Abstract
Androgen deprivation therapy (ADT) is the main treatment approach in advanced prostate cancer and in recent years has primarily involved the use of gonadotropin-releasing hormone (GnRH) agonists. However, despite their efficacy, GnRH agonists have several drawbacks associated with their mode of action. These include an initial testosterone surge and testosterone microsurges on repeat administration. GnRH antagonists provide an alternative approach to ADT with a more direct mode of action that involves immediate blockade of GnRH receptors. Antagonists produce a more rapid suppression of testosterone (and prostate-specific antigen [PSA]) without a testosterone surge or microsurges and appear to offer an effective and well tolerated option for the hormonal treatment of prostate cancer. Comparisons with GnRH agonists have shown GnRH antagonists to be at least as effective in achieving and maintaining castrate testosterone levels in patients with prostate cancer. Furthermore, with antagonists, the lack of an initial testosterone surge (which may cause clinical flare) may allow more rapid relief of symptoms related to prostate cancer, avoid the need for concomitant antiandrogens to prevent clinical flare (so avoiding any antiandrogen-associated adverse events) and allow GnRH antagonist use in patients with high tumour burden and/or acute problems such as spinal cord compression. Although several antagonists have been investigated, only degarelix and abarelix are currently available for clinical use in prostate cancer. Currently, degarelix is the most extensively studied and widely available agent in this class. Degarelix is one of a newer generation of antagonists which, in a comprehensive and ongoing clinical development programme, has been shown to provide rapid, profound and sustained testosterone suppression without the systemic allergic reactions associated with earlier antagonists. This review examines the currently available data on GnRH antagonists in prostate cancer.
Keywords: abarelix, degarelix, GnRH agonist, GnRH antagonist, prostate cancer
Introduction
Around 70 years ago, Huggins and Hodges first demonstrated the responsiveness of metastatic prostate cancer (PCa) to androgen suppression [Huggins and Hodges, 1941]. Since then, androgen deprivation therapy (ADT) has formed the mainstay of treatment for advanced disease. ADT originally involved orchiectomy or estrogens; however, these modalities were superseded by gonadotropin-releasing hormone (GnRH) agonists. The agonists avoid the emotional and psychological effects of surgical castration and in recent decades have become the most common approach to ADT [Heidenreich et al. 2010]. However, these agents have drawbacks associated with their mode of action, including an initial testosterone surge, which can delay castration and may stimulate PCa cells, leading to an exacerbation of clinical symptoms (flare) [Thompson, 2001; Van Poppel and Nilsson, 2008]; agonists can also cause testosterone microsurges on repeat administration.
More recently, a new approach to ADT has emerged in the form of GnRH antagonism. This involves direct and immediate blockade of GnRH receptors, preventing release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) and producing rapid suppression of testosterone without an initial surge. The first antagonist to undergo clinical development was abarelix, which was approved for clinical use in the USA at the end of 2003 [Mongiat-Artus and Teillac, 2004]. However, in response to reports of systemic allergic reactions, an extensive risk management programme was imposed and in 2005 abarelix was voluntarily withdrawn from the US market [Huhtaniemi et al. 2009]. The drug is still available in Germany for the initiation of medical castration in advanced or metastatic hormone-dependent PCa when androgen suppression is necessary [Kirby et al. 2009]. Launch of abarelix across Europe is planned following the approval of the drug in 11 additional European Union countries in August 2010 (see http://www.specialityeuropeanpharma.com/sepproducts.html).
More recently, degarelix, the first of a new generation of GnRH antagonists, has been developed for the treatment of PCa. In a comprehensive and ongoing clinical development programme, degarelix provided fast, profound and sustained testosterone suppression without the systemic allergic reactions associated with abarelix. Degarelix is indicated for the treatment of advanced hormone-dependent PCa and is currently approved for use in many countries in North and South America and Europe. This review will examine currently available data on GnRH antagonists in PCa.
Mechanism of action
GnRH antagonists induce castration by a different mechanism to that of GnRH agonists. Agonists produce intense initial stimulation of GnRH receptors, causing a marked rise in LH and FSH and, as a consequence, testosterone. Eventually, agonist-induced overstimulation overrides the natural pulsatile control of LH release, leading to receptor desensitization or downregulation, which suppresses LH and FSH secretion and reduces testosterone to castrate levels [Heidenreich et al. 2010]. In contrast, GnRH antagonists act more directly, competitively binding to and blocking pituitary GnRH receptors and causing an immediate blockade of LH and FSH secretion [Heidenreich et al. 2010; Van Poppel and Nilsson, 2008]. This produces rapid testosterone suppression, without any initial stimulation or surge.
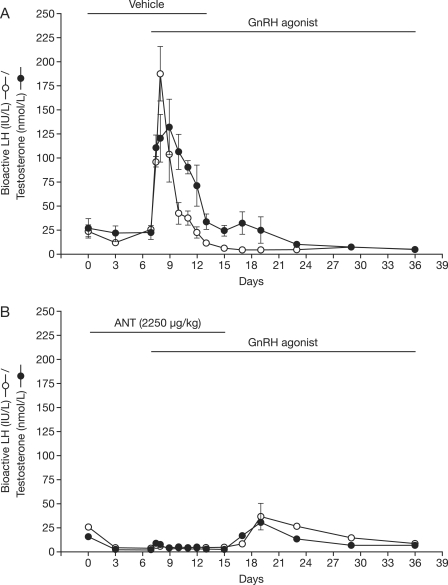
Animal studies show not only that GnRH antagonist administration suppresses LH and testosterone to castrate levels but also that administration of a GnRH antagonist before an agonist will blunt the characteristic agonist-induced LH and testosterone surge (Figure 1) [Pinski et al. 1992; Sharma et al. 1992]. However, after the antagonist is removed, there is an increase in testosterone and LH, presumably agonist driven, to above castration levels. Although the increase is more modest than that with agonist alone, it appears to be more prolonged. Thus agonist-induced testosterone suppression appears to require at least some level of receptor activation to induce downregulation, and thus some degree of initial testosterone induction is likely – it appears that the agonist-induced testosterone surge may be blunted but not eliminated by prior administration of an antagonist.
Figure 1.
Mean (+SE) of serum bioactive luteinizing hormone (LH; open circles) and testosterone (filled circles) of adult male monkeys (five per group) receiving an implant loaded with the gonadotropin (GnRH) agonist (buserelin) on day 7. On days 0–15 animals were treated, in addition to the GnRH agonist, with GnRH antagonist vehicle (upper panel) or Nal-Glu GnRH antagonist at a dose of 2250 µg/kg daily (bottom panel). ANT, antagonist. (Reproduced with permission from Sharma et al. [1992]).
Abarelix
Clinical efficacy
A phase II open-label trial in 242 patients with PCa requiring initial hormonal treatment compared the efficacy of abarelix (n = 209) with that of GnRH agonists (n = 33) ± antiandrogen. Abarelix 100 mg was administered intramuscularly on days 1 and 15 followed by 50 mg on day 29 and every 28 days thereafter; results were reported only up to day 27 [Tomera et al. 2001]. Phase III studies included two US trials and a European trial. In a 3-month US trial, 269 men with locally advanced and metastatic disease were randomized to abarelix 100 mg (n = 180) or leuprolide 7.5 mg (n = 89) monthly; the abarelix group received an additional injection on day 15 [McLeod et al. 2001]. In the second US trial, 255 patients with stage D1/D2 disease were randomized to abarelix 100 mg monthly (n = 170) or leuprolide 7.5 mg monthly and bicalutamide 50 mg daily (n = 85) over 24 weeks [Trachtenberg et al. 2002]. In the European phase III trial, 177 patients with advanced or metastatic cancer were treated for 48 weeks with abarelix 100 mg 4-weekly or goserelin 3.6 mg 4-weekly plus bicalutamide 50 mg daily [Selvaggi et al. 2001].
In phase II/III trials, abarelix effectively suppressed testosterone to castrate levels in patients with PCa but did not cause an associated testosterone surge. After 27–29 days, castration was achieved in ≥93% of patients receiving abarelix [Trachtenberg et al. 2002; McLeod et al. 2001; Selvaggi et al. 2001; Tomera et al. 2001]. Abarelix was as effective in achieving castration as GnRH agonists ± antiandrogens but was associated with a more rapid suppression of testosterone [Tomera et al. 2001]. A US phase III trial (n = 269) showed that in men who had achieved castration by day 29, castration was maintained in 98.8% of the abarelix group and 97.7% of the leuprolide group [McLeod et al. 2001]. In the European phase III trial (n = 177), castration was achieved by day 84 in 99% of patients receiving abarelix and 100% of those receiving goserelin/bicalutamide [Selvaggi et al. 2001].
Prostate-specific antigen (PSA) reductions during phase III trials were similar for abarelix and GnRH agonists ± antiandrogen, although in two trials this was initially more rapid with abarelix [Mongiat-Artus and Teillac, 2004; McLeod et al. 2001]. Abarelix was associated with rapid decreases in LH and FSH levels in US phase III trials [Trachtenberg et al. 2002; McLeod et al. 2001].
Long-term clinical efficacy
In a US phase III trial, long-term ability to achieve and maintain castration was maintained at 24 weeks in a similar proportion of abarelix- and GnRH agonist/antiandrogen-treated patients (90.4% vs. 83.8% of patients, respectively) [Trachtenberg et al. 2002]. However, based on data from two randomized, open-label, active-comparator trials, the abarelix package insert warned that effectiveness of testosterone suppression decreases with continued dosing in some patients and that effectiveness beyond 12 months had not been established (see http://www.druglib.com/druginfo/plenaxis). Indeed, waning of castration rates after 24 weeks appeared to occur more frequently with abarelix than active controls [Mongiat-Artus and Teillac, 2004]. In the phase III European trial, escape from castration was more common with abarelix (22%) than agonist/antiandrogen (8%) and time to escape was significantly shorter with abarelix (p = 0.007) [Debruyne et al. 2006].
Safety
In phase III studies, abarelix displayed a safety profile generally comparable to that of leuprolide ± bicalutamide [Debruyne et al. 2006; Trachenberg et al. 2002; McLeod et al. 2001]. However, allergic reactions were a major concern with abarelix [Mongiat-Artus and Teillac 2004]. Overall, immediate-onset systemic allergic reactions were observed in 15/1397 (1.1%) patients with PCa (mostly in men without advanced symptomatic disease) who received abarelix [Mongiat-Artus and Teillac, 2004]. The cumulative risk of these reactions increased with duration of treatment. Furthermore, in a trial involving patients with advanced symptomatic PCa, 3.7% of patients experienced immediate-onset systemic allergic reactions (see http://www.druglib.com/druginfo/plenaxis).
Degarelix
Degarelix is a third-generation GnRH antagonist synthetically modified with a view to reducing the histamine-releasing activity associated with previous GnRH antagonists.
Preclinical studies
In preclinical studies, degarelix demonstrated a fast and sustained dose-dependent and reversible suppression of the pituitary-gonadal axis, manifested by decreases in LH and testosterone levels, with a longer duration of action than other antagonists [Broqua et al. 2002]. Degarelix also inhibited tumour growth to a similar extent to surgical castration [Princivalle et al. 2007]. In animal studies [Broqua et al. 2002] and an ex vivo human skin model [Koechling et al. 2010], degarelix displayed only weak histamine-releasing properties, having the lowest propensity for histamine release among the GnRH antagonists tested.
Pharmacokinetics
Pharmacokinetic analysis indicates that upon subcutaneous administration degarelix forms a depot from which the drug distributes to the rest of the body [Steinberg, 2009]. In rats, degarelix release from the depot was characterized by a short initial burst followed by plasma concentrations decaying with a half-life of several weeks [White et al. 2007].
Following subcutaneous administration of degarelix 240 mg to PCa patients in the phase III trial (CS21), AUC0–28 days was 635 ng/day per ml and Cmax was 66.0 ng/ml and occurred at tmax = 40 h. Degarelix is eliminated biphasically, with a median terminal half-life of ∼43 days for the starting dose and 28 days for the maintenance dose.
Clinical efficacy
Long-term phase II dose-finding trials
The efficacy and safety of degarelix has been evaluated in two randomized, 1-year dose-finding trials in Europe/South Africa [Van Poppel et al. 2008] and North America [Gittelman et al. 2008]. Degarelix produced fast, profound and sustained suppression of testosterone and PSA, with no evidence of a testosterone surge or systemic allergic reactions. The most effective starting dose was 240 mg and the most suitable maintenance doses were 80 mg and 160 mg; these doses were investigated further in a large phase III trial.
Phase III efficacy trial (CS21)
The efficacy of degarelix was compared with the GnRH agonist leuprolide in a pivotal 1-year randomized phase III trial (CS21) in patients with histologically confirmed PCa (all stages) for whom ADT was indicated [Klotz et al. 2008]. Patients (n = 610) received degarelix 240 mg for 1 month followed by monthly doses of 80 mg (n = 207) or 160 mg (n = 202), or leuprolide 7.5 mg/month (n = 201) for 1 year. Concomitant antiandrogen was available as flare protection in the leuprolide group at the investigator’s discretion.
