Abstract
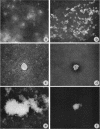
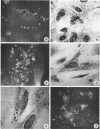
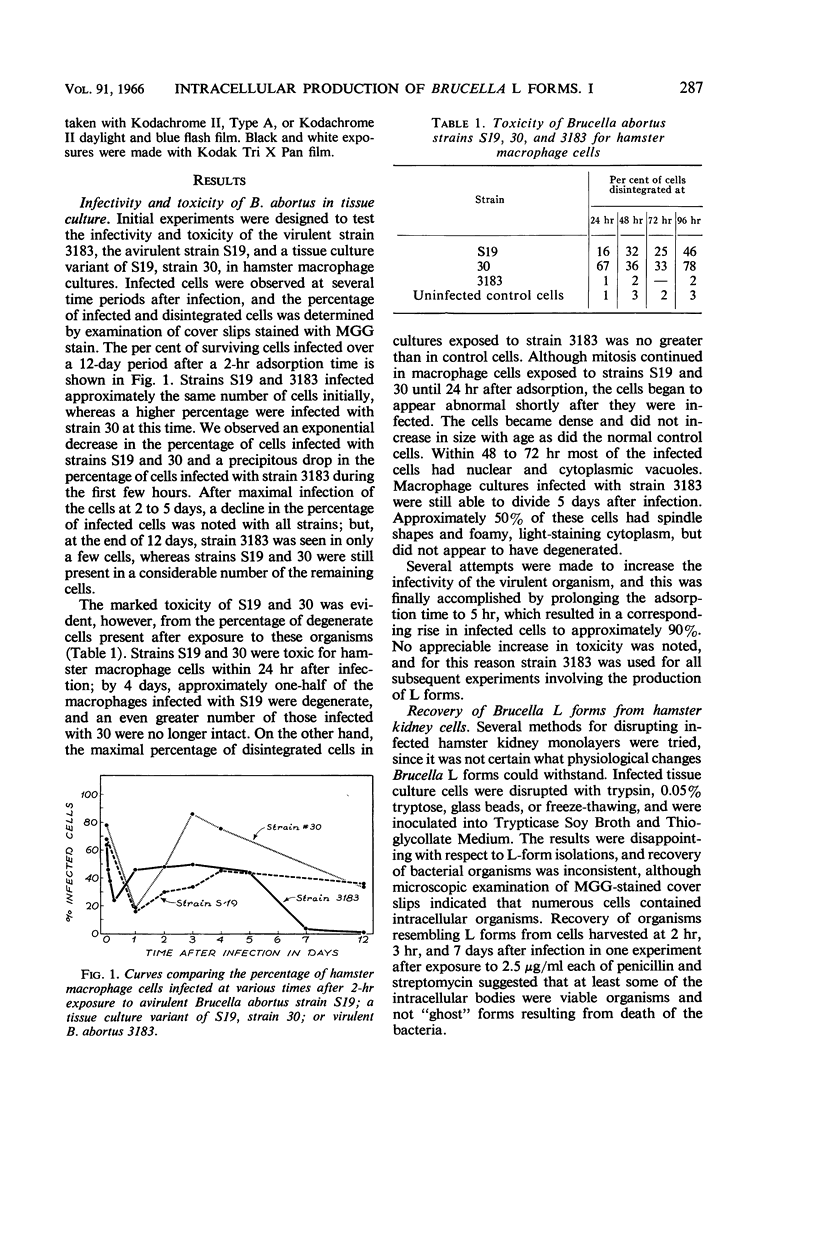
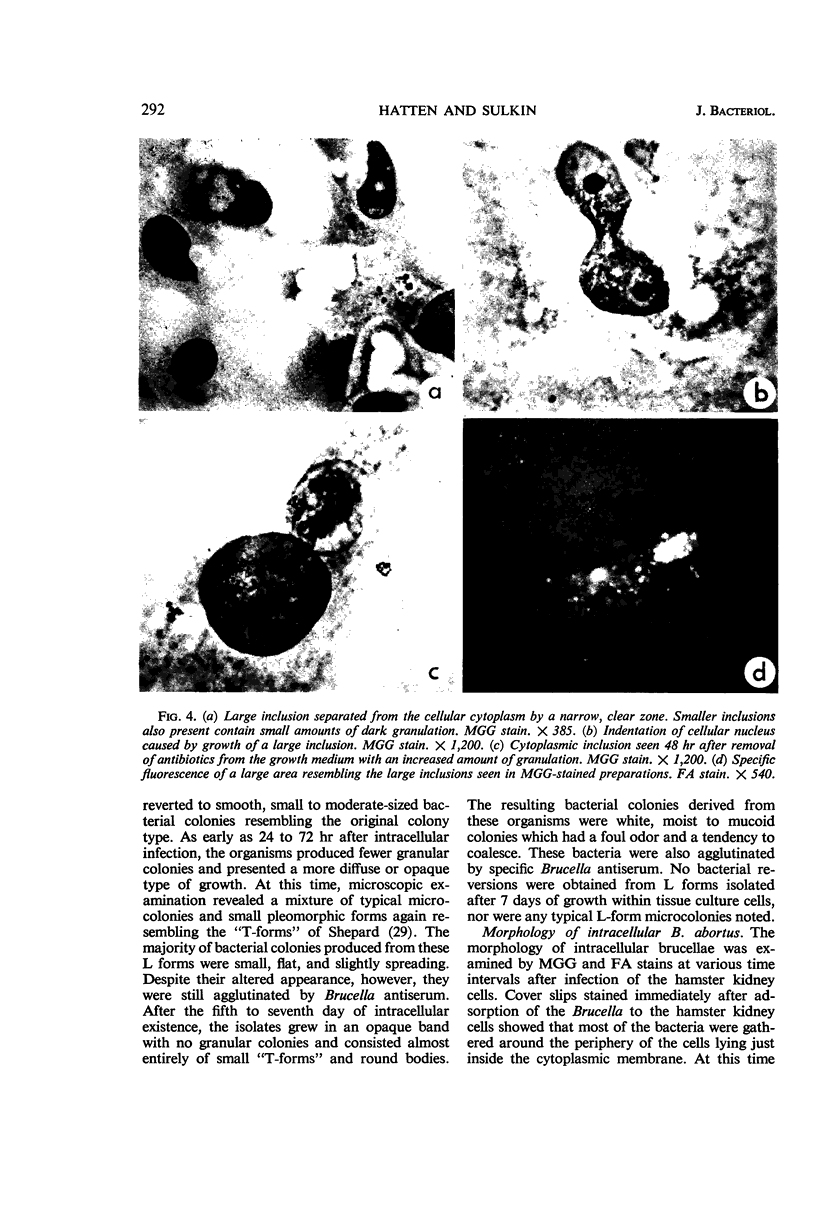
Hatten, Betty A. (The University of Texas Southwestern Medical School, Dallas), and S. Edward Sulkin. Intracellular production of Brucella L forms. I. Recovery of L forms from tissue culture cells infected with Brucella abortus. J. Bacteriol. 91:285–296. 1966.—Infectivity of virulent Brucella abortus strain 3183 was less for hamster macrophages after a 2-hr adsorption period than for an attenuated strain (S19) and its tissue culture variant (30). Both strains S19 and 30 were very toxic for the cells, but 3183 was not toxic. Two types of L forms were recovered from a large percentage of hamster kidney cell cultures when disintegration of infected cells was accelerated by tissue culture medium of high pH. One type grew in finely granular microcolonies, was isolated from cells infected for short periods of time, and often reverted to the bacterial form. The other type occurred in small irregularly shaped forms which later developed into round bodies. Both stained specifically with fluorescein-conjugated B. abortus antiserum. Semisolid media containing 0.7% agar provided optimal subsurface L-form growth. L forms also grew well in Thioglycollate Medium but grew poorly in other liquid media. Surface L-form growth was supported by several agar media, but CO2 was required for optimal growth. Monolayers infected with strain 3183 and examined immediately after adsorption contained occasional small, round bodies. Bizarre forms increased in number with time and, after 24 to 72 hr, large pink-staining inclusions were often present which persisted for several days. Also appearing at about the same time were smaller, dark-staining forms which were first seen in clusters but later dispersed and finally occurred in chainlike configurations. Direct fluorescent-antibody stains of infected cells established that the intracellular forms were related to the infecting strain of B. abortus.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARILE M. F., MALIZIA W. F., RIGGS D. B. Incidence and detection of pleuropneumonia-like organisms in cell cultures by fluorescent antibody and cultural procedures. J Bacteriol. 1962 Jul;84:130–136. doi: 10.1128/jb.84.1.130-136.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAUN W., POMALES-LEBRON A., STINEBRING W. R. Interactions between mononuclear phagocytes and Brucella abortus strains of different virulence. Proc Soc Exp Biol Med. 1958 Feb;97(2):393–397. doi: 10.3181/00379727-97-23752. [DOI] [PubMed] [Google Scholar]
- CARRERE L., ROUX J. Obtention de formes L de Brucella melitensis. Ann Inst Pasteur (Paris) 1953 Apr;84(4):796–798. [PubMed] [Google Scholar]
- CLARK H. F., SHEPARD C. C. A DIALYSIS TECHNIQUE FOR PREPARING FLUORESCENT ANTIBODY. Virology. 1963 Aug;20:642–644. doi: 10.1016/0042-6822(63)90292-7. [DOI] [PubMed] [Google Scholar]
- DIENES L., BANDUR B. M., MADOFF S. DEVELOPMENT OF L-TYPE GROWTH IN NEISSERIA GONORRHOEAE CULTURES. J Bacteriol. 1964 Jun;87:1471–1476. doi: 10.1128/jb.87.6.1471-1476.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIENES L., WEINBERGER H. J. The L forms of bacteria. Bacteriol Rev. 1951 Dec;15(4):245–288. doi: 10.1128/br.15.4.245-288.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIERCKS F. H., HAMMON W. M. Hamster kidney cell tissue cultures for propagation of Japanese B encephalitis virus. Proc Soc Exp Biol Med. 1958 Mar;97(3):627–632. doi: 10.3181/00379727-97-23827. [DOI] [PubMed] [Google Scholar]
- FARID Z., MIALE A., Jr, OMAR M. S., VAN PEENEN P. F. Antibiotic treatment of acute brucellosis caused by Brucella melitensis. J Trop Med Hyg. 1961 Jul;64:157–163. [PubMed] [Google Scholar]
- FREEMAN B. A., KROSS D. J., CIRCO R. Host-parasite relationships in brucellosis. II. Destruction of macrophage cultures by Brucella of different virulence. J. J Infect Dis. 1961 May-Jun;108:333–338. doi: 10.1093/infdis/108.3.333. [DOI] [PubMed] [Google Scholar]
- GOODLOW R. J., MIKA L. A., BRAUN W. The effect of metabolites upon growth and variation of Brucella abortus. J Bacteriol. 1950 Sep;60(3):291–300. doi: 10.1128/jb.60.3.291-300.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLAND J. J., PICKETT M. J. Intracellular behavior of Brucella variants in chick embryo cells in tissue culture. Proc Soc Exp Biol Med. 1956 Dec;93(3):476–479. doi: 10.3181/00379727-93-22792. [DOI] [PubMed] [Google Scholar]
- KAGAN B. M., MOLANDER C. W., WEINBERGER H. J. Induction and cultivation of staphylococcal L forms in the presence of methicillin. J Bacteriol. 1962 May;83:1162–1163. doi: 10.1128/jb.83.5.1162-1163.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLIENEBERGER-NOBEL E. Filterable forms of bacteria. Bacteriol Rev. 1951 Jun;15(2):77–103. doi: 10.1128/br.15.2.77-103.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAVILLAUREIX J. Action de formes L pathogènes sur des cultures de cellules cancéreuses. C R Hebd Seances Acad Sci. 1957 Feb 18;244(8):1098–1101. [PubMed] [Google Scholar]
- MADOFF S., DIENES L. L forms from pneumococci. J Bacteriol. 1958 Sep;76(3):245–250. doi: 10.1128/jb.76.3.245-250.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDERMOTT W. Microbial persistence. Yale J Biol Med. 1958 Feb;30(4):257–291. [PMC free article] [PubMed] [Google Scholar]
- NELSON E. L., PICKETT M. J. The recovery of L forms of Brucella and their relation to Brucella phage. J Infect Dis. 1951 Nov-Dec;89(3):226–232. doi: 10.1093/infdis/89.3.226. [DOI] [PubMed] [Google Scholar]
- RAZIN S., ARGAMAN M. Lysis of Mycoplasma, bacterial protoplasts, spheroplasts and L-forms by various agents. J Gen Microbiol. 1963 Jan;30:155–172. doi: 10.1099/00221287-30-1-155. [DOI] [PubMed] [Google Scholar]
- RENOUX G., SUIRE A. SPONTANEOUS LYSIS AND PHAGE-CARRIER STATE IN BRUCELLA CULTURES. J Bacteriol. 1963 Oct;86:642–647. doi: 10.1128/jb.86.4.642-647.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUBIO-HUERTOS M., GONZALEZ-VAZQUEZ C. Morphology and pathogenicity of L forms of Clostridium tetani induced by glycine. Ann N Y Acad Sci. 1960 Jan 15;79:626–631. doi: 10.1111/j.1749-6632.1960.tb42732.x. [DOI] [PubMed] [Google Scholar]
- SANDERS E., HUDDLESON I. F. The influence of environmental conditions on the growth and dissociation of Brucella abortus. Am J Vet Res. 1956 Apr;17(63):324–330. [PubMed] [Google Scholar]
- SCHEIBEL I., ASSANDRI J. Isolation of toxigenic L-phase variants from Cl. tetani. Acta Pathol Microbiol Scand. 1959;46:333–338. doi: 10.1111/j.1699-0463.1959.tb01103.x. [DOI] [PubMed] [Google Scholar]
- SHAFFER J. M., KUCERA C. J., SPINK W. W. The protection of intracellular brucella against therapeutic agents and the bactericidal action of serum. J Exp Med. 1953 Jan;97(1):77–90. doi: 10.1084/jem.97.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEPARD M. C. Recovery, propagation, and characteristics of T-strain PPLO isolated from human cases of nongonococcal urethritis. Ann N Y Acad Sci. 1960 Jan 15;79:397–402. doi: 10.1111/j.1749-6632.1960.tb42704.x. [DOI] [PubMed] [Google Scholar]
- SMADEL J. E. Intracellular infections. Bull N Y Acad Med. 1963 Mar;39:158–172. [PMC free article] [PubMed] [Google Scholar]
- SPINK W. W. Current status of therapy of brucellosis in human beings. J Am Med Assoc. 1960 Feb 13;172:697–698. doi: 10.1001/jama.1960.63020070004016. [DOI] [PubMed] [Google Scholar]
- STINEBRING W. R. Characteristics of intracellularly grown Brucella abortus. J Infect Dis. 1962 Jul-Aug;111:17–24. doi: 10.1093/infdis/111.1.17. [DOI] [PubMed] [Google Scholar]
- STINEBRING W. R., KESSEL R. Continuous growth of Brucella abortus in mononuclear phagocytes of rats and guinea pigs. Proc Soc Exp Biol Med. 1959 Jul;101(3):412–415. doi: 10.3181/00379727-101-24962. [DOI] [PubMed] [Google Scholar]
- TULASNE R., LAVILLAUREIX J. Pouvoir pathogène expérimental, pour la souris, d'une souche de formes L des bactéries. C R Seances Soc Biol Fil. 1954 Dec;148(23-24):2080–2082. [PubMed] [Google Scholar]