Abstract
Stem cell-encapsulating hydrogel microbeads of several hundred microns in size suitable for injection, that could quickly degrade to release the cells, are currently unavailable. The objectives of this study were to: (1) develop oxidized alginate-fibrin microbeads encapsulating human umbilical cord mesenchymal stem cells (hUCMSCs); (2) investigate microbead degradation, cell release, and osteogenic differentiation of the released cells for the first time. Three types of microbeads were fabricated to encapsulate hUCMSCs: (1) Alginate microbeads; (2) oxidized alginate microbeads; (3) oxidized alginate-fibrin microbeads. Microbeads with sizes of about 100–500 µm were fabricated with 1×106 hUCMSCs/mL of alginate. For the alginate group, there was little microbead degradation, with very few cells released at 21 d. For oxidized alginate, the microbeads started to slightly degrade at 14 d. In contrast, the oxidized alginate-fibrin microbeads started to degrade at 4 d and released the cells. At 7 d, the number of released cells greatly increased and showed a healthy polygonal morphology. At 21 d, the oxidized alginate-fibrin group had a live cell density that was 4-fold that of the oxidized alginate group, and 15-fold that of the alginate group. The released cells had osteodifferentiation, exhibiting highly elevated bone marker gene expressions of ALP, OC, collagen I, and Runx2. Alizarin staining confirmed the synthesis of bone minerals by hUCMSCs, with the mineral concentration at 21 d being 10-fold that at 7 d. In conclusion, fast-degradable alginate-fibrin microbeads with hUCMSC encapsulation were developed that could start to degrade and release the cells at 4 d. The released hUCMSCs had excellent proliferation, osteodifferentiation, and bone mineral synthesis. The alginate-fibrin microbeads are promising to deliver stem cells inside injectable scaffolds to promote tissue regeneration.
Keywords: alginate-fibrin microbeads, fast degradable hydrogel, human umbilical cord stem cells, cell release, osteodifferentiation and mineralization, tissue engineering
1. Introduction
Tissue engineering offers immense promise to millions of patients suffering from debilitating diseases [1–7]. The need for bone repair arises due to skeletal diseases, congenital malformations, trauma, and tumor resections [8–11]. Musculoskeletal conditions cost the USA more than $200 billion annually [12]. Stem cells guided for osteogenic differentiation and delivered via various scaffolds are promising to meet the increasing need for bone regeneration [13–17].
A hydrogel is a three-dimensional polymer network, crosslinked chemically, physically or ionically, with water as the predominant dispersal medium [18]. Alginate has been used for cell delivery because it can be cross-linked under mild conditions, and alginate hydrogels are highly hydrated with good biocompatibility [19]. Alginate is a natural polymer derived primarily from brown seaweed and bacteria. The degradation of alginate is slow and uncontrollable, which is undesirable for tissue engineering applications. To overcome this problem, previous studies developed alginate hydrogels that were hydrolytically degradable via partial oxidation of the alginate [20]. In certain applications, it is desirable to use a fast-degradable hydrogel. In recent studies [21,22], cells were encapsulated in alginate microbeads, and the microbeads were mixed into a calcium phosphate cement (CPC), which served as a moderate load-bearing scaffold for cell delivery. The alginate microbeads protected the cells from the mixing and injection forces as well as the CPC setting reaction [21]. After injection and CPC setting reaction, it is desirable for the alginate microbeads to quickly degrade to release the cells throughout the CPC scaffold, while concomitantly creating macropores for cell migration and proliferation. However, the previous alginate microbeads did not degrade and failed to release the cells. Therefore, there is a need to develop cell-encapsulating microbeads that can quickly degrade to release the cells.
Fibrin is a natural fibrous protein involved in the clotting of blood. It is polymerized from fibrinogen and thrombin into a mesh structure that can form a hemostatic clot (in conjunction with platelets) over a wound site [23]. Fibrin scaffolds can be engineered as a tissue substitute that is biocompatible and biodegradable [24]. Proliferation and differentiation of the stem cells can be achieved in a fibrin matrix, and fibrin alone or in combination with other materials has been used as scaffolds to regenerate adipose tissue, bone, cartilage, etc. [24]. In previous studies, fibrin microbeads were produced in hot oil, and the cells were seeded on the surface of the beads, but the cells were not encapsulated inside the beads [25]. This is because it is difficult to construct cell-encapsulated fibrin microbeads due to the sticky nature of fibrin. Other studies fabricated cell-encapsulating fibrin beads of about 3 mm in diameter, by first preparing an alginate-fibrin mixture and then extracting the alginate [26,27]. However, these large beads, when mixed into a paste such as calcium phosphate cement, would not be injectable because the beads are larger than the needle size. Indeed, previous studies on fibrin beads did not mention injection. Furthermore, these fibrin beads appeared not fast degradable, since these beads were cultured for 40 d without reporting cell release from the beads [28].
A literature search revealed no report on stem cell-encapsulating alginate-fibrin microbeads with diameters of several hundred µm, which can degrade rapidly and release the cells in a few days. These fine microbeads would be injectable with a calcium phosphate cement or another paste such as an injectable polymer. Once delivered, these microbeads could degrade inside the scaffold to release the cells and create macropores of several hundred µm in size, which are suitable for cell migration and tissue ingrowth. In addition, the influx of nutrients and the outflux of biological metabolites produced by the encapsulated cells would be easier due to the small size and high surface area of the microbeads than the 3-mm beads.
Accordingly, the objectives of this study were to develop stem cell-encapsulating alginate-fibrin microbeads that could quickly degrade and release the cells, and to investigate the proliferation and osteogenic differentiation of cells released from microbeads. The hypotheses are: (1) While alginate and oxidized alginate microbeads would fail to release the cells, the oxidized alginate-fibrin microbeads would rapidly degrade and release the cells; (2) Cells would not proliferate when encapsulated in alginate and oxidized alginate microbeads, but would rapidly proliferate when using oxidized alginate-fibrin microbeads; (3) Cells released from the microbeads could differentiate into the osteogenic lineage and synthesize bone minerals in vitro.
2. MATERIALS AND METHODS
2.1. hUCMSC culture
hUCMSCs were obtained commercially (ScienCell, Carlsbad, CA). They were harvested from the Wharton’s Jelly in cords of healthy babies, using an established method [29,30]. The use of hUCMSCs was approved by the University of Maryland. Cells were cultured in a low-glucose Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin (Invitrogen, Carlsbad, CA) (control media). At 80–90% confluence, hUCMSCs were detached and passaged, and passage 4 cells were used. The osteogenic media consisted of the control media plus 100 nM dexamethasone, 10 mM β-glycerophosphate, 0.05 mM ascorbic acid, and 10 nM 1α,25-Dihydroxyvitamin (Sigma, St. Louis, MO) [31–33].
2.2. Synthesis of hydrogel microbeads with hUCMSC encapsulation
Hydrogels are appealing as carriers for cell encapsulation and delivery because of their structural similarity to the extracellular matrix of tissues and their high water and fluid content for cell survival. Alginate has been used to encapsulate cells because it is non-cytotoxic and can form an ionically-crosslinked network under mild conditions [33–35]. Previous studies encapsulated stem cells in alginate microbeads with good viability [22,33]. In the present study, three types of alginate-based microbeads were fabricated to encapsulate the hUCMSCs: (1) Alginate microbeads; (2) oxidized alginate microbeads; (3) oxidized alginate-fibrin microbeads.
For type 1, a 1.2 % (by mass) sodium alginate solution was prepared by dissolving alginate (UP LVG, 64% guluronic acid, MW = 75,000–220,000 g/mol, ProNova, Oslo, Norway) in saline (155 mmol/L NaCl), following previous studies [33,35]. hUCMSCs were added to the alginate solution at a density of 1 million cells/mL of alginate. The alginate-cell solution was loaded into a syringe which was connected to a bead-generating device (Var J1, Nisco, Zurich, Switzerland). Nitrogen gas was fed to the gas inlet and a pressure of 8 psi was established to form a coaxial air flow to break up the alginate droplets. The droplets fell into a well of 100 mmol/L calcium chloride solution and crosslinked to form microbeads [33]. A microscope (Eclipse TE-2000S, Nikon, Melville, NY) was used to measure the sizes of the microbeads.