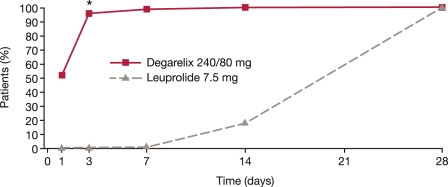
Both degarelix doses were as effective as leuprolide at reducing serum testosterone throughout the trial. Treatment response (testosterone suppression ≤0.5 ng/ml between 28 and 364 days) was achieved by similar proportions of patients in all three groups: 96.4–98.3% (intention-to-treat population). Degarelix was significantly faster than leuprolide at suppressing testosterone and PSA. By day 3, testosterone levels ≤0.5 ng/ml were achieved by 96.1% in the degarelix 240/80 groups compared with 0% in the leuprolide group [Klotz et al. 2008]; by day 14 these values were 100 and 18.2%, respectively (Figure 2) [Boccon-Gibod et al. 2008]. Testosterone surge (increase of ≥15% from baseline on any 2 days during the first 2 weeks of treatment) was reported in 80% of patients receiving leuprolide versus 0% of those receiving degarelix. Eight patients (4%) receiving leuprolide had testosterone microsurges (increases of >0.25 ng/ml) in the week following the ninth injection; no testosterone microsurges were detected with degarelix [Klotz et al. 2008].
Figure 2.
Percentage of patients with testosterone ≤0.5 ng/ml during the first month of treatment in the phase III comparative study CS21. *p < 0.001 versus leuprolide (pairwise comparisons by Fisher’s exact test) [Adapted from Persson et al. 2009].
PSA was also suppressed more rapidly with degarelix: by day 14, median PSA had fallen by 64% and 65% in the 240/80 and 240/160 groups, respectively, compared with 18% for leuprolide; by day 28 these values were 85%, 83% and 68%. Degarelix also rapidly reduced median LH and FSH levels, which remained suppressed until the end of the trial. With leuprolide, however, there was an initial increase in LH and FSH, and FSH levels did not fall to the same extent as in the degarelix arms [Klotz et al. 2008].
Secondary endpoints from phase III efficacy trial
Prostate-specific antigen
Analyses of PSA data from the CS21 trial showed a significantly lower risk of PSA failure or death for patients receiving degarelix 240/80 mg compared with leuprolide 7.5 mg (p = 0.05; log-rank) during the first year of treatment. PSA failure occurred mainly in patients with advanced disease and exclusively in those with baseline PSA >20 ng/ml [Tombal et al. 2010].
Patients with baseline PSA >20 ng/ml showed a significantly longer time to PSA failure with degarelix (p = 0.04; log-rank) [Tombal et al. 2010]. In patients with baseline PSA >50 ng/ml, 29.2% of those receiving degarelix and 40.0% of those receiving leuprolide experienced PSA failure (the difference was not statistically significant; p = 0.10).
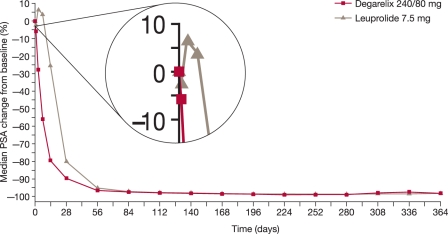
In patients with metastatic disease, 21.6% of those in the degarelix 240/80 mg group and 36.2% of those in the leuprolide group experienced PSA failure (the difference was not statistically significant; p = 0.156). An initial increase in PSA observed in patients with metastatic disease receiving leuprolide was not seen in the degarelix group [Tombal et al. 2010] (Figure 3). In the locally advanced subgroup, the proportion of patients with PSA failure was similar between treatment groups.
Figure 3.
Median percentage change in prostate-specific antigen (PSA) from baseline for degarelix 240/80 mg versus leuprolide.
Overall, the proportion of patients achieving PSA < 4 ng/ml over time was similar in both groups, although this was achieved more rapidly with degarelix. PSA suppression < 4 ng/ml at day 28 was achieved by 59% of patients receiving degarelix versus 34% receiving leuprolide (p < 0.0001). Corresponding proportions at day 364 were 83% and 78% (difference not statistically significant; p = 0.339). For patients with metastatic disease, a higher proportion of those receiving degarelix achieved PSA < 4 ng/ml over the study period [Tombal et al. 2010].
Overall, these results may suggest the hypothesis that degarelix at 240/80 mg offers improved PSA control compared with leuprolide.
Serum alkaline phosphatase
Elevated levels of the bone markers serum alkaline phosphatase (S-ALP) and bone-specific alkaline phosphatase have been associated with progression of skeletal metastases in PCa [Lein et al. 2007; Lorente et al. 1996] and are significant predictors of early death [Robinson et al. 2008; Johansen et al. 2007; Ramankulov et al. 2007; Jung et al. 2004].
In CS21, S-ALP levels in both treatment groups were maintained around baseline in patients with localized and locally advanced disease [Schröder et al. 2010]. In patients with metastatic disease, baseline S-ALP levels were high, reflecting the presence of skeletal metastases. In this cohort, after initial peaks in both groups, S-ALP was suppressed by both treatments, falling below baseline by day 56 with degarelix 240/80 mg and by day 84 with leuprolide. At day 364, reduction in S-ALP was significantly greater with degarelix 240/80 mg than with leuprolide (mean S-ALP at day 364: leuprolide 179 IU/l, degarelix 96 IU/l, p = 0.014). In metastatic disease, the late rise in S-ALP levels seen during leuprolide treatment, which might suggest therapy failure, was not apparent with degarelix.
Analysis by baseline PSA showed S-ALP levels 3–4 times higher in patients with baseline PSA ≥50 ng/ml versus < 50 ng/ml. After initial peaks in both groups, patients with baseline PSA ≥50 ng/ml had greater absolute reductions in S-ALP and reductions from baseline with degarelix 240/80 mg than with leuprolide. Furthermore, there was a late rise in S-ALP with leuprolide (returning to baseline levels before the trial end), which was not observed with degarelix (S-ALP levels remained below baseline at trial end).
In patients with baseline PSA ≥50 ng/ml, the difference in suppression at day 364 between degarelix and leuprolide was statistically significant (83 vs. 163 IU/l; p = 0.007). In patients with PSA < 50 ng/ml, S-ALP was maintained around baseline levels, irrespective of treatment.
These results may allow the generation of a hypothesis that suggests better S-ALP control and prolonged control of skeletal metastases with degarelix compared with the GnRH agonist leuprolide over a 1-year treatment period.
Effect of concomitant antiandrogen therapy. In CS21, 11% of patients (23/201) in the leuprolide group received concomitant bicalutamide for flare protection. A testosterone surge occurred in 81% of patients (144/178) receiving leuprolide without bicalutamide compared with 74% (17/23) of those receiving leuprolide and bicalutamide [Klotz et al. 2008].
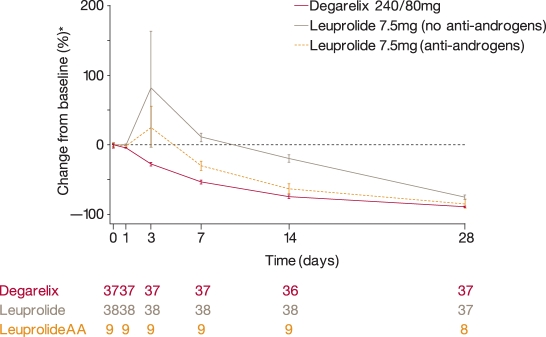
PSA reduction from baseline was more rapid in patients receiving leuprolide/bicalutamide than in those receiving leuprolide alone. Thus, for patients starting antiandrogen therapy on/before day 7, median PSA was reduced by 61.7% on day 14 and 89.1% on day 28, compared with reductions of 15.3% and 61.7%, respectively, for patients not on antiandrogens. Indeed, PSA reduction from baseline in patients receiving leuprolide/bicalutamide was similar to that with degarelix [Klotz et al. 2008]. In patients with metastatic disease, there was an initial PSA surge in patients receiving leuprolide without concomitant antiandrogens; a smaller PSA surge was noted in leuprolide patients who were receiving concomitant antiandrogens (Ferring, data on file) (Figure 4). No PSA surge was observed with degarelix 240/80 mg in the metastatic patient cohort.
Figure 4.
Mean (± standard error) percentage change in prostate-specific antigen from baseline during the first 28 days of treatment in patients with metastatic disease receiving degarelix 240/80 mg and in those who received leuprolide with or without antiandrogens (AA) in the phase III CS21 trial.
The probability of testosterone ≤0.5 ng/ml from day 28 to day 364 (intention-to-treat analysis) was similar among patients who received no antiandrogen therapy (96.5%) and those starting antiandrogens on/before day 7 (95%) (Ferring, data on file).
Thus, since addition of an antiandrogen to a GnRH agonist does not always prevent testosterone surge, a GnRH antagonist may be a more reliable approach to surge and, as a consequence, flare protection.
Effect of baseline body weight on efficacy
During CS21, patients were stratified by body weight (<90 kg and ≥90 kg). Of 620 patients randomized to treatment, the majority (501/620) were < 90 kg. There were no discernable trends among patients receiving degarelix 240/80 mg indicating an effect of body weight on any of the following efficacy endpoints: the proportion of patients with testosterone ≤0.5 ng/ml from day 28 to day 364; the proportion of patients with testosterone ≤0.5 ng/ml on day 3; the proportion of patients with testosterone surge during the first 2 weeks of treatment; and the percentage change in PSA from baseline to day 14 and to day 28 (Ferring, data on file). Thus, no dose adjustments are required for heavier patients (i.e. body weight ≥90 kg) receiving degarelix 240/80 mg.
Long-term extension of phase III clinical trial
Patients completing CS21 were eligible to enter an ongoing 5-year extension study (CS21A) of safety and efficacy in patients continuing on degarelix or crossing over from leuprolide to degarelix after 1 year [Persson et al. 2010; Plekhanov et al. 2010]. Patients initially receiving degarelix continued with the same monthly maintenance dose (160 mg or 80 mg), while those previously receiving leuprolide 7.5 mg were randomized to a starting dose of degarelix 240 mg followed by monthly maintenance doses of 80 mg or 160 mg.
Beyond 1 year, the risk of PSA failure or death decreased in patients switched from leuprolide to degarelix. At a median follow up of 27.5 months, there was a significant improvement in PSA progression-free survival hazard rates from 0.20 events/year in the first year to 0.08 events/year following switch in the leuprolide/degarelix group (p = 0.003); corresponding hazard rates for the continuous degarelix 240/80 mg group were 0.11 and 0.14 events/year (difference not statistically significant; p = 0.464), demonstrating a consistent effect of degarelix over time. The same pattern of hazard change was observed in the subgroup of patients with baseline PSA levels >20 ng/ml. After a median follow up of 27.5 months, PSA progression-free survival hazard rates improved from 0.38 events/year in the first year to 0.19 events/year following the switch to degarelix in leuprolide patients (p = 0.031); corresponding hazard rates for degarelix were 0.23 and 0.23 events/year (difference not statistically significant; p = 0.988) [Plekhanov et al. 2010].
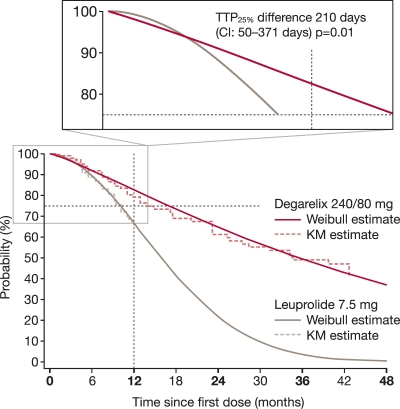
The time for 25% of patients with baseline PSA >20 ng/ml to experience PSA failure or death (TTP25%) was numerically longer with degarelix than leuprolide (407 vs. 303 days; difference not statistically significant, p = 0.085) over 1 year. An even greater difference was seen when analysing TTP25% using degarelix data beyond 1 year: 514 versus 303 days (p = 0.01) for degarelix versus leuprolide [Plekhanov et al. 2010] (Figure 5).
Figure 5.
The probability of freedom from prostate-specific antigen (PSA) failure or death over time for patients with baseline PSA ≥20 ng/ml in the CS21 and CS21a trials. The magnified area of the graph shows the time for 25% of patients with baseline PSA ≥20 ng/ml to experience PSA failure or death (TTP25%).
Comparison of degarelix with abarelix
Although there are no trials directly comparing the GnRH antagonists abarelix and degarelix, indirect comparisons between studies suggest that achievement of castration with degarelix may be more rapid than with abarelix [European Medicines Agency, 2010; Trachtenberg et al. 2002; McLeod, et al. 2001].
Safety
Overall safety
Degarelix was well tolerated during its clinical development programme. In the 1-year phase II and III studies, most adverse events were related to androgen deprivation (i.e. hot flushes, weight increase, etc.). The most frequent adverse events were hot flushes, injection-site pain, and fatigue [Gittelman et al. 2008; Klotz et al. 2008; Van Poppel et al. 2008]. Most events were mild to moderate in intensity; there were no dose-dependent side effects or systemic allergic reactions.
In the 1-year phase III study [Klotz et al. 2008], both degarelix 240/80 mg or 240/160 mg and leuprolide had a similar incidence of adverse events. As in phase II studies, there were no systemic allergic reactions with degarelix. Degarelix was associated with a higher rate of injection-site reactions than leuprolide (40% in pooled degarelix arms vs. < 1%, respectively, p < 0.001), although these occurred predominantly after the first injection and were mostly mild or moderate. Moreover, the difference in injection-site reaction rates may reflect different routes of administration (subcutaneous with degarelix vs. intramuscular with leuprolide) and injection volume. Local injection-site reactions have also been noted with GnRH agonists when administered subcutaneously (see http://products.sanofi-aventis.us/eligard/eligard.pdf) [Oka et al. 2006]. While there was also a higher incidence of chills reported with degarelix, there was a significantly lower incidence of musculoskeletal events (arthralgia) and urinary tract infections with degarelix than leuprolide. In total, five patients (2%) died in each of the degarelix groups, compared with nine (4%) in the leuprolide group; none of the deaths were considered to be related to the study drugs.
Musculoskeletal adverse events
Musculoskeletal adverse events were reported in 17% versus 26% (p = 0.001) of patients in the degarelix and leuprolide groups, respectively, during the 1-year phase III trial [Crawford et al. 2010a]. Results from the long-term extension study (CS21A) showed that beyond 1 year, the musculoskeletal event rate was similar between patients continuing on degarelix (16%) and those switching from leuprolide to degarelix (18%, difference not statistically significant, p = 0.75) [Crawford et al. 2010b]. These observations suggest a lower incidence of musculoskeletal adverse events with degarelix than with leuprolide.
The significantly lower musculoskeletal adverse event rate with degarelix during CS21 and the improved musculoskeletal adverse event rate for those switching to degarelix during CS21A, together with the better S-ALP control than leuprolide in patients with metastatic disease [Schroder et al. 2010] support the hypothesis that degarelix may further prolong control of skeletal metastases compared with GnRH agonists during long-term treatment.
Cardiovascular safety
A recent analysis evaluated the comparative cardiovascular safety of degarelix 240/80 mg or 240/160 mg and leuprolide in the phase III CS21 trial [Smith et al. 2010]. Outcomes included QT interval and cardiovascular adverse events. Overall, degarelix and leuprolide displayed similar cardiovascular safety profiles. There were no significant differences between treatment groups for mean change in Fridericia’s correction of QT (QTcF) during the trial. Marked prolongation of the QTc interval was uncommon (≤1%) with either agent. There were no significant differences in rates of new ischaemic heart disease or arrhythmias. Supraventricular arrhythmias were the most common type of arrhythmia (pooled degarelix group 2%, leuprolide 4%). Other arrhythmias occurred in ≤1% of patients in each group. The most frequently reported cardiac disorder was ischaemic heart disease (occurring in 4% of patients treated with degarelix and 10% of those on leuprolide).
Recently, the US Food and Drug Administration required manufacturers of GnRH agonists to include new safety warnings on their labels regarding the increased risk of diabetes and certain cardiovascular diseases [US Food and Drug Administration, 2010]. In contrast, most (but not all) studies have reported that orchiectomy is not associated with greater risk of cardiovascular events [Levine et al. 2010; Alibhai et al. 2009; Keating et al. 2006]. These observations raise the possibility that cardiovascular risk may vary for different forms of ADT.
Additional analyses of men treated with degarelix showed a greater risk of cardiovascular events was associated with established cardiovascular disease; risk was also influenced by modifiable risk factors (e.g. obesity and alcohol consumption) but not variation in degarelix doses or testosterone values [Klotz et al. 2010]. This appears to suggest that the cardiovascular risk in patients treated with degarelix may be driven by normal aging.
Ongoing trials
The currently available data on the use of degarelix in advanced PCa, including evidence of the lack of clinical flare and subsequent testosterone microsurges, has led to interest in its potential use in a number of additional clinical settings, which are being investigated in several ongoing clinical trials. These include: comparison of the safety and efficacy of degarelix given intermittently versus continuous ADT with leuprolide or degarelix in patients with PCa with prior treatment failure after localized treatment (CS37; ClinicalTrials.gov identifier: NCT00928434); comparison of degarelix with goserelin/bicalutamide, in terms of prostate size reduction, in patients of intermediate to high risk who require neoadjuvant hormone therapy before radiotherapy (curative intent) (CS30; ClinicalTrials.gov identifier: NCT00833248); comparison of degarelix with goserelin/bicalutamide, in terms of prostate volume reduction, in patients who are candidates for medical castration (CS31; ClinicalTrials.gov identifier: NCT00884273); comparison of the efficacy and safety of degarelix 3-month depot with goserelin in patients requiring ADT (CS35; ClinicalTrials.gov identifier: NCT00946920); an uncontrolled trial of degarelix as intermittent androgen deprivation for ≥1 cycle (CS29; ClinicalTrials.gov identifier: NCT00801242); an uncontrolled, exploratory trial of degarelix as second-line hormonal treatment after PSA failure in GnRH agonist-treated patients with PCa (CS27; ClinicalTrials.gov identifier: NCT00738673). The results of these studies should help to provide invaluable data on the efficacy, safety and quality of life associated with degarelix use in a variety of therapeutic settings in PCa.
Other GnRH antagonists
Other GnRH antagonists, primarily in the early stages of development, have been investigated in PCa over the years [Huirne et al. 2001] but only degarelix and abarelix are available for clinical use.
Cetrorelix is used to prevent premature ovulation in controlled ovarian stimulation before assisted reproductive technologies (see http://www. merckserono.com/en/products/fertility/infertility/cetrotide/cetrotide.html); however, development in PCa has been discontinued. In prostate tumour xenografts in a nude mouse model, final volume and weight of tumours were significantly reduced after 8 weeks’ treatment with cetrorelix [Lamharzi et al. 1998]. Cetrorelix also inhibited growth of an androgen-independent PCa cell line in vivo [Jungwirth et al. 1997]. Primary cell cultures from human PCa showed a reduction in cell growth rate and increase in DNA-fragmented cell number with cetrorelix [Castellón et al. 2006]. Several phase I/II studies were conducted with cetrorelix in patients with advanced PCa [Reissman et al. 2000; Gonzalez-Barcena et al. 1995, 1994]. In one study, after 6 weeks cetrorelix had reduced testosterone to castrate levels with a significant decrease in bone pain, relief in urinary outflow obstruction, and reversal of the signs of prostatism [Gonzalez-Barcena et al. 1994]. In patients with advanced PCa and metastases in the spinal cord, neurological symptoms regressed and bladder function improved after 3 months of cetrorelix treatment [Gonzalez-Barcena et al. 1995].
The GnRH antagonist acyline has been studied in humans [Amory et al. 2009; Page et al. 2006; Herbst et al. 2004, 2002] and is currently in phase I development for oncology (see http://www.merrionpharma.com/content/portfolio/pipeline.asp). After subcutaneous injection in healthy volunteers, acyline produces a rapid and sustained suppression of testosterone that can persist for up to 2 weeks [Herbst et al. 2004]. Recently, oral administration of acyline in a proprietary formulation (GIPET; GI enhancing permeability technology) that enhances the oral bioavailability of peptides significantly suppressed testosterone and gonadotropins in volunteers, without untoward side effects, and was speculated as possibly having utility in the management of PCa [Amory et al. 2009].
Preliminary phase II data with teverelix (in phase II development for PCa) appeared to show suppression of testosterone to castrate levels [Reuters, 2008] although these data are not yet available in a peer-reviewed publication.
Ozarelix is in phase II development for the treatment of PCa (see http://www.sppirx.com/drugs_proprietary.html). It is associated with immediate and complete in vivo testosterone suppression, without the risk of surge/flare [Festuccia et al. 2010]. Ozarelix showed antiproliferative effects and produced an accumulation of cells in G2/M cell cycle phase in an in vitro study of two androgen-independent PCa cell models that are unresponsive to androgen stimulation [Festuccia et al. 2010]. The authors considered that their results may suggest that GnRH antagonists might have an anticancer effect in androgen-independent prostate models.
Summary
The pharmacological profile of GnRH antagonists – fast suppression of testosterone and PSA in the absence of the testosterone surge/flare associated with agonists – provides a promising new treatment option for PCa. Although several antagonists have been investigated in recent years, only degarelix and abarelix are currently available for clinical use in PCa. Moreover, abarelix has been associated with the risk of systemic allergic reactions and, although currently available in Germany, was withdrawn in the US in 2005 [Huhtaniemi et al. 2009]. By contrast, degarelix, the first of a new generation of GnRH antagonists, has been shown in an extensive clinical development programme to retain the efficacy benefits of these agents without the systemic allergic reactions associated with the earlier members of the class.
Clinical comparisons with GnRH agonists, the current standard of care in advanced PCa, show that GnRH antagonists, such as degarelix, are at least as effective in achieving and maintaining castrate testosterone levels in patients with PCa. The absence of a testosterone surge with antagonists avoids initial stimulation of the cancer and worsening of clinical status, and allows more rapid relief of symptoms related to PCa. Because of this, concomitant antiandrogens are not required to prevent clinical flare during antagonist therapy, thereby avoiding antiandrogen-associated adverse events. In addition, the absence of the risk of clinical flare allows GnRH antagonist use in patients with high tumour burden and/or acute problems such as spinal cord compression.
In addition to the significantly faster suppression of testosterone and PSA with the antagonist degarelix than with the agonist leuprolide, degarelix also appears to offer better disease control during the first treatment year (fewer PSA failures, a significantly lower risk of PSA failure or death, lower S-ALP levels and fewer musculoskeletal adverse events). Furthermore, Initial longer-term data suggest that these benefits extend beyond 1 year, supporting the use of degarelix as first-line ADT. The full results from the ongoing extension study are awaited to help clarify the long-term safety and efficacy of degarelix. Although switching from an agonist to an antagonist appears to offer clinical benefits, preclinical data appear to suggest that switching from an antagonist to an agonist is still likely to be accompanied by a testosterone surge and its associated negative clinical implications.
The fast onset of action and lack of testosterone surge with the antagonist degarelix may be beneficial in a range of other clinical settings, such as neoadjuvant to radiation therapy and intermittent ADT, both of which are under investigation. Data from ongoing studies of degarelix in these and other clinical settings may help clarify those PCa patients most likely to benefit from the use of GnRH antagonists.
Conclusion
GnRH antagonists represent a pharmacological method of castration for patients with PCa that offers a more direct and logical mechanism of action than GnRH agonists. With their faster onset of testosterone suppression and absence of surge/flare, these agents appear to offer an effective and well tolerated alternative for the hormonal treatment of PCa. Currently, degarelix is the most extensively studied and widely available agent in this class.
Acknowledgements
Medical writing assistance (funded by Ferring Pharmaceuticals) was provided by Thomas Lavelle of Bioscript Stirling Ltd.
Conflict of interest statement
Professor Boccon-Gibod is an advisor to Ferring Pharmaceuticals. Egbert van der Meulen and Bo-Eric Persson are employees of Ferring Pharmaceuticals.
References
- Alibhai S.M., Duong-Hua M., Sutradhar R., Fleshner N.E., Warde P., Cheung A.M., et al. (2009) Impact of androgen deprivation therapy on cardiovascular disease and diabetes. J Clin Oncol 27: 3452–3458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amory J.K., Leonard T.W., Page S.T., O'Toole E., McKenna M.J., Bremner W.J. (2009) Oral administration of the GnRH antagonist acyline, in a GIPET-enhanced tablet form, acutely suppresses serum testosterone in normal men: single-dose pharmacokinetics and pharmacodynamics. Cancer Chemother Pharmacol 64: 641–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccon-Gibod L., Klotz L., Schröder H., Andreou C., Persson B.E., Cantor P., et al. (2008) Degarelix compared to leuprolide depot 7.5 mg in a 12-month randomised, open-label, parallel-group phase III study in prostate cancer patients. Eur Urol (Suppl. 7): 205–205 abstract 53718555585 [Google Scholar]
- Broqua P., Riviere P.J., Conn P.M., Rivier J.E., Aubert M.L., Junien J.L. (2002) Pharmacological profile of a new, potent, and longacting gonadotropin-releasing hormone antagonist: degarelix. J Pharmacol Exp Ther 301: 95–102 [DOI] [PubMed] [Google Scholar]
- Castellón E., Clementi M., Hitschfeld C., Sánchez C., Benítez D., Sáenz L., et al. (2006) Effect of leuprolide and cetrorelix on cell growth, apoptosis, and GnRH receptor expression in primary cell cultures from human prostate carcinoma. Cancer Invest 24: 261–268 [DOI] [PubMed] [Google Scholar]
- Crawford E.D., Moul J.W., Shore N.D., Olesen T.K., Persson B.E. (2010a) Switching from leuprolide to degarelix vs continuous degarelix treatment – effects on long-term prostate-specific antigen control. J Urol 183(4 Suppl): e262–e262 abstract 670 [Google Scholar]
- Crawford E.D., Moul J.W., Shore N.D., Olesen T.K., Persson B.E. (2010b) Prostate-specific antigen and serum alkaline phosphatase levels in prostate cancer patients receiving degarelix or leuprolide. J Urol 183(4 Suppl): e338–e338 abstract 866 [DOI] [PubMed] [Google Scholar]
- Debruyne F., Bhat G., Garnick M.B. (2006) Abarelix for injectable suspension: first-in-class gonadotropin-releasing hormone antagonist for prostate cancer. Future Oncol 2: 677–696 [DOI] [PubMed] [Google Scholar]
- European Medicines Agency (2010) Summary of Product Characteristics. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_ _Product_Information/human/000986/WC500023252.pdf (accessed 3 June 2011)
- Festuccia C., Dondi D., Piccolella M., Locatelli A., Gravina G.L., Tombolini V., et al. (2010) Ozarelix, a fourth generation GnRH antagonist, induces apoptosis in hormone refractory androgen receptor negative prostate cancer cells modulating expression and activity of death receptors. Prostate 70: 1340–1349 [DOI] [PubMed] [Google Scholar]
- Gittelman M., Pommerville P.J., Persson B.E., Jensen J.K., Olesen T.K. (2008) on behalf of the Degarelix Study Group A 1-year, open-label, randomized phase II dose-finding study of degarelix, a novel gonadotropin-releasing hormone (GnRH) receptor blocker, in the treatment of prostate cancer in North America. J Urol 180: 1986–1992 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Barcena D., Cardenas-Cornejo I., Vadillo-Buenfil M., Comaru-Schally A.M., Cortez-Morales A., Schally A.V., et al. (1995) Luteinizing hormone-releasing hormone antagonist cetrorelix as primary single therapy in patients with advanced prostatic cancer and paraplegia due to metastatic invasion of spinal cord. Urology 45: 275–281 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Barcena D., Vadillo-Buenfil M., Gomez-Orta F., Fuentes Garcia M., Cardenas-Cornejo I., Graef-Sanchez A., et al. (1994) Responses to the antagonistic analog of LH-RH (SB-75, cetrorelix) in patients with benign prostatic hyperplasia and prostatic cancer. Prostate 24: 84–92 [DOI] [PubMed] [Google Scholar]
- Heidenreich, A., Bolla, M., Joniau, S., Mason, M.D., Matveev, V.B., Mottet, N. et al. (2010) Guidelines on Prostate Cancer. European Association of Urology. Available at: http://www.uroweb.org/gls/pdf/Prostate%20Cancer%202010%20June%2017th.pdf (accessed 6 June 2011)
- Herbst K.L., Anawalt B.D., Amory J.K., Bremner W.J. (2002) Acyline: the first study in humans of a potent, new gonadotropin-releasing hormone antagonist. J Clin Endocrinol Metab 87: 3215–3220 [DOI] [PubMed] [Google Scholar]
- Herbst K.L., Coviello A.D., Page S.T., Amory J.K., Anawalt B.D., Bremner W.J. (2004) Acyline, a gonadotropin releasing-hormone antagonist suppresses gonadotropins and testosterone for 15 days after a single dose. J Clin Endocrinol Metab 89: 5959–5965 [DOI] [PubMed] [Google Scholar]
- Huggins C., Hodges C.V. (1941) Studies on prostatic cancer. I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res 1: 293–297 [DOI] [PubMed] [Google Scholar]
- Huhtaniemi I., White R., McArdle C.A., Persson B.E. (2009) Will GnRH antagonists improve prostate cancer treatment? Trends Endocrinol Metab 20: 43–50 [DOI] [PubMed] [Google Scholar]
- Huirne J.A.F., Lambalk C.B. (2001) Gonadotropin-releasing-hormone-receptor antagonists. Lancet 358: 1793–1803 [DOI] [PubMed] [Google Scholar]
- Johansen J.S., Brasso K., Iversen P., Teisner B., Garnero P., Price P.A., et al. (2007) Changes of biochemical markers of bone turnover and YKL-40 following hormonal treatment for metastatic prostate cancer are related to survival. Clin Cancer Res 13: 3244–3249 [DOI] [PubMed] [Google Scholar]
- Jung K., Lein M., Stephan C., Von Hösslin K., Semjonow A., Sinha P., et al. (2004) Comparison of 10 serum bone turnover markers in prostate carcinoma patients with bone metastatic spread: diagnostic and prognostic implications. Int J Cancer 111: 783–791 [DOI] [PubMed] [Google Scholar]
- Jungwirth A., Galvan G., Pinski J., Halmos G., Szepeshazi K., Cai R.Z., et al. (1997) Luteinizing hormone-releasing hormone antagonist cetrorelix (SB-75) and bombesin antagonist RC-3940-II inhibit the growth of androgen-independent PC-3 prostate cancer in nude mice. Prostate 32: 164–172 [DOI] [PubMed] [Google Scholar]
- Keating N.L., O’Malley J., Smith M.R. (2006) Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol 24: 4448–4456 [DOI] [PubMed] [Google Scholar]
- Kirby R.S., Fitzpatrick J.M., Clarke N. (2009) Abarelix and other gonadotrophin-releasing hormone antagonists in prostate cancer. BJU Int 104: 1580–1584 [DOI] [PubMed] [Google Scholar]
- Klotz L., Boccon-Gibod L., Shore N.D., Andreou C., Persson B.E., Cantor P., et al. (2008) The efficacy and safety of degarelix: a 12-month, comparative, randomized, open-label, parallel-group phase III study in prostate cancer patients. BJU Int 102: 1531–1538 [DOI] [PubMed] [Google Scholar]
- Klotz L., Smith M.R., van der Meulen E.A., Tanko L.B. (2010) Baseline cardiovascular risk factors predict cardiovascular (CV) events during androgen deprivation therapy (ADT) with degarelix. Ann Oncol 21(Suppl. 8): viii 276, abstract 883 P–viii 276, abstract 883 P [Google Scholar]
- Koechling W., Hjortkjaer R., Tankó L.B. (2010) Degarelix, a novel GnRH antagonist, causes minimal histamine release compared with cetrorelix, abarelix and ganirelix in an ex vivo model of human skin samples. Br J Clin Pharmacol 70: 580–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamharzi N., Schally A.V., Koppan M. (1998) Luteinizing hormone-releasing hormone (LH-RH) antagonist cetrorelix inhibits growth of DU-145 human androgen-independent prostate carcinoma in nude mice and suppresses the levels and mRNA expression of IGF-II in tumors. Regul Pept 77: 185–192 [DOI] [PubMed] [Google Scholar]
- Lein M., Wirth M., Miller K., Eickenberg H.U., Weissbach L., Schmidt K., et al. (2007) Serial markers of bone turnover in men with metastatic prostate cancer treated with zoledronic acid for detection of bone metastases progression. Eur Urol 52: 1381–1387 [DOI] [PubMed] [Google Scholar]
- Levine G.N., D'Amico A.V., Berger P., Clark P.E., Eckel R.H., Keating N.L., et al. (2010) Androgen-deprivation therapy in prostate cancer and cardiovascular risk: a science advisory from the American Heart Association, American Cancer Society and American Urological Association: endorsed by the American Society for Radiation Oncology. Circulation 121: 833–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorente J.A., Morote J., Raventos C., Encabo G., Valenzuela H. (1996) Clinical efficacy of bone alkaline phosphatase and prostate specific antigen in the diagnosis of bone metastasis in prostate cancer. J Urol 155: 1348–1351 [PubMed] [Google Scholar]
- McLeod D., Zinner N., Tomera K., Gleason D., Fotheringham N., Campion M., et al. (2001) A phase 3, multicenter, open-label, randomized study of abarelix versus leuprolide acetate in men with prostate cancer. Urology 58: 756–761 [DOI] [PubMed] [Google Scholar]
- Mongiat-Artus P., Teillac P. (2004) Abarelix: the first gonadotrophin-releasing hormone antagonist for the treatment of prostate cancer. Expert Opin Pharmacother 5: 2171–2179 [DOI] [PubMed] [Google Scholar]
- Oka D., Shiba M., Arai Y., Nakayama M., Takayama H., Inoue H., et al. (2006) Skin reactions to 3-month depot type of luteinizing hormone-releasing hormone agonist therapy. Jpn Med Assoc J 49: 48–54 [Google Scholar]
- Page S.T., Amory J.K., Anawalt B.D., Irwig M., Brockenbrough A., Matsumoto A.M., et al. (2006) Testosterone gel combined with depomedroxyprogesterone acetate (DMPA) is an effective male hormonal contraceptive regimen but is not enhanced by the addition of the GnRH antagonist acyline. J Clin Endocrinol Metab 91: 4374–4380 [DOI] [PubMed] [Google Scholar]
- Persson B.E., Kold Olesen T., Jensen J.K. (2009) Degarelix: a new approach for the treatment of prostate cancer. Neuroendocrinology 90: 235–244 [DOI] [PubMed] [Google Scholar]
- Persson B.E., Olesen T.K., Jensen J., Mason M. (2010) Effects of switching from leuprolide to degarelix on long-term prostate-specific antigen (PSA) and serum alkaline phosphatase (S-ALP) control. Ann Oncol 21(Suppl. 8): viii 280–viii 280 abstract 893 P [Google Scholar]
- Pinski J., Yano T., Miller G., Schally A.V. (1992) Blockade of the LH response induced by the agonist D-Trp-6-LHRH in rats by a highly potent LH-RH antagonist SB-75. Prostate 20: 213–224 [DOI] [PubMed] [Google Scholar]
- Plekhanov A., Crawford E.D., Olesen T.K., Van Der Meulen E.A., Persson B.E. (2010) Switching from leuprolide to degarelix vs. continuous degarelix treatment – effects on long-term PSA control. Eur Urol 9(Suppl. 6): 538–538 abstract N12 [Google Scholar]
- Princivalle M., Broqua P., White R., Meyer J., Mayer G., Elliott L., et al. (2007) Rapid suppression of plasma testosterone levels and tumor growth in the Dunning rat model treated with degarelix, a new gonadotropin-releasing hormone antagonist. J Pharmacol Exp Ther 320: 1113–1118 [DOI] [PubMed] [Google Scholar]
- Ramankulov A., Lein M., Kristiansen G., Loening S.A., Jung K. (2007) Plasma osteopontin in comparison with bone markers as indicator of bone metastasis and survival outcome in patients with prostate cancer. Prostate 67: 330–340 [DOI] [PubMed] [Google Scholar]
- Reissmann T., Schally A.V., Bouchard P., Riethmiiller H., Engel J. (2000) The LHRH antagonist cetrorelix: a review. Hum Reprod Update 6: 322–331 [DOI] [PubMed] [Google Scholar]
- Robinson D., Sandblom G., Johansson R., Garmo H., Stattin P., Mommsen S., et al. (2008) Prediction of survival of metastatic prostate cancer based on early serial measurements of prostate specific antigen and alkaline phosphatase. J Urol 179: 117–122 [DOI] [PubMed] [Google Scholar]
- Reuters (2008). Key developments, Ardana plc: Ardana plc announces preliminary phase II repeat dose results for teverelix LA in prostate cancer. Available at: http://www.reuters.com/article/2008/04/09/idUS59548+09-Apr-2008+RNS20080409 (accessed 4 January 2011)
- Schröder F.H., Tombal B., Miller K., Boccon-Gibod L., Shore N.D., Crawford E.D., et al. (2010) Alkaline phosphatase changes in prostate cancer patients receiving degarelix or leuprolide: results from a 12-month, comparative, phase III study (CS21). BJU Int 106: 182–187 [DOI] [PubMed] [Google Scholar]
- Selvaggi F., Khoe G.S.S., Van Cangh P., Jung J.-L., Schulman C.C., Vallancien G., et al. (2001) Comparison of abarelix depot (A-D) and goserelin (G) plus bicalutamide (B) in advanced prostate cancer: results of a multicentre, open-label, randomised, phase III study. Eur Urol 39(Suppl. 5): 78–78 [Google Scholar]
- Sharma O.P., Weinbauer G.F., Behre H.M., Nieschlag E. (1992) The gonadotropin-releasing hormone (GnRH) agonist-induced initial rise of bioactive LH and testosterone can be blunted in a dose-dependent manner by GnRH antagonist in the non-human primate. Urol Res 20: 317–321 [DOI] [PubMed] [Google Scholar]
- Smith M.R., Klotz L., Persson B.E., Olesen T.K., Wilde A.A. (2010) Cardiovascular safety of degarelix: results from a 12-month, comparative, randomized, open label, parallel-group phase III trial in patients with prostate cancer. J Urol 184: 2313–2319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg M. (2009) Degarelix: a gonadotropin-releasing hormone antagonist for the management of prostate cancer. Clin Ther 31: 2312–2331 [DOI] [PubMed] [Google Scholar]
- Thompson I.M. (2001) Flare associated with LHRH-agonist therapy. Rev Urol 3(Suppl. 3): S10–S14 [PMC free article] [PubMed] [Google Scholar]
- Tombal B., Miller K., Boccon-Gibod L., Schröder F., Shore N., Crawford E.D., et al. (2010) Additional analysis of the secondary end point of biochemical recurrence rate in a phase 3 trial (CS21) comparing degarelix 80 mg versus leuprolide in prostate cancer patients segmented by baseline characteristics. Eur Urol 57: 836–842 [DOI] [PubMed] [Google Scholar]
- Tomera K., Gleason D., Gittelman M., Moseley W., Zinner N., Murdoch M., et al. (2001) The gonadotropin-releasing hormone antagonist abarelix depot versus luteinizing hormone releasing hormone agonists leuprolide or goserelin: initial results of endocrinological and biochemical efficacies in patients with prostate cancer. J Urol 165: 1585–1589 [PubMed] [Google Scholar]
- Trachtenberg J., Gittleman M., Steidle C., Barzell W., Friedel W., Pessis D., et al. (2002) A phase 3, multicenter, open label, randomized study of abarelix versus leuprolide plus daily antiandrogen in men with prostate cancer. J Urol 167: 1670–1674 [DOI] [PubMed] [Google Scholar]
- US Food and Drug Administration (2010) FDA Drug Safety Communication: Update to ongoing safety review of GnRH agonists and notification to manufacturers of GnRH agonists to add new safety information to labeling regarding increased risk of diabetes and certain cardiovascular diseases. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm229986.htm (accessed 6 June 2011)
- Van Poppel H., Nilsson S. (2008) Testosterone surge: rationale for gonadotropin-releasing hormone blockers? Urology 71: 1001–1006 [DOI] [PubMed] [Google Scholar]
- Van Poppel H., Tombal B., de la Rosette J.J., Persson B.E., Jensen J.K., Olesen T.K. (2008) on behalf of the Degarelix Study Group Degarelix: a novel gonadotropin-releasing hormone (GnRH) receptor blocker – results from a 1-yr, multicentre, randomised, phase II dose-finding study in the treatment of prostate cancer. Eur Urol 54: 805–815 [DOI] [PubMed] [Google Scholar]
- White, R., Schwach, G., and Schteingart, C.D. (2007) Degarelix, a unique, sustained-release depot GnRH blocker with a long duration of action. 1st European Multidisciplinary Meeting on Urological Cancers, Barcelona, 2–4 November 2007. Abstract P92. Available at: http://www.emucbarcelona2007.org/fileadmin/user_upload/downloads/EMUC_Binnen.pdf (accessed 3 June 2011)