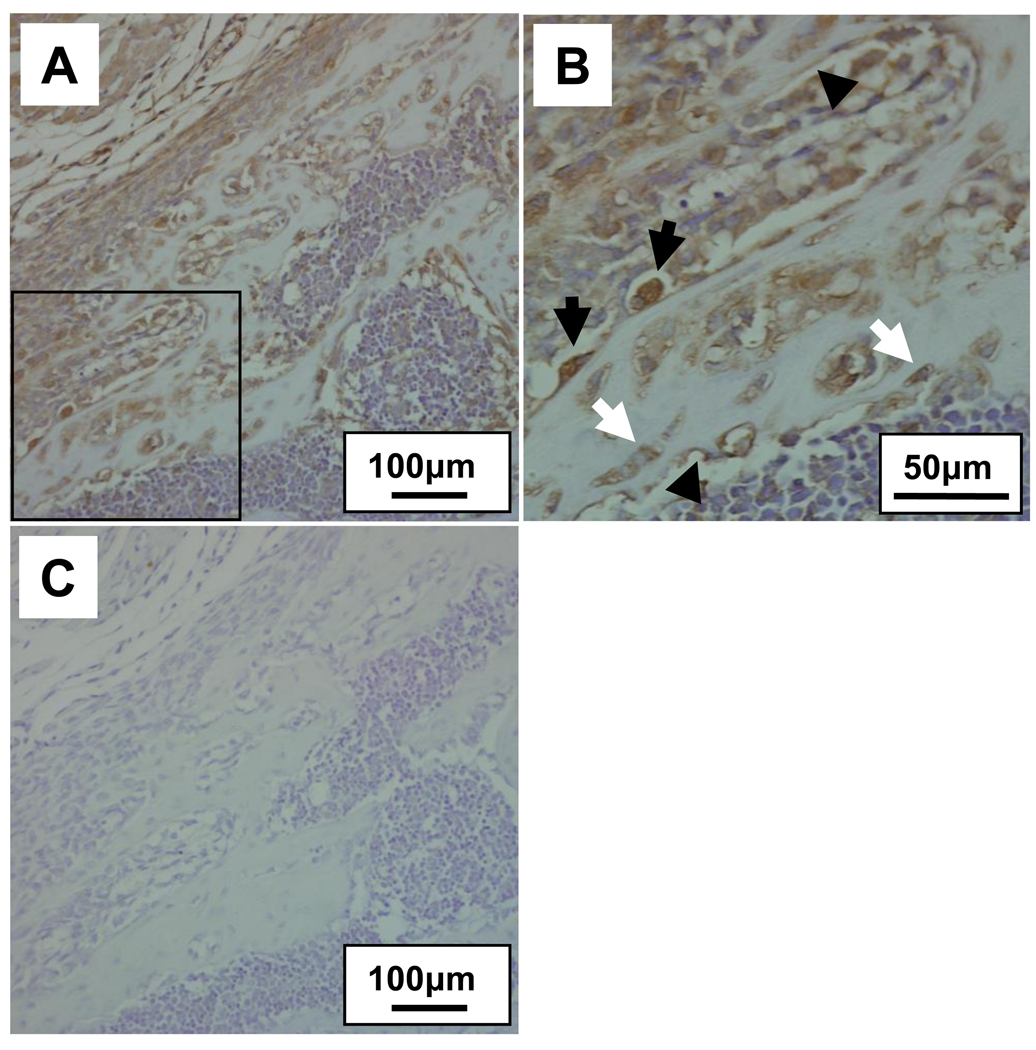
Using anti-Podnl antibody we further examined the distribution of Podnl protein in mouse bone. As shown in Fig. 4, osteoblasts on the bone surface, actively producing bone matrix and newly formed bone matrix were immunostained, and osteocytes were slightly stained (Fig. 4A and B). The anti-Podnl antibody with the synthetic peptide was also used as a negative control and no immunoreactivities were observed (Fig. 4C).
Fig. 4.
Immunohistological detection of Podnl in mouse bone. The mouse newborn alveolar bones were immunostained (Fig. A; lower magnification by 20X) and the immunoreactivity to anti-Podnl antibody was clearly observed in osteoblasts (indicated by black arrows) associated with bone, newly formed bone matrix (arrow heads) and osteocytes (white arrows) (Fig. 4B; higher magnification of open boxed area in Fig. 4A, 40X). No immunoreactivities were observed when anti-Podnl antibody was preincubated with the synthetic peptide used for antibody generation (Fig. 4C).
The potential functions of class I SLRP family members in mineralized tissues have been well investigated. DCN and its closely related SLRP member, biglycan (BGN), are two of the major class I SLRP members found in bone. DCN- and BGN-knockout (KO) mice have been generated and the phenotypes were characterized where BGN-KO mice displayed a decreased bone mass while DCN-KO mice did not. However, when genes for DCN and BGN are both disrupted, severe bone phenotypes, e.g. markedly retarded bone growth and osteopenia, occur, and these phenotypes are much more severe than those of the BGN single deficient animals [22], suggesting the critical importance and some redundancy of these two class I SLRP members in bone formation. The presence of the class II SLRPs, e.g. lumican and osteoadherin, and class III, e.g. osteoglycin, in bone, has been reported [23,24,25], but their quantities and functions in bone are still not well understood. The expression pattern of Podnl in mineralized matrix and osteoblasts strongly indicate the potential modulatory role of this novel SLRP in matrix mineralization. The highest expression levels of Podnl mRNA expression in MC cells were observed when matrix mineralization started and fully proceeded in vitro. This suggests its involvement in matrix mineralization. In support with this notion, Podnl protein in bone was detected primarily in the fraction that is associated with mineralized insoluble collagen matrix. Since the emerging SLRP function in bone biology and pathophysiology becomes clear, i.e. BGN promotes osteoblast differentiation [9,11,12], whereas DCN inhibits mineralization [10,13], the effect of Podnl on mineralized tissues may be through another signaling pathway and is currently under investigation.
Highlights.
-
>
A novel SLRP member, Podnl was identified.
-
>
Podnl was highly expressed in mineralized tissues/osteoblasts.
-
>
Podnl is present in bone, associated with insoluble matrix.
-
>
Podnl is a secreted glycoprotein.
Acknowledgements
This study was supported by grants from the National Institutes of Health (NIDCR-R21DE020909 and DE10489, M.Y.), University Research Council Grant (University of North Carolina at Chapel Hill, Y.M.) and Boston University Henry Goldman School of Dental Medicine (Y.M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Accession number
The nucleotide sequences for mouse Podocan-like gene have been deposited in the GeneBank database under GenBank Accession number DQ303463.
References
- 1.Yamauchi M, Woodley DT, Mechanic GL. Aging and cross-linking of skin collagen. Biochem Biophys Res Commun. 1988;152:898–903. doi: 10.1016/s0006-291x(88)80124-4. [DOI] [PubMed] [Google Scholar]
- 2.Uzawa K, Grzesik WJ, Nishiura T, Kuznetsov SA, Robey PG, Brenner DA, Yamauchi M. Differential expression of human lysyl hydroxylase genes, lysine hydroxylation, and cross-linking of type I collagen during osteoblastic differentiation in vitro. J Bone Miner Res. 1999;14:1272–1280. doi: 10.1359/jbmr.1999.14.8.1272. [DOI] [PubMed] [Google Scholar]
- 3.Pornprasertsuk S, Duarte WR, Mochida Y, Yamauchi M. Lysyl hydroxylase-2b directs collagen crosslinking pathways in MC3T3-E1 cells. J Bone Miner Res. 2004;19:1349–1355. doi: 10.1359/JBMR.040323. [DOI] [PubMed] [Google Scholar]
- 4.Saito M, Fujii K, Marumo K. Degree of mineralization-related collagen crosslinking in the femoral neck cancellous bone in cases of hip fracture and controls. Calcif Tissue Int. 2006;79:160–168. doi: 10.1007/s00223-006-0035-1. [DOI] [PubMed] [Google Scholar]
- 5.Vogel KG, Trotter JA. The effect of proteoglycans on the morphology of collagen fibrils formed in vitro. Coll Relat Res. 1987;7:105–114. doi: 10.1016/s0174-173x(87)80002-x. [DOI] [PubMed] [Google Scholar]
- 6.Vogel KG, Paulsson M, Heinegard D. Specific inhibition of type I and type II collagen fibrillogenesis by the small proteoglycan of tendon. Biochem J. 1984;223:587–597. doi: 10.1042/bj2230587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vogel KG, Koob TJ, Fisher LW. Characterization and interactions of a fragment of the core protein of the small proteoglycan (PGII) from bovine tendon. Biochem Biophys Res Commun. 1987;148:658–663. doi: 10.1016/0006-291x(87)90927-2. [DOI] [PubMed] [Google Scholar]
- 8.Hedbom E, Heinegard D. Interaction of a 59-kDa connective tissue matrix protein with collagen I and collagen II. J Biol Chem. 1989;264:6898–6905. [PubMed] [Google Scholar]
- 9.Chen XD, Fisher LW, Robey PG, Young MF. The small leucine-rich proteoglycan biglycan modulates BMP-4-induced osteoblast differentiation. Faseb J. 2004;18:948–958. doi: 10.1096/fj.03-0899com. [DOI] [PubMed] [Google Scholar]
- 10.Mochida Y, Duarte WR, Tanzawa H, Paschalis EP, Yamauchi M. Decorin modulates matrix mineralization in vitro. Biochem Biophys Res Commun. 2003;305:6–9. doi: 10.1016/s0006-291x(03)00693-4. [DOI] [PubMed] [Google Scholar]
- 11.Mochida Y, Parisuthiman D, Yamauchi M. Biglycan is a positive modulator of BMP-2 induced osteoblast differentiation. Adv Exp Med Biol. 2006;585:101–113. doi: 10.1007/978-0-387-34133-0_7. [DOI] [PubMed] [Google Scholar]
- 12.Parisuthiman D, Mochida Y, Duarte WR, Yamauchi M. Biglycan modulates osteoblast differentiation and matrix mineralization. J Bone Miner Res. 2005;20:1878–1886. doi: 10.1359/JBMR.050612. [DOI] [PubMed] [Google Scholar]
- 13.Mochida Y, Parisuthiman D, Pornprasertsuk-Damrongsri S, Atsawasuwan P, Sricholpech M, Boskey AL, Yamauchi M. Decorin modulates collagen matrix assembly and mineralization. Matrix Biol. 2009;28:44–52. doi: 10.1016/j.matbio.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iozzo RV. The family of the small leucine-rich proteoglycans: key regulators of matrix assembly and cellular growth. Crit Rev Biochem Mol Biol. 1997;32:141–174. doi: 10.3109/10409239709108551. [DOI] [PubMed] [Google Scholar]
- 15.Iozzo RV. Matrix proteoglycans: from molecular design to cellular function. Annu Rev Biochem. 1998;67:609–652. doi: 10.1146/annurev.biochem.67.1.609. [DOI] [PubMed] [Google Scholar]
- 16.Iozzo RV. The biology of the small leucine-rich proteoglycans. Functional network of interactive proteins. J Biol Chem. 1999;274:18843–18846. doi: 10.1074/jbc.274.27.18843. [DOI] [PubMed] [Google Scholar]
- 17.Schaefer L, Iozzo RV. Biological functions of the small leucine-rich proteoglycans: from genetics to signal transduction. J Biol Chem. 2008 doi: 10.1074/jbc.R800020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mochida Y, Parisuthiman D, Kaku M, Hanai J, Sukhatme VP, Yamauchi M. Nephrocan, a novel member of the small leucine-rich repeat protein family, is an inhibitor of transforming growth factor-beta signaling. J Biol Chem. 2006;281:36044–36051. doi: 10.1074/jbc.M604787200. [DOI] [PubMed] [Google Scholar]
- 19.Termine JD, Belcourt AB, Conn KM, Kleinman HK. Mineral and collagen-binding proteins of fetal calf bone. J Biol Chem. 1981;256:10403–10408. [PubMed] [Google Scholar]
- 20.Cheng H, Caterson B, Neame PJ, Lester GE, Yamauchi M. Differential distribution of lumican and fibromodulin in tooth cementum. Connect Tissue Res. 1996;34:87–96. doi: 10.3109/03008209609021494. [DOI] [PubMed] [Google Scholar]
- 21.Matsushima N, Ohyanagi T, Tanaka T, Kretsinger RH. Super-motifs and evolution of tandem leucine-rich repeats within the small proteoglycans--biglycan, decorin, lumican, fibromodulin, PRELP, keratocan, osteoadherin, epiphycan, and osteoglycin. Proteins. 2000;38:210–225. doi: 10.1002/(sici)1097-0134(20000201)38:2<210::aid-prot9>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 22.Corsi A, Xu T, Chen XD, Boyde A, Liang J, Mankani M, Sommer B, Iozzo RV, Eichstetter I, Robey PG, Bianco P, Young MF. Phenotypic effects of biglycan deficiency are linked to collagen fibril abnormalities, are synergized by decorin deficiency, and mimic Ehlers-Danloslike changes in bone and other connective tissues. J Bone Miner Res. 2002;17:1180–1189. doi: 10.1359/jbmr.2002.17.7.1180. [DOI] [PubMed] [Google Scholar]
- 23.Raouf A, Ganss B, McMahon C, Vary C, Roughley PJ, Seth A. Lumican is a major proteoglycan component of the bone matrix. Matrix Biol. 2002;21:361–367. doi: 10.1016/s0945-053x(02)00027-6. [DOI] [PubMed] [Google Scholar]
- 24.Ramstad VE, Franzen A, Heinegard D, Wendel M, Reinholt FP. Ultrastructural distribution of osteoadherin in rat bone shows a pattern similar to that of bone sialoprotein. Calcif Tissue Int. 2003;72:57–64. doi: 10.1007/s00223-002-2047-9. [DOI] [PubMed] [Google Scholar]
- 25.Fernandez B, Kampmann A, Pipp F, Zimmermann R, Schaper W. Osteoglycin expression and localization in rabbit tissues and atherosclerotic plaques. Mol Cell Biochem. 2003;246:3–11. [PubMed] [Google Scholar]