For type 2, alginate was oxidized to increase its degradability. Oxidation was done using sodium periodate at the correct stoichiometric ratio of sodium periodate/alginate to have certain percentages of alginate oxidation [20]. The percentage of oxidation (%) was the number of oxidized uronate residues per 100 uronate units in the alginate chain. In a previous study, alginate of up to 5% oxidation was synthesized [20]. In our preliminary studies, the microbeads using 5% oxidized alginate failed to degrade after 21 d in the culture media. This slow degradation may be desirable for other applications, but is too slow for releasing cells in a CPC scaffold. The microbeads using 10% oxidized alginate were too weak to be handled. Hence, in the present study, alginate at 7.5% oxidation was synthesized. The alginate oxidation followed previous procedures [20]. Briefly, 1% by mass of sodium alginate was dissolved in distilled water. 1.51 mL of 0.25 mol/L sodium periodate (Sigma) was added to 100 mL of alginate solution, which was stirred to react in the dark at room temperature. At 24 h, the oxidization reaction was stopped by adding 1 g of ethylene glycol and then 2.5 g of sodium chloride. Ethanol of 200 mL was added to precipitate the product, which was then collected by centrifugation. The precipitates were re-dissolved in 100 mL of water and precipitated with 200 mL of ethanol. The second precipitates were collected and dissolved in 30 mL of water. The final product was freeze dried for 24 h, and used to make the microbeads. The oxidized alginate thus obtained was dissolved in saline at a concentration of 1.2%, and the hUCMSC-encapsulating microbeads were made as described for type 1. They are referred to as “oxidized alginate microbeads”.
For type 3, fibrin was added to the oxidized alginate to obtain oxidized alginate-fibrin microbeads. An oxidized alginate solution at a concentration of 1.2% by mass in saline was prepared as described for type 2. Fibrinogen from bovine plasma (Sigma) was added at a concentration of 0.1% to the alginate solution and incubated at 37 °C for 2 h to yield a mixed alginate-fibrinogen solution. The fibrinogen concentration of 0.1% was selected because in preliminary studies, fibrinogen > 0.1% yielded microbeads that were sticking to each other because fibrin was sticky. Fibrinogen concentration < 0.1% resulted in microbeads that were not fast degradable. hUCMSCs were added to the alginate-fibrinogen solution at a density of 1 × 106 cells/mL. For cross-linking, a solution containing 125 mL of 100 mmol/L calcium chloride plus 125 NIH units of thrombin (Sigma) was prepared. When the alginate-fibrinogen droplets were sprayed into this solution, calcium chloride caused the alginate to crosslink, while the reaction between fibrinogen and thrombin produced fibrin. This yielded hUCMSC-encapsulating microbeads that are referred to as “oxidized alginate-fibrin microbeads”.
2.3. Viability of hUCMSCs
One mL of microbeads was used for five wells of a 6-well plate. This yielded 0.2 mL of microbeads encapsulating 200,000 cells in each well, to which was added 2 mL of osteogenic media. Twenty-five wells were prepared, with five wells (n = 5) each culturing for 1, 4, 7, 14 and 21 d. Three types of microbeads totaled 75 wells for the live/dead assay. Cells were stained with a live/dead kit (Invitrogen) and observed via epifluorescence microscopy (Eclipse TE-2000S, Nikon, Melville, NY). Three images were taken at random locations for each sample, with five samples yielding 15 images for each type of microbeads at each time point. The live and dead cells were counted. The percentage of live cells was: PLive = NLive/(NLive + NDead), where NLive = the number of live cells, and NDead = the number of dead cells [36]. The live cell density, DLive, was calculated: DLive = NLive/A, where A is the area of the view field for NLive.
2.4. Osteogenic differentiation of hUCMSCs
Only type 3 degraded rapidly and released the cells with enhanced proliferation. Hence, type 3 microbeads were used for measurement of osteogenic differentiation. A quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR, 7900HT, Applied Biosystems, Foster City, CA) method was used. After culturing in the osteogenic media for 4, 7, 14 and 21 d (n = 5), the total cellular RNA of the cells were extracted with TRIzol reagent (Invitrogen) and reverse-transcribed into cDNA using a High-Capacity cDNA Archive kit, following previous studies [21]. TaqMan gene expression assay kits, including two pre-designed specific primers and probes, were used to measure the transcript levels of the proposed genes on human alkaline phosphatase (ALP, Hs00758162_m1), osteocalcin (OC, Hs00609452_g1), collagen type I (Hs00164004), Runx2 (Hs00231692_m1), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH, Hs99999905). Relative expression for each target gene was evaluated using the 2-ΔΔCt method [37]. Ct values of target genes were normalized by the Ct of the TaqMan human housekeeping gene GAPDH to obtain the ΔCt values. The Ct of hUCMSCs cultured on tissue culture polystyrene in the control media for 1 d served as the calibrator [21,38].
2.5. hUCMSC Mineralization
The oxidized alginate-fibrin microbeads with hUCMSCs were used. At 4, 7, 14 and 21 d (n = 5), Alizarin Red S (ARS) staining was performed to visualize bone mineralization [17]. Cells were washed with PBS, fixed with 10% formaldehyde, and stained with ARS (Millipore, Billerica, MA) for 5 min, which stained calcium-rich deposits made by the cells into a red color. Controls were included with hUCMSCs cultured in control media. An osteogenesis assay kit (Millipore, Billerica, MA) was used to extract the stained minerals and measure the Alizarin Red concentration at OD405, following the manufacture’s instructions. Time periods of up to 21 d were selected because in previous studies, a great increase in calcium content during in vitro cell cultures was found between 12 d to 21 d [39].
One-way and two-way ANOVA were performed to detect significant effects of the variables. Tukey’s multiple comparison tests were used at a p value of 0.05.
3. Results
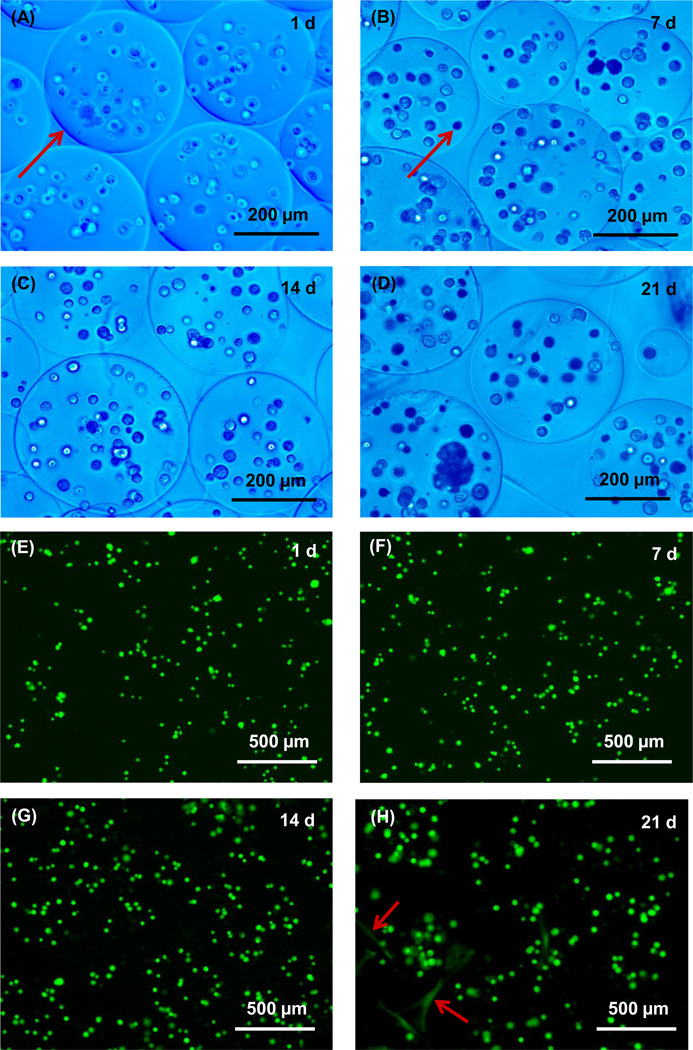
Fig. 1A–D show optical photos for alginate microbeads encapsulating hUCMSCs at 1, 7, 14 and 21 d, respectively. Because the microbeads were nearly transparent and difficult to see, a blue filter was used which enhanced the contrast and clarity of the microbeads. Measurement of 100 random microbeads showed that the microbead diameter ranged from about 98 to 495 µm, with a mean of 311 µm. Typical fluorescent photos of live/dead staining are shown in Fig. 1E–H at 1, 7, 14 and 21 d, respectively. The live cells appeared as small green dots in the hydrogel. There was no noticeable microbead degradation or cell release from 1 d to 14 d. At 21 d, only a few cells were released and showed a spreading and polygonal morphology (arrows in H).
