Abstract
A potential treatment for paralysis resulting from spinal cord injury is to route control signals from the brain around the injury via artificial connections. Such signals could then control electrical stimulation of muscles, thereby restoring volitional movement to paralyzed limbs1–3. In previously separate experiments, activity of motor cortex neurons related to actual or imagined movements has been used to control computer cursors and robotic arms4–10, and paralyzed muscles have been activated by functional electrical stimulation (FES)11–13. Here we show that monkeys can directly control stimulation of muscles using the activity of neurons in motor cortex, thereby restoring goal-directed movements to a transiently paralyzed arm. Moreover, neurons could control functional stimulation equally well regardless of any prior association to movement, a finding that significantly expands the source of control signals for brain-machine interfaces. Monkeys learned to utilize these artificial connections from cortical cells to muscles to generate bidirectional wrist torques, and controlled multiple neuron-muscle pairs simultaneously. Such direct transforms from cortical activity to muscle stimulation could be implemented by autonomous electronic circuitry, creating a relatively natural neuroprosthesis. These results are the first demonstration that direct artificial connections between cortical cells and muscles can compensate for interrupted physiological pathways and restore volitional control of movement to paralyzed limbs.
Spinal cord injury impairs neural pathways between the brain and limbs, but spares both the motor cortex and muscles. Recent studies have shown that quadriplegic patients could volitionally modulate activity of neurons in hand area of motor cortex, even several years after paralysis6, and that monkeys could use cortical activity to control a robotic arm to acquire targets4 and feed themselves5. These and other brain-machine interface studies used sophisticated algorithms to decode task-related activity of neural populations and calculate requisite control parameters for external devices4–6,8–10. An alternate strategy to restore limb function is to directly connect cortical cell activity to control stimulation of a patient’s paralyzed muscles (Figure 1A). Here we show that monkeys can learn to use direct artificial connections from arbitrary motor cortex cells to grade stimulation delivered to multiple muscles and restore goal-directed movement to a paralyzed arm.
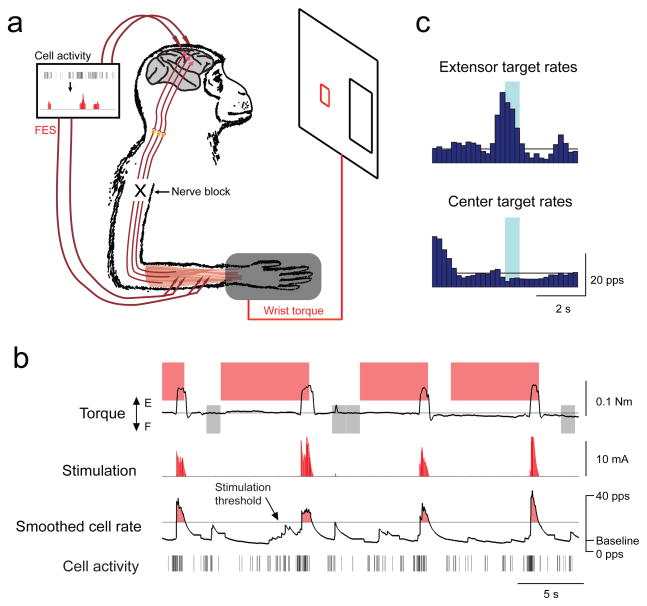
Figure 1. Brain-controlled functional electrical stimulation (FES) of muscle.
(A) Schematic shows cortical cell activity converted to FES during peripheral nerve block. (B) Example of motor cortex cell activity controlling FES of paralyzed wrist extensors. Extensor (red shading) and center (grey shading) wrist torque targets were randomly presented. Monkeys learned to modