Abstract
In this study, we report on the presence of efflux transporter activity before oocyte maturation in sea stars and its upregulation after maturation. This activity is similar to the multidrug resistance (MDR) activity mediated by ATP binding cassette (ABC) efflux transporters. In sea star oocytes the efflux activity, as measured by exclusion of calcein-am, increased two-fold 3 h post-maturation. Experiments using specific and non-specific dyes and inhibitors demonstrated that the increase in transporter activity involves an ABCB protein, P-glycoprotein (P-gp), and an ABCC protein similar to the MDR-associated protein (MRP)-like transporters. Western blots using an antibody directed against mammalian P-gp recognized a 45 kDa protein in sea star oocytes that increased in abundance during maturation. An antibody directed against sea urchin ABCC proteins (MRP) recognized three proteins in immature oocytes and two in mature oocytes. Experiments using inhibitors suggest that translation and microtubule function are both required for post-maturation increases in transporter activity. Immunolabeling revealed translocation of stored ABCB proteins to the plasma cell membrane during maturation, and this translocation coincided with increased transport activity. These MDR transporters serve protective roles in oocytes and eggs, as demonstrated by sensitization of the oocytes to the maturation inhibitor, vinblastine, by MRP and PGP-specific transporter inhibitors.
Keywords: multidrug resistance, multidrug resistance associated protein, oocyte maturation, permeability glycoprotein, sea star, translocation
Introduction
Efflux transporters are an evolutionarily conserved group of transmembrane proteins belonging to the ATP-binding cassette (ABC) superfamily. These proteins are ubiquitous and are especially well-known for their role in mediating the phenotype of multidrug resistance (MDR) seen in cancer cells and various pathogens. Major MDR efflux transporters include the permeability glycoproteins (P-gp) and multidrug resistance associated proteins (MRP) (Borst et al. 2000; Dean et al. 2001). Efflux transporters export moderately hydrophobic compounds, lipids, conjugated organic anions, hormones and xenobiotics (reviewed in Krishna & Mayer 2000 and Kruh & Belinsky 2003). Efflux transporters transport hydrophobic compounds from exocrine and endocrine glands (Sarkadi et al. 1994), function during epithelial microvillar transport (reviewed in Lange & Gartzke 2001), and are involved in the developmental physiology of plants (Arabidopsis) and molds (Dictyostelium) (Good & Kuspa 2000; Gaedeke et al. 2001; Friml et al. 2003).
MDR transport is utilized by freshwater and marine species including fish, molluscs, sponges, sea urchins and echiuroid worms (Epel 1998; Kurelec 1992; Smital & Kurelec 1998; Hamdoun et al. 2004; Smital et al. 2004) and are protective, transporting cellular products and xenobiotics across the plasma membrane (Epel 1998; Toomey & Epel 1993; Kurelec 1997; Hamdoun et al. 2002). Transporter expression in aquatic model systems is induced by exposure to various environmental toxicants (Minier et al. 1993; Smital & Kurelec 1998). In invertebrates, P-gp-mediated efflux is the main focus of most research on efflux transporters, although recent evidence confirms the vital role of MRP-mediated efflux in several species (Hamdoun et al. 2004; reviewed in Leslie et al. 2001).
In both vertebrates and invertebrates, MDR transporters are expressed in a variety of reproductive and developmental cell types. In humans, both MRP and P-gp are expressed in trophoblast cells and other placental tissues (Utoguchi et al. 2000; Atkinson et al. 2003). Murine oocytes and early embryos express P-gp, which is thought to function as a protectant against toxicants (Elbling et al. 1993). Porcine oocytes express mRNA alone for both P-gp (MDR1) and MRP (MRP1) transporters (Takebayashi et al. 2001). Amphibian embryos express P-gp proteins and exhibit active P-gp mediated dye efflux (Bonfanti et al. 1998). In invertebrates, embryos from diverse phyla express MDR transporters including nematode, annelid, mollusc and echinoderm (Broeks et al. 1996; Hamdoun et al. 2002; and 2004; Toomey & Epel 1993; McFadzen et al. 2000; Minier et al. 2002).
While less is known about the function of MDR-like transport activities in reproduction and development, studies indicate that ABC transporters play important roles. These include regulation of dauer larval development in C. elegans (Yabe et al. 2005), auxin-mediated development in Arabidopsis (Noh et al. 2001; Klein et al. 2003), and stalk cell differentiation in Dictyostelium development (Good & Kuspa 2000). In sea urchins, MRP- and P-gp-like transporters are present in eggs and early embryos, but the majority of transport activity is MRP-like and appears after fertilization by post-translational upregulation of existing protein stores (Hamdoun et al. 2004). These MDR transporters appear to have an as yet unknown regulatory role in mitotic progression and their transport activity also protects from toxic exogenous compounds (Hamdoun et al. 2004).
The membrane reorganization at fertilization involves changes analogous to those seen during sea star oocyte maturation (Jaffe & Terasaki 1993, 1994). Sea star oocytes are arrested in the prophase of meiosis I in the ovaries and are induced to mature by 1-methyladenine (1-MA) released from the surrounding follicular cells at spawning. The primary characteristics of oocyte maturation are germinal vesicle breakdown, completion of meiosis I and II and female pronuclear formation. Sea star oocyte maturation is mediated by signaling molecules including cAMP, kinases, phospholipases and phosphatases (reviewed in Meijer & Mordret 1994; Kishimoto 1998) and results in reorganization of the cytoplasmic contents, including endoplasmic reticulum, ribosomes (Terasaki 1994) and cortical granules (Santella et al. 1999) using F-actin (Heil-Chapdelaine & Otto 1996) and microtubules (Ookata et al. 1993). Oocyte maturation, as determined by germinal vesicle breakdown, is completed within 1 h of 1-MA addition. Injection of a higher concentration of 1-MA into female sea stars will induce spawning within 1–3 h, during which the mature eggs separate from the supportive follicular cells and are released (Meijer & Guerrier 1984). This study describes MDR transport activity in sea star oocytes and eggs, and demonstrates an increase in this MDR-mediated efflux at oocyte maturation.
Materials and methods
Reagents and chemicals
1-Methyladenine, actinomycin D, cycloheximide, 5,6-Dichloro-1-b-D-ribofuranosylbenzimidazole (DRB), emetine, colchicine, reversin (R-205), rhodamine B, 1benzoyl-5-methoxy-2-methylindole-3-acetic acid (BMMA), Nonidet P-40, horseradish peroxidase (HRP)-conjugated goat antimouse antibody and HRP-conjugated rabbit antigoat antibody were purchased from Sigma-Aldrich Chemical Company (St. Louis, MO, USA). Calcein-acetoxymethylester (c-am), Alexa Fluor 488-goat antimouse IgG and Alexa Fluor 488-rabbit antigoat IgG were purchased from Molecular Probes (Eugene, OR, USA). The MRP inhibitor, MK571, was purchased from Cayman Chemical (Ann Arbor, MI, USA) and the P-gp inhibitor, PSC833, was a gift from Norvartis (Basel, Switzerland). The primary antibody to P-gp, C219, was purchased from Signet Laboratories (Dedham, MA, USA). The primary antibody to MRP (T-24) was constructed by Santa Cruz Biotechnology (Santa Cruz, CA, USA), using multiple conserved amino acid sequences isolated from MRP genes of the sea urchin, Strongylocentrotus purpuratus. Stocks of each chemical were made in either dimethyl sulfoxide or methanol, except 1-MA which was stored in filtered seawater (FSW). Paraformaldehyde for oocyte and egg fixation was purchased from Polysciences (Warrington, PA, USA) and made into a 4% solution with FSW.
Animals and oocyte isolation
Adult Asterina miniata and Pisaster ochraceous were collected during their respective reproductive cycles and maintained at the Bodega Marine Laboratory (BML) in continuous flow-through, sand-filtered Bodega Bay seawater (13°C ± 2 C) with weekly feedings of invertebrate food pellets, shrimp or mussels. Immature oocytes were isolated by boring a quarter inch hole in the upper arm near the disc of 2–3 females and removing pieces of the intact ovaries (to avoid confusion, immature oocytes will be called oocytes and mature eggs will be called eggs).
Ovarian pieces were transferred to cold Ca-free ASW and shaken to force the release of oocytes from follicular cells and the ovaries. Oocytes were separated from the ovarian pieces by filtration through a 250 μm mesh screen and washed three times in 0.7 μm FSW. Oocytes were examined under a phase-contrast microscope for the presence of germinal vesicles and overall appearance, and tested for germinal vesicle breakdown (GVBD) after addition of 1-MA. GVBD is a standard measurement of oocyte maturation in the sea star. The germinal vesicle is visible in the immature oocyte and disappears within an hour after hormone stimulation. During GVBD, the nuclear envelope separates from the cytoplasmic matrix and becomes ragged or misshapen and eventually disintegrates (Meijer & Guerrier 1984). Inhibitors of oocyte maturation would decrease the percentage of oocytes maturing and undergoing GVBD.
Dye accumulation before and after oocyte maturation
Isolated oocytes were diluted into 15 mL conical tubes filled with 9 mL FSW then 1 mL of 1 mm 1-MA in FSW (final concentration 100 μm) was added to tubes labeled for mature eggs for a final volume of 10 mL. Oocytes were allowed to mature for 1–5 h (hours post-maturation: hpm) before dye was added. Vehicle controls of dimethylsulfoxide (DMSO) or methanol were conducted. A dose–response for c-am was determined for both oocytes and eggs (0.1–2 μm, Km = 0.5 μm for immature oocytes). Experimental c-am concentrations were either 0.25 μm or 0.5 μm. Saturation with MDR inhibitors with immature oocytes occurred at concentrations higher than 0.5 μm. The lower c-am concentration of 0.25 μm was used during certain experiments to clearly discern the relative changes in dye accumulation. At the appropriate maturational time points, c-am was added to 15 mL conical tubes containing oocytes or eggs and incubated for 2 h at 15°C on a slow shaker table. After the incubation period and settling, oocytes and eggs were transferred to immunoslides and viewed using a fluorescent microscope. Fluorescent images of 20–30 immature oocytes and mature eggs were collected using an Olympus BX50WI fixed stage microscope, equipped with a xenon illumination source, fluorescence objective lenses, and a Roper Scientific CoolSnap HQ cooled CCD camera interfaced to a computer through the imaging program, Metamorph (Molecular Devices, Downingtown, PA, USA). Using Metamorph, filters used included an excitation wavelength of 490 nm and an emission wavelength of 535 nm for all c-am experiments, with optimal filter combinations used for other dyes. The mean pixel fluorescence intensity of individual oocytes and eggs was measured and averaged for each time point.
Experiments to determine if transcription, translation or the cytoskeleton are involved in the regulation of transport activity
For experiments involving cell function inhibitors, oocytes were isolated and prepared for incubation. Maturation was induced by 1-MA and cell function inhibitors were added 20 min later to ensure maturation was initiated. Transcriptional inhibitors, actinomycin D (AD) and DRB, were used at 1 μm and 100 μm, respectively. Translational inhibitors, cycloheximide (CY) and emetine (EM), were used at 2.5 μm and 100 μm, respectively. The microtubule inhibitor, colchicine (COL), was used at 100 μm. Samples were incubated for 2 h with the cell function inhibitors. Samples were washed with FSW because the cell function inhibitors are substrates for MDR transporters (Litman et al. 2001). C-am (0.25 μm) was added to the tubes and incubated for 2 h at 15°C. Samples were prepared for and analyzed by fluorescence microscopy.
Dose response curve to MDR inhibitors
After confirmation of dye efflux activity, a dose–response curve for reversin 205 (R205), an inhibitor of MDR-mediated dye efflux, was determined for both oocytes and eggs. MDR inhibitors are used to verify functional MDR transport and, if transport activity is present, should result in an increase in fluorescence in relation to the untreated control. R205 (1–20 μm) was added with 0.25 μm c-am to tubes containing oocytes and eggs (1 hpm) and incubated for 2 h at 15°C. The samples were analyzed by fluorescence microscopy after the incubation period. A dose of 3 μm R205 was determined to be the most appropriate final concentration based on the fact that this was the lowest concentration where maximal fluorescence was obtained (data not shown). All further experiments with R205 used this concentration unless noted. A dose–response curve for c-am (0.25 μm) dye accumulation using PSC833, MK571 and BMMA was conducted to determine the appropriate concentration of these inhibitors. Concentrations of rhodamine B were obtained from published data (Toomey & Epel 1993; Hamdoun et al. 2002).
Dye accumulation kinetics in immature oocytes and mature eggs
To determine the dye accumulation kinetics during oocyte maturation transition, oocytes were attached to two polylysine-coated two-chambered glass slides. Oocytes were incubated in 1% bovine serum albumin (BSA) in FSW for 15 min at 15°C and then washed three times in FSW. The chambered slides were blocked to prevent binding of c-am to polylysine-coated slides in order to maintain soluble concentrations of c-am. A solution (3 mL) of FSW and 0.5 μm c-am dye was added at t = 0 and fluorescence images of the same 7–10 oocytes were started at 10-min intervals for 60 min. At t = 60, 30 μL of 10 mm 1-MA was added to three of the chambers and images of fluorescence continued for over 2 h at 10-min intervals and then at 15-min intervals for the final 30 min. Specific MDR inhibitors were added to two chambers to determine the type of transporter involved in the change in dye efflux during maturation. During experiments with the specific inhibitors, images were collected every 15 min for 2.5 h (t = 150). At t = 150, the P-gp-specific inhibitor, PSC833 (10 μm), and the MRP-specific inhibitor, MK571 (10 μm), were individually added to one of the chambers containing matured eggs. Images were collected at 10-min intervals for another 60 min (t = 210) and analyzed accordingly by MetaMorph software (Universal Imaging).
Comparison of dye accumulation with specific and non-specific dyes and inhibitors
To compare the different transporters functioning in each maturational state, samples of oocytes and eggs (1 hpm) were incubated with c-am (0.25 mm) for 2 h with various transport inhibitors. The specific inhibitors used were PSC833 (5 μm for c-am), MK571 (5 μm for c-am) and BMMA (10 μm for c-am), a putative MRP1 inhibitor (Maguire et al. 2001). Other inhibitors or substrates were utilized in the dye accumulation experiments such as R205 (3 μm for c-am) and rhodamine B (RhoB, 1 μm for c-am). Rhodamine B was chosen because it is not a substrate in systems where MRP is the primary transporter such as the sea urchin egg (Toomey & Epel 1993; Hamdoun et al. 2002, 2004), and rhodamine 123, a related dye, is believed to be P-gp specific (Twentyman et al. 1994).
Immunoblotting
For immunoblots with C219, samples of oocytes, eggs (1, 3 and 5 hpm) and eggs matured in the presence of cell function inhibitors for 5 hpm from both species were collected into conical tubes, hand centrifuged and flash frozen in liquid nitrogen after removal of all FSW. Samples were pulverized using a mortar and pestle chilled with liquid nitrogen and homogenized with 23× volume of ice-cold Tris-HEPES-EDTA sucrose buffer with general protease inhibitor cocktail (Sigma Chemical, St. Louis, MO, USA). Protein concentrations of the homogenates were determined using the Pierce standard bicinchoninc acid (BCA) protein assay. Solubilized homogenates (10 μg of total protein/sample) were loaded onto 10% sodium dodecyl sulfate (SDS) polyacrylamide gels and run at 155 V for 90 min at room temperature. Proteins were transferred to nitrocellulose for 1 h at 115 V in a cooled chamber. Blots were blocked for 2 h in 4% non-fat milk in Tris-buffered saline (10 μm Tris base, 150 μm NaCl, pH 7.4) and stored overnight in Tris-buffered saline with 1% Tween. P-gp protein was labeled by incubating blots for 2 h with the mouse monoclonal antibody C219 at 1 μg/mL, followed by the horse radish peroxidase (HRP)-conjugated goat antimouse (1:5000) secondary antibody for 1 h. HRP-labeled P-gp protein was visualized by reaction with Pierce (Rockford, IL, USA) chemiluminescent reagent according to the manufacturer's instructions and photographed using an EpiChem Digital Darkroom (UVP, Upland, CA, USA). Fish (Gillicthys mirabilis) gill homogenate was used as a positive control as it is known to express relatively high levels of P-gp (Hemmer et al. 1995; Tutundjian et al. 2002). Embryos from the sea urchin, Lytechinus anamesus, which do not express a C219-reactive P-gp (Toomey & Epel 1993; Hamdoun et al. 2002), served as a negative control.
For immunoblots with T-24 MRP antibody, samples of oocytes and eggs (1, 3 and 5 hpm) were collected, washed and concentrated to 50 μL volume using a table-top picofuge. Excess FSW was removed and the samples were transferred to a glass mini-homogenizer. The ice-cold homogenization buffer contained 200 μL THE-sucrose buffer with 1 mm PMSF and 50 μm 2× protease inhibitor cocktail. Samples were homogenized on ice for several minutes, avoiding bubbles. Samples were transferred to 2 mL microfuge tubes and centrifuged for 5 min at 5000 r.p.m. at 4°C. The supernatant was removed and prepared for gel electrophoresis by adding an equal volume 2× Laemilli buffer. The pellet was resuspended in the homogenization buffer, mixed and prepared for gel electrophoresis. Protein concentrations of homogenates were determined using a standard BCA protein assay. Solubilized homogenates (10 μg of total protein/sample) were loaded onto 10% SDS polyacrylamide gels and run at 155 V for 90 min at room temperature (RT). Proteins were transferred to nitrocellulose for 1 h at 115 V in a cooled chamber. Blots were blocked for 2 h in 4% non-fat milk in Tris-buffered saline (TBS) and incubated overnight with primary T-24 MRP antibody (1:1000) in TBS at 4°C. The blots were incubated in the HRP-conjugated rabbit antigoat (1:5000) secondary antibody for 2 h at RT. HRP-labeled MRP protein was visualized by reaction with chemiluminescent reagent according to the manufacturer's instructions and photographed using a EpiChem Digital Darkroom. Homogenized mouse liver was used as a positive control.
Immunocytochemistry
To determine the movement of the P-gp transporter within the oocyte during maturation, we labeled fixed and permeabilized oocytes and eggs (1, 3 and 5 hpm) with C219. The oocytes were extracted from female sea stars and prepared as above. Immature oocytes were immediately fixed in 4% paraformaldehyde in FSW and stored at 4°C overnight. At the appropriate time points after the addition of 1-MA, the eggs were also fixed and stored. Subsamples of eggs matured in the presence of either AD (1 μm), CY (2.5 μm) or COL (100 μm) for 5 h were also fixed and stored overnight. The next day samples were washed three times in Ca-ASW for 10 min followed by 1 h incubation in 1% NP-40 in phosphate-buffered saline (PBS) to permeabilize the cell membrane. The samples were washed for another 3 × 10 min in PBS and transferred to microfuge tubes. Samples were blocked in BSA (4% in PBS) for 1 h and then incubated for 1 h in primary antibody C219 in PBS (1:50) at RT. The samples were washed 1 × 5 min in PBS and incubated for 30 min in a 1:10 dilution of goat serum and washed 3 × 10 min in PBS. The oocytes and eggs were finally incubated for 1 h in a 1:500 dilution of the secondary antibody, Alexa 488 goat antimouse, with gentle mixing in the dark. Samples were washed 1 × 5 min in PBS and resuspended in glycerol mounting media and stored at −20°C. A secondary control (no primary) of oocytes was also prepared and stored.
Immunocytochemistry using the T-24 MRP antibody was similar to the C219 protocol until the BSA blocking step. After the PBS wash cycle, the samples were blocked in bulk in 15 mL conical tubes in 4% BSA (PBS) overnight at 4°C (100 μL of 1% Na Azide was added for long-term storage). After overnight incubation in BSA, subsamples were transferred to microfuge tubes and washed for 1 × 10 min in PBS. Primary T-24 MRP antibody (1:100 dilution in PBS) was added to the sample while in fresh PBS for 3–4 h at RT with gentle mixing. The primary antibody was removed followed by another 1 × 5 min wash in PBS. The sample was blocked in a 1:10 dilution of rabbit serum in PBS for 1 h and washed for 3 × 10 min in 1% BSA in PBS. The secondary antibody, Alexa 488 rabbit antigoat (1:500), was added to the samples in fresh PBS for 2 h with gentle mixing in the dark. The secondary was removed and, after a 1 × 5 min wash in PBS, glycerol mounting media was added to the samples, mixed and stored at −20°C. A secondary control was also prepared.
All samples were viewed using an Olympus Fluoview 500 laser scanning confocal microscope (equipped with AOTF for complete channel separation) mounted on an Olympus BX60WI fixed stage upright microscope (Olympus, New York, NY, USA). Samples were imaged using a water immersion 10× fluorescence objective lens. Images (both fluorescence at 488 nm excitation and differential interference contrast) were collected as single scans or as Z-series with 5 μm optical sections followed by Z-series projection using Fluoview software (Olympus).
Data analysis
Fluorescence intensity was measured for individual oocytes and eggs using image analysis macros provided with MetaMorph (Universal Imaging) software. All absolute measurements were averaged (± 1 SD). Data were reported either as absolute fluorescence with differences between treatments analyzed using either two sample paired t-tests of means or analysis of variance (ANOVA) (P < 0.05), or data was reported as a relative fluorescence (treatment/control) and averaged over the three experiments (± 1 SD; P < 0.05). Protein band intensity on immunoblots was analyzed using UVP Labworks (UVP, Upland, CA, USA).
Results
Changes in MDR protein expression and activity during oocyte maturation
C-am is a non-fluorescent MRP and P-gp substrate (Essodaigui et al. 1998). C-am is membrane permeable but, once inside the cell, esterases hydrolyze the acetoxymethylester creating membrane impermeant, fluorescent calcein which accumulates in the cell. Free calcein is also a MDR substrate but the km is 5000-fold lower than c-am (Essodaigui et al. 1998). Cells with little or no transport activity accumulate high levels of calcein and fluoresce brightly. In cells with a higher level of transport activity, c-am is effluxed before esterases cleave the c-am ester bond and the cells will accumulate less calcein.
To assess the activity of dye transport during oocyte maturation, oocytes and eggs from P. ochraceous were exposed to c-am for 2 h and the amount of dye accumulation was analyzed using fluorescence microscopy (Fig. 1A). The amount of dye accumulation in oocytes was twice the amount recorded in eggs 3 hpm. To test if the dye accumulation was related to P-gp-mediated transport, oocytes and eggs were incubated with Reversin 205 (R205), a small peptide inhibitor of P-gp (Sharom 1999). The ratio of treated (R205)/untreated oocytes and eggs was 1.64 ± 0.24 and 2.64 ± 0.47, respectively. Oocytes do have some functional MDR activity, as evidenced by an increase in dye accumulation (ratio > 1.0) during co-incubation with R205. The decrease in dye accumulation in eggs relative to oocytes was mostly due to the increase in transporter activity, as demonstrated by the statistically similar inhibition of transport activity in treated eggs (3 hpm) and treated oocytes (P > 0.05).
Fig. 1.
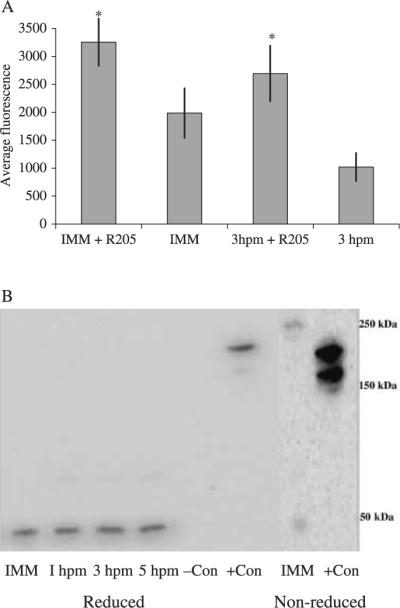
Changes in dye accumulation during oocyte maturation and protein analysis using the monoclonal P-gp antibody, C219, from immature oocyte and maturing egg samples. (A) Immature oocytes and mature eggs (1 and 5 hpm) were incubated with 0.5 μm c-am with or without 3 μm R205 for 2 h at 15°C. Mean pixel fluorescence (± SD) intensity of 20–30 individual oocytes and eggs was measured and averaged for each maturational time point. *Denotes significant differences using the paired t-test between inhibitor pairs (P < 0.05). Ratios of treated/untreated immature oocytes and mature eggs were 1.64 ± 0.24 and 2.64 ± 0.47, respectively. Data is from oocytes extracted from P. ochraceous. (B) Sea star oocyte and egg protein samples analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel and immunoblot using the monoclonal P-gp antibody, C219. Reduced samples of controls, immature oocytes (IMM) and mature eggs (1–5 hpm) and non-reduced samples of immature oocytes and positive controls were analyzed on separate blots. Data is from oocytes extracted from P. ochraceous.
The increase in dye efflux (decreasing dye accumulation) after the 2 h incubation period correlated with an increase in the intensity of the bands on the immunoblots using the P-gp antibody, C219 (Fig. 1B). The intensity of the 3 and 5 hpm bands was 79% and 103% greater than the oocyte band, respectively. The monoclonal antibody C219 specifically binds to a highly conserved amino acid sequence (VQEALD) near the ATP-binding cassette domain (Georges et al. 1990) and generally recognizes proteins in the 150–180 kDa range (Litman et al. 2001). In the sea star, the antibody recognized a protein in the 45 kDa range. When protein samples from P. ochraceous oocytes and eggs (1–3 hpm) were not reduced by the addition of β-mercaptoethanol to the Laemilli buffer, two diffuse bands at 220 kDa and 45 kDa were apparent (Fig. 1B).
Is MDR activity required for oocyte maturation?
Functional MDR activity did not appear to be involved in oocyte maturation because oocytes from A. miniata incubated with R205 (3 μm) or specific P-gp/MRP inhibitors, PSC833 (10 μm) or MK571 (10 μm), did not spontaneously mature and oocytes were not inhibited from maturation by the addition of 1-MA in the presence of these inhibitors. However, MDR transport activity is a protective mechanism during sea star oocyte maturation, as evidenced by the decrease in the percentage of GVBD (oocyte maturation) when oocytes were incubated with vinblastine, an inhibitor of sea star oocyte maturation (Meijer & Guerrier 1984), and the specific transport inhibitors. Vinblastine (300 μm), used alone, decreased GVBD by 42% ± 8%, but vinblastine incubated with PSC833 (10 μm) or MK571 (10 μm) decreased the percentage of GVBD by 69% ± 5% and 73% ± 7%, respectively. Both specific inhibitors significantly increased inhibition of maturation by vinblastine.
Mechanism of activation of MDR activity during oocyte maturation
To determine the mechanism by which MDR activity increases during maturation, oocytes from A. miniata were incubated in the presence of transcriptional, translational and microtubule inhibitors. Several of the cell function inhibitors (colchicine, emetine) are known to inhibit oocyte maturation (Meijer & Guerrier 1984); therefore, the inhibitors are added at the end of the hormone-dependent period of oocyte maturation (20 min after 1-MA addition). Data are expressed as relative fluorescence values that are dependent on the base level of c-am accumulation. A ratio greater than 1.0 ± 1 was considered significant. Eggs from A. miniata matured in the presence of inhibitors of translation (cycloheximide and emetine) and microtubule function (colchicine) accumulated c-am at levels similar to oocytes and significantly higher than eggs (4 hpm; Fig. 2A). Transcriptional inhibitors (actinomycin D and DRB) did not interfere with the increase in dye efflux in the maturing eggs. The concentration of colchicine used for inhibiting the increase in dye efflux activity was high because colchicine is a substrate for MDR transporters (Loe et al. 1996; Litman et al. 2001).
Fig. 2.
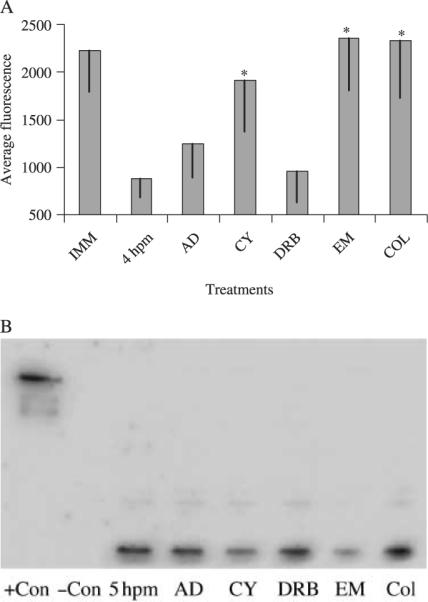
Effects of cell function inhibitors on the decrease in dye accumulation after oocyte maturation and protein analysis using the monoclonal P-gp antibody, C219, from samples of oocytes, eggs and eggs incubated with cell function inhibitors. (A) Oocytes were incubated for 2 h with cell function inhibitors 20 min after 1-methyladenine (1-MA) addition. C-am (0.25 μm) was added to the tubes and incubated for 2 h at 15°C. Transcriptional inhibitors, actinomycin D (AD) and DRB, were used at 1 μm and 100 μm, respectively. Translational inhibitors, cycloheximide (CY) and emetine (EM), were used at 2.5 μm and 100 μm, respectively. The microtubule inhibitor, colchicine (COL), was used at 100 μm. Mean (± SD) pixel fluorescence intensity of 20–30 individual oocytes and eggs was measured and averaged for each maturational time point. *Denotes significant differences using the paired t-test between the inhibitor and the untreated mature eggs (P < 0.05). Data is from oocytes extracted from A. miniata. (B) Sea star oocyte and egg protein samples analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel and immunoblot using the monoclonal P-gp antibody, C219. Samples of controls, 5 hpm mature eggs and mature eggs incubated with cell function inhibitors during maturation. Data is from oocytes extracted from P. ochraceous.
An immunoblot of oocyte and egg protein samples from P. ochraceous treated with the cell function inhibitors using C219 also demonstrated the importance of translation in the increase in P-gp associated activity (Fig. 2B). Bands from eggs treated with cycloheximide and emetine were 41.1% and 21.1% less intense than the 5 hpm samples, respectively. Eggs treated with colchicine did not have a decrease in band intensity but rather increased to 183.3% of the control. Besides the possibility of technical error in determining protein concentration or gel loading, it is unknown why colchicine caused higher band intensity in the immunoblots with C219 because colchicine is not known to interfere with protein translation.
Determination of the type(s) of ABC efflux transporter activity present
A dose–response with either of the P-gp/MRP-mediated transport inhibitors, PSC833 or MK571, in oocytes and eggs (3 hpm) from A. miniata was conducted to determine the major efflux activity present in the eggs (Fig. 3A). Specific inhibitors of P-gp and MRP-mediated transport have been developed, including PSC833 and MK571. Concentrations in the low micromolar range of both PSC833 and MK571 are known to inhibit the activity of P-gp and MRP transporters, respectively (reviewed in Krishna & Mayer 2000). PSC833 was a more potent inhibitor of c-am efflux in both oocytes and eggs than MK571 at all experimental concentrations except at 20 μm. However, PSC833 and MK571 inhibited c-am efflux in eggs significantly more than in oocytes. At 20 μm, many of the eggs burst after the 2 h incubation period for both specific inhibitors but specific inhibitors did not inhibit oocyte maturation at 20 μm. Figure 3(B) shows the dose–response of oocytes and eggs incubated with 0.25 μm c-am and a range of concentrations of R205 (1–20 μm) as well as the dose–response of eggs to BMMA. In oocytes, R025 (1 μm) inhibited dye efflux significantly, almost reaching the experimental detection limit, but did have a significant effect in eggs until 3–5 μm. BMMA did not inhibit c-am efflux in oocytes.
Fig. 3.
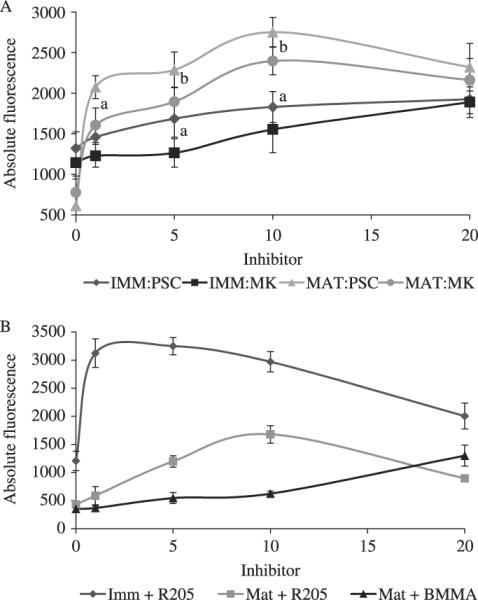
Dose–responses of immature oocytes and mature eggs when incubated with PSC833, MK571, R205 and BMMA (mature only). Immature oocytes and mature eggs were (1 hpm) incubated with c-am (0.25 μm) for 2 h with the multidrug resistance (MDR) inhibitors. (A) PSC833 (1–20 μm), a P-gp inhibitor, and MK571 (1–20 μm), a multidrug resistance associated protein (MRP) inhibitor; and (B) R205 (1–20 μm), a general MDR inhibitor, and BMMA (1–20 μm), a putative MRP1 inhibitor. Mean pixel fluorescence intensity of 20–30 individual oocytes and eggs was measured and averaged for each maturational time point. (a) Denotes significant differences using the paired t-test between data points at that concentration between the two inhibitors (P < 0.05), and (b) denotes significant differences using the paired t-test between data points (P < 0.1). Data is from oocytes extracted from A. miniata.
Figure 4 shows the kinetics of dye accumulation during the transition from immature oocyte to mature egg and the involvement of P-gp and MRP-like transporters. In the oocytes from A. miniata, dye accumulated at a steady rate for 2.5 h (Fig. 4A). Dye accumulation continued at a slower rate for the next 1.5 h. In the maturing eggs, dye accumulation was similar to the oocytes until 1 h after 1-MA addition (t = 120). The eggs completed GVBD at that time and the rate of dye accumulation significantly decreased to ~0 units/min for 60 min and slowly increased after t = 180. When specific inhibitors were added 1.5 h after 1-MA (t = 150), dye accumulation increased at a similar rate as early immature oocytes (~25 units/min; Fig. 4B). The slopes of the curve during the time-lapse dye accumulation experiments were significantly different between the untreated eggs (~4 units/min) and the specific inhibitor-treated eggs (~25 units/min) (Fig. 2B; P < 0.05). Increases in dye accumulation with PSC833 or MK571 (10 μm of each) were not significantly different.
Fig. 4.
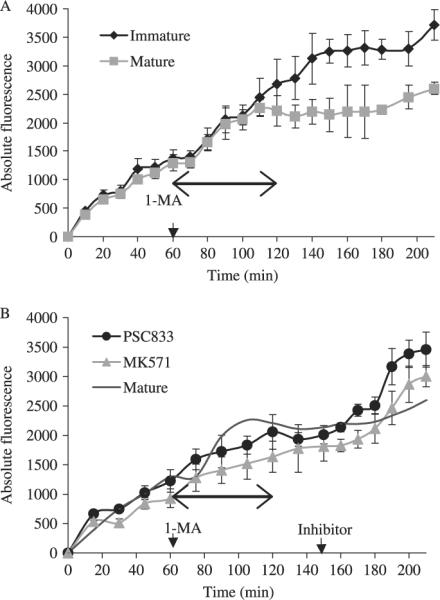
Time-lapse kinetics of c-am accumulation in immature oocytes and mature eggs with and without specific inhibitors (mean ± SD). (A) Images of fluorescence from the same 7–10 oocytes/eggs after the addition of 0.5 μm c-am were taken at 10-min intervals for 60 min. At t = 60, 1 mm 1-methyladenine (1-MA) was added to the appropriate chambers. Recordings continued for over 2 h at 10-min intervals and then at 15-min intervals for the final 30 min. (B) For experiments with PSC833 and MK571, images were taken every 15 min for 2.5 h (t = 150) of the same 7–10 oocytes/eggs. At t = 150 (arrow), PSC833 10 μm and MK571 (10 μm) were individually added to one of the chambers containing matured eggs. Recordings were taken at 10-min intervals for another 60 min (t = 210) and analyzed according to MetaMorph. Arrowed line indicates the duration of oocyte maturation determined by germinal vesicle breakdown. Comparisons of the time lapse experiments and the other dye accumulation experiments is cautioned because of the discreet snapshot nature of the dye accumulation experiments compared to the continuous monitoring of dye accumulation in the time lapse experiments. Data are from oocytes isolated from A. miniata.
To further elucidate what type of MDR transporters are involved in the dye efflux of both oocytes and eggs from A. miniata, oocytes and eggs were incubated in c-am and an assortment of inhibitors or substrates (R205, MK571, PSC833, BMMA and RhoB). Dye accumulation data was expressed as relative fluorescence values (control fluorescence noted on figures). All inhibitors increased c-am accumulation (compared to control values) significantly (P < 0.05) in both oocytes and eggs, except for BMMA in oocytes. The relative c-am accumulation due to PSC833 and MK571 treatment at 5 μm was significantly less than dye accumulation with R205 (3 μm, Fig. 5A; P < 0.05). The relative differences between R205 and PSC833 in inhibiting dye efflux in the oocytes may be a result of differences in affinities between the two P-gp inhibitors. C-am efflux in the oocytes was inhibited two- to three-fold by R205 (Fig. 5A). BMMA, a putative MRP1 inhibitor (Maguire et al. 2001), did not inhibit c-am efflux significantly in the oocytes, suggesting that little or no MRP1-mediated dye efflux was occurring in the oocytes. In the egg, c-am efflux is inhibited significantly by all treatments, including BMMA (Fig. 5B). After maturation, c-am accumulation with R025, PSC833 and MK571 was not significantly different (Fig. 5B).
Fig. 5.
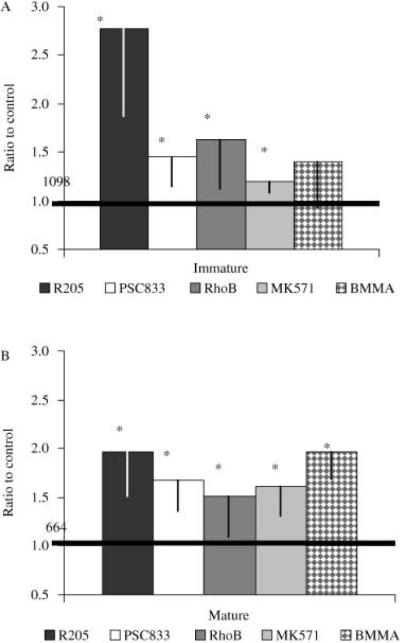
Dye accumulation in immature oocytes and mature eggs using c-am incubated with specific and non-specific inhibitors of multidrug resistance (MDR) transport. Relative fluorescence of immature oocytes (A) and mature eggs (B; 1 hpm) were incubated with c-am (0.25 μm) for 2 h with R205 (3 μm), PSC833 (5 μm), RhoB (1 μm), a P-gp substrate, MK571 (5 μm) or BMMA (10 μm). Mean pixel fluorescence intensity of 20–30 individual oocytes and eggs was measured and averaged for each maturational time point. Darkened line marks the level of control fluorescence that is given in absolute fluorescence units. *Denotes significant differences relative to the control absolute fluorescence (P < 0.05). Data is from oocytes extracted from A. miniata.
Localization of the P-gp transporter before and after maturation
To determine the location of the P-gp-like transporter in the oocyte and its movement during maturation, oocytes and eggs were fixed and incubated with a P-gp-specific antibody and viewed using confocal microscopy. In oocytes from P. ochraceous, P-gp was localized throughout the cytoplasm near the periphery of the cell. The germinal vesicle was the only portion of the oocyte without labeling (Fig. 6A). At 1 hpm, P-gp was primarily located at the periphery of the maturing egg although a number of foci remained localized to the cytoplasm. A Z-series through an oocyte demonstrates the cytoplasmic distribution of the protein surrounding the germinal vesicle. Another Z-series through an egg (1 hpm) illustrates localization of P-gp at or near the cell surface (Fig. 6B). During the next 4–5 h an increase in antibody labeling occurred, suggesting that protein translation and translocation to the periphery occurred within the 5 h time period (Fig. 7). In the eggs fixed at 4 hpm, more P-gp localized to the cytoplasm than the 3 or 5 hpm eggs (data not shown).
Fig. 6.
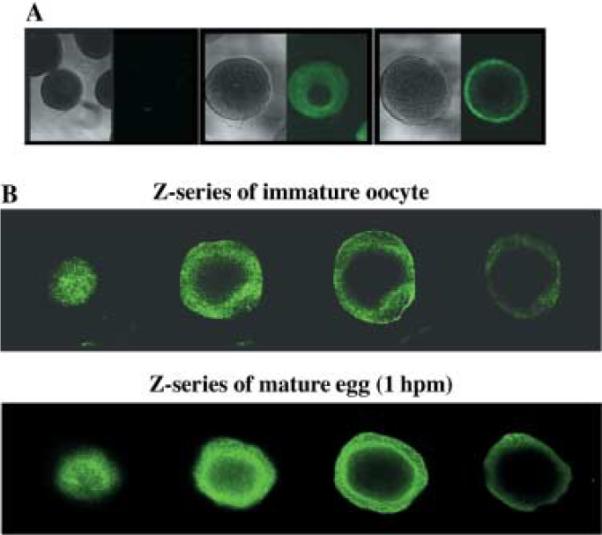
Immunocytochemistry of immature oocytes and mature eggs using the monoclonal antibody, C219. (A) Fixed and permeabilized immature oocytes and mature eggs (1 hpm) were incubated with the P-gp monoclonal antibody, C219. A secondary control (no primary) of oocytes was also prepared. (B) Z-series through an immature oocyte (top row) and mature egg (bottom row) labeled with C219. Samples were viewed using an Olympus Fluoview 500 laser scanning microscope (Olympus, New York, NY, USA). Photomicrographs were analyzed using Fluoview (Olympus) software. Data are from oocytes isolated from P. ochraceous.
Fig. 7.
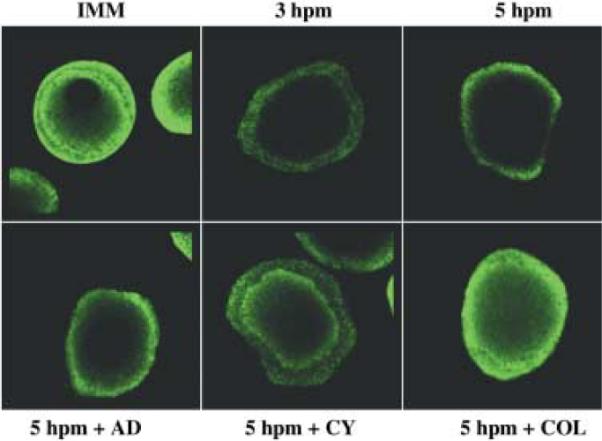
Immunocytochemistry of mature eggs incubated with cell function inhibitors using the monoclonal antibody, C219. Fixed and permeabilized immature oocyte (IMM), eggs (3 and 5 hpm) matured in the presence of cell function inhibitors were labeled with the P-gp monoclonal antibody, C219. Eggs 5 hpm were matured in the presence of actinomycin D (AD, 1 μm), cycloheximide (CY, 2.5 μm) or colchicine (COL, 100 μm). Samples were viewed using an Olympus Fluoview 500 laser scanning microscope (Olympus, New York, NY, USA). Photomicrographs were analyzed using Fluoview (Olympus) software. Data are from oocytes isolated from P. ochraceous.
In eggs exposed to cell function inhibitors during the maturation process (until 5 hpm), the increase in cortical/surface P-gp may have been a result of both translation and trafficking through the vesicle-to-membrane translocation pathway (Fig. 7). Cycloheximide (CY) inhibited the amount of antibody recognition when compared to the 5 hpm (Fig. 7). Colchicine (COL) inhibited the translocation of the protein to the periphery and resulted in increased antibody recognition in the cytoplasm compared to the 5 hpm (Fig. 7). Actinomycin D (AD) did not result in any obvious differences compared to the 5 hpm (Fig. 7).
Localization of the MRP protein(s) before and after maturation
Protein analysis using the sea urchin T-24 MRP antibody reveals three bands in oocytes with affinity for the antibody. These bands are located at approximately 180 kDa, 80 kDa and 65 kDa (Fig. 8A). In eggs (5 hpm) from A. miniata, the lower molecular weight band had disappeared, leaving only the 180 kDa and 80 kDa bands. The 180 kDa and 80 kDa band intensities did not appear to be different before and after maturation. MRPs usually migrate around 190 kDa on SDS-polyacrylamide gel electrophoresis (PAGE) (Litman et al. 2001), which is similar to one of the polypeptide bands observed in the oocyte.
Fig. 8.

(A) Sea star oocyte and egg protein samples analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel and immunoblot using the T-24 MRP antibody designed from sea urchin MRP sequences. Reduced samples of positive control (mouse liver), immature oocytes (IMM) and mature eggs (5 hpm) are shown. Data are from oocytes isolated from A. miniata. (B) Immunocytochemistry of immature oocytes, mature eggs and mature eggs incubated with cell function inhibitors using a polyclonal spMRP antibody designed using sea urchin sequences. Fixed and permeabilized immature oocytes, mature eggs (1 and 5 hpm) and 5 hpm mature eggs matured with cell function inhibitors were labeled with the P-gp monoclonal antibody, C219. Eggs 5 hpm were matured in the presence of actinomycin D (AD, 1 μm), cycloheximide (CY, 2.5 μm) or colchicine (COL, 100 μm). A secondary antibody-only negative control (no primary antibody) of oocytes was also included. Samples were viewed using an Olympus Fluoview 500 laser scanning microscope (Olympus, New York, NY, USA). Photomicrographs were analyzed using Fluoview (Olympus) software. Data are from oocytes isolated from A. miniata.
To determine changes in the location of MRP-like transporter proteins in the oocyte and egg, oocytes and maturing eggs from A. miniata were fixed and incubated with T-24 MRP antibody. Unlike C219, the transporters localized with T-24 MRP were distinctly localized throughout the cytoplasm in the oocyte without evidence of a germinal vesicle (Fig. 8B). At 1 hpm, MRP was translocated to the periphery in a similar manner as P-gp (Fig. 8B). At 5 hpm, the labeled proteins increased in density and again were localized throughout the cytoplasm of the egg (Fig. 8B). AD did not interfere with the increase in density and translocation (Fig. 8B). CY very slightly decreased the amount of MRP labeling after 5 h, and COL appeared to decrease the translocation of the protein to the periphery after 5 h (Fig. 8B).
Discussion
This study has shown that MDR-like efflux activity is present in immature sea star oocytes and increases two-fold within 3 h following in vitro maturation. A dramatic increase in MDR activity also occurs within 60 min of treatment with 1-MA. Upregulation of MDR activity is associated with both P-gp and MRP-like transport activities and entails both the microtubule-dependent translocation of stored MDR transporters to the periphery and another cycle of translation and translocation of nascent transporter proteins.
Evidence for P-gp-like activity
Three lines of evidence support the conclusion that sea star oocytes and eggs possess a P-gp transporter(s). First, dye accumulation studies demonstrate that the relative amount of dye efflux activity increased two-fold 3 h after the addition of 1-MA (Fig. 1). Dye accumulation did not saturate the detection method even in the presence of 10 μm R205. Increases in c-am accumulation by the highly specific P-gp inhibitor, PSC833, as well as another P-gp-specific substrate, rhodamine B, indicate that P-gp transporters are functioning in oocytes and eggs. The inhibition of dye efflux activity by these P-gp-specific inhibitors (Hamdoun et al. 2004; reviewed in Twentyman et al. 1994; Krishna & Mayer 2000; Litman et al. 2001) indicate that functional P-gp transport activity is present. Second, the completion of maturation of the sea star oocyte coincides with an increase in active efflux of the c-am, and this correlates with the translocation of C219 label to the egg surface, strongly suggesting insertion of a new P-gp-like transporter in the egg plasma membrane. Third, the well characterized, P-gp-specific C219 antibody did label a single polypeptide on western blots, however, there was the unexpected finding that the molecular weight was substantially lower than previously characterized P-gps in other systems. This may indicate that sea star oocytes/eggs possess a different P-gp-like transporter, or the finding is the result of the use of reducing conditions during SDS-PAGE, because non-reduced samples showed a 225 kDa band, similar in molecular weight to other P-gp transporters.
In oocytes, the MDR inhibitor, R205, appears to saturate the MDR-mediated transport of c-am. Oocytes incubated for 2 h in 0.5 μm c-am and R205 (3 μm) would often exhibit fluorescence that was at or above signal saturation of the CCD sensor at a given exposure time (100 ms). The saturation of MDR transport activity by R205 in oocytes was demonstrated by the absence of further increases with more R205 and suggests a relatively low number of functional R205-sensitive transporters. Furthermore, the data suggest that the primary transporter in the oocyte is P-gp. The distinct labeling of the P-gp-like protein at the surface of the oocyte along with the inhibition of c-am efflux by PSC833 and RhoB, both known to be P-gp specific, indicate that functional P-gp transport activity is the primary transporter at this stage.
The sea star P-gp-like transporter has two characteristics that are different from vertebrate P-gp transporters. First, the C219-reactive protein migrates at 45 kDa (Fig. 1) rather than in the 150–200 kDa range that vertebrate P-gp transporters migrate to on SDS-PAGE (Litman et al. 2001). Second, the presence of two bands in the non-reduced oocyte samples suggests a linkage by disulfide bonds in the C219-reactive protein that is similar to the breast cancer resistance protein (BCRP, Doyle & Ross 2003). Mitoxantrone, a known substrate for BCRP, did not affect c-am accumulation in either oocytes or eggs, indicating that the C219-reactive protein is not a BCRP-related transporter but rather a novel P-gp-like transporter. We cannot rule out the possibility that the C219 antibody is recognizing another protein with a homologous amino acid sequence similar to C219 (Georges et al. 1990), or that highly specific proteolytic degradation resulted in a single lower molecular weight polypeptide. However, as mentioned above, the timing of the movement of the detected proteins by immunocyto-chemistry and the increase in P-gp specific transport activity, as demonstrated in the time-lapse experiments, support the conclusion that C219 is recognizing a P-gp-like transporter in the sea star. Partial sequencing of the sea star P-gp and an analysis of the secondary structure for cysteine bridges is necessary to conclusively answer this question.
Evidence for MRP activity
Three lines of evidence strongly suggest that sea star eggs possess MRP transport activity. First, increases in c-am accumulation in the presence of the MRP-specific inhibitor, MK571, occurred in oocytes and eggs. Second, the MRP-specific antibody, T-24, showed that MRP protein was translocated to the egg surface when dye transport activity increased. Third, the T-24 antibody recognized several polypeptides on western blots, suggesting that at least two MRPs (or MRP isoforms) may be present.
Oocytes appeared to have very low MRP activity as compared to P-gp activity. The MRP-specific inhibitors, MK571 and BMMA, did not dramatically affect c-am accumulation in the oocyte. BMMA, an MRP1 inhibitor, had no effect on dye accumulation, indicating that MRP1 is not functional at that stage (Fig. 5). In the oocytes, the low intensity labeling of MRP-like transporters at the cell membrane by T-24 MRP antibody also confirms that MRP-mediated efflux is not a major contributor to dye efflux. This is consistent with the suggestion that P-gp is the primary transporter in the oocyte.
The MDR inhibitor, R205, inhibited c-am transport effectively. R205 is known to interact with P-gp transporters (Sharom et al. 1999) but its interactions with MRP are unknown. The data in this paper suggest that, at least in the sea star oocyte, R205 does competitively inhibit MRP transporters, yet is a superior substrate for P-gp transporters as compared to MRP. Data using these specific inhibitors should be carefully interpreted because there is an inherent risk in using dyes and inhibitors termed `transporter-specific', based on mammalian cell studies, in experiments with invertebrate systems. We do not assume that the use of specific dyes and inhibitors determines with absolute certainty the presence and function of specific MDR transporters. However, a combined approach with inhibitors, dyes and antibodies collectively suggests their presence and functional activities.
Changes in transport activity following oocyte maturation
The relative amount of c-am efflux activity increased two-fold 3 h after the addition of 1-MA. Increases in c-am accumulation by PSC833 and rhodamine B indicate that P-gp transporters are functioning in the eggs. The greater relative inhibition of dye efflux by PSC833 in the eggs compared to oocytes suggests an increase in the amount of P-gp-like transporters. As described above, an increase in P-gp-mediated efflux activity is supported by an increase in band intensity of the C219-labeled protein and the increase in labeling of the protein at the cell surface 3–5 hpm. Increases in c-am accumulation due to MK571 indicate that MRP-like transporters are functioning, together with P-gp transporters, in the eggs. The change in efficacy of BMMA in eggs versus oocytes further substantiates an increase in the MRP-like transporter population. It is possible that different MRPs (MRP2–9) could be involved in the increase in transport activity at maturation because MK571 is not specific for specific MRP transporters.
The completion of maturation in the sea star coincides with the increase in active efflux of the dye and correlates with the translocation of both the P-gp and MRP-like transporters. MDR transporters are likely stored in cytoplasmic vesicles or the endoplasmic reticulum, which is altered during the reorganization of the oocyte during maturation (Jaffe & Terasaki 1994), and transported during cytoplasmic restructuring by microtubules and microfilaments. This is consistent with reported translocation of oocyte cytoplasmic constituents during oocyte maturation (Santella et al. 1999). The storage of MDR transporters in the cytoplasm and the subsequent translocation to a functional state in the cell membrane has not been reported in an immature oocyte, although there is functional evidence of storage in the mature sea urchin egg (Hamdoun et al. 2004). It is suspected, although not determined, that the transporters in the oocyte cytoplasm are not functional until they reach the plasma membrane.
Storage and translocation of transporter proteins in the cytoplasm of various cell types has been previously reported (reviewed in Santamarina-Fojo et al. 2001; Bryant et al. 2002). Translocation is involved in the upregulation of MRP1 in bilirubin removal from neurons (Gennuso et al. 2004) and in microvillar natural defense function (reviewed in Lange & Gartzke 2001). The proposed translocation of sea star MDR transporter during maturation is similar to the storage and translocation of cortical granules duration maturation (Santella et al. 1999) or the activation of amino acid transporters during fertilization in the sea urchin (Epel 1972). The hormone-induced translocation of stored MDR transporter proteins in response to 1-MA is analogous to the insulin-induced translocation of the GLUT4 transporter in human cells (Watson & Pessin 2001).
Data from this study indicate that translation of new transporter proteins is partially responsible for the increase in dye efflux during oocyte maturation. The role of translation is evident by the negative effect of translational inhibitors, cycloheximide and emetine, on protein band intensity as well as fluorescence intensity (Figs 2, 7 and 8). Increases in immunoblot band intensity do not occur until 2–4 h after the initial transport of the existing P-gp transporter to the periphery. Nascent transporters are produced after maturation is complete, thus increasing the basal level of C219-reactive protein.
The role of MDR transporters in the immature sea star oocyte and their subsequent upregulation is as yet undetermined. Transporter activities appear unnecessary for oocyte maturation to occur even though MDR transporters are expressed in oocytes while in the female sea star ovary. Furthermore, oocyte maturation is not activated or inhibited by the inhibition of MDR transporters. Because the internal body cavity of sea stars is known to equilibrate with the external environment (Prusch & Whoriskey 1976), potentially allowing for the exposure of oocytes to toxicants, the transporters may function in a secondary protective role in the oocyte in ovario by exporting endogenous or exogenous compounds. The secondary protective role hypothesis is supported by data demonstrating an increase in the inhibition of GVBD by vinblastine when oocytes are co-incubated with a specific MDR inhibitor.
The change in MDR transport activity during sea star oocyte maturation has several major similarities and differences with the change in MDR transport activity during sea urchin fertilization (Hamdoun et al. 2004). In both systems, there is a dramatic increase in MDR transport activity in the final state versus the initial (egg > oocyte; two-cell embryo > spawned egg), coinciding with the process of cytoplasmic reorganization. Both systems utilize stored transporter proteins that are eventually translocated to the periphery and involve increases in MRP-mediated transport activity during the transition. Finally, in both systems the transport activity is responsible for protection against drugs that inhibit cell division and maturation (Hamdoun et al. 2004; present study).
A major difference in transport activity between sea star oocyte maturation and sea urchin fertilization is the role of translation in sea star oocyte maturation and the apparent lack thereof at sea urchin fertilization. In sea star oocytes, translation plays a significant role in the increase of MDR activity after maturation, while in the sea urchin egg, the upregulation of MDR efflux is mediated post-translationally after fertilization. Another major difference between these two echinoderms is the type of predominant transporter. In sea urchin eggs, MRP is the major transporter whereas in sea star immature oocytes, P-gp predominates. The difference in transporter population between these echinoderm species may be a reflection of dietary and benthic habitat differences.
In summary, MDR transport is functional in immature oocytes from the ovaries of female sea stars. A P-gp-like transporter appears to be the dominant MDR transporter in the immature oocyte and may be fully functional in ovario. At maturation, both P-gp-like and MRP-like transporters are expressed and transported to the plasma membrane within hours of maturation. Mature eggs are usually spawned within hours of maturation and fertilized within 2–3 h of maturation in the external environment. The post-maturation increase described here may serve to prime the egg for external fertilization. The increase in MDR activity at 5 hpm may not be relevant to natural conditions and is possibly an artifact of delayed fertilization. The increase in MRP-mediated transport may be only relevant after fertilization and during early development both by transporting endogenous compounds for controlling cell regulation and as a secondary role of protection in an adverse aqueous environment (Hamdoun et al. 2004). In the sea star, the purpose of MDR transport in the immature oocytes and mature eggs is not completely known, but they may function in the defense against toxic xenobiotic compounds as well as play an endogenous role in maturation itself.
Acknowledgements
This publication was supported by the National Sea Grant College Program of the US Department of Commerce's National Oceanic and Atmospheric Administration under NOAA Grant number NA06-RG0142, Project R/CZ-168 through the California Sea Grant College Program. The views expressed herein do not necessarily reflect the views of any of those organizations. T.A. Roepke was a Sea Grant Trainee and was supported by UC Toxic Substance Research & Teaching Program: Coastal Toxicology Component and by a NIEHS Pre-doctoral Traineeship, Grant number Arial5 T32 ES07059. A.M. Hamdoun was supported by NIH NRSA Fellowship number 1-F32-HD-47136 and NICHD Training Grant (AH) T32 HD071131. We would like to thank Nature McGinn for assistance with dye accumulation experiments and for critical review of the manuscript. We also wish to thank the BML Animal Resource Group for collection and maintenance of the sea stars.
Footnotes
Bodega Marine Laboratory Contribution number 2357.
References
- Atkinson DE, Greenwood SL, Sibley CP, Glazier JD, Fairbairn LJ. Role of MDR1 and MRP1 in trophob-last cells, elucidated using retroviral gene transfer. Am. J. Physiol. Cell Physiol. 2003;285:C584–C591. doi: 10.1152/ajpcell.00418.2002. [DOI] [PubMed] [Google Scholar]
- Bonfanti P, Colombo A, Camatini M. Identification of a multixenobiotic resistance mechanism in Xenopus laevis embryos. Chemosphere. 1998;37:2751–2760. doi: 10.1016/s0045-6535(98)00318-x. [DOI] [PubMed] [Google Scholar]
- Borst P, Evers R, Kool M, Wihnholds J. A family of drug transporters: The multidrug resistance-associated proteins. J. Natl Cancer Inst. 2000;92:1295–1302. doi: 10.1093/jnci/92.16.1295. [DOI] [PubMed] [Google Scholar]
- Broeks A, Gerrard B, Allikmets R, Dean M, Plasterk R. Homologues of the human multidrug resistance genes MRP and MDR contribute to heavy metal resistance in the soil nematode Caenohabditis elegans. EMBO J. 1996;15:6132–6143. [PMC free article] [PubMed] [Google Scholar]
- Bryant NJ, Govers R, James DE. Regulated transport of the glucose transporter glut4. Nat. Rev. 2002;3:267–277. doi: 10.1038/nrm782. [DOI] [PubMed] [Google Scholar]
- Dean M, Rzhetsky A, Allikmets R. The human ATP-binding cassette (ABC) transporter superfamily. Genome Res. 2001;11:1156–1166. doi: 10.1101/gr.184901. [DOI] [PubMed] [Google Scholar]
- Doyle LA, Ross DD. Multidrug resistance mediated by the breast cancer resistance protein BCRP (ABCG2) Oncogene. 2003;22:7340–7358. doi: 10.1038/sj.onc.1206938. [DOI] [PubMed] [Google Scholar]
- Elbling L, Berger W, Rehberger A, Waldhor T, Micksche M. P-glycoprotein regulates chemosensitivity in early developmental stages of the mouse. FASEB J. 1993;7:1499–1506. doi: 10.1096/fasebj.7.15.7903262. [DOI] [PubMed] [Google Scholar]
- Epel D. Activation of an NA+-dependent amino acid transport system upon fertilization of sea urchin eggs. Exp. Cell Res. 1972;72:74–89. doi: 10.1016/0014-4827(72)90569-1. [DOI] [PubMed] [Google Scholar]
- Epel D. Use of multidrug transporters as first lines of defense against toxins in aquatic organisms. Comp. Biochem. Physiol. 1998;120:23–28. [Google Scholar]
- Essodaigui M, Broxterman HJ, Garnier-Suillerot A. Kinetic analysis of calcein and calcein-acetoxymethylester efflux mediated by the multidrug resistance protein and P-glycoprotein. Biochemistry. 1998;37:2243–2250. doi: 10.1021/bi9718043. [DOI] [PubMed] [Google Scholar]
- Friml J, Vieten A, Sauer M, et al. Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature. 2003;426:147–53. doi: 10.1038/nature02085. [DOI] [PubMed] [Google Scholar]
- Gaedeke N, Klein M, Kolukisaoglu U, et al. The Arabidopsis thaliana ABC transporter AtMRP5 controls root development and stomata movement. EMBO J. 2001;20:1875–1887. doi: 10.1093/emboj/20.8.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennuso F, Fernettie C, Tirolo C, et al. Bilirubin protects astrocytes from its own toxicity by inducing up-regulation and translocation of multidrug resistance-associated protein 1 (Mrp1) Proc. Natl Acad. Sci. 2004;101:2470–2475. doi: 10.1073/pnas.0308452100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges E, Bradley G, Gariepy J, Ling V. Detection of P-glycoprotein isoforms by gene specific monoclonal antibodies. Proc. Natl Acad. Sci. 1990;87:152–156. doi: 10.1073/pnas.87.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good JR, Kuspa A. Evidence that a cell-type-specific efflux pump regulated cell differentiation in Dictyostelium. Dev. Biol. 2000;220:53–61. doi: 10.1006/dbio.2000.9611. [DOI] [PubMed] [Google Scholar]
- Hamdoun AM, Cherr GN, Roepke TA, Epel D. Activation of multidrug efflux transporter activity at fertilization in sea urchin embryos (Strongylocentrotus purpurpatus) Dev. Biol. 2004;276:452–462. doi: 10.1016/j.ydbio.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Hamdoun AM, Griffin FJ, Cherr GN. Tolerance to biodegraded crude oil in marine invertebrate embryos and larvae is associated with expression of a multixenobiotic resistance transporter. Aquat. Toxicol. 2002;61:127–140. doi: 10.1016/s0166-445x(02)00050-4. [DOI] [PubMed] [Google Scholar]
- Heil-Chapdelaine RA, Otto JJ. Characterization of changes in F-actin during maturation of starfish oocytes. Dev. Biol. 1996;177:204–216. doi: 10.1006/dbio.1996.0156. [DOI] [PubMed] [Google Scholar]
- Hemmer MJ, Courtney LA, Ortego LS. Immunohistochemical detection of P-glycoprotein in teleost tissues using mammalian polyclonal and monoclonal antibodies. J. Exp. Zool. 1995;272(1):69–77. doi: 10.1002/jez.1402720109. [DOI] [PubMed] [Google Scholar]
- Jaffe LA, Terasaki M. Structural changes of the endoplasmic reticulum of sea urchin eggs during fertilization. Dev. Biol. 1993;156:566–573. doi: 10.1006/dbio.1993.1103. [DOI] [PubMed] [Google Scholar]
- Jaffe LA, Terasaki M. Structural changes in the endoplasmic reticulum of starfish oocytes during meiotic maturation and fertilization. Dev. Biol. 1994;164:579–587. doi: 10.1006/dbio.1994.1225. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. Cell cycle arrest and release in starfish oocytes and eggs. Semin. Cell Dev. Biol. 1998;9:549–557. doi: 10.1006/scdb.1998.0249. [DOI] [PubMed] [Google Scholar]
- Klein M, Perfus-Barbeoch L, Frelet A, et al. The plant multidrug resistance ABC transporter AtMRP5 is involved in guard cell hormonal signalling and water use. Plant J. 2003;33(1):119–129. doi: 10.1046/j.1365-313x.2003.016012.x. [DOI] [PubMed] [Google Scholar]
- Krishna R, Mayer LD. Multidrug resistance (MDR) in cancer mechanisms, reversal using modulators of MDR and the role of MDR modulators in influencing the pharmacokinetics of anticancer drugs. Pharm. Sci. 2000;11:265–283. doi: 10.1016/s0928-0987(00)00114-7. [DOI] [PubMed] [Google Scholar]
- Kruh GD, Belinsky MG. The MRP family of drug efflux pumps. Oncogene. 2003;22:7537–7552. doi: 10.1038/sj.onc.1206953. [DOI] [PubMed] [Google Scholar]
- Kurelec B. The multixenobiotic resistance mechanism in aquatic organisms. Crit. Rev. Toxicol. 1992;22:23–43. doi: 10.3109/10408449209145320. [DOI] [PubMed] [Google Scholar]
- Kurelec B. A new type of hazardous chemical: the chemosensitizers of multixenobiotic resistance. Environ. Health Perspect. 1997;105(Suppl. 4):855–860. doi: 10.1289/ehp.97105s4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange K, Gartzke J. Microvillar cell surface as a natural defense system against xenobiotics: a new interpretation of multidrug resistance. Am. J. Physiol. Cell Physiol. 2001;281:C369–C385. doi: 10.1152/ajpcell.2001.281.2.C369. [DOI] [PubMed] [Google Scholar]
- Leslie EM, Deeley RG, Cole SPC. Toxicological relevance of the multidrug resistance protein 1, MRP1 (ABCC1) and related transporters. Toxicology. 2001;167:3–23. doi: 10.1016/s0300-483x(01)00454-1. [DOI] [PubMed] [Google Scholar]
- Litman T, Druley TE, Stein WD, Bates SE. From MDR to MXR: New understanding of multidrug resistance systems, their properties and clinical significance. Cell. Mol. Life Sci. 2001;58:931–959. doi: 10.1007/PL00000912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loe DW, Almquist KC, Deeley RG, Cole SP. Multidrug resistance protein (MRP)-mediated transport of leukotriene C4 and chemotherapeutic agents in membrane vesicles: Demonstration of glutathione-dependent vincristine transport. J. Biol. Chem. 1996;271:9675–9682. doi: 10.1074/jbc.271.16.9675. [DOI] [PubMed] [Google Scholar]
- Maguire AR, Plunkett SJ, Papot S, Clynes M, O'Connor R, Touhey S. Synthesis of indomethacin analogues for evaluation as modulators of MRP activity. Bioorg. Med. Chem. 2001;9:745–762. doi: 10.1016/s0968-0896(00)00292-3. [DOI] [PubMed] [Google Scholar]
- McFadzen I, Eufemia N, Heath C, Epel D, Moore M, Lowe D. Multidrug resistance in the embryos and larvae of the mussel Mytilus edulis. Mar. Environ. Res. 2000;50:319–323. doi: 10.1016/s0141-1136(00)00057-x. [DOI] [PubMed] [Google Scholar]
- Meijer L, Guerrier P. Maturation and fertilization in starfish oocytes. Int. Rev. Cytol. 1984;86:129–196. doi: 10.1016/s0074-7696(08)60179-5. [DOI] [PubMed] [Google Scholar]
- Meijer L, Mordret G. Starfish oocyte maturation: from prophase to metaphase. Semin. Dev. Biol. 1994;5:165–171. [Google Scholar]
- Minier C, Akcha F, Galgani F. P-glycoprotein expression in Crassostrea gigas and Mytilus edulis in polluted seawater. Comp. Biochem. Physiol. Part A. 1993;106B:1029–1036. doi: 10.1016/0305-0491(93)90068-g. [DOI] [PubMed] [Google Scholar]
- Minier C, Lelong C, Djemel N, et al. Expression and activity of a multixenobiotic resistance system in the Pacific oyster Crassostrea gigas. Mar. Environ. Res. 2002;54:455–459. doi: 10.1016/s0141-1136(02)00195-2. [DOI] [PubMed] [Google Scholar]
- Noh B, Murphy AS, Spalding EP. Multidrug resistance-like genes of Arabidopsis required for auxin transport and auxin-mediated development. Plant Cell. 2001;13:2441–2454. doi: 10.1105/tpc.010350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ookata K, Hisanaga S, Okumura E, Kishimoto T. Association of p34cdc2/cyclin B complex with microtubules in starfish oocytes. J. Cell Sci. 1993;105(Pt 4):873–881. doi: 10.1242/jcs.105.4.873. [DOI] [PubMed] [Google Scholar]
- Prusch RD, Whoriskey F. Maintenance of fluid volume in the starfish water vascular system. Nature. 1976;262:577–578. doi: 10.1038/262577a0. [DOI] [PubMed] [Google Scholar]
- Santamarina-Fojo S, Remaley AT, Neufeld EB, Brewer HB., Jr. Regulation and intracellular trafficking of the ABCA1 transporter. J. Lipid Res. 2001;42:1339–1345. [PubMed] [Google Scholar]
- Santella L, De Riso L, Gragnaniello G, Kyozuka K. Cortical granule translocation during maturation of starfish oocytes requires cytoskeletal rearrangement triggered by Ins-P3-mediated Ca2+ release. Exp. Cell Res. 1999;248:567–574. doi: 10.1006/excr.1999.4425. [DOI] [PubMed] [Google Scholar]
- Sarkadi B, Muller M, Homolya L, et al. Interaction of bioactive hydrophobic peptides with the human multidrug transporter. FASEB J. 1994;8:766–770. doi: 10.1096/fasebj.8.10.7914178. [DOI] [PubMed] [Google Scholar]
- Sharom FJ, Yu X, Lu P, et al. Interaction of the P-glycoprotein multidrug transporter (MDR1) with high affinity peptide chemosensitizers in isolated membranes, reconstituted systems and intact cells. Chem. Pharmacol. 1999;58:571–586. doi: 10.1016/s0006-2952(99)00139-2. [DOI] [PubMed] [Google Scholar]
- Smital T, Kurelec B. The chemosensitizers of multixenobiotic resistance mechanism in aquatic invertebrates: a new class of pollutants. Mutat. Res. 1998;399:43–53. doi: 10.1016/s0027-5107(97)00265-0. [DOI] [PubMed] [Google Scholar]
- Smital T, Luckenbach T, Sauerborn R, Hamdoun AM, Vega RL, Epel D. Emerging contaminants-pesticides, PPCPs, microbial degradation products and natural substances as inhibitors of multixenobiotic defense in aquatic organisms. Mutat. Res. 2004;552:101–117. doi: 10.1016/j.mrfmmm.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Takebayashi Y, Nakayama K, Fujioka T, et al. Expression of multidrug resistance associated transporters (MDR1, MRP1, LRP and BCRP) in porcine oocytes. Int. J. Mol. Med. 2001;7:397–400. doi: 10.3892/ijmm.7.4.397. [DOI] [PubMed] [Google Scholar]
- Terasaki M. Redistribution of cytoplasmic components during germinal vesicle breakdown in starfish oocytes. J. Cell Sci. 1994;107:1797–1805. doi: 10.1242/jcs.107.7.1797. [DOI] [PubMed] [Google Scholar]
- Toomey BH, Epel D. Multixenobiotic resistance in Urechis caupo embryos: protection from environmental toxins. Biol. Bull. 1993;185:355–364. doi: 10.2307/1542476. [DOI] [PubMed] [Google Scholar]
- Tutundjian R, Cachot J, Leboulenger F, Minier C. Genetic and immunological characterisation of a multixeno-biotic resistance system in the turbot (Scophthalmus maximus) Comp. Biochem. Physiol. Part B: Biochem. Mol. Biol. 2002;132B(2):463–471. doi: 10.1016/s1096-4959(02)00058-1. [DOI] [PubMed] [Google Scholar]
- Twentyman PR, Rhodes T, Rayner S. A comparison of rhodamine 123 accumulation and efflux in cells with P-glycoprotein-mediated and MRP-associated multidrug resistance phenotypes. Eur. J. Cancer. 1994;30:1360–13690. doi: 10.1016/0959-8049(94)90187-2. [DOI] [PubMed] [Google Scholar]
- Utoguchi N, Gurudatt AC, Avery M, Audus KL. Functional expression of P-glycoprotein in primary cultures of human cytotrophoblasts and BeWo cells. Reprod. Toxicol. 2000;14:217–224. doi: 10.1016/s0890-6238(00)00071-x. [DOI] [PubMed] [Google Scholar]
- Watson RT, Pessin JE. Subcellular compartmentalization and trafficking of the insulin-responsive glucose transporter, GLUT4. Exp. Cell Res. 2001;271:75–83. doi: 10.1006/excr.2001.5375. [DOI] [PubMed] [Google Scholar]
- Yabe Y, Suzuki N, Furukawa T, Ishihara T, Katsura I. Multidrug resistance-associated protein MRP-1 regulates dauer diapause by its export activity in Caenorhabditis elegans. Development. 2005;132:3197–3207. doi: 10.1242/dev.01909. [DOI] [PubMed] [Google Scholar]
- Functional expression of P-glycoprotein in primary cultures of human cytotrophoblasts and BeWo cells. Reprod. Toxicol. 14:217–224. doi: 10.1016/s0890-6238(00)00071-x. [DOI] [PubMed] [Google Scholar]
- Watson RT, Pessin JE. Subcellular compartmentalization and trafficking of the insulin-responsive glucose transporter, GLUT4. Exp. Cell Res. 2001;271:75–83. doi: 10.1006/excr.2001.5375. [DOI] [PubMed] [Google Scholar]
- Yabe Y, Suzuki N, Furukawa T, Ishihara T, Katsura I. Multidrug resistance-associated protein MRP-1 regulates dauer diapause by its export activity in Caenorhabditis elegans. Development. 2005;132:3197–3207. doi: 10.1242/dev.01909. [DOI] [PubMed] [Google Scholar]