Figure 1.
Optical photos of hUCMSC-encapsulating alginate microbeads (type 1) cultured for (A) 1 d, (B) 7 d, (C) 14d, and (D) 21 d. A blue filter was used to enhance the contrast of the microbeads. The arrow in (A) indicates the boundary of a relatively large microbead. Most of the microbeads appeared nearly spherical. The arrow in (B) indicates cells inside a microbead. (E–H) Typical fluorescent images of live/dead staining at 1, 7, 14 and 21 d, respectively. The arrows in (H) indicate a few cells that were released from the microbeads and attached to TCPS. Five samples were cultured at each time point (n = 5).
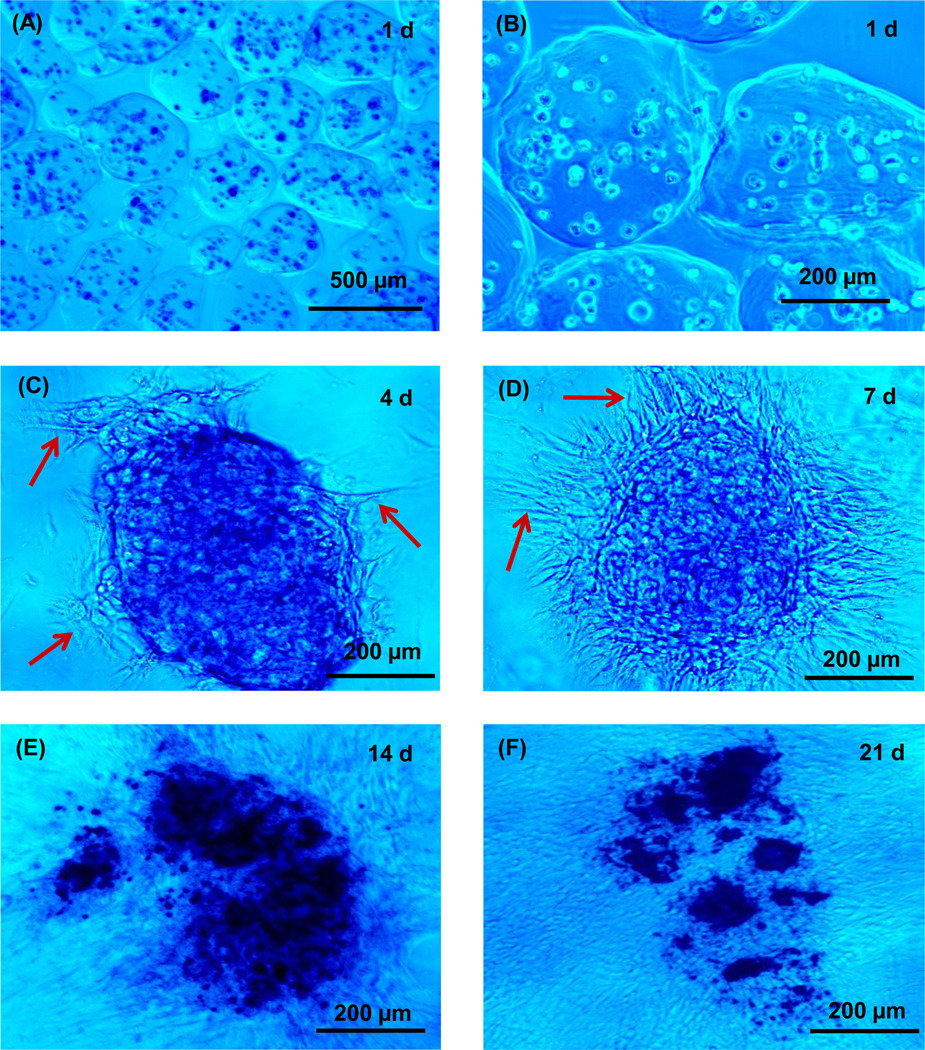
Optical photos of the oxidized alginate microbeads are shown in Fig. 2A–D. The oxidized alginate microbeads appeared to be weaker mechanically than the non-modified alginate microbeads. Many of the microbeads appeared to be elongated and not spherical, likely because the gel was not as stiff as the unmodified alginate. Because the microbeads were not spherical, we measured the length (the longest dimension) and the thickness (perpendicular to the length). Measurement of 100 randomly-chosen microbeads showed that the length ranged from about 87 to 580 µm, with a mean of 335 µm. The thickness ranged from 75 to 345 µm, with a mean of 232 µm. Typical fluorescent photos are shown in Fig. 2E–H. There is slight microbead degradation and cell release at 14 and 21 d, with most cells showing as green dots but some cells showing a spreading morphology.
Figure 2.
Optical photos of hUCMSC-encapsulating oxidized alginate microbeads (type 2) at (A) 1 d, (B) 7 d, (C) 14 d, and (D) 21 d. The microbeads started to degrade at 14 d. At 21 d, some microbeads started to fall apart (arrows in D). (E–H) Fluorescent images of live/dead staining at 1, 7, 14 and 21 d, respectively. The live cells appeared as small green dots at 1 and 7 d. Many cells still appeared as green dots at 14 and 21 d. However, there were cells with a spreading morphology at 14 and 21 d, indicative of cell release from the microbeads and attaching to the tissue culture polystyrene.
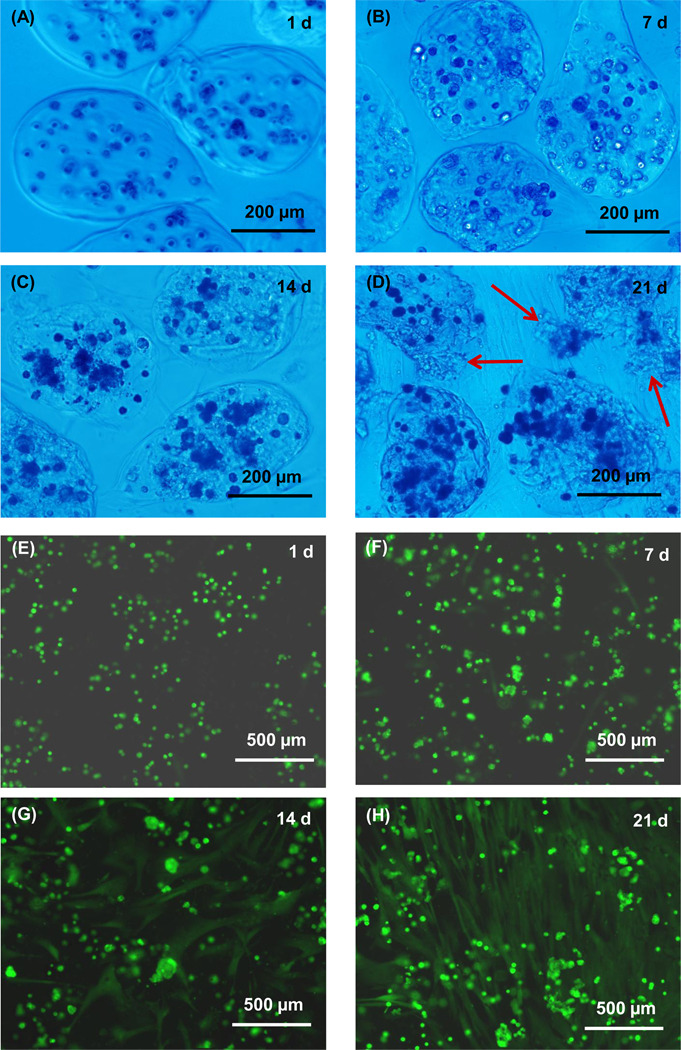
The microbead degradation and cell release are much more pronounced in Fig. 3 for the oxidized alginate-fibrin microbeads: (A) 1 d with low magnification, (B) 1 d with high magnification, (C), 4 d, (D) 7 d, (E) 14 d, and (F) 21 d. Although the microbeads deviated from the spherical shape, they were intact and did not degrade at 1 d. Measurement of 100 random microbeads showed that the length ranged from about 105 to 566 µm, with a mean of 314 µm. The thickness ranged from 93 to 411 µm, with a mean of 261 µm. At 4 d, the microbeads started to degrade. The degradation accelerated at 7 d and numerous cells were released. The microbeads broke down at 14 d and became small pieces at 21 d.
