Summary
Background
In the Medical Research Council (MRC) COIN trial, the epidermal growth factor receptor (EGFR)-targeted antibody cetuximab was added to standard chemotherapy in first-line treatment of advanced colorectal cancer with the aim of assessing effect on overall survival.
Methods
In this randomised controlled trial, patients who were fit for but had not received previous chemotherapy for advanced colorectal cancer were randomly assigned to oxaliplatin and fluoropyrimidine chemotherapy (arm A), the same combination plus cetuximab (arm B), or intermittent chemotherapy (arm C). The choice of fluoropyrimidine therapy (capecitabine or infused fluouroracil plus leucovorin) was decided before randomisation. Randomisation was done centrally (via telephone) by the MRC Clinical Trials Unit using minimisation. Treatment allocation was not masked. The comparison of arms A and C is described in a companion paper. Here, we present the comparison of arm A and B, for which the primary outcome was overall survival in patients with KRAS wild-type tumours. Analysis was by intention to treat. Further analyses with respect to NRAS, BRAF, and EGFR status were done. The trial is registered, ISRCTN27286448.
Findings
1630 patients were randomly assigned to treatment groups (815 to standard therapy and 815 to addition of cetuximab). Tumour samples from 1316 (81%) patients were used for somatic molecular analyses; 565 (43%) had KRAS mutations. In patients with KRAS wild-type tumours (arm A, n=367; arm B, n=362), overall survival did not differ between treatment groups (median survival 17·9 months [IQR 10·3–29·2] in the control group vs 17·0 months [9·4–30·1] in the cetuximab group; HR 1·04, 95% CI 0·87–1·23, p=0·67). Similarly, there was no effect on progression-free survival (8·6 months [IQR 5·0–12·5] in the control group vs 8·6 months [5·1–13·8] in the cetuximab group; HR 0·96, 0·82–1·12, p=0·60). Overall response rate increased from 57% (n=209) with chemotherapy alone to 64% (n=232) with addition of cetuximab (p=0·049). Grade 3 and higher skin and gastrointestinal toxic effects were increased with cetuximab (14 vs 114 and 67 vs 97 patients in the control group vs the cetuximab group with KRAS wild-type tumours, respectively). Overall survival differs by somatic mutation status irrespective of treatment received: BRAF mutant, 8·8 months (IQR 4·5–27·4); KRAS mutant, 14·4 months (8·5–24·0); all wild-type, 20·1 months (11·5–31·7).
Interpretation
This trial has not confirmed a benefit of addition of cetuximab to oxaliplatin-based chemotherapy in first-line treatment of patients with advanced colorectal cancer. Cetuximab increases response rate, with no evidence of benefit in progression-free or overall survival in KRAS wild-type patients or even in patients selected by additional mutational analysis of their tumours. The use of cetuximab in combination with oxaliplatin and capecitabine in first-line chemotherapy in patients with widespread metastases cannot be recommended.
Funding
Cancer Research UK, Cancer Research Wales, UK Medical Research Council, Merck KGgA.
Introduction
The introduction and biomarker refinement of treatments targeting the epidermal growth factor receptor (EGFR) has been one of the most promising developments in oncology treatment in the past 5 years. The benefit of EGFR tyrosine kinase inhibitors (gefitinib and erlotinib) in lung cancer is limited to patients whose tumours contain a mutation at the drug-binding site in the ATP-binding domain of the receptor, and has been seminal in the biomarker-defined enrichment of the responsive populations.1,2 In colorectal cancer, no such mutations in EGFR occur, but clinical benefit has been shown with monoclonal antibodies, which bind to the extracellular receptor domain inhibiting ligand binding (notably epidermal growth factor, amphiregulin, and epiregulin) and receptor dimerisation.3 This clinical benefit, apparently limited to patients whose tumours contain no evidence of a mutation in KRAS, was first noted in non-randomised studies4 and was subsequently confirmed in randomised trials of antibody monotherapy in patients who were refractory to chemotherapy.5,6 This finding is plausible because KRAS encodes a G protein, which is a key link in the signal transduction pathway (RAS–RAF–MAP kinase) from receptor to nucleus, and the observed mutations result in constitutive activation of the pathway unlikely to be affected by cell surface receptor binding. Other activating mutations such as those in BRAF and NRAS in colorectal cancers might have similar negative effects on the efficacy of EGFR-targeted therapy.7
We present the results of the Medical Research Council (MRC) COIN trial, which was the largest trial of the addition of an EGFR-targeted monoclonal antibody (cetuximab) to chemotherapy (in this case a regimen of oxaliplatin and a fluoropyrimidine) in the first-line treatment of advanced colorectal cancer, and in which the effect was prospectively analysed primarily in relation to the mutational status of KRAS in tumour tissue, and secondarily in relation to the mutational status of BRAF, NRAS, and KRAS in tumour tissue. The COIN trial also assessed the effect of preplanned treatment interruptions in oxaliplatin and fluoropyrimidine combination chemotherapy on overall survival; these results are reported in a companion paper.8
Methods
Trial design and participants
The COIN trial protocol is available on the MRC Clinical Trials Unit website. Patients were eligible to participate in the trial if they had given written informed consent, had histologically confirmed adenocarcinoma of the colon or rectum, inoperable metastatic or locoregional measurable disease, had received no previous chemotherapy for metastatic disease, and had WHO performance status 0–2 and good organ function.
COIN was approved by national research ethics committees in the UK and Ireland and both the Medicines and Healthcare Regulatory Agency and Irish Medicines Board. The trial was undertaken by the MRC Clinical Trials Unit, following the principles of Good Clinical Research Practice, and overseen by an independent trial steering committee. Confidential interim analyses were reviewed at least annually by an independent data monitoring committee.
Randomisation and masking
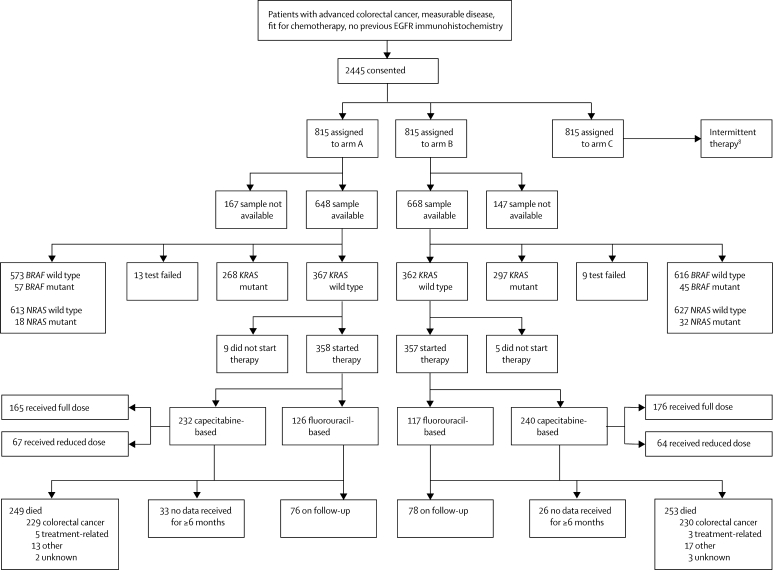
Patients were randomly assigned with minimisation by the MRC Clinical Trials Unit via telephone (1:1:1 ratio) to receive continuous chemotherapy (control, arm A) or one of two research interventions: continuous chemotherapy plus cetuximab (arm B) or intermittent chemotherapy (arm C; figure 1). The minimisation factors were hospital, WHO performance status, chemotherapy regimen, previous adjuvant chemotherapy, liver metastases, and peritoneal metastases. Treatment allocation was not masked. The arm A versus B results are reported in this paper and the arm A versus C results are reported separately.8
Figure 1.
Trial profile
EGFR=epidermal growth factor receptor.
Procedures
Oncologists chose between two chemotherapy regimens according to local hospital policy or patient preference: oxaliplatin plus capecitabine, or oxaliplatin plus fluorouracil and folinic acid. Oxaliplatin plus capecitabine was given as a 3-weekly regimen of intravenous oxaliplatin 130 mg/m2 over 2 h followed by oral capecitabine twice a day for 2 weeks. The dose of capecitabine was 1000 mg/m2 orally twice a day, but was reduced to 850 mg/m2 twice a day in a protocol amendment for patients in arm B only after 1775 (73%) patients had been randomly assigned to all groups, when an analysis of toxic effects showed that the rate of grade 3 or 4 diarrhoea was higher than expected (30%).9 Oxaliplatin plus fluorouracil and folinic acid was given as a 2-weekly regimen of intravenous L-folinic acid 175 mg or D,L-folinic acid 350 mg over 2 h given concurrently with oxaliplatin 85 mg/m2 over 2 h, followed by intravenous bolus fluorouracil 400 mg/m2 then fluorouracil 2400 mg/m2 infusion over 46 h administered via an ambulatory pump and a central venous line. In arm B, cetuximab was given as an initial intravenous dose of 400 mg/m2 over 2 h and subsequently at 250 mg/m2 over 1 h once a week. Treatment was continued until disease progression, development of cumulative toxic effects, or patient choice. Patients were allowed to discontinue one or more agents within the regimen as a result of toxic effects, while continuing on the remaining agent or agents.
CT scans were done within 4 weeks before start of treatment, and were repeated every 12 weeks and assessed on the basis of RECIST (version 1.0) criteria.10 Because overall survival was the primary outcome measure of the trial, responses were not confirmed by repeat scans and external radiological review was not undertaken. Symptoms were scored with National Cancer Institute Common Terminology Criteria for Adverse Events (version 3.0).11 Serious adverse events and deaths, together with an assessment of causality, were continuously reported and assessed by an expert practising oncologist on behalf of the MRC.
In view of the emerging consensus that EGFR immunohistochemistry was not a reliable predictor of response to EGFR-targeted therapy,12 all patients irrespective of EGFR status were eligible for the COIN trial. However, all patients were asked to provide a tumour sample for future analysis of EGFR status. Consultation with the UK Human Tissue Authority and the research ethics committee concluded that the KRAS test could be done on all patients in addition to the EGFR test. Patients were also asked to provide additional consent for future bowel cancer research. Research staff at randomising sites requested patients' tumour samples, which were stored at the Wales Cancer Bank.
Sections of the formalin-fixed, paraffin-embedded tumour blocks were stained with haematoxylin and eosin, optical images were stored, and tumour material was macrodissected, cored, or laser-capture microdissected for the DNA analyses. Tissue microarrays were constructed for immunohistochemical analysis of EGFR. DNA was extracted with QIAmp DNA Microkits (Qiagen, Hilden, Germany). KRAS mutations in codons 12, 13, and 61 and BRAF mutations in codon 600 were screened with pyrosequencing. Additionally, KRAS (all three codons), BRAF (codons 594 and 600), and NRAS (codons 12 and 61) mutations were screened with MALDI-TOF mass array (Sequenom, San Diego, CA, USA).7 For KRAS, more than 1000 samples were successfully analysed by both techniques with greater than 99% genotype call concordance, and for BRAF more than 850 samples were analysed by both methods and greater than 98% of genotype calls were consistent. For discordant genotype calls, Sanger sequencing was used to establish genotype (webappendix p 1–3).
EGFR immunohistochemistry analysis was done on triplicate 0·6 mm cores in 25 tissue microarrays with validated control tissues stained with the standard US Food and Drug Administration approved EGFR pharmDx assay (DAKO, Glostrup, Denmark) at the University College London advanced diagnostics reference laboratory (London, UK). The cutoff points examined for positive versus negative tumours were 0 versus the rest, less than 10% versus 10% or more, and less than 20% versus 20% or more of total tumour cells showing membrane staining (webappendix p 3).
The original objective was to establish whether the addition of cetuximab improved overall survival in patients with advanced colorectal cancer. However, shortly after COIN completed recruitment, external evidence showed that anti-EGFR antibodies were unlikely to benefit patients with this disease whose tumours carry KRAS mutations.5,6 The decision was taken to revise the primary research hypothesis before any analysis was done and before KRAS mutation data for COIN patients became available. The revised statistical plan was reviewed and approved by the independent data monitoring committee and independent trial steering committee. The revised primary objective thus became to determine whether the addition of cetuximab to continuous chemotherapy resulted in improved overall survival in patients with KRAS wild-type tumours. The secondary objectives were to evaluate whether the research intervention resulted in improved overall survival in four groups: (1) patients with tumours wild-type for all of KRAS, BRAF, and NRAS; (2) patients with KRAS mutant tumours; (3) patients with tumours mutant for any of KRAS, BRAF, or NRAS; and (4) all patients randomly allocated to treatment groups. Progression-free survival, response, and toxic effects were all evaluated in each of these patient groups. At the time of analysis for both overall and progression-free survival, survivors were censored at the date they were last known to be alive.
Statistical analysis
Originally the sample size for the arm B versus A comparison was 1614 patients, 807 in each group to parallel the arm C versus A comparison (see protocol for details of arm C vs A sample size calculation). With 1614 patients, a 6·4% advantage in overall survival at 2 years (from 20% to 26·4%; hazard ratio [HR] 0·828) could be detected with 90% power. When the primary objective of the arm B versus A comparison was prospectively revised to focus on the KRAS wild-type population, the primary analysis of arm A versus B was planned to take place when 511 overall survival events had occurred in patients with KRAS wild-type tumours. In this molecularly selected cohort, a higher HR of 0·76 could be detected at 87% power with a two-sided α of 0·05.
Analyses were undertaken according to a predefined statistical analysis plan, which was approved in advance by the COIN trial management group before the database was locked (Sept 2, 2009). All patients randomly assigned to treatment group were included in the analyses, on the basis of the intention-to-treat principle. All p values are two-sided and were not adjusted for multiple testing. Time-to-event curves for analysis of overall and progression-free survival were estimated with the Kaplan-Meier method. HRs, confidence intervals, and p values were estimated with the log-rank method.
We compared worst toxic effects experienced overall between treatment groups using a χ2 test, or Fisher's exact test in case of low event rates (n<5). Exploratory analyses to identify predictive factors were done with a Cox proportional hazards model entering treatment group, the potential predictive factor, and a treatment-predictive factor interaction term. Interaction tests were done with likelihood-ratio tests of the null hypothesis that the interaction coefficient is zero. Stata (version 11.1) was used for all analyses.
The trial is registered, ISRCTN27286448.
Role of the funding source
The trial was conceived and developed by the National Cancer Research Institute advanced colorectal clinical studies group. The MRC was the overall sponsor of the study, with some responsibilities for the sites in Ireland delegated to the Irish Clinical Oncology Research Group. The MRC, through its employees in the MRC Clinical Trials Unit, are authors on the paper and were integral to the collection and analysis of the paper and the writing of the report. The corresponding author had full access to the data and had final responsibility for the decision to submit for publication.
Results
Between March 9, 2005, and May 9, 2008, 2445 patients were randomly assigned to treatment groups at 111 centres in the UK and Ireland, of whom 1630 patients were assigned to the arm A versus B comparison (figure 1). Their baseline characteristics are shown in table 1 and were well balanced between the two trial groups and across the different subgroups defined by KRAS mutation status.
Table 1.
Baseline characteristics for all patients and by KRAS mutation status
|
All patients |
KRAS wild-type group |
KRAS mutant group |
|||||
|---|---|---|---|---|---|---|---|
| Arm A (N=815) | Arm B (N=815) | Arm A (N=367) | Arm B (N=362) | Arm A (N=268) | Arm B (N=297) | ||
| Sex | |||||||
| Male | 525 (64%) | 543 (67%) | 245 (67%) | 253 (70%) | 169 (63%) | 192 (65%) | |
| Female | 290 (36%) | 272 (33%) | 122 (33%) | 109 (30%) | 99 (37%) | 105 (35%) | |
| Age | |||||||
| Median (years) | 63 (56–69) | 63 (58–70) | 63 (56–69) | 64 (59–70) | 63 (56–69) | 64 (58–70) | |
| >75 years | 74 (9%) | 72 (9%) | 31 (8%) | 30 (8%) | 28 (10%) | 27 (9%) | |
| WHO performance status | |||||||
| 0 | 375 (46%) | 376 (46%) | 177 (48%) | 171 (47%) | 114 (43%) | 135 (45%) | |
| 1 | 378 (46%) | 377 (46%) | 166 (45%) | 171 (47%) | 131 (49%) | 135 (45%) | |
| 2 | 62 (8%) | 62 (8%) | 24 (7%) | 20 (6%) | 23 (9%) | 27 (9%) | |
| Site of primary tumour | |||||||
| Colon | 453 (56%) | 444 (54%) | 210 (57%) | 197 (54%) | 145 (54%) | 163 (55%) | |
| Rectum | 243 (30%) | 262 (32%) | 101 (28%) | 119 (33%) | 90 (34%) | 93 (31%) | |
| Rectosigmoid junction | 113 (14%) | 106 (13%) | 56 (15%) | 46 (13%) | 32 (12%) | 41 (14%) | |
| Status of primary tumour | |||||||
| Resected | 445 (55%) | 420 (52%) | 218 (59%) | 188 (52%) | 157 (59%) | 173 (58%) | |
| Unresected | 331 (41%) | 346 (42%) | 131 (36%) | 148 (41%) | 97 (36%) | 107 (36%) | |
| Local recurrence | 39 (5%) | 49 (6%) | 18 (5%) | 26 (7%) | 14 (5%) | 17 (6%) | |
| Timing of metastases | |||||||
| Metachronous | 249 (31%) | 239 (29%) | 125 (34%) | 112 (31%) | 84 (31%) | 85 (29%) | |
| Synchronous | 552 (68%) | 569 (70%) | 236 (64%) | 246 (68%) | 180 (67%) | 212 (71%) | |
| No metastases | 7 (1%) | 6 (1%) | 4 (1%) | 5 (1%) | 3 (1%) | 0 | |
| Type of metastases | |||||||
| Liver only | 174 (21%) | 194 (24%) | 91 (25%) | 87 (24%) | 44 (16%) | 74 (25%) | |
| Liver plus others | 436 (53%) | 418 (51%) | 180 (49%) | 190 (52%) | 151 (56%) | 155 (52%) | |
| Non-liver | 198 (24%) | 197 (24%) | 92 (25%) | 80 (22%) | 70 (26%) | 68 (23%) | |
| Number of metastatic sites | |||||||
| 1 | 283 (35%) | 305 (37%) | 140 (38%) | 131 (36%) | 82 (31%) | 113 (38%) | |
| 2 | 326 (40%) | 311 (38%) | 140 (38%) | 139 (38%) | 116 (43%) | 115 (39%) | |
| >2 | 199 (24%) | 193 (24%) | 83 (23%) | 87 (24%) | 87 (32%) | 69 (23%) | |
| Previous treatment for metastases | |||||||
| Radiotherapy | 26 (3%) | 24 (3%) | 10 (3%) | 9 (2%) | 7 (3%) | 12 (4%) | |
| Surgery | 142 (17%) | 130 (16%) | 64 (17%) | 57 (16%) | 55 (21%) | 52 (18%) | |
| Alkaline phosphatase <300 U/L | 670 (82%) | 696 (85%) | 309 (84%) | 309 (85%) | 224 (84%) | 257 (87%) | |
| Platelet count <400 000 per μL | 564 (69%) | 549 (67%) | 259 (71%) | 251 (69%) | 189 (71%) | 189 (64%) | |
| White blood cell count <10 000 per L | 577 (71%) | 574 (70%) | 265 (72%) | 252 (70%) | 199 (74%) | 217 (73%) | |
Data are n (%) or median (IQR).
Samples from 1316 (81%) patients were suitable for analysis of KRAS, BRAF, and NRAS mutation status (samples from 141 patients [9%] were not available and 173 [11%] samples contained insufficient tumour material for processing). Mutations were detected in KRAS in 565 (43%) patients, BRAF in 102 (8%), and NRAS in 50 (4%) with greater than 98% success rates (webappendix p 3). Figure 1 shows the full results by mutation site and by trial group. 706 (54%) patients carried a KRAS, BRAF, or NRAS mutation (no patients had KRAS and BRAF mutations or BRAF and NRAS mutations, and 11 patients carried both KRAS and NRAS mutations) and 581 (44%) patients were all wild type.
37 patients (2%) did not start trial therapy because of clinical deterioration after randomisation, patient choice, or because they were subsequently found to be ineligible. Figure 1 shows choice of chemotherapy (capecitabine-based or fluorouracil-based). 153 (19%) patients in the cetuximab group (arm B) started therapy with the lower dose of capecitabine of 850 mg/m2. The median duration of follow-up among surviving patients with KRAS wild-type tumours randomly assigned to the control group was 21 months (IQR 18–29) and to the cetuximab group was 23 months (17–29), and was similar for the whole population.
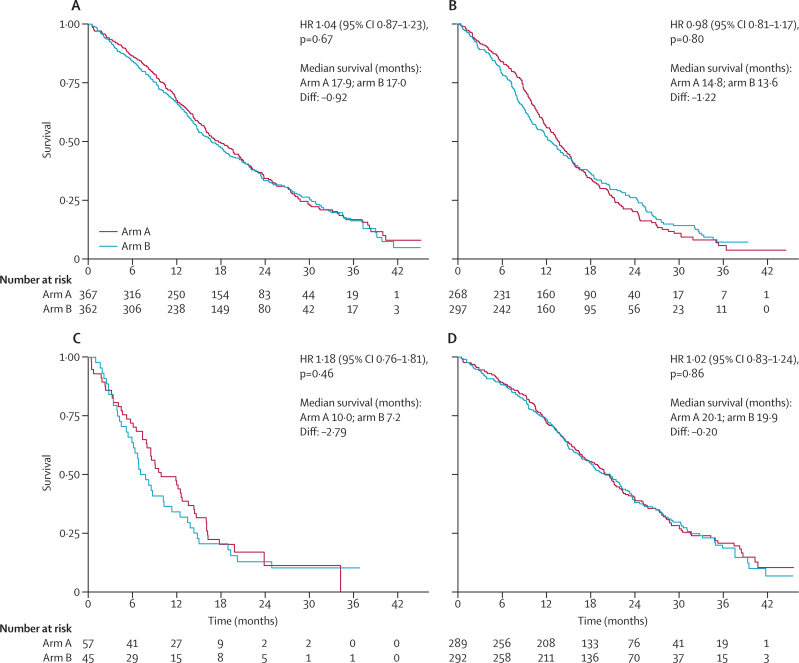
At the time of analysis, there was no evidence of a difference in overall survival between treatment groups. 257 (71%) patients with KRAS wild-type tumours had died in each group, with a median survival of 17·9 months (IQR 10·3–29·2) in the control group and 17·0 months (9·4–30·1) in the cetuximab group (HR 1·04, 95% CI 0·87–1·23; p=0·67; figure 2). Of these deaths, 467 (90·9%) were due to colorectal cancer, eight (1·6%) were treatment-related, 32 (6·2%) were due to other causes, and the causes for seven (1·4%) are still unknown. There was no evidence of an effect of the addition of cetuximab to oxaliplatin-based chemotherapy on overall survival in any of the prespecified cohorts—eg, median survival in patients with KRAS mutant tumours was 14·8 months (IQR 9·5–22·8) in the control group and 13·6 months (8·0–26·1) in the cetuximab group (HR 0·98, 95% CI 0·81–1·17, p=0·80; figure 2, webappendix p 7).
Figure 2.
Kaplan-Meier overall survival curves for patients with (A) KRAS wild-type, (B) KRAS mutant, (C) BRAF mutant and KRAS wild-type, and (D) KRAS, NRAS, and BRAF all wild-type tumours
HR=hazard ratio.
We noted no evidence of an effect of cetuximab on the risk of progression in the KRAS wild-type group (median progression-free survival was 8·6 months in both groups, IQR 5·0–12·5 in the control group, 5·1–13·8 in the cetuximab group; HR 0·96, 95% CI 0·82–1·12, p=0·60; webappendix p 8). No evidence of a difference in progression-free survival was seen among all patients nor in any of the genetically defined groups. In COIN, 110 patients had the KRAS Gly13Asp mutation that was recently reported13 to correlate with some benefit from the addition of cetuximab in chemorefractory patients. In these 110 patients, the HR for effect of cetuximab on progression-free survival was 1·11 (95% CI 0·76–1·63, p=0·60), compared with an HR of 1·05 (0·87–1·27, p=0·61) in patients with other KRAS mutations. In those treated with infusional fluorouracil, 30 patients had KRAS Gly13Asp mutations and the HR was 1·07 (95% CI 0·51–2·24, p=0·86) compared with 1·03 (0·74–1·44, p=0·85) in patients with other KRAS mutations.
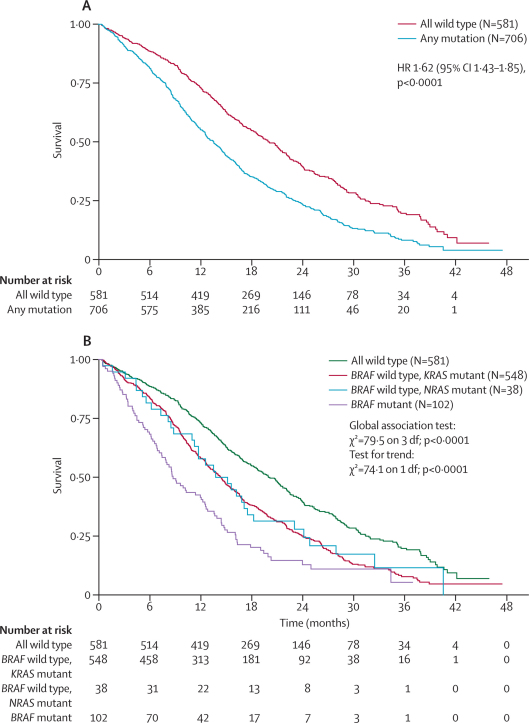
Irrespective of treatment received, median overall survival was shorter in patients who had mutations in any of the three oncogenes (n=706, 13·6 months, IQR 7·9–23·1) than among those whose tumours were wild type for all of KRAS, NRAS, and BRAF (n=581, 20·1 months, IQR 11·5–31·7; log-rank test p<0·0001, figure 3). If the mutational type was then segregated, median overall survival was shorter in patients whose tumours had mutations in BRAF (n=102, 8·8 months, IQR 4·5–16·1) than in those with BRAF wild-type tumours but a mutation in KRAS (n=548, 14·4 months, 8·5–24·0) or NRAS (n=38, 13·8 months, 8·2–24·1). A global test for differences was highly significant (p<0·0001), as was a test for trend (p<0·0001, figure 3). The overall survival for the primary analysis cohort, patients with KRAS wild-type tumours, was 17·5 months (n=729, IQR 9·6–30·3). This group is not shown in either figure because it contains prognostically mixed cohorts—ie, it also includes some patients with BRAF and NRAS mutant tumours.
Figure 3.
Tumour mutational status as a prognostic factor for overall survival
HR=hazard ratio.
A prognostic effect of patients' tumour mutation status was also noted on progression-free survival, with median progression-free survival ranging from 5·6 months (IQR 2·8–8·6) for patients with BRAF mutant tumours to 9·0 months (5·7–14·1) for those tumours wild type for all of KRAS, NRAS, and BRAF. These effects remained strongly significant when added into a multivariate model adjusted for other important prognostic variables. For progression-free survival, all three mutations remained independently significant (p<0·0001, p<0·0001, and p=0·0088 for KRAS, BRAF, and NRAS, respectively), whereas for overall survival, KRAS and BRAF remained significant (p<0·0001 for both), but NRAS did not (p=0·15). Among the other variables in the multivariate model were the four prognostic indicators suggested by Köhne and colleagues13—ie, white blood cell count, number of metastatic sites, alkaline phosphatase, and WHO performance status. For both overall and progression-free survival, all four remained independently significant at the 5% level.
An agreed score for EGFR immunohistochemistry was available for 1065 (65%) patients. Reasons for unavailability of scores included no tissue block received, no satisfactory score achievable, and tissue regarded as unsatisfactory for analysis by immunohistochemistry. The cutoff point of less than 10% versus 10% or more of cells showing EGFR membrane staining was selected as being the most reproducible and widely used index for scoring of EGFR positivity. This approach identified 310 (58%) of 533 patients in the control group and 329 (62%) of 532 patients in the cetuximab group as having EGFR-positive tumours. Positive EGFR immunohistochemistry was a poor prognostic factor in the KRAS wild-type cohort (for progression-free survival, HR 1·27, 95% CI 1·07–1·52, p=0·0078 in univariate analysis and HR 1·25, 1·05–1·50, p=0·015 after adjustment for prognostic factors found to be significant for progression-free survival in a separate analysis using a backwards stepwise procedure). In the KRAS wild-type cohort, there was no evidence of a predictive effect of EGFR immunohistochemistry irrespective of cutoff point. However, in the KRAS mutant cohort, patients with more than 10% of tumour cells EGFR positive had a detrimental effect on progression-free survival from the addition of cetuximab (HR 1·28, 95% CI 1·00–1·63, p=0·047, n=285). By contrast, no such effect was identified in the EGFR less than 10% cohort (HR 0·87, 0·64–1·19, p=0·37, n=169).
A complete or partial response was reported in 209 (57%) KRAS wild-type patients in the control group and in 232 (64%) KRAS wild-type patients receiving cetuximab (OR 1·35, 95% CI 1·00–1·82, p=0·049). The addition of cetuximab resulted in reduced dose intensity in KRAS wild-type patients over the first 24 weeks (for fluorouracil-based therapy: median 78% in the control group [IQR 70–87] vs 73% [66–82] in the cetuximab group, p=0·031; for capecitabine-based therapy: 85% [74–92] vs 79% [67–88], p=0·0021). In patients receiving fluorouracil-based therapy, this difference was noted mainly in the dose intensity of infused fluorouracil (p=0·02; webappendix p 4). In those receiving capecitabine-based therapy, dose intensity was different for both oxaliplatin and capecitabine, but these differences largely disappeared after the capecitabine dose reduction in arm B.
The addition of cetuximab (arm B) did not have a noticeable effect on time on treatment in either the capecitabine-based or fluorouracil-based therapy groups. In both arms combined, the overall median duration of treatment was 29 weeks (IQR 16–41) in patients receiving fluorouracil-based therapy, but 25 weeks (IQR 13–35) in those given capecitabine-based therapy (p=0·0028 adjusting for treatment group; webappendix p 4).
Addition of cetuximab increased the incidence of skin (rash, nail changes, and hand–foot syndrome), gastrointestinal (diarrhoea, stomatitis), and other toxic effects (anaemia, lethargy, and hypomagnesaemia; table 2). In both treatment groups there were increased toxic effects with fluorouracil-based therapy for neutropenia, peripheral neuropathy, and stomatitis compared with the patients on capecitabine-based therapy. With the addition of cetuximab (arm B), diarrhoea and skin toxic effects were reported more often in patients on capecitabine-based therapy than on fluorouracil-based therapy, and peripheral neuropathy was less common in patients treated with fluorouracil-based therapy. In the KRAS wild-type cohort, similar differences in toxic effects were noted, but were not statistically significant (webappendix p 5). After the dose reduction in the subgroup of patients receiving capecitabine-based therapy and cetuximab,9 the incidence of grade 3 or higher diarrhoea fell from 30% (116/381; p<0·0001 vs no cetuximab) to 16% (25/153; p=0·25 vs no cetuximab). Other toxic effects were also lowered (data not shown).
Table 2.
Grade 3 or higher toxic effects in all patients randomly assigned to treatment groups, by chemotherapy regimen over entire treatment period
|
Arm A |
Arm B |
p value for AvsB |
||||||
|---|---|---|---|---|---|---|---|---|
| Fluorouracil-based therapy (N=279) | Capecitabine-based therapy (N=536) | p value | Fluorouracil-based therapy (N=281) | Capecitabine-based therapy (N=534) | p value | Fluorouracil-based therapy | Capecitabine-based therapy | |
| Platelet count | 7 (3%) | 16 (3%) | 0·70 | 7 (2%) | 16 (3%) | 0·68 | 0·99 | 0·99 |
| Haemoglobin | 6 (2%) | 7 (1%) | 0·36 | 21 (7%) | 17 (3%) | 0·0058* | 0·0033† | 0·038† |
| White blood cell count | 28 (10%) | 6 (1%) | 0·0001* | 33 (12%) | 3 (1%) | 0·0001* | 0·52 | 0·32 |
| Neutrophil count | 86 (31%) | 21 (4%) | 0·0001* | 88 (31%) | 13 (2%) | 0·0001* | 0·90 | 0·17 |
| Nausea | 13 (5%) | 37 (7%) | 0·21 | 17 (6%) | 48 (9%) | 0·14 | 0·58 | 0·21 |
| Vomiting | 10 (4%) | 27 (5%) | 0·34 | 18 (6%) | 38 (7%) | 0·70 | 0·17 | 0·16 |
| Diarrhoea | 31 (11%) | 82 (15%) | 0·10 | 55 (20%) | 141 (26%) | 0·030† | 0·0055† | 0·0001† |
| Hand–foot syndrome | 7 (3%) | 25 (5%) | 0·13 | 18 (6%) | 67 (13%) | 0·0064† | 0·026† | 0·0001† |
| Nail changes | 0 | 0 | .. | 6 (2%) | 11 (2%) | 0·94 | 0·014† | 0·00045† |
| Skin rash | 0 | 1 (<1%) | 0·99 | 56 (20%) | 108 (20%) | 0·92 | 0·0001† | 0·0001† |
| Peripheral neuropathy | 63 (23%) | 86 (16%) | 0·022* | 38 (14%) | 73 (14%) | 0·95 | 0·0053* | 0·28 |
| Hypomagnesaemia | 0 | 0 | .. | 16 (6%) | 16 (3%) | 0·059 | 0·0001† | 0·0001† |
| Anorexia | 12 (4%) | 31 (6%) | 0·37 | 28 (10%) | 43 (8%) | 0·36 | 0·014† | 0·14 |
| Alopecia | 1 (<1%) | 2 (<1%) | 0·99 | 0 | 1 (<1%) | 0·99 | 0·32 | 0·57 |
| Pain | 34 (12%) | 75 (14%) | 0·47 | 34 (12%) | 72 (13%) | 0·58 | 0·98 | 0·81 |
| Stomatitis | 13 (5%) | 4 (1%) | 0·00040* | 27 (10%) | 18 (3%) | 0·00021* | 0·023† | 0·0023† |
| Lethargy | 51 (18%) | 98 (18%) | 0·99 | 81 (29%) | 128 (24%) | 0·13 | 0·0033† | 0·023† |
| Vein pain | 0 | 8 (1%) | 0·056 | 0 | 3 (1%) | 0·56 | .. | 0·13 |
Data are n (%) or p value.
More toxic effects in arm A than in arm B, or in patients on fluorouracil-based therapy than on capecitabine-based therapy (p<0·05).
More toxic effects in arm B than arm A, or on capecitabine-based therapy than on fluorouracil-based therapy (p<0·05).
Among all patients randomly assigned to treatment groups, treatment-related deaths were reported in ten patients in the control group and nine in the cetuximab group. Of the nine patients taking cetuximab, eight were in the capecitabine-based therapy plus cetuximab subgroup. Seven of the deaths occurred before the capecitabine dose reduction and were predominantly related to gastrointestinal toxic effects. In the control group, the ten deaths were split evenly between capecitabine-based and fluorouracil-based therapy, with no pattern to the causative toxic effects noted.
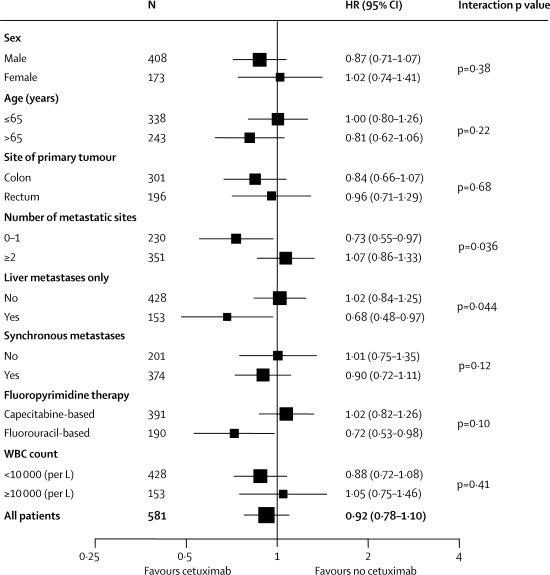
16 additional factors were explored for predictive value of progression-free survival among the all-wild-type patients, because this subset was judged the most likely to be responsive. Improved progression-free survival with cetuximab was seen in patients treated with fluorouracil-based therapy (HR 0·72, 95% CI 0·53–0·98, p=0·037), but not in those treated with capecitabine-based therapy (HR 1·02, 0·82–1·26, p=0·88; p=0·10 for interaction; see webappendix p 9 for data for KRAS wild-type patients). Additionally, patients with no or one metastatic site had improved progression-free survival with cetuximab (HR 0·73, 0·55–0·97, p=0·030) whereas those with two or more metastatic sites did not (HR 1·07, 0·86–1·33, p=0·56; p=0·036 for interaction; figure 4). Similar results were obtained for patients with liver metastases only versus more widespread metastatic disease. Further analyses showed that both number of metastatic sites or liver-only metastases and choice of fluoropyrimidine therapy had significant (at the 10% level) interactions with treatment group. Fluoropyrimidine therapy was the weaker of the two, but neither was substantially affected by adjustment for important prognostic factors, or for each other. Thus, progression-free survival benefit was restricted to patients with KRAS wild-type tumours and zero or one metastatic site treated with fluorouracil-based therapy (n=96, HR 0·55, 95% CI 0·35–0·87, p=0·011).
Figure 4.
Exploratory predictive factor analyses
HR=hazard ratio.
In analysis of surgery for metastases, no increase in potentially curative liver resections was identified, with resection rates among KRAS wild-type patients who had liver-only metastases at baseline of 13% (n=12/91) in the control group and 15% (n=13/87) in the cetuximab group (p=0·74). At the time of analysis, second-line therapy was administered to significantly fewer patients in the cetuximab group (386 [56%] of 695) than in the control group (448 [62%] of 724; p=0·015) in patients who were eligible to receive further treatment (patients who were alive, had completed COIN protocol treatment, and were not lost to follow-up). Patients treated with fluorouracil-based rather than capecitabine-based therapy were also more likely to receive second-line therapy (table 3).
Table 3.
Second-line therapy received by KRAS wild-type patients, by chemotherapy received
| Arm A | Arm B | p value | ||
|---|---|---|---|---|
| AllKRASwild-type patients | ||||
| Total eligible for second-line therapy* | 323 | 311 | .. | |
| Therapy received | ||||
| Any second-line therapy | 210 (65%) | 169 (54%) | 0·0061 | |
| Irinotecan | 171 (53%) | 132 (42%) | 0·0082 | |
| Fluoropyrimidine | 155 (48%) | 121 (39%) | 0·021 | |
| Oxaliplatin | 60 (19%) | 46 (15%) | 0·20 | |
| EGFR-targeted therapy | 16 (5%) | 21 (7%) | 0·33 | |
| Other therapy | 10 (3%) | 8 (3%) | 0·69 | |
| Patients receiving fluorouracil-based therapy | ||||
| Total eligible for second-line therapy* | 113 | 98 | .. | |
| Therapy received | ||||
| Any second-line therapy | 78 (69%) | 53 (54%) | 0·026 | |
| Irinotecan | 65 (58%) | 40 (41%) | 0·015 | |
| Fluoropyrimidine | 68 (60%) | 44 (45%) | 0·027 | |
| Oxaliplatin | 22 (19%) | 14 (14%) | 0·32 | |
| EGFR-targeted therapy | 7 (6%) | 5 (5%) | 0·78 | |
| Other therapy | 2 (2%) | 1 (1%) | 0·99 | |
| Patients receiving capecitabine-based therapy | ||||
| Total eligible for second-line therapy* | 210 | 213 | .. | |
| Therapy received | ||||
| Any second-line therapy | 132 (63%) | 116 (54%) | 0·080 | |
| Irinotecan | 106 (50%) | 92 (43%) | 0·13 | |
| Fluoropyrimidine | 87 (41%) | 77 (36%) | 0·27 | |
| Oxaliplatin | 38 (18%) | 32 (15%) | 0·40 | |
| EGFR-targeted therapy | 9 (4%) | 16 (8%) | 0·16 | |
| Other therapy | 8 (4%) | 7 (3%) | 0·77 | |
Data are n (%) or p value. EGFR=epidermal growth factor receptor.
Patients deemed eligible for second-line therapy were those that survived at least 14 days after coming off-trial.
Discussion
COIN is the largest trial of the addition of an EGFR-targeted monoclonal antibody to first-line combination chemotherapy in patients with advanced colorectal cancer. No benefit could be shown with the addition of cetuximab to oxaliplatin-based chemotherapy. This finding holds true both for the primary outcome measure of overall survival and for the secondary outcome measure of progression-free survival for the KRAS wild-type cohort. In a predefined secondary analysis, we postulated that the group of patients with no mutation in the genes tested within the RAS–RAF–MAP kinase pathway would be the most likely to show a benefit. However, even patients with tumours wild type for all three genes did not show any evidence of a benefit from the addition of cetuximab. Conversely, we have recorded no evidence of a detrimental effect for patients with KRAS mutant tumours. De Roock and colleagues14 have suggested that the KRAS Gly13Asp mutation might not predict lack of benefit with use of EGFR inhibitors. In COIN, this mutation was identified in 110 patients, but was not associated with any difference in outcome with the addition of cetuximab. We do, however, show that these mutations have a strong prognostic effect, with median survival ranging from 8·8 months for patients with BRAF mutant tumours, about 14 months for patients with KRAS or NRAS mutant tumours, to 20·1 months for patients with tumours that were all wild type. These factors remain strongly prognostic in a multivariate analysis, and consideration should therefore be given in future trials to their use as stratification factors or inclusion or exclusion criteria.
The overall survival of patients in COIN was inferior to other trials of similar design in this setting for chemotherapy versus chemotherapy plus anti-EGFR therapy.15–22 Evidence that patients with colorectal cancer in the UK (and Denmark) have 5–10% inferior survival compared with patients from Canada, Australia, and Scandinavia has recently been published.23 This effect was ascribed to more advanced disease stage at presentation, reflected especially in inferior 1-year survival. In COIN, the patients were drawn from 111 hospitals across the UK and Ireland and thus are more broadly representative of patients with advanced colorectal cancer than are those in other trials in which recruitment is from more selected centres. The COIN trial was not intended for patients who receive first-line chemotherapy in anticipation of possible resection of metastases, and the trial cohort therefore had fairly advanced disease with a substantial proportion of patients having unresected primary tumours and synchronous metastatic disease. These factors affect survival in the control group as well as the experimental group. However, the characteristics of the population were not fundamentally different from those in other trials of EGFR-targeted antibodies so, although contributing to the shorter overall survival, they do not adequately account for the failure to detect the expected improvement with addition of cetuximab. Less aggressive use of effective treatments has been a criticism of the UK health system. However, combination chemotherapy with or without cetuximab used until disease progression is thought to be aggressive first-line therapy. The fact that bevacizumab is not approved for reimbursement in the UK National Health Service means that the small increase in overall survival from the use of this agent will not be reflected in the survival of COIN patients. A significant reduction in the use of second-line therapy was also noted in the cetuximab group (56% vs 62%). This finding could be a consequence of the increased toxic effects noted with addition of cetuximab, but by itself is unlikely to account for the absence of survival benefit in view of the lack of overall benefit in progression-free survival and the small difference shown.
One major factor that could affect the benefit of the addition of cetuximab to chemotherapy is the precise nature of the agents used in combination. The addition of bevacizumab to cetuximab and combination chemotherapy seems to be detrimental.15,16 The only phase 3 trial in first-line therapy showing an overall survival benefit to date used irinotecan and infusional fluorouracil as the chemotherapy backbone.17 By comparison, the trials using oxaliplatin have not shown improved overall survival and this failure has raised the possibility of a negative interaction between oxaliplatin and cetuximab.19–21
A broad set of predefined exploratory analyses have been done in an attempt to understand the results of the COIN trial. The only group for which some evidence of a potential benefit was suggested were those patients who have three coincident factors: KRAS wild-type tumours, treatment with infused fluorouracil rather than capecitabine, and a limited distribution of metastatic disease (either zero or one metastatic site vs two or more sites, or liver metastases only vs more widespread disease). This cohort generally conforms to those patients identified in guidance from the UK National Institute for Health and Clinical Excellence for the use of cetuximab, which was issued shortly before the trial was analysed.24 For patients with KRAS wild-type tumours treated with infused fluorouracil, the benefit in progression-free survival (HR for fluorouracil-based therapy was 0·77, p=0·06, compared with HR for capecitabine-based therapy of 1·06, p=0·56, p for interaction 0·07; webappendix p 9) was consistent with other trials.15–22 This finding again suggests the potential importance of the agents used in combination when using EGFR-targeted therapies.
Within the COIN trial, after identification of increased toxic effects in patients treated with capecitabine, oxaliplatin, and cetuximab, a capecitabine dose reduction was mandated in the cetuximab group only.9 This change successfully reduced levels of toxic effects to rates similar to those in the control group and improved the dose of oxaliplatin delivered, but obviously reduced the exposure of those 19% of patients in arm B to fluoropyrimidine. The lack of benefit in patients treated with capecitabine could be accounted for at least in part by the increase in toxic effects recorded, and the resulting reduction in dose intensity of the chemotherapy administered or by other undetermined factors. However, we emphasise that these subgroup findings need validation.
This trial was one of the first not to require positive EGFR immunohistochemistry as a patient selection characteristic. 22% of patients were completely negative and 40% had less than 10% EGFR membrane staining. In COIN, there is no evidence to suggest that EGFR immunohistochemistry staining is a predictive factor for clinical benefit of the addition of cetuximab in the KRAS wild-type population. This finding does not support the requirement for EGFR testing in the current cetuximab licence. Outcomes in patients with KRAS mutant tumours were heterogeneous with respect to EGFR staining, such that those with greater than 10% EGFR staining experienced a detrimental effect on addition of cetuximab as shown in some other studies,17 but this effect was not recorded in patients with less than 10% EGFR staining.
The results of the COIN trial are unexpected and add to the variance seen in other trials evaluating the use of EGFR monoclonal antibodies in combination with chemotherapy in the first-line treatment of advanced colorectal cancer (panel). The overall lack of benefit of the addition of cetuximab to oxaliplatin and fluoropyrimidine combinations seen in the COIN trial, even in the absence of bevacizumab, is likely to be attributable to the specific toxic effect profile of the combination of the oxaliplatin and capecitabine chemotherapy backbone, with which no benefit was seen in any subgroup when cetuximab was added. By contrast, in patients treated with oxaliplatin and infusional fluorouracil, similar benefits in progression-free survival were seen as in other studies. The potent prognostic effect of BRAF, KRAS, and NRAS mutations on the outcome of patients with advanced colorectal cancer shows the fundamental importance of these changes and emphasises the need to stratify future trials for these factors and to seek specific therapeutic approaches within these molecular subgroups.
Panel. Research in context.
Systematic review
Several phase 3 trials have assessed the benefit of the addition of epidermal-growth-factor-receptor-targeted therapies to standard therapy for advanced colorectal cancer with varying results.5,6,15–18,20,21 Systematic review suggests that this therapy is associated with improved progression-free survival, but with the greatest advantage seen in patients who have received previous chemotherapy. The benefits are confined to patients with no evidence of mutation in the KRAS oncogene.
Interpretation
The outcome of this study is contrary to expectation showing no benefit from the addition of cetuximab in patients with KRAS wild-type tumours. There is an interaction with the choice of chemotherapy used, such that patients choosing the oral fluorouracil prodrug capecitabine plus oxaliplatin gained no benefit in any subgroup. This finding could be attributable to overlapping gastrointestinal toxic effects and as a result this triple combination cannot be recommended. By contrast, the results with infusional fluorouracil plus oxaliplatin treatment mirrored those of other studies showing a small benefit with the addition of cetuximab.
Acknowledgments
Acknowledgments
We thank the 2445 patients and their families who participated in COIN; Tony Lai for use of laboratory space in the Wales Heart Research Institute, Cardiff University for the somatic mutation profiling; and Natacha Lays and Gilian Peuteman for technical support. The trial was funded through the peer-reviewed Cancer Research UK Clinical Trials Advisory and Awards Committee, the MRC, and an unrestricted educational grant from Merck kGaA. Additional support in the form of discounted products was provided by Sanofi, Wyeth, and Baxter. Data collection at UK sites was supported by staff funding from the National Cancer Research Networks. The somatic profiling was co-supported by Cancer Research Wales (CGS, PhD studentship) and Merck. BC was supported by a PhD grant of the Institute for the Promotion of Innovation through Science and Technology in Flanders (IWT-Vlaanderen). JB was supported in part by the University College London Hospitals and University College London Comprehensive Biomedical Research Centre. MJK has received a grant from the Irish Health Research Board. In addition to the individuals named in the list of COIN Trial Investigators, we acknowledge the contributions of a large number of clinicians, research nurses, data managers, and other clinical and support staff at the participating centres.
COIN Trial Investigators
Trial management group T S Maughan (chair), M T Seymour, R H Wilson, R A Adams, H Wasan, A Madi, J Cassidy, MJ Kennedy, E Hodgkinson, P Rogers, M Pope.
MRC Clinical Trials Unit A M Meade, R Kaplan, D Fisher, S L Kenny, J K Mitchell, L Nichols, L Harper, B Sydes, E Kay, L Thompson, L Clement, C Courtney, G Griffiths, C Murphy, M Parmar.
Genetic analysis C G Smith, R Harris, S Idziaszczyk, B Claes, D Lambrechts, J P Cheadle.
EGFR immunohistochemistry B Jasani, S Storkel, R Adams, M James.
Data monitoring committee J Northover (chair), J Brown, M Aapro, R Stout.
Trial steering committee C Parker (current chair), M Mason, R Rudd, P W M Johnson, J Whelan.
Sponsor Medical Research Council.
Clinical investigators (Institution [number of patients contributed]) C Lowdell, R H Phillips, R Ahmad, P Riddle, A Creak (Charing Cross Hospital, London, UK [81]); J Cassidy, A McDonald, A Waterston, N Mohammed, J White, H Yosef, A Hennessy (Beatson Oncology Centre, Glasgow, UK [81]); E M Bessell, V Potter (Nottingham City Hospital, Nottingham, UK [72]); M Seymour, A Anthoney, A Melcher, R Cooper, D Sebag-Montefiore (St James' University Hospital, Leeds, UK [69]); P Ross, M Leslie, N Maisey, A Gaya, G Mikhaeel (Guy's and St Thomas's Hospitals, London, UK [67]); J Summers, M Hill, R James (Maidstone Hospital, Maidstone, UK [64]); T S Maugham, A Brewster, T Crosby, S Mukherjee, N Iqbal (Velindre Hospital, Cardiff, UK [63]); J Wadsley, D Furniss, S Pledge, J Hornbuckle, S Clenton (Weston Park Hospital, Sheffield, UK [63]); R Glynne-Jones, M Harrison, S Mawdsley, N Anyamene, R Hughes (Mount Vernon Hospital, UK [57]); D Propper, C Cottrill (St Bartholomew's Hospital, London, UK [57]); D Smith, S Myint, N Ali, M Iqbal, P Clark, B Haylock (Clatterbridge Centre for Oncology, UK [50]); T Iveson, A Bateman, C Baughan (Southampton Hospital, UK [46]); S Falk, K Hopkins (Bristol Oncology Centre, Bristol, UK [45]); J Bridgewater, S Karp (North Middlesex Hospital, London, UK [44]); C Topham, G Middleton, S Essapen, S Cummins (Royal Surrey County Hospital, UK [44]); B Sizer (Essex County Hospital, UK [42]); S Gollins (Glan Clwyd Hospital, UK [42]); A Maraveyas (Castle Hill Hospital, Hull, UK [41]); S Tahir (Broomfield Hospital, Essex, UK [36]); J Bridgewater (Princess Alexandra Hospital, UK [36]); C Blesing (Great Western Hospital, Swindon, UK [34]); H Wasan (Hammersmith Hospital, London, UK [34]); M Tighe, S Falk (Musgrove Park Hospital, Taunton [34]); R Ellis (Royal Cornwall Hospital, UK [34]); J Dent, JK Joffe (Huddersfield Royal Infirmary, UK [33]); S Shepherd, K Benstead, D Farrugia (Cheltenham General Hospital, UK [32]); P Mack, A Hamid, M Butt, R Roy (Diana, Princess of Wales Hospital, Grimsby, UK [31]); T Hickish (Royal Bournemouth Hospital, UK [31]); H Yosef (Hairmyres Hospital, UK [29]); D Tsang, P Leonard, J Prejbisz (Southend Hospital, UK [28]); J Ledermann, J Bridgewater (University College London Hospital, London, UK [27]); T Hickish, R Osborne (Poole Hospital, Dorset, UK [26]); A O'Callaghan, S R Muthuramalingam (Portsmouth Oncology Centre, Queen Alexandra Hospital, UK [25]); F Adab (University Hospital of North Staffordshire NHS Trust, UK [25]); N J Wadd, J Van der Voet, D Wilson (James Cook University Hospital, UK [24]); J Wagstaff, C Askill (Singleton Hospital, UK [24]); I Pedley, A Azzabi (Sunderland Royal Hospital, UK [24]); E Marshall (Whiston Hospital, UK [24]); R James (Kent and Canterbury Hospital, UK [23]); F Daniel (Derriford Hospital, Plymouth, UK [22]); R Osborne (Dorset County Hospital, UK [22]); T J Iveson (Salisbury District Hospital, UK [22]); D Jodrell, C McLean, S Clive, L Dawson, H A Philips (Western General Hospital, Edinburgh, UK [22]); S Falk (Yeovil District Hospital, UK [22]); R James (Queen Elizabeth, The Queen Mother Hospital, UK [21]); P Chakraborti, R Kulkarni (Royal Derby Hospital, UK [21]); L M Samuel, G MacDonald (Aberdeen Royal Infirmary, UK [20]); C Bradley, C Twelves (Bradford Royal Infirmary, UK [20]); S Giridharan, F Adab (Staffordshire General Hospital, UK [20]); S Myint (Southport & Formby District General Hospital, UK [18]); S Gollins (Wrexham Maelor Hospital, UK [18]); N Stuart, C Bale (Ysbyty Gwynedd Hospital, UK [18]); (William Harvey Hospital, UK [17]); J Valle, M Saunders, G Wilson (Christie Hospital, Manchester, UK [16]); A Weaver, A Jones (Churchill Hospital, Oxford, UK [16]); K McAdam, C Jephcott (Peterborough District Hospital, UK [16]); S Cleator (St Mary's Hospital, London, UK [16]); A Webb (Worthing Hospital, UK [16]); J Glaholm (Good Hope Hospital, UK [15]); R Hughes, P M Mulholland (Lister Hospital, UK [15]); N Steven (Queen Elizabeth, University Hospital, Birmingham, UK [15]); A Mayer, T Meyer (Royal Free Hospital [15]); N Lo, G Cogill, L Toy (Torbay Hospital, UK [15]); M Wilkins (West Wales General Hospital, UK [15]); C Wilson, C Palmer (Addenbrooke's Hospital, UK [14]); I Geh (Birmingham Heartlands Hospital, UK [14]); R Thomas (Bedford Hospital NHS Trust [13]); J Nicoll (Cumberland Infirmary, UK [13]); P Chakraborti (Queens Hospital, Burton Upon Trent [13]); A Azzabi (South Tyneside Hospital, UK [13]); R Wilson, M Eatock (Belfast City Hospital, UK [12]); A Hartley (Manor Hospital, Walsall, UK [12]); J Gildersleve, A Freebairn (Berkshire Cancer Centre, Berkshire, UK [12]); G P Deutsch, A Webb, M Wilkins (Royal Sussex County Hospital, Brighton, UK [12]); F McKinna (Eastbourne District General Hospital [11]); D Cunningham, I Chau (Royal Marsden, Sutton, UK [11]); S Susnerwala, M Wise, A Birtle (Royal Preston Hospital, UK [11]); A Hamid (Scunthorpe General Hospital, UK [11]); F J Lofts (St George's Hospital, London, UK [11]); J Kennedy (St James' Hospital, Dublin, Ireland [11]); D Whillis (Raigmore Hospital, UK [10]); S Susnerwala (Blackpool Victoria Hospital, UK [9]); L T Tan, C Palmer (Hinchingbrooke Hospital, UK [9]); N Maisey (Queen Elizabeth Hospital, UK [9]); M Keane (University College Hospital, Galway, Ireland [9]); C Macmillan, K Patel (Northampton General Hospital, UK [8]); D Farrugia (Worcestershire Royal Hospital, UK [8]); G Bertelli, V Vigneswaran (Withybush General Hospital, UK [7]); D Smith (Aintree, University Hospital, UK [6]); R McDermott (AMNCH, Tallaght Hospital, Dublin, Ireland [6]); S O'Reilly (Mercy Hospital, Cork, Ireland [6]); V Hall (Royal Hampshire County Hospital, Winchester, UK [6]); C Hamilton, A Dhadda (Scarborough Hospital, UK [6]); C Baughan (St Mary's Hospital, Isle of Wight, UK [6]); H Ford, M Moody (West Suffolk Hospital, UK [6]); M Tomlinson (Weston General Hospital, Weston Super Mare, UK [6]); L Grogan, O Breathnach (Beaumont Hospital, Dublin, Ireland [4]); R Soomal (Ipswich Hospital NHS Trust, UK [4]); J McCaffrey (Mater Misericordiae Private Hospital, Dublin, Ireland [4]); J McCaffrey (Mater Misericordiae Public Hospital, Dublin, Ireland [4]); C Rees (North Hampshire Hospital, Basingstoke, UK [4]); P J Atherton (North Tyneside General Hospital, UK [4]); S Beesley (Conquest Hospital, UK [3]); W Dobrowsky (Wansbeck General Hospital, UK [3]); G Leonard (Waterford Regional Hospital, Waterford, Ireland [3]); R Gupta (Midwestern Regional Hospital, Limerick, Ireland [2]); J Stewart (Milton Keynes General Hospital, UK [2]); D Cunningham (Royal Marsden Hospital, London, UK [2]).
Contributors
TSM was chief investigator of the trial, co-wrote the protocol, chaired the trial management group, and co-wrote the report. MTS, RHW, and AMM contributed to the trial design and the writing of the protocol. RK, AMM, DF, SLK, JKM, and EK were responsible for data and trial management, statistical analysis and interpretation, and report preparation. RAA and AM were the COIN trial fellows, contributed to trial management, reviewed all safety events, and contributed to the laboratory research. RHW and MTS also contributed to trial management. JPC, CGS, SI, RH, MDJ, and RAA were responsible for the collation of tumour samples, tumour preparation for analysis, DNA extraction and mutational analysis, and report preparation. CGS, BC, and DL did the Sequenom analysis in Leuven. BJ, RAA, and MDJ manufactured the tissue microarrays and were responsible for the EGFR immunohistochemistry. JB was the oncologist responsible for recruitment and treatment of the largest number of patients (70), and MJK was the lead oncologist for the sites in Ireland and contributed to trial management. All authors reviewed all the data and commented on the report.
Conflicts of interest
TSM and RAA have received travel, accommodation, and lecture fees from Roche and Merck Serono. AMM, DF, SLK, JKM, EK, and RK are employed by the UK MRC, which is also the trial sponsor. MTS has received travel expenses and an educational grant from Roche. JB has received an honorarium payment and travel expenses for attendance at educational meetings from Merck. BJ has received consultancy fees from Dako Ltd, Roche, and AstraZeneca. MJK has received travel expenses from Amgen. CGS, SI, RH, MDJ, BC, DL, RHW, AM, and JPC all declare that they have no conflicts of interest.
Web Extra Material
References
- 1.Lynch TJ, Bell DW, Sordella R. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 2.Paez JG, Jänne PA, Lee JC. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 3.Khambata-Ford S, Garrett CR, Meropol NJ. Expression of epiregulin and amphiregulin and K-ras mutation status predict disease control in metastatic colorectal cancer patients treated with cetuximab. J Clin Oncol. 2007;25:3230–3237. doi: 10.1200/JCO.2006.10.5437. [DOI] [PubMed] [Google Scholar]
- 4.Lièvre A, Bachet JB, Le Corre D. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66:3992–3995. doi: 10.1158/0008-5472.CAN-06-0191. [DOI] [PubMed] [Google Scholar]
- 5.Karapetis CS, Khambata-Ford S, Jonker DJ. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757–1765. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- 6.Amado RG, Wolf M, Peeters M. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626–1634. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- 7.De Roock W, Claes B, Bernasconi D. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol. 2010;11:753–762. doi: 10.1016/S1470-2045(10)70130-3. [DOI] [PubMed] [Google Scholar]
- 8.Adams RA, Meade AM, Seymour MT, on behalf of the MRC COIN Trial Investigators Intermittent versus continuous oxaliplatin and fluoropyrimidine combination chemotherapy for first-line treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet Oncol. 2011 doi: 10.1016/S1470-2045(11)70102-4. published online June 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adams RA, Meade AM, Madi A. Toxicity associated with combination oxaliplatin plus fluoropyrimidine with or without cetuximab in the MRC COIN trial experience. Br J Cancer. 2009;100:251–258. doi: 10.1038/sj.bjc.6604877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Therasse P, Arbuck SG, Eisenhauer EA. New guidelines to evaluate the response to treatment in solid tumors (RECIST Guidelines) J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 11.National Cancer Institute Common Toxicity Criteria for Adverse Events (version 3.0) http://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf (accessed May 18, 2011).
- 12.Chung KY, Shia J, Kemeny NE. Cetuximab shows activity in colorectal cancer patients with tumors that do not express the epidermal growth factor receptor by immunohistochemistry. J Clin Oncol. 2005;23:1803–1810. doi: 10.1200/JCO.2005.08.037. [DOI] [PubMed] [Google Scholar]
- 13.Köhne CH, Cunningham D, Di Costanzo F. Clinical determinants of survival in patients with 5-fluorouracil based treatment for metastatic colorectal cancer: results of a multivariate analysis of 3825 patients. Ann Oncol. 2002;13:308–317. doi: 10.1093/annonc/mdf034. [DOI] [PubMed] [Google Scholar]
- 14.De Roock W, Jonker DJ, Di Nicolantonio F. Association of KRAS p.G13D mutation with outcome in patients with chemotherapy refractory metastatic colorectal cancer treated with cetuximab. JAMA. 2010;304:1812–1820. doi: 10.1001/jama.2010.1535. [DOI] [PubMed] [Google Scholar]
- 15.Tol J, Koopman M, Cats A. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med. 2009;360:563–572. doi: 10.1056/NEJMoa0808268. [DOI] [PubMed] [Google Scholar]
- 16.Hecht JR, Mitchell E, Chidiac T. A randomized phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol. 2009;27:672–680. doi: 10.1200/JCO.2008.19.8135. [DOI] [PubMed] [Google Scholar]
- 17.Van Cutsem E, Köhne C-H, Hitre E. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 18.Peeters M, Price TJ, Cervantes A. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol. 2010;28:4706–4713. doi: 10.1200/JCO.2009.27.6055. [DOI] [PubMed] [Google Scholar]
- 19.Bokemeyer C, Bondarenko I, Hartmann JT. KRAS status and efficacy of first-line treatment of patients with metastatic colorectal cancer (mCRC) with FOLFOX with or without cetuximab: the OPUS experience. Proc Am Soc Clin Oncol. 2008;26(suppl) abstr 4000. [Google Scholar]
- 20.Douillard JY, Siena S, Cassidy J. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010;28:4697–4705. doi: 10.1200/JCO.2009.27.4860. [DOI] [PubMed] [Google Scholar]
- 21.Tveit K, Guren T, Glimelius B. Randomized phase III study of 5-fluorouracil/folinate/oxaliplatin given continuously or intermittently with or without cetuximab, as first-line treatment of metastatic colorectal cancer: the NORDIC VII study ( NCT00145314) by the Nordic Colorectal Cancer Biomodulation Group. Proc Am Soc Clin Oncol. 2011;29(suppl) abstr 365. [Google Scholar]
- 22.Borner M, Koeberle D, Von Moos R. Adding cetuximab to capecitabine plus oxaliplatin (XELOX) in first-line treatment of metastatic colorectal cancer: a randomized phase II trial of the Swiss Group for Clinical Cancer Research SAKK. Ann Oncol. 2008;19:1288–1292. doi: 10.1093/annonc/mdn058. [DOI] [PubMed] [Google Scholar]
- 23.Coleman MP, Forman D, Bryant H, the ICBP Module 1 Working Group Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995–2007 (the International Cancer Benchmarking Partnership): an analysis of population-based cancer registry data. Lancet. 2011;377:127–138. doi: 10.1016/S0140-6736(10)62231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Institute for Health and Clinical Excellence . Cetuximab for the first-line treatment of metastatic colorectal cancer. TA176. National Institute for Health and Clinical Excellence; London, UK: 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.