Abstract
The National Institutes of Health (NIH) classification of graft-versus-host disease (GVHD) is a significant improvement over prior classifications, and has prognostic implications. We hypothesized that the NIH classification of GVHD would predict the survival of patients with GVHD treated with extracorporeal photopheresis (ECP). Sixty-four patients with steroid refractory/dependent GVHD treated with ECP were studied. The 3-year overall survival (OS) was 36% (95% confidence interval [CI] 13–59). Progressive GVHD was seen in 39% of patients with any acute GVHD (aGVHD) (classic acute, recurrent acute, overlap) compared to 3% of patients with classic chronic GVHD (cGVHD) (P = .002). OS was superior for patients with classic cGVHD (median survival, not reached) compared to overlap GVHD (median survival, 395 days, 95% CI 101 to not reached) and aGVHD (delayed, recurrent or persistent) (median survival, 72 days, 95% CI 39–152). In univariate analyses, significant predictors of survival after ECP included GVHD subtype, bilirubin, platelet count, and steroid dose. In multivariate analyses overlap plus classic cGVHD was an independent prognostic feature predictive of superior survival (hazard ratio [HR] 0.34, 95% CI 0.14–0.8, p = .014). This study suggests that NIH classification can predict outcome after ECP for steroid refractory/dependent GVHD.
Keywords: Hematopoietic cell transplantation, Graft-versus-host disease, NIH classification, Photopheresis
INTRODUCTION
Graft-versus-host disease (GVHD), a common complication occurring after allogeneic stem cell transplant (SCT), has been historically classified as acute (aGVHD) (onset within first 100 days of allogeneic SCT) and chronic (cGVHD) (onset after 100 days of allogeneic SCT) [1,2]. cGVHD has protean manifestations that resemble a variety of autoimmune disorders [3,4], and has been previously classified as limited and extensive, although the prognostic significance of this classification is unclear [5]. The National Institutes of Health (NIH) published consensus criteria for diagnosis and classification of cGVHD, and relies on the phenotype of GVHD rather than timing of GVHD. GVHD is classified as classic aGVHD (onset before 100 days), recurrent aGVHD, delayed aGVHD (onset after day 100), persistent aGVHD, overlap GVHD (features of both aGVHD and cGVHD), and classic cGVHD [6].
A variety of agents has been studied for steroid dependent/refractory GVHD [7–15]. Extracorporeal photopheresis (ECP) is effective in the treatment of GVHD, and has been used with variable efficacy [16–20]. The target organ responsiveness varies widely, and depends on type of GVHD (acute or chronic), and previous treatment (steroid-refractory or dependent). A recent randomized study showed that ECP decreases the steroid usage in a subset of patients with GVHD [21]. None of the previous studies of ECP for GVHD treatment have incorporated the NIH classification. It is very likely that many patients enrolled in “cGVHD” ECP studies, have recurrent aGVHD or overlap GVHD. We and others have shown the prognostic value of this classification in retrospective series, and have demonstrated that patients with classic and overlap cGVHD have the best survival compared to other phenotypes [22,23]. As most transplant centers are using the NIH classification for GVHD, it is important to validate the prognostic ability of the NIH classification to predict the outcome of GVHD treated with ECP to allow better patient selection for ECP and allow rational development of clinical trials pertaining to the use of ECP.
This is the first report showing that the outcome of patients with GVHD treated with ECP is influenced by the clinical phenotype as proposed by the NIH classification.
PATIENTS AND METHODS
Sixty-four consecutive patients who started ECP (1997–2008) for treatment of GVHD after an allogeneic SCT were included in the study cohort. All patients underwent SCT on standard of care or institutional review board (IRB) approved protocols. All patients signed IRB-approved data consents. ECP specific parameters, including frequency, number of treatment sessions, and duration were abstracted from medical charts. Detailed reviews of GVHD characteristics were done, and GVHD was reclassified in accordance with the classification as proposed by the NIH consensus criteria and as described previously [22]. Details regarding immunosuppressive therapy (IST) and steroid usage were obtained, including dose at start of ECP and at month 2, and at subsequent 2 monthly intervals until the end of ECP. A decrease in the steroid dose while on ECP was considered as a surrogate marker of a patient deriving clinical benefit from ECP and was analyzed.
Transplant Regimens
Table 1 summarizes the SCT characteristics. Standard GVHD prophylaxis regimens with cyclosporine (CsA)/tacrolimus and methotrexate (MTX) (myeloablative (MA) transplants) or mycophenolate moefetil (MMF) (reduced intensity conditioning [RIC] and nonmyeloablative [NMA] transplants) were used. All patients received standard institutional care regarding antimicrobial prophylaxis, cytomegalovirus (CMV) monitoring, and treatment.
Table 1.
Baseline Characteristics
| Number | Percentage | |
|---|---|---|
| Age (median, range, years) | 45 (23–67) | |
| Sex (recipient, male/female) | 39/25 | 61/39 |
| Disease | ||
| Acute myelogenous leukemia | 15 | 23 |
| Acute lymphoblastic leukemia | 6 | 9 |
| Chronic myelogenous leukemia | 10 | 16 |
| Chronic lymphocytic leukemia | 4 | 6 |
| Multiple myeloma | 7 | 11 |
| Myelodysplastic syndrome | 8 | 13 |
| Lymphoma | 8 | 13 |
| Other | 8 | 9 |
| Disease risk | ||
| Low | 24 | 38 |
| Intermediate | 6 | 9 |
| High | 29 | 45 |
| Stem cell source | ||
| Bone marrow | 18 | 28 |
| Peripheral blood stem cells | 44 | 69 |
| Other | 2 | 3 |
| Donor | ||
| Related | 34 | 53 |
| Unrelated | 30 | 47 |
| Regimen | ||
| Ablative | 42 | 66 |
| Reduced intensity/nonmyeloablative | 22 | 34 |
| CMV | ||
| Recipient (+/−) | 38/21 | 60/33 |
| Donor (+/−) | 27/32 | 42/50 |
CMV indicates cytomegalovirus.
Some percentages do not add up to 100 because of missing data.
Ablative regimens: cyclophosphamide 120 mg/kg and total body irradiation (TBI) 1200 cGy; cyclophosphamide 120 mg/kg, and busulfan 16 mg/kg; cyclophosphamide 7200 mg/m2, etoposide 2000 mg/m2, BCNU 400 mg/m2.
Reduced-intensity regimens: fludarabine 90 mg/m2 and busulfan 8 mg/kg with TBI 400 cGy; fludarabine 90 mg/m2, and busulfan 8 mg/kg, fludarabine 90 mg/m2 and busulfan 8 mg/kg with antithymocyte globulin, fludarabine 90 mg/m2, and melphalan 140 mg/m2 with antithymocyte globulin. Nonmyeloablative: fludarabine 90 mg/m2 and TBI 200 cGy.
GVHD Treatment
For treatment of aGVHD the following general principles were used: grade II or higher aGVHD was treated with 1–2 mg/kg of methylprednisolone or an equivalent dose of prednisone for 7 to 14 days, followed by a taper of 10% every 5 to 7 days. Treatment of steroid refractory aGVHD was variable, and included use of ECP, and other agents like antithymocyte globulin (ATG), and infliximab.
Treatment of cGVHD was variable, but followed some general principles. Patients with isolated mouth, ocular, or minimal skin cGVHD were treated with topical steroids. Patients with significant cGVHD were treated with calcineurin inhibitor along with systemic steroids (prednisone). Although duration and dosing of steroids were not standardized, patients typically received treatment until all symptoms of cGVHD were resolved or stabilized, and subsequent tapers of IST were attempted. Patients were started on second-line agents at the discretion of the treating physician.
Treatment with ECP
Patients were started on ECP if they were steroid dependent (recurrence of GVHD with attempts at steroid taper), steroid intolerant (eg, severe myopathy), steroid refractory, or had concomitant infections that would preclude a primary steroid based therapy (eg, multiple reactivations of CMV). Patients were treated on a weekly basis with 2 pheresis sessions a week. This was continued for 3 to 4 weeks and then decreased to an every 2- to 3-week interval. The goal was to get patients to an every 4-week frequency.
Responses were assessed retrospectively by a single investigator (B.S.). Responses were classified as complete response (CR) if all clinical characteristics of GVHD had normalized. Partial response (PR) was defined as improvement, but not a complete resolution of clinical and laboratory characteristics. Progressive disease (PD) was defined as worsening of clinical characteristics or new organ involvement. Stable disease (SD) was defined as not meeting criteria for PR or PD. Steroid and other IST dose reductions were not used to assess response. All patients in the study cohort were assigned a response status, unless documentation to assess a response was missing (N = 2). Responses were assessed at 2 months after initiating ECP. If a patient had progressive GVHD, ECP was discontinued. In patients who had clinical benefit (CR, PR, or SD) from ECP, systemic steroids were gradually tapered before attempting to stop ECP.
Statistical Analysis
Descriptive statistics, including median and ranges for continuous variables, as well as percentages and frequencies for categoric variables, were calculated. Groups with nominal outcome were compared using chi-square test or Fisher exact test; groups with continuous outcomes were compared using Mann-Whitney U-test. Spearman correlation was used to assess the correlation between 2 continuous or ordinal variables. Wilcoxon signed-rank test was used to compare paired continuous variables. Overall survival (OS) (day of SCT to the day of death or last follow-up) and ECP-specific survival (the day of onset of ECP to the day of death or last follow-up) was calculated. Kaplan-Meier survival curves were calculated for each cohort (or clinical group), and were compared using the log-rank test. Because of the small sample size, in some analyses, patients with any acute feature (aGVHD and overlap GVHD) were combined and compared with classic cGVHD. Cox regression models were constructed for multivariate analyses for time to event endpoints. Cumulative incidence of nonrelapse mortality (NRM) was estimated using the Kaplan-Meier method with adjusting for relapse as a competing risk event. All reported P-values were 2 tailed and considered significant at P < .05. Analyses were performed using SPSS version 13, SAS system version 9.1 and R version 2.1.1.
RESULTS
Patient characteristics are shown in Table 1. The median follow-up of the surviving patients from initiation of ECP was 725 days (range: 192 to not reached). The 3-year OS of the entire cohort was 36% (95% CI 13–59).
GVHD Subtype and IST
GVHD at onset of ECP was classified per NIH classification as: classic aGVHD (N = 15, 23%), recurrent aGVHD (N = 5, 8%), persistent aGVHD (N = 0), overlap GVHD (N = 12, 19%), and classic cGVHD (N = 31, 50%). One patient had inadequate records for accurate assessment of GVHD subtype. Of the 20 patients with various subtypes of aGVHD, the maximum grades of aGVHD by Glucksberg criteria at onset of ECP were as follows: 18 patients (90%) had grade II–IV and 10 patients (50%) had grade IIIIV. Among patients with cGVHD (n = 43), the subscale scores were captured. The mean sum of subscale scores was 5.74 (range: 2–14). The subscale scores were not significantly different in overlap compared to classic cGVHD (P = .73). The scores for the various subscales are as outlined in Table 2. cGVHD was stratified as mild (N = 4, 9%), moderate (N = 23, 52%), and severe (N = 16, 36%) at ECP onset, and was similar in patients with overlap (17%, 33%, and 50%) and classic chronic (7%, 61%, and 29%) GVHD (P = .51). The median number of recurrences of GVHD prior to initiation of ECP was 2 (range: 0–6 recurrences), and was similar across all subtypes. Patients with any features of aGVHD had a median platelet count of 48.5 × 109/L compared to patients with classic cGVHD (203 × 109/L, P < .001). The median bilirubin level was significantly different at time of ECP initiation among the various GVHD subtypes (any acute: 1.35 mg/dL versus classic chronic: 0.6 mg/dL, P = .002).
Table 2.
Chronic GVHD Subscale
| Subscale | Score (Number/Percentage)
|
|||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Karnofsky performance status | 0 | 25 (58) | 14 (33) | 2 (5) |
| Skin | 12 (28) | 4 (9) | 14 (33) | 11 (26) |
| Gastrointestinal | 31 (72) | 6 (14) | 6 (14) | 0 |
| Ocular | 27 (63) | 5 (12) | 11 (25) | 0 |
| Mouth | 24 (56) | 17 (40) | 2 (4) | 0 |
| Pulmonary | 35 (81) | 5 (12) | 1 (2) | 2 (5) |
| Joints/fascia | 32 (75) | 9 (21) | 1 (2) | 1 (2) |
| Liver | 20 (46) | 14 (33) | 7 (16) | 2 (5) |
| Genitourinary (females only, N = 20) | 14 (70) | 4 (20) | 0 | 0 |
GVHD indicates graft-versus-host disease.
Some percentages do not add up to 100 because of missing data.
The median steroid doses were 1.35 mg/kg, 0.7 mg/kg and 0.2 mg/kg in patients with aGVHD (recurrent, delayed, or persistent), overlap, and classic cGVHD. Patients with manifestations of aGVHD had a significantly higher steroid dose than classic cGVHD (P < .001).
ECP and Outcome
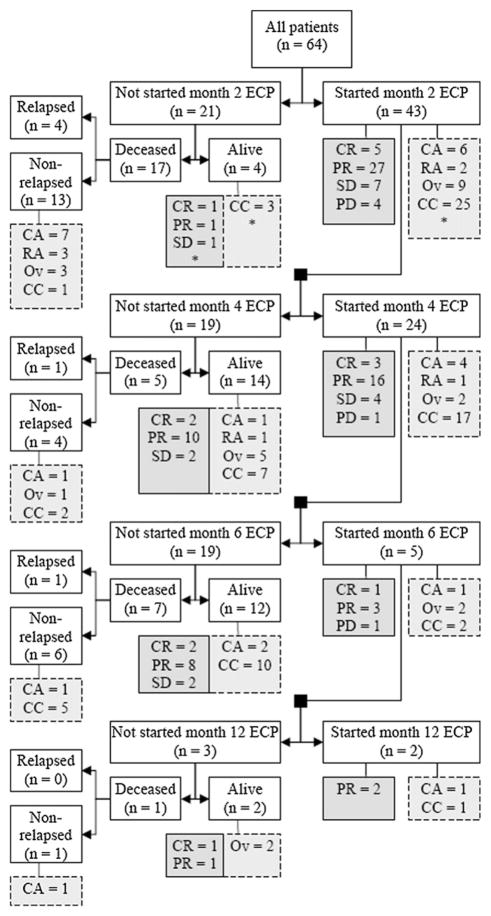
Patients were started on ECP at a median of 228 days post-SCT (range: 39–2943 days). Fifteen patients (23%) started ECP prior to day 100, at a median of 50 days (range: 39–97 days). Patients with classic cGVHD started ECP significantly late compared to other subtypes (median, 786 versus 108 days, P < .001). The median number of ECP treatments was 12 (range: 1–83 treatments). Patients with classic cGVHD received more ECP treatments than other subtype (14 versus 9, P = .02). ECP was initiated for steroid refractory GVHD without any recurrences in 5 (8%) patients, and after 1, 2, or 3 recurrences of GVHD in 20 (31%), 13 (20%), and 13 (20%). ECP was used in 11 patients (17%) who had 4 or greater recurrences. In 2 patients, adequate detailed records were not available. The number of recurrences of GVHD prior to ECP for patients with classic aGVHD, recurrent aGVHD, overlap, and classic cGVHD were 0 (20%), 1 (30%), 2 (30%), and 3 (20%), respectively. Figure 1 is a flow diagram indicating the number of patients with responses at various time points during the ECP and details regarding reasons for stopping ECP. Of the 64 patients treated with ECP, 21 patients did not start month 2 of therapy. Four of these patients are alive (CR = 1, PR = 1, SD = 1, missing = 1). Similarly, of the 43 patients that started month 2 of therapy, 19 did not start month 4. Fourteen of 19 patients are alive (CR = 2, PR = 10, SD = 2). The continuation of ECP was determined by a variety of factors, including response, degree of systemic immmunosuppression, logistics of being able to continue ECP, and treating-physician decision.
Figure 1.
Flow diagram summarizing disposition of patients during the period of study analyses. The dark gray boxes with solid lines show the response of GVHD to ECP (CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease). The light gray boxes with the dashed lines show the GVHD subtype (CA, classic acute; RA, recurrent acute; OV, overlap chronic; CC, classic chronic).*Reflects 1 missing patient.
Response to ECP
CR, PR, and SD was attained in 7 (11%), 30 (47%), and 12 (19%) patients, respectively, and 13 (20%) had PD. One patient (3%) was not evaluable because of missing records. Forty-three patients (67%) were on ECP at 2 months after initiation of therapy with a response rate of 74% (32 of 43). Response rate of patients who started month 4, 6, and 12 of ECP were 79% (19 of 24), 80% (4 of 5), and 100% (2 of 2), respectively. Patients with < 1 mg/kg steroid dose at onset of ECP had a significantly higher incidence of response compared to those with ≥1 mg/kg (90% versus 56%, P = .003).
Table 3 describes the details of responses as stratified by GVHD type at ECP onset. PD was seen in 39% of patients with any aGVHD (classic acute, recurrent acute, overlap) compared to 3% of patients with classic cGVHD (P = .002). When GVHD was classified as chronic (classic plus overlap) versus acute (classic, recurrent), progression of GVHD was significantly lower in patients with cGVHD (14% versus 42%, P = .010).
Table 3.
Responses to ECP Stratified by GVHD Subtype
| GVHD Type | Response (Number/Percentage)
|
|||
|---|---|---|---|---|
| CR | PR | SD | PD | |
| Classic acute (N = 15) | 2 (13) | 4 (27) | — | 8 (53) |
| Recurrent acute (N = 5) | — | 3 (60) | 2 (40) | — |
| Overlap (N = 12) | 2 (17) | 6 (50) | — | 4 (33) |
| Classic chronic (N = 31) | 3 (10) | 17 (55) | 10 (32) | 1 (3) |
GVHD indicates graft-versus-host disease; ECP, extracorporeal photopheresis; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
One patient was not evaluable because of missing records in the classic acute group.
ECP use led to significant decrease in steroid doses in patients with chronic (classic plus overlap) GVHD (mean dose pre-ECP, 0.52 mg/kg versus 0.37 mg/kg post-ECP, p = .009).
Subscale Score and Response
Subscale scores for all domains were assigned to patients with overlap and chronic classic GVHD (N = 43). Severity scores at ECP onset did not predict response. Only KPS (0–1 versus 2–3) and GI subscale scores (0–1 versus 2–3) were associated with GVHD progression (4% versus 25%, P = .067; 8% versus 50%, P = .027). Subscale scores were lower (better) at last follow-up compared to pre-ECP for lung (P = .035) and joints/fascia (P = .057). Although severity did not change significantly with ECP, the overall score was significantly lower in responders compared to nonresponders (P = .0056).
Survival after ECP
Thirty-four patients are surviving at the time of analyses (30 patients deceased; nonrelapse, 24; relapse, 6), with an actuarial 3 year OS of 36% (95% CI 13–59). OS was superior for patients with classic cGVHD(median survival, not reached) compared to overlap GVHD (median survival, 395 days, 95% CI 101 to not reached) and aGVHD (delayed, recurrent or persistent) (median survival, 72 days, 95% CI 39–152) (P < .0001) (Figure 2A). Patients with aGVHD (classic acute, recurrent acute) had a significantly decreased OS compared to cGVHD (classic plus overlap) (P < .001) (Figure 2B). This difference in OS (from ECP onset) was more apparent when patients with any aGVHD features (recurrent acute, delayed acute, and overlap chronic) were compared with classic cGVHD (3-year OS < 25% versus 52%, P < .0001) (Figure 2C). The numbers of GVHD recurrences prior to initiation of ECP were not significant in predicting OS (0–1 vs. ≥2, P = .39; 0–2 vs. ≥3, P = .19). Response to ECP was predictive of OS. The OS of responders (CR or PR) (median survival, not reached), stabilization of GVHD (median survival, 213 days, 95% CI 0–1058) or progression (median survival, 36 days, 95% CI 14–58) was significantly different (P < .0001) (Figure 3). Steroid dose at initiation of ECP was an important predictor of OS (≤1 mg/kg vs. >1 mg/kg, not reached versus 108 days, P = .008).
Figure 2.
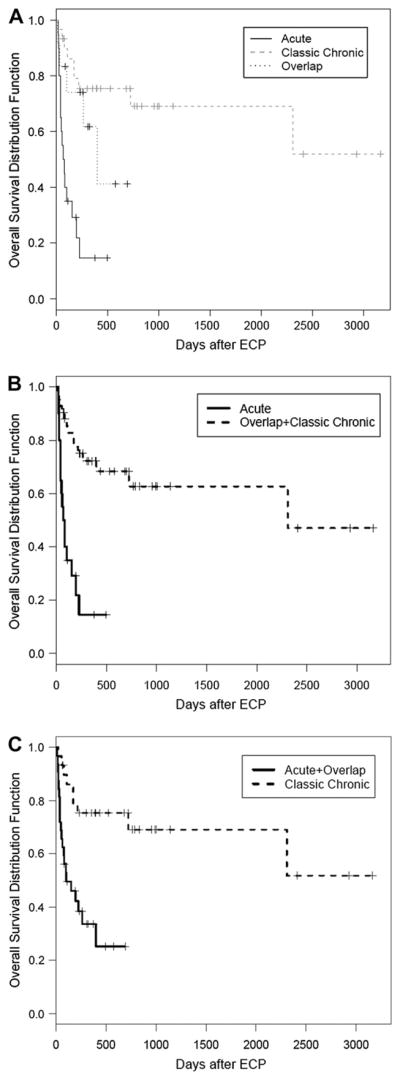
OS (from initiation of ECP) stratified by GVHD type. (A) Patients with aGVHD (persistent acute, recurrent acute, delayed acute) overlap cGVHD, and classic cGVHD had significantly different OS (P = .001). (B) aGVHD versus overlap plus classic cGVHD. (C) Any aGVHD (classic, recurrent, overlap) versus classic cGVHD.
Figure 3.
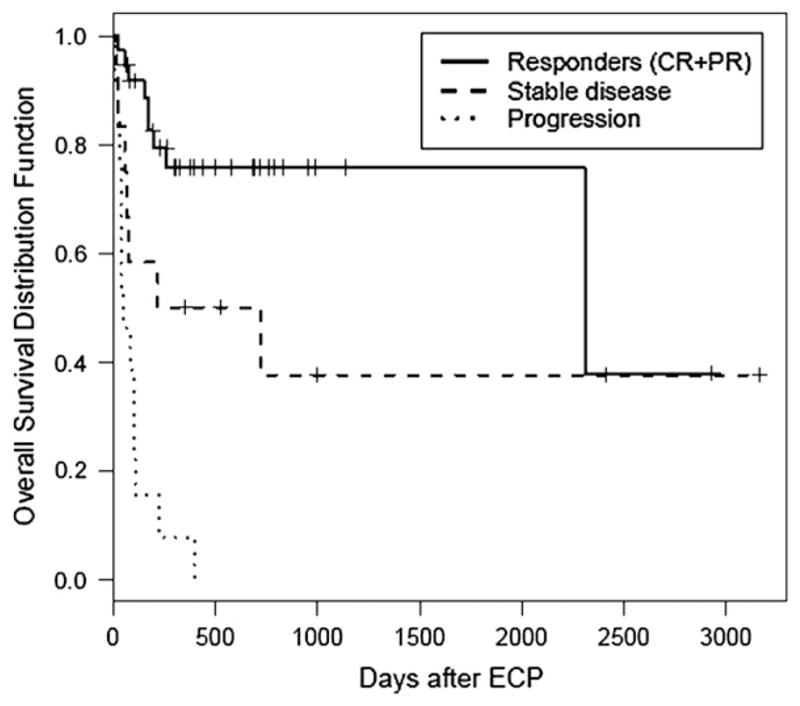
OS (from initiation of ECP) stratified by best response to ECP. The overall survival of patients with response (CR or PR) (median survival, not reached), stabilization of GVHD (median survival, 217 days) or progression (median survival, 39 days) was significantly different (P < .0001). CR, complete response; PR, partial response.
Other Risk Factors Predicting Survival
The KPS subscale at onset of ECP was predictive of OS (score 0–1 versus 2–3, median, not reached versus 152 days, 95% CI 0–491, P = .026). Similarly, lower subscale scores (0–1) compared to a higher subscale score (≥2) were associated with improved OS for liver (median, not reached versus 409 days, 95% CI 43–750, P = .027), GI tract (median, not reached versus 116 days, 95% CI 0–221, P = .010), and lung (not reached versus 39 days, 95% CI 0–134, P = .033). However, higher skin subscale scores (2–3 versus 0–1) was associated with superior OS (median, not reached versus 431 days, 95% CI 0–862, P = .05). This probably reflects the higher percentage of patients with cGVHD who had skin involvement.
Platelet count of ≤100 × 109/L or >100 × 109/L at the time of initiation of ECP was an important predictor of OS (68 days versus not reached, P < .001). Bilirubin level of ≤3 mg/dL or >3 mg/dL at the time of onset of ECP was associated with significant difference in OS (431 versus 32 days, P < .001). Stem cell source, donor status, absolute lymphocyte count at day 30, CD3+ and CD34+ cells per kg (of recipient body weight) in the infused graft, and age of ≤40 years or >40 years or as a continuous variable had no impact on OS.
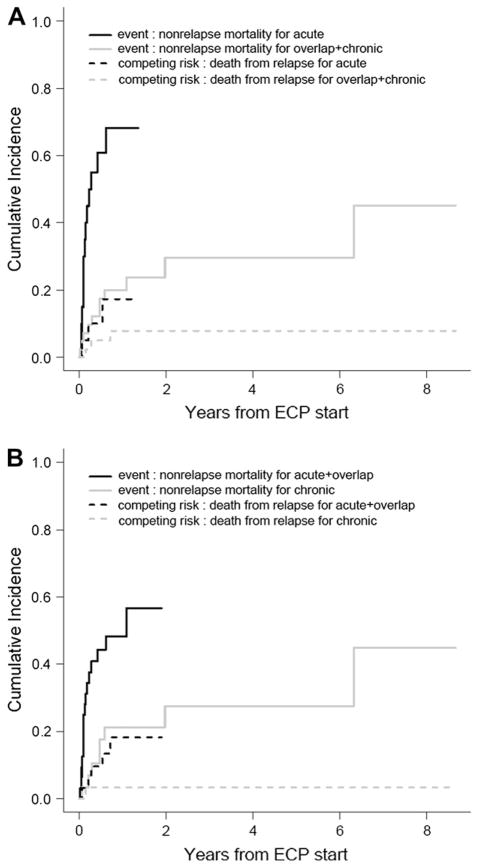
NRM
The 2-year cumulative NRM with relapse as a competing risk for the entire cohort was 42% (95% CI 27%–56%). NRM with relapse as a competing risk was significantly higher in patients with aGVHD (classic acute plus recurrent) compared to cGVHD (overlap plus classic) (68% versus 29%, P < .001) (Figure 4A). Patients with classic cGVHD had a significantly lower NRM compared to other types (57% versus 28%, P = .007) (Figure 4B).
Figure 4.
NRM with death from relapse as a competing risk. (A) NRM of patients with aGVHD (classic acute, recurrent acute) compared with cGVHD (overlap plus classic chronic) (P < .0001). (B) NRM of patients with any aGVHD (classic acute, recurrent, overlap) compared with classic cGVHD (P = .007).
Multivariable Analysis
Bilirubin level and platelet count were both predictors of survival in univariate analyses, but were correlated with each other (Spearman correlation = −0.696, P < .001). Steroid dose was correlated with GVHD type (Spearman correlation = −0.611, P < .001). Platelet count, donor status, and GVHD type were the 3 covariates that were analyzed in the final multivariable model. GVHD type was categorized as classic cGVHD versus other subtypes in the first model and as classic cGVHD plus overlap versus all other subtypes in the second model. Table 4 summarizes the details of the multivariable model. GVHD type was an independent predictor of survival in the second model (hazard ratio [HR] 0.34, 95% CI 0.14–0.8, P = .0141). Platelet count was a predictor of survival in both models.
Table 4.
Multivariable Model
| Variable | Reference | Hazard Ratio (95% CI) | P-Value |
|---|---|---|---|
| Model 1 | |||
| GVHD type* | Acute plus overlap | 0.63 (0.22–1.79) | .381 |
| Donor status | Related | 0.52 (0.23–1.16) | .108 |
| Platelet count† (×109/L) | 39.25 | 0.1 (0.03–0.32) | <.001 |
| Model 2 | |||
| GVHD type‡ | Acute | 0.34 (0.14–0.8) | .014 |
| Donor status | Related | 0.56 (0.26–1.22) | .146 |
| Platelet count† (×109/L) | 39.25 | 0.11 (0.04–0.33) | <.001 |
GVHD indicates graft-versus-host diesase; CI, confidence interval.
GVHD type: acute (classic acute, recurrent acute) plus overlap compared to classic chronic.
Platelet count: reference was 39.25 × 109/L. This was calculated as the 25th percentile from the range of platelet count and was compared with the 75th percentile.
GVHD type: acute (classic acute, recurrent acute) compared to overlap plus classic chronic.
DISCUSSION
In this study, we show that the outcome of GVHD when treated with ECP is influenced by the clinical phenotype of GVHD as proposed by the NIH classification. ECP was used as second line or in further advanced cases of GVHD. Patients with classic cGVHD had the best survival compared to patients with other subtypes of GVHD after ECP.
ECP has been used to treat GVHD typically for steroid refractory, dependent or for “cGVHD” (GVHD after day 100). In a pilot study of 21 patients, ECP was used for treatment of steroid refractory aGVHD [17]. In this cohort of patients, 60% achieved a CR after 3 months of ECP. A high proportion of patients with skin only, liver only, or liver and skin involvement had CRs. There were no responses to ECP seen with GI GVHD or when all 3 target organs were involved. This suggests that even in a seemingly homogenous cohort of steroid refractory aGVHD, response to ECP varies with organ involvement. Patients who had a CR with ECP had a significant better survival compared to nonresponders. Results appear better when ECP is initiated early in the course of steroid refractory GVHD [16]. In this setting, GI GVHD responded to ECP in 62% of the patients. In our cohort, 20 patients had aGVHD (classic aGVHD = 15, recurrent aGVHD = 5), and 10 patients had 2 or more GVHD recurrences. Grade II and IV aGVHD was present in 50% of patients at time of ECP initiation with 70% of patients with GI GVHD (20% with grade III–IV). In this high-risk group of patients, ECP was initiated at a median of 79 days after transplant and an OS of 20% (5/20) was seen.
The role of ECP may be more established in treating cGVHD (GVHD after day 100). In a pilot study of 15 patients, target organ sites that preferentially responded to ECP included skin, liver, and oral mucosa [16]. These studies predated the development of the NIH classification, and some patients with skin and liver could represent patients with recurrent aGVHD, delayed aGVHD, or overlap GVHD. We have previously shown that 36%and 37% of patients with limited and extensive cGVHD are reclassified as recurrent aGVHD, late aGVHD, or persistent aGVHD [22].
KPS is a known predictor of outcome in patients with cGVHD. Previous studies have shown that patients with a KPS of >90% have an excellent survival (14/15 patients alive) [16]. In our study, KPS (score 0–1 versus 2–3) was predictive of response and survival. Lower subscale scores for KPS, liver, lung, and gastrointestinal tract predicted a superior survival. Paradoxically, higher skin subscale scores (2–3 versus 0–1) predicted a superior survival. The incidence of higher skin subscale score was not significantly different in patients with overlap compared to classic cGVHD. It is known that “sclerotic” phenotype of cGVHD may respond preferentially to ECP [20]. In the NIH classification, the higher skin scores (2–3) include not only extent of rash ≥19% body surface area, but also include features of sclerosis. Thus, the higher skin scores at ECP onset probably reflect the superior outcome of patients with sclerotic GVHD with ECP. Retrospective assessment of overall clinical response of cGVHD is not reliable, often because of lack of detailed documentation and difficulty in estimating the residual damage from cGVHD versus ongoing activity. Although, the NIH consensus guidelines have proposed detailed response criteria, these are not applicable for retrospective studies [24].
Steroid dose (<1 mg/kg or ≥1 mg/kg) at onset of ECP was predictive of both response and survival. Although, patients with cGVHD had a significantly lower steroid dose than other subtypes at onset of ECP, they continued to have a significant decrease in steroid dose after ECP. It has been shown in a randomized study of ECP versus no ECP, that a >50% reduction in steroid dose along with a >25% reduction in total skin score, was seen in 8% of patients compared to no patients in the control arm (P = .04) [21].
The precise biologic mechanism of action of ECP remains elusive. This has hampered the development of biomarkers to predict ECP responsiveness. Until this is achieved, clinical factors and GVHD phenotype to predict ECP responsiveness have to be relied upon. This study identifies a subset of patients with cGVHD (viz. classic cGVHD and overlap GVHD) that benefits from ECP. Patients with steroid dependent/refractory classic cGVHD have a significantly improved survival with ECP compared to other GVHD subtypes. Future trials should study early intervention with ECP in this cohort of patients to try and decrease steroid and other IST-related adverse effects. It is possible that modulation of T-regulatory cells by ECP effect responses [25–27]. IST agents that promote T-regulatory cell survival may be potentially beneficial in combination with ECP. A phase II/III trial studying such an approach is being planned by Bone Marrow Transplant Clinical Trial Network for new-onset cGVHD with suboptimal response to steroids.
In summary, our data shows that the recently proposed NIH classification of GVHD helps in selecting patients with steroid dependent/refractory GVHD more likely to benefit from ECP and predicting response to ECP. Patients with classic cGVHD and overlap GVHD have a superior outcome when treated with ECP. This is in contrast with the inability of ECP to effectively salvage steroid dependent/refractory patients with classic aGVHD or recurrent aGVHD. This subset of patients should be considered for clinical trials investigating novel agents or in combination with ECP.
Acknowledgments
We thank Carey Clifton, RN, ACNP, and Leigh Ann Vaughan, ACNP, for their patient care and support of the Long Term Transplant Clinic.
Footnotes
Contribution: M.J. designed the study, analyzed and interpreted data, and wrote the article. B.S. assessed response on all patients and wrote the article. G.S. coordinated photopheresis on most patients. A.K., W.C., B.E., S.G., J.G., and F.S. contributed to clinical care, and commented on the manuscript. S.D. helped in data accumulation. H.C. helped analyze data.
Financial disclosure: M.J. has received research funding from Therakos. All other authors declare no conflict of interest.
References
- 1.Couriel D, Caldera H, Champlin R, Komanduri K. Acute graft-versus-host disease: pathophysiology, clinical manifestations, and management. Cancer. 2004;101:1936–1946. doi: 10.1002/cncr.20613. [DOI] [PubMed] [Google Scholar]
- 2.Lee SJ, Vogelsang G, Flowers ME. Chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2003;9:215–233. doi: 10.1053/bbmt.2003.50026. [DOI] [PubMed] [Google Scholar]
- 3.Graze PR, Gale RP. Chronic graft versus host disease: a syndrome of disordered immunity. Am J Med. 1979;66:611–620. doi: 10.1016/0002-9343(79)91171-9. [DOI] [PubMed] [Google Scholar]
- 4.Wingard JR, Piantadosi S, Vogelsang GB, et al. Predictors of death from chronic graft-versus-host disease after bone marrow transplantation. Blood. 1989;74:1428–1435. [PubMed] [Google Scholar]
- 5.Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69:204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 6.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Basara N, Kiehl MG, Blau W, et al. Mycophenolate mofetil in the treatment of acute and chronic GVHD in hematopoietic stem cell transplant patients: four years of experience. Transplant Proc. 2001;33:2121–2123. doi: 10.1016/s0041-1345(01)01968-6. [DOI] [PubMed] [Google Scholar]
- 8.Lopez F, Parker P, Nademanee A, et al. Efficacy of mycophenolate mofetil in the treatment of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2005;11:307–313. doi: 10.1016/j.bbmt.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 9.Couriel DR, Saliba R, Escalon MP, et al. Sirolimus in combination with tacrolimus and corticosteroids for the treatment of resistant chronic graft-versus-host disease. Br J Haematol. 2005;130:409–417. doi: 10.1111/j.1365-2141.2005.05616.x. [DOI] [PubMed] [Google Scholar]
- 10.Margolis J, Vogelsang G. An old drug for a new disease: pentostatin (Nipent) in acute graft-versus-host disease. Semin Oncol. 2000;27:72–77. [PubMed] [Google Scholar]
- 11.Vogelsang GB. Advances in the treatment of graft-versus-host disease. Leukemia. 2000;14:509–510. doi: 10.1038/sj.leu.2401687. [DOI] [PubMed] [Google Scholar]
- 12.Ho VT, Zahrieh D, Hochberg E, et al. Safety and efficacy of denileukin diftitox in patients with steroid-refractory acute graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Blood. 2004;104:1224–1226. doi: 10.1182/blood-2004-01-0028. [DOI] [PubMed] [Google Scholar]
- 13.Cutler C, Miklos D, Kim HT, et al. Rituximab for steroid-refractory chronic graft-versus-host disease. Blood. 2006;108:756–762. doi: 10.1182/blood-2006-01-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ratanatharathorn V, Ayash L, Reynolds C, et al. Treatment of chronic graft-versus-host disease with anti-CD20 chimericmonoclonal antibody. Biol Blood Marrow Transplant. 2003;9:505–511. doi: 10.1016/s1083-8791(03)00216-7. [DOI] [PubMed] [Google Scholar]
- 15.Gomez-Almaguer D, Ruiz-Arguelles GJ, Tarin-Argaza LC, et al. Alemtuzumab for the treatment of steroid-refractory acute graft-versus-host disease. Biol Blood Marrow Transplant. 2008;14:10–15. doi: 10.1016/j.bbmt.2007.08.052. [DOI] [PubMed] [Google Scholar]
- 16.Greinix HT, Volc-Platzer B, Rabitsch W, et al. Successful use of extracorporeal photochemotherapy in the treatment of severe acute and chronic graft-versus-host disease. Blood. 1998;92:3098–3104. [PubMed] [Google Scholar]
- 17.Greinix HT, Volc-Platzer B, Kalhs P, et al. Extracorporeal photochemotherapy in the treatment of severe steroid-refractory acute graft-versus-host disease: a pilot study. Blood. 2000;96:2426–2431. [PubMed] [Google Scholar]
- 18.Greinix HT, Volc-Platzer B, Knobler RM. Extracorporeal photochemotherapy in the treatment of severe graft-versus-host disease. Leuk Lymphoma. 2000;36:425–434. doi: 10.3109/10428190009148389. [DOI] [PubMed] [Google Scholar]
- 19.Greinix HT, Knobler RM, Worel N, et al. The effect of intensified extracorporeal photochemotherapy on long-term survival in patients with severe acute graft-versus-host disease. Haematologica. 2006;91:405–408. [PubMed] [Google Scholar]
- 20.Couriel DR, Hosing C, Saliba R, et al. Extracorporeal photochemotherapy for the treatment of steroid-resistant chronic GVHD. Blood. 2006;107:3074–3080. doi: 10.1182/blood-2005-09-3907. [DOI] [PubMed] [Google Scholar]
- 21.Flowers ME, Apperley JF, van BK, et al. A multicenter prospective phase 2 randomized study of extracorporeal photopheresis for treatment of chronic graft-versus-host disease. Blood. 2008;112:2667–2674. doi: 10.1182/blood-2008-03-141481. [DOI] [PubMed] [Google Scholar]
- 22.Jagasia M, Giglia J, Chinratanalab W, et al. Incidence and outcome of chronic graft-versus-host disease using National Institutes of Health consensus criteria. Biol Blood Marrow Transplant. 2007;13:1207–1215. doi: 10.1016/j.bbmt.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Arora M, Nagaraj S, Witte J, et al. New classification of chronic GVHD: added clarity from the consensus diagnoses. Bone Marrow Transplant. 2009;43:149–153. doi: 10.1038/bmt.2008.305. [DOI] [PubMed] [Google Scholar]
- 24.Pavletic SZ, Martin P, Lee SJ, et al. Measuring therapeutic response in chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: IV. Response Criteria Working Group report. Biol Blood Marrow Transplant. 2006;12:252–266. doi: 10.1016/j.bbmt.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 25.Bladon J, Taylor P. Extracorporeal photopheresis normalizes some lymphocyte subsets (including T regulatory cells) in chronic graft-versus-host-disease. Ther Apher Dial. 2008;12:311–318. doi: 10.1111/j.1744-9987.2008.00593.x. [DOI] [PubMed] [Google Scholar]
- 26.Lamioni A, Carsetti R, Legato A, et al. Induction of regulatory T cells after prophylactic treatment with photopheresis in renal transplant recipients. Transplantation. 2007;83:1393–1396. doi: 10.1097/01.tp.0000261635.30578.d8. [DOI] [PubMed] [Google Scholar]
- 27.Rubegni P, Sbano P, Cevenini G, et al. CD4+ CD25+ lymphocyte subsets in chronic graft versus host disease patients undergoing extracorporeal photochemotherapy. Int J Immunopathol Pharmacol. 2007;20:801–807. doi: 10.1177/039463200702000416. [DOI] [PubMed] [Google Scholar]