Figure 3.
Optical photos of hUCMSC-encapsulating oxidized alginate-fibrin microbeads (type 3) at (A) 1 d with low magnification, (B) 1 d with higher magnification, (C), 4 d, (D) 7 d, (E) 14 d, and (F) 21 d. The microbeads were intact and did not degrade at 1 d. At 4 d, they started to degrade, with arrows in (C) indicating the breakdown of the microbead boundary and the release of the encapsulated hUCMSCs. The degradation accelerated at 7 d and numerous cells were released (arrows in D). The microbeads fell apart in (E) and became small pieces in (F).
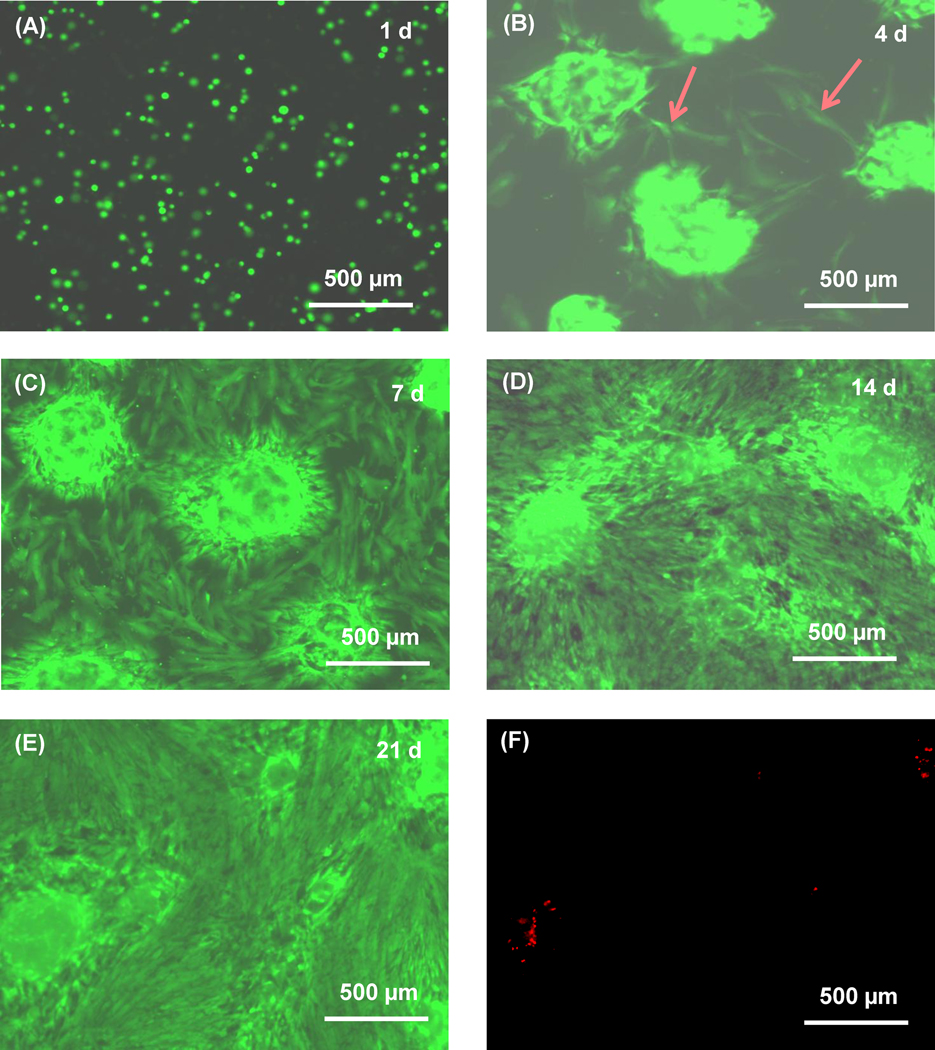
The fluorescent live/dead staining photos for the oxidized alginate-fibrin microbeads are shown in Fig. 4. At 1 d, the cells appeared as small green dots, similar to those for type 1. However, at 4 d, some cells were released from the microbeads and showed a spreading morphology. The number of released cells greatly increased from 7 to 21 d. The released cells proliferated, forming a confluent monolayer at 21 d. In addition, the cells developed a healthy polygonal morphology, indicating that they had attached to the tissue culture polystyrene. Dead cells were stained red and were very few at all time points, with an example in (F) at 21 d.
Figure 4.
Typical fluorescent live/dead staining images for the oxidized alginate-fibrin microbeads (type 3). Live cells were stained green at (A) 1 d, (B) 4 d, (C) 7 d, (D) 14 d, (E) 21 d. (F) Dead cells were stained red at 21 d. At 1 d, the cells appeared as green dots. At 4 d, some cells were released from the microbeads and showed a spreading morphology. At 7 d, the number of released cells greatly increased and attached on the tissue culture polystyrene, showing a healthy polygonal morphology. The released cells proliferated, forming a confluent monolayer at 21 d. Dead cells were few from 1 d to 21 d.
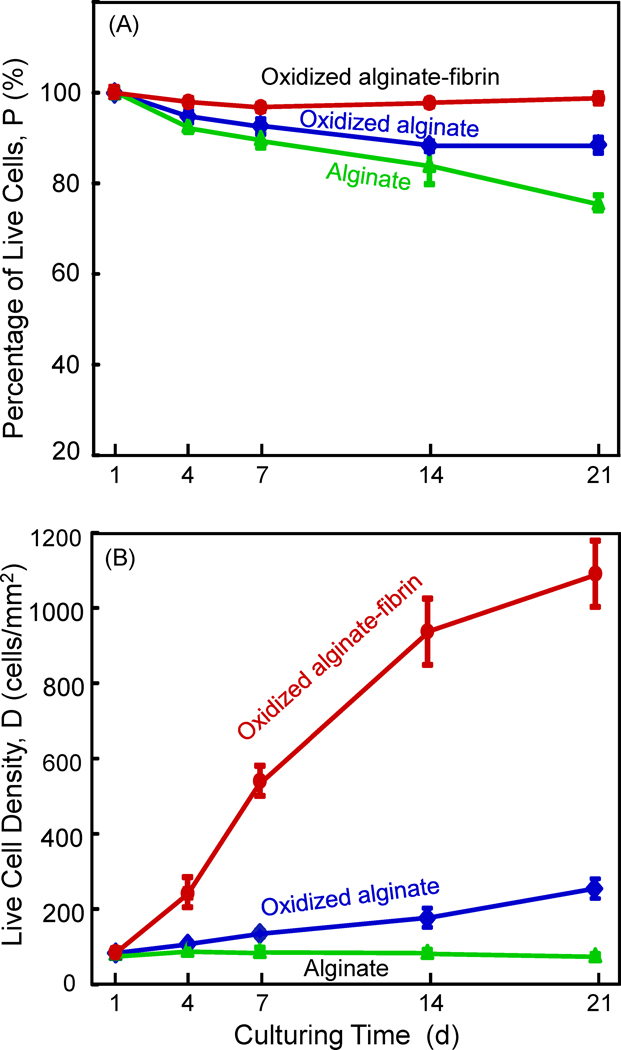
In Fig. 5A, the percentage of live cells for the oxidized alginate-fibrin group was the highest, followed by the oxidized alginate group, with the alginate group being the lowest. In (B), the number of live cells per area was nearly constant for alginate microbeads, and slightly increased with time for the oxidized alginate microbeads. In contrast, cells rapidly proliferated for the oxidized alginate-fibrin group, yielding a substantial increase in live cell density. At 21 d, the oxidized alginate-fibrin group had a live cell density that was approximately 4-fold that of the oxidized alginate group, and 15-fold that of the alginate group.
Figure 5.
hUCMSC viability: (A) Percentage of live cells, and (B) live cell density. Each value is the mean of five measurements, with the error bar showing one standard deviation (mean ± sd; n = 5).
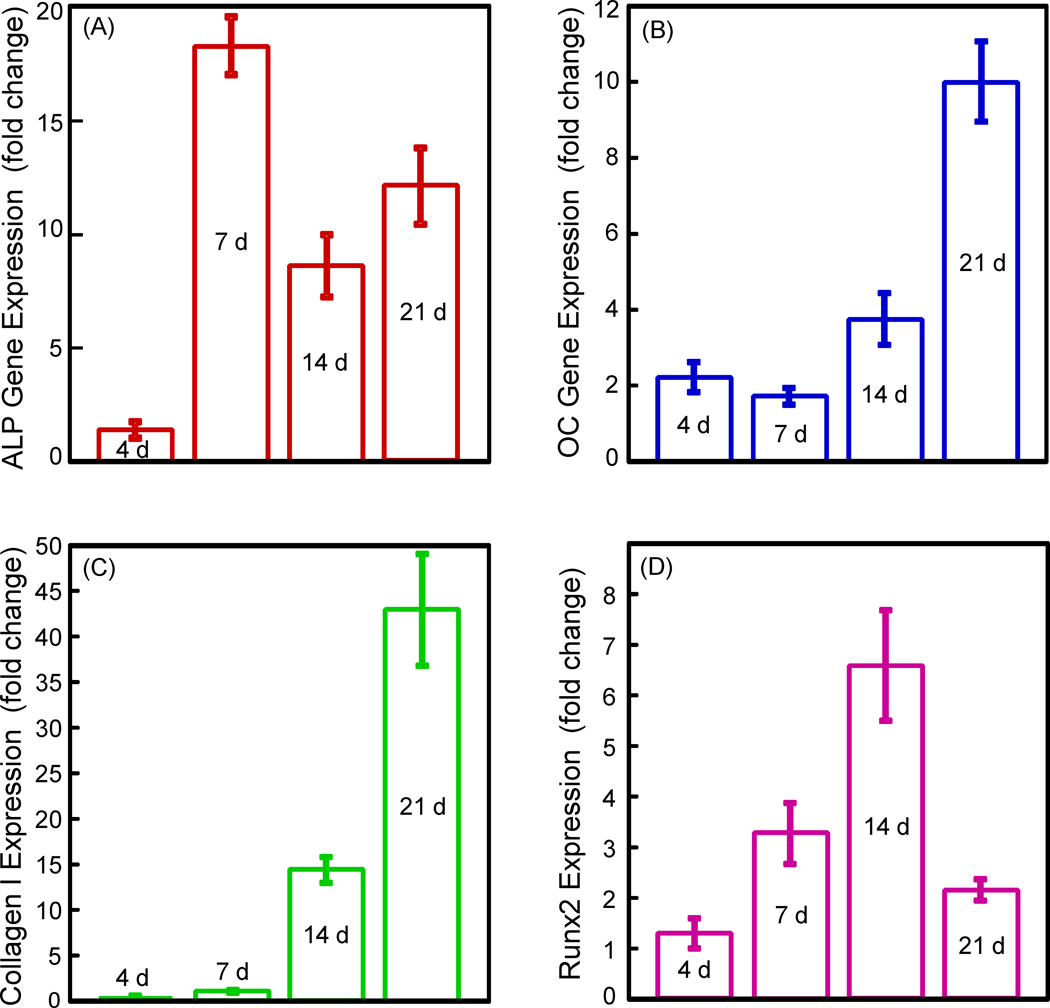
The oxidized alginate-fibrin group with successful cell release was selected for the RT-PCR experiment, and the results are plotted in Fig. 6. The ALP gene expression was minimal at 4 d, greatly increased at 7 d (p < 0.05), and then decreased at 14 d and 21 d. ALP at 7 d was 18-fold that at 4 d. The OC expression started to increase at 14 d and greatly increased at 21 d. So did the collagen I expression. Runx2 peaked at 14 d. These data indicate that hUMSCs released from the microbeads had differentiated into the osteogenic lineage.
Figure 6.
RT-PCR results for the oxidized alginate-fibrin group. Each value is mean ± sd; n = 5. hUCMSCs cultured for 1 d in control media without the osteogenic supplements served as control and its value was set as being 1. When cultured in osteogenic media, the ALP gene expression peaked at 7 d. Runx2 peaked at 14 d. OC and collagen I peaked at 21 d. Therefore, the hUMSCs released from the oxidized alginate-fibrin microbeads had differentiated into the osteogenic lineage.
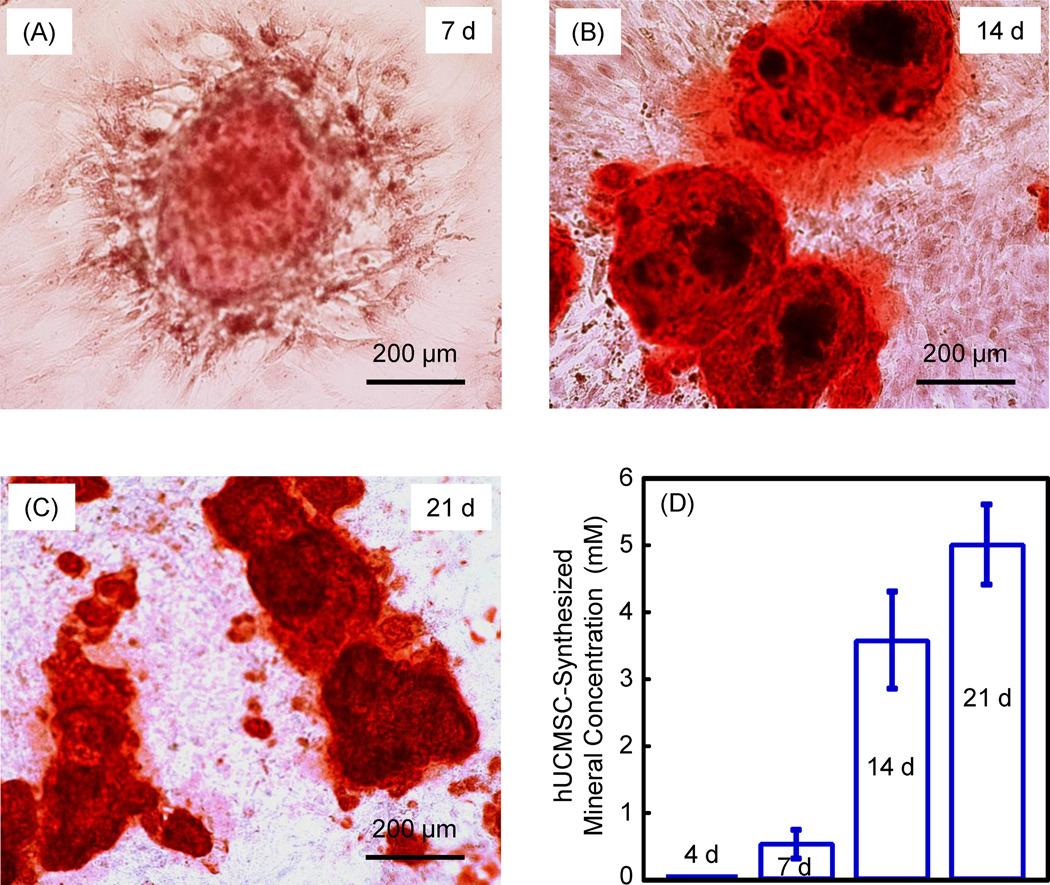
Results on hUCMSC mineralization are shown in Fig. 7. There was little mineral staining at 1 and 4 d (not shown). There was noticeable mineral synthesis in (A) at 7 d. The mineral staining greatly increased at 14 d and 21 d (B and C). In (D), the mineral concentration was measured by an osteogenesis assay. The mineral synthesized by the hUCMSCs was minimal at 4 d, then increased significantly at 14 and 21 d (p < 0.05).
Figure 7.
Mineralization of hUMSCs released from the oxidized alginate-fibrin microbeads at: (A) 7 d, (B) 14 d, and (C) 21 d. Culture was performed in osteogenic media. The ARS stained the calcium minerals into a red color. (D) The mineral concentration was measured by an osteogenesis assay (n = 5). These results demonstrate that the hUCMSCs released from the degradable microbeads successfully synthesized bone minerals.
4. Discussion
It is desirable to have fast-degradable hydrogel microbeads with stem cell encapsulation, so that after delivery, the microbeads could quickly release the cells to enhance cell function. However, microbeads with sizes of several hundred microns suitable for injection, that can quickly degrade and release the cells, are not available. In the present study, fast-degradable alginate-fibrin microbeads with hUCMSC encapsulation were developed for the first time that could start to degrade and release the cells at 4 d to enhance cell proliferation. The hUCMSCs were successfully released from the microbeads and differentiated down the osteogenic lineage. Umbilical cords can provide an inexpensive and inexhaustible stem cell source, without the invasive procedure needed to harvest bone marrow MSCs. In previous studies, hUCMSCs were derived for tissue engineering applications [16,29–31,40,41]. hUCMSCs were shown to be primitive MSCs with a high plasticity and developmental flexibility [42]. Preliminary animal studies indicated no immunorejection [43]. Recent studies showed that hUCMSCs had good potential for bone tissue engineering [22,31,32,41,44]. These studies are supported by the promising results of the present study on the use of hUCMSCs.
The hydrogel microbeads alone are useful for stem cell delivery and tissue engineering. In addition, it is useful to incorporate the cell-encapsulating microbeads into a paste vehicle for placement or injection delivery, with the microbeads providing protection to the cells from the mixing and injection forces. The paste could be a calcium phosphate cement, a polymer, a slow-degrading hydrogel, or any other injectable vehicle. After the paste hardens to form a scaffold, it is desirable for the microbeads inside the scaffold to degrade quickly to release the cells, while concomitantly creating macropores in the scaffold to enhance cell migration and proliferation.
A literature search indicated that: (1) Fibrin beads of about 3 mm in diameter were made in previous studies to encapsulate cells [26,27]; (2) fibrin microbeads of 50–300 µm in diameter were pre-fabricated in hot oil and then seeded with cells on the surfaces, with no cell encapsulation [25]; (3) alginate bulk scaffolds were developed for tissue engineering [2]; (4) alginate beads of 3.6 mm and 2.2 mm diameters were made for cell encapsulation, but the beads were not readily degradable and no cell release from the beads was achieved [34,35]; (5) RGD-modified alginate microbeads of 1 mm in diameter were fabricated, but they did not degrade, and the authors did not mention cell release [45]; (6) alginate microbeads of 207 µm were developed to encapsulate cells, but the microbeads were not degradable and no cell release was obtained [21]. Hence, there has been no report on cell-encapsulating hydrogel microbeads, with several hundred microns in size, that can quickly degrade and release the cells. Based on the literature search, this is the first report on alginate-fibrin microbeads, with sizes of several hundred microns which are suitable for injection delivery, that could degrade to release the cells as early as at day 4. Furthermore, the released hUCMSCs had excellent proliferation, osteogenic differentiation, and synthesis of bone minerals.
Alginate microbeads (type 1) showed little degradation in the culture media at 21 d, with few cell release and little proliferation. In fluorescent images, the live cells showed as green dots with no spreading morphology from 1 d to 21 d, with only a few cells released at 21 d. The MSCs are anchorage-dependent and need a bioactive surface in order to attach and spread [46,47]. In previous studies, researchers used peptides to modify the hydrogels and obtained cell attachment and spreading in the bioactive gels [4,6,18,48]. However, even in the bioactive alginate, the MSCs had no significant proliferation. This is because encapsulating and wrapping the cells inside alginate caused contact inhibition, therefore the cell proliferation became arrested [47]. As a result, cells encapsulated in alginate had no proliferation, which is consistent with Fig. 1 and Fig. 5B for the alginate group in the present study. Another point worth noting is that it is generally beneficial to functionalize the hydrogel, such as via incorporating peptides, to enhance cell attachment and function, especially when the cells would reside inside the hydrogel carrier for a month or longer [4,6,18,48]. Because the purpose of the present study was to render the hydrogel fast-degradable and to release the cells in several days, the alginate hydrogel was not functionalized. Further study should functionalize the fast-degradable alginate and determine if the cell viability and proliferation could be further improved.
Type 2, the oxidized alginate, started to show partial microbead degradation with limited cell release from the microbeads at 14 d and 21 d. The fluorescent images showed the live cells as green dots at 1 d and 7 d. However, at 14 d and 21 d, while most cells still appeared as green dots, an increasing number of cells showed a spreading and polygonal morphology. This was accompanied by the proliferation of the released cells, with an increase in the number of live cells at 14 d and 21 d (Fig. 5B). Previous studies used partial oxidation of the alginate, the modification of alginate with cell adhesion peptides, the creation of macropores, and the incorporation of growth factors to increase cell viability [2,45,49]. In another study, a macroporous alginate scaffold of 2 × 2 × 5 mm was fabricated and then seeded with cells, and the cells could migrate out of the scaffold [2]. Cells were seeded on the pre-formed bulk scaffold, hence the cells were not encapsulated and no alginate beads were made [2]. The present study showed that oxidized alginate microbeads could be developed to degrade after 2 weeks to release the cells and promote proliferation.
In dramatic contrast, type 3, the oxidized alginate-fibrin microbeads, started to release the hUCMSCs at 4 d, with numerous cell release at 7 d. This resulted in rapid cell proliferation, yielding a live cell density that was 4-fold that of the oxidized alginate group, and 15-fold that of the alginate group, while the initial cell encapsulation density was the same for the three groups. Therefore, adding a small amount of fibrin to the oxidized alginate greatly enhanced microbead degradation, cell release, and proliferation. In our preliminary studies, fibrin concentrations of 0.2%, 0.3%, 0.5%, 1.0%, 2.0% and 2.5% were examined. However, they resulted in many fibrin clots in the alginate. The microbeads were sticking together because the fibrin served as “glue”, and the microbeads were too weak to maintain their integrity when handled. On the other hand, when a low fibrin concentration of 0.05% was used, the microbead degradation and cell release were delayed. Therefore, an intermediate fibrin concentration of 0.1% in alginate was used in this study, which appeared to be optimal for microbead integrity as well as its degradation and cell release. In addition, a preliminary experiment was performed to examine if the oxidized alginate-fibrin microbeads with 0.1% fibrin could survive the injection process. The microbeads were mixed with a calcium phosphate cement paste at 50% by volume of microbeads, and injected using a syringe with a 10-gauge tip, following the methods as described in a previous study [21]. After the paste was extruded, the calcium phosphate cement was washed away with water and the microbeads were collected. The microbeads were examined in a microscope and appeared to be similar to the microbeads before injection. This indicates that the oxidized alginate-fibrin microbeads survived the injection process without significant damage. Further study is needed to examine the stem cell proliferation and differentiation after being injected with the calcium phosphate cement. The present study showed that alginate-fibrin microbeads with hUCMSC encapsulation could be developed to possess initial microbead integrity for handling, and then with a fast degradation that could release the cells starting at 4 d.
The released cells from the oxidized alginate-fibrin microbeads differentiated into the osteogenic lineage. The ALP peaked at 7 d, and then decreased at 14 and 21 d, although the ALP expressions at 14 and 21 d were still about 10-fold higher than that at 4 d. ALP is an enzyme expressed by MSCs during osteogenesis and is a well-defined marker for their differentiation [14,15,38,50–53]. At the early stage of osteogenic differentiation, the ALP expression is first upregulated. Then, as the cascade of events for the differentiation continues, other markers such as Runx 2, OC and collagen type I become upregulated, while the ALP is decreased. This is consistent with Fig. 6 that showed that while the ALP peaked at 7 d, Runx2 peaked at 14 d, and OC and collagen I peaked at 21 d. Previous studies also showed the ALP peaking at 4 to 16 d [14,15,38,50–52]. One study showed that the ALP peaked at 8 d, then decreased at 16 d [50]. In another study, the ALP peaked at 4 d, and then decreased at 8 d [38]. That same study reported that the OC peaked at 8 d, later than the 4 d for ALP [38]. While the peaking time appeared to vary for different systems under different culture and measurement conditions, it is consistent that for osteogenic differentiation of MSCs, the ALP peaks first which is followed by other markers such as OC. The hUCMSCs released from the oxidized alginate-fibrin microbeads synthesized bone minerals after 7 d. The ARS staining and the mineral concentration measurement both showed more mineralization at 14 and 21 d. The mineralization at 21 d was 10-fold that at 7 d. Therefore, these novel alginate-fibrin microbeads could quickly release the hUCMSCs for proliferation, osteogenic differentiation and mineralization.
These stem cell-encapsulating alginate-fibrin microbeads are promising to deliver stem cells in scaffolds for tissue engineering applications. When the microbeads degrade, the cells are released throughout the volume of the scaffold, in contrast to cell seeding only on the surface for a pre-formed scaffold. In addition, the degradation of the microbeads can create macropores in the scaffold to further increase porosity and enhance cell migration and fluid circulation. These stem cell-encapsulating alginate-fibrin microbeads could be useful in delivering stem cells in calcium phosphate carriers and polymer carriers. They could also deliver stem cells in a slow-degrading hydrogel, to form a construct of fast-degrading microbeads inside a slow-degrading hydrogel matrix, where the cells could be released into the macropores created by the microbead degradation. Further studies are needed to incorporate these fast-degradable microbeads into an injecatble calcium phosphate cement to investigate cell release and proliferation inside the scaffold. Further studies are also needed to incorporate these fast-degradable microbeads into an injectable polymeric scaffold to investigate cell function in the scaffold.
5. Conclusion
Stem cell-encapsulating alginate-fibrin microbeads of several hundred microns in diameter were developed that could quickly degrade and release the cells. The encapsulated hUCMSCs were released from the microbeads starting at 4 d, and the released cells showed excellent proliferation, osteogenic differentiation, and synthesized bone minerals. A fibrin concentration of 0.1% in alginate appeared to be optimal for obtaining microbeads with mechanical integrity to be handled without damaging the microbeads and the cells, and yet be able to quickly degrade and release the cells. The microbead diameter of 207 µm is suitable for mixing with an injectable paste. After 21 d, the oxidized alginate-fibrin microbead group had a live cell density that was 4-fold that of the oxidized alginate group, and 15-fold that of the alginate group. The released hUCMSCs had greatly increased bone marker gene expressions from 7 d to 21 d, compared to 4 d. hUCMSC mineralization increased by 10-fold from 7 d to 21 d. For potential applications, the fast-degradable alginate-fibrin microbeads could have a wide range of applications, including cell/growth factor delivery in calcium phosphate cements and polymer pastes, where the microbeads could quickly degrade and release the cells throughout the entire scaffold. This would concomitantly create macropores in the scaffold to enhance cell migration and tissue ingrowth. Further studies are needed to investigate microbead degradation, cell release, and osteogenic differentiation inside the scaffolds for bone tissue engineering.
ACKNOWLEDGMENTS
We thank Dr. Michael D. Weir for fruitful discussions and experimental help. This study was supported by NIH R01 grants DE17974 and DE14190 (HX), Maryland Stem Cell Fund (HX), and the University of Maryland Dental School.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lavik E, Langer R. Tissue engineering: Current state and perspectives. Applied Microbiol & Biotech. 2004;65:1–8. doi: 10.1007/s00253-004-1580-z. [DOI] [PubMed] [Google Scholar]
- 2.Hill E, Boontheekul T, Mooney DJ. Designing scaffolds to enhance transplanted myoblast survival and migration. Tissue Eng. 2006;12:1295–1304. doi: 10.1089/ten.2006.12.1295. [DOI] [PubMed] [Google Scholar]
- 3.Mao JJ, Giannobile WV, Helms JA, Hollister SJ, Krebsbach PH, Longaker MT, Shi S. Craniofacial tissue engineering by stem cells. J Dent Res. 2006;85:966–979. doi: 10.1177/154405910608501101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salinas CN, Anseth KS. The influence of the RGD peptide motif and its contextual presentation in PEG gels on human mesenchymal stem cell viability. J Tissue Eng Reg Med. 2008;2:296–304. doi: 10.1002/term.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atala A. Engineering organs. Curr Opin Biotechnol. 2009;20:575–592. doi: 10.1016/j.copbio.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Kretlow JD, Young S, Klouda L, Wong M, Mikos AG. Injectable biomaterials for regenerating complex craniofacial tissues. Adv Mater. 2009;21:3368–3393. doi: 10.1002/adma.200802009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aoki T, Ohnishi H, Oda Y, Tadokoro M, Sasao M, Kato H, Hattori K, Ohgushi H. Generation of induced pluripotent stem cells from human adipose-derived stem cells without c-MYC. Tissue Eng A. 2010;16:2197–2206. doi: 10.1089/ten.TEA.2009.0747. [DOI] [PubMed] [Google Scholar]
- 8.Gomes ME, Mikos AG, Reis RL. Injectable Polymeric Scaffolds for Bone Tissue Engineering. In: Reis RL, San Roman J, editors. Biodegradable Systems in Tissue Engineering and Regenerative Medicine. Boca Raton, FL: CRC Press; 2004. pp. 29–38. [Google Scholar]
- 9.Mikos AG, Herring SW, Ochareon P, Elisseeff J, Lu HH, Kandel R, et al. Engineering complex tissues. Tissue Eng. 2006;12:3307–3339. doi: 10.1089/ten.2006.12.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson PC, Mikos AG, Fisher JP, Jansen JA. Strategic directions in tissue engineering. Tissue Eng. 2007;13:2827–2837. doi: 10.1089/ten.2007.0335. [DOI] [PubMed] [Google Scholar]
- 11.Mao JJ, Vunjak-Novakovic G, Mikos AG, Atala A. Regenerative medicine: Translational approaches and tissue engineering. Boston, MA: Artech House; 2007. [Google Scholar]
- 12.Praemer A, Furner S, Rice DP. Musculoskeletal conditions in the United States. Rosemont, IL: Amer Acad Orthop Surg; 1999. [Google Scholar]
- 13.Jansen JA, Vehof JWM, Ruhe PQ, Kroeze-Deutman H, Kuboki Y, Takita H, et al. Growth factor-loaded scaffolds for bone engineering. J Controlled Release. 2005;101:127–136. doi: 10.1016/j.jconrel.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Benoit DSW, Nuttelman CR, Collins SD, Anseth KS. Synthesis and characterization of a fluvastatin-releasing hydrogel delivery system to modulate hMSC differentiation and function for bone regeneration. Biomaterials. 2006;27:6102–6110. doi: 10.1016/j.biomaterials.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 15.Reilly GC, Radin S, Chen AT, Ducheyne P. Differential alkaline phosphatase responses of rat and human bone marrow derived mesenchymal stem cells to 45S5 bioactive glass. Biomaterials. 2007;28:4091–4097. doi: 10.1016/j.biomaterials.2007.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarugaser R, Lickorish D, Baksh D, Hosseini MM, Davies JE. Human umbilical cord perivascular (HUCPV) cells: A source of mesenchymal progenitors. Stem Cells. 2005;23:220–229. doi: 10.1634/stemcells.2004-0166. [DOI] [PubMed] [Google Scholar]
- 17.Varghese S, Hwang NS, Ferran A, Hillel A, Theprungsirikul P, Canver AC, et al. Engineering musculoskeletal tissues with human embryonic germ cell derivatives. Stem Cells. 2010;28:765–774. doi: 10.1002/stem.325. [DOI] [PubMed] [Google Scholar]
- 18.Drury JL, Mooney DJ. Review. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24:4337–4351. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 19.Drury JL, Dennis RG, Mooney DJ. The tensile properties of alginate hydrogels. Biomaterials. 2004;25:3187–3199. doi: 10.1016/j.biomaterials.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Bouhadir KH, Lee KY, Alsberg E, Damm KL, Anderson KW, Mooney DJ. Degradation of partially oxidized alginate and its potential application for tissue engineering. Biotech Progress. 2001;17:945–950. doi: 10.1021/bp010070p. [DOI] [PubMed] [Google Scholar]
- 21.Zhao L, Weir MD, Xu HHK. An injectable calcium phosphate - alginate hydrogel - umbilical cord mesenchymal stem cell paste for bone tissue engineering. Biomaterials. 2010;31:6502–6510. doi: 10.1016/j.biomaterials.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu HHK, Zhao L, Weir MD. Stem cell-calcium phosphate constructs for bone engineering. J Dent Res. 2010;89:1482–1488. doi: 10.1177/0022034510384623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Atrah HI. Fibrin glue. British Med J. 1994;308:933–934. doi: 10.1136/bmj.308.6934.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahmed TA, Dare EV, Hincke M. Fibrin: a versatile scaffold for tissue engineering applications. Tissue Eng B. 2008;14:199–215. doi: 10.1089/ten.teb.2007.0435. [DOI] [PubMed] [Google Scholar]
- 25.Gorodetsky R, Levdansky L, Gaberman E, Gurevitch O, Lubzens E, McBride WH. Fibrin microbeads (FMB) loaded with mesenchymal cells support their long term survival while sealed at room temperature. Tissue Eng C. 2011 doi: 10.1089/ten.tec.2010.0644. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perka C, Arnold U, Spitzer RS, Lindenhayn K. The use of fibrin beads for tissue engineering and subsequential transplantation. Tissue Eng. 2001;7:359–361. doi: 10.1089/10763270152044215. [DOI] [PubMed] [Google Scholar]
- 27.Spitzer RS, Perka C, Lindenhayn K, Zippel H. Matrix engineering for osteogenic differentiation of rabbit periosteal cells using alpha-tricalcium phosphate particles in a three-dimensional fibrin culture. J Biomed Mater Res. 2002;59:690–696. doi: 10.1002/jbm.1277. [DOI] [PubMed] [Google Scholar]
- 28.Arnold U, Schweitzer S, Lindenhayn K, Perka C. Optimization of bone engineering by means of growth factors in a three-dimensional matrix. J Biomed Mater Res A. 2003;67:260–269. doi: 10.1002/jbm.a.10577. [DOI] [PubMed] [Google Scholar]
- 29.Wang HS, Hung SC, Peng ST. Mesenchymal stem cells in the Wharton’s jelly of the human umbilical cord. Stem Cells. 2004;22:1330–1337. doi: 10.1634/stemcells.2004-0013. [DOI] [PubMed] [Google Scholar]
- 30.Bailey MM, Wang L, Bode CJ, Mitchell KE, Detamore MS. A comparison of human umbilical cord matrix stem cells and temporomandibular joint condylar chondrocytes for tissue engineering temporomandibular joint condylar cartilage. Tissue Eng. 2007;13:2003–2010. doi: 10.1089/ten.2006.0150. [DOI] [PubMed] [Google Scholar]
- 31.Baksh D, Yao R, Tuan RS. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells. 2007;25:1384–1392. doi: 10.1634/stemcells.2006-0709. [DOI] [PubMed] [Google Scholar]
- 32.Wang L, Singh M, Bonewald LF, Detamore MS. Signaling strategies for osteogenic differentiation of human umbilical cord mesenchymal stromal cells for 3D bone tissue engineering. J Tissue Eng Reg Med. 2009;3:398–404. doi: 10.1002/term.176. [DOI] [PubMed] [Google Scholar]
- 33.Zhao L, Weir MD, Xu HHK. Human umbilical cord stem cell encapsulation in calcium phosphate scaffolds for bone engineering. Biomaterials. 2010;31:3848–3857. doi: 10.1016/j.biomaterials.2010.01.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simon CG, Guthrie WF, Wang FW. Cell seeding into calcium phosphate cement. J Biomed Mater Res A. 2004;68:628–639. doi: 10.1002/jbm.a.20008. [DOI] [PubMed] [Google Scholar]
- 35.Weir MD, Xu HHK, Simon CG., Jr. Strong calcium phosphate cement-chitosan-mesh construct containing cell-encapsulating hydrogel beads for bone tissue engineering. J Biomed Mater Res A. 2006;77:487–496. doi: 10.1002/jbm.a.30626. [DOI] [PubMed] [Google Scholar]
- 36.Xu HHK, Zhao L, Detamore MS, Takagi S, Chow LC. Umbilical cord stem cell seeding on fast-resorbable calcium phosphate bone cement. Tissue Eng A. 2010;16:2743–2753. doi: 10.1089/ten.tea.2009.0757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 38.Kim K, Dean D, Mikos AG, Fisher JP. Effect of initial cell seeding density on early osteogenic signal expression of rat bone marrow stromal cells cultured on cross-linked poly(propylene fumarate) disks. Biomacromolecules. 2009;10:1810–1817. doi: 10.1021/bm900240k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang YH, Liu Y, Maye P, Rowe DW. Examination of mineralized nodule formation in living osteoblastic cultures using fluorescent dyes. Biotech Progress. 2006;22:1697–1701. doi: 10.1021/bp060274b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jäger M, Degistirici O, Knipper A, Fischer J, Sager M, Krauspe R. Bone healing and migration of cord blood-derived stem cells into a critical size femoral defect after xenotransplantation. J Bone Miner Res. 2007;22:1224–1233. doi: 10.1359/jbmr.070414. [DOI] [PubMed] [Google Scholar]
- 41.Wang L, Dormer NH, Bonewald LF, Detamore MS. Osteogenic differentiation of human umbilical cord mesenchymal stromal cells in polyglycolic acid scaffolds. Tissue Eng A. 2010;16:1937–1948. doi: 10.1089/ten.TEA.2009.0706. [DOI] [PubMed] [Google Scholar]
- 42.Can A, Karahuseyinoglu S. Concise review: Human umbilical cord stroma with regard to the source of fetus-derived stem cells. Stem Cells. 2007;25:2886–2895. doi: 10.1634/stemcells.2007-0417. [DOI] [PubMed] [Google Scholar]
- 43.Weiss ML, Medicetty S, Bledsoe AR, Rachakatla RS, Choi M, Merchav S, et al. Human umbilical cord matrix stem cells: Preliminary characterization and effect of transplantation in a rodent model of Parkinson’s disease. Stem Cells. 2006;24:781–792. doi: 10.1634/stemcells.2005-0330. [DOI] [PubMed] [Google Scholar]
- 44.Diao Y, Ma Q, Cui F, Zhong Y. Human umbilical cord mesenchymal stem cells: osteogenesis in vivo as seed cells for bone tissue engineering. J Biomed Mater Res A. 2009;91:123–131. doi: 10.1002/jbm.a.32186. [DOI] [PubMed] [Google Scholar]
- 45.Evangelista MB, Hsiong SX, Fernandes R, Sampaio P, Kong HJ, Barrias CC, et al. Upregulation of bone cell differentiation through immobilization within a synthetic extracellular matrix. Biomaterials. 2007;28:3644–3655. doi: 10.1016/j.biomaterials.2007.04.028. [DOI] [PubMed] [Google Scholar]
- 46.Nuttelman CR, Tripodi MC, Anseth KS. In vitro osteogenic differentiation of human mesenchymal stem cells photoencapsulated in PEG hydrogels. J Biomed Mater Res A. 2004;68:773–782. doi: 10.1002/jbm.a.20112. [DOI] [PubMed] [Google Scholar]
- 47.Markusen JF, Mason C, Hull DA, Town MA, Tabor AB, Clements M, et al. Behavior of adult human mesenchymal stem cells entrapped in alginate-GRGDY beads. Tissue Eng. 2006;12:821–830. doi: 10.1089/ten.2006.12.821. [DOI] [PubMed] [Google Scholar]
- 48.Kong HJ, Smith MK, Mooney DJ. Designing alginate hydrogels to maintain viability of immobilized cells. Biomaterials. 2003;24:4023–4029. doi: 10.1016/s0142-9612(03)00295-3. [DOI] [PubMed] [Google Scholar]
- 49.Boontheekul T, Kong HJ, Mooney DJ. Controlling alginate gel degradation utilizing partial oxidation and bimodal molecular weight distribution. Biomaterials. 2005;26:2455–2465. doi: 10.1016/j.biomaterials.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 50.Datta N, Pha QP, Sharma U, Sikavitsas VI, Jansen JA, Mikos AG. In vitro generated extracellular matrix and fluid shear stress synergistically enhance 3D osteoblastic differentiation. Proc Natl Acad Sci USA. 2006;103:2488–2493. doi: 10.1073/pnas.0505661103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Radin S, Reilly G, Bhargave G, Leboy PS, Ducheyne P. Osteogenic effects of bioactive glass on bone marrow stromal cells. J Biomed Mater Res A. 2005;73:21–29. doi: 10.1002/jbm.a.30241. [DOI] [PubMed] [Google Scholar]
- 52.Leach JK, Kaigler D, Wang Z, Krebsbach PH, Mooney DJ. Coating of VEGF-releasing scaffolds with bioactive glass for angiogenesis and bone regeneration. Biomaterials. 2006;27:3249–3255. doi: 10.1016/j.biomaterials.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 53.Sundelacruz S, Kaplan DL. Stem cell- and scaffold-based tissue engineering approaches to osteochondral regenerative medicine. Seminar Cell Dev Bio. 2009;20:646–655. doi: 10.1016/j.semcdb.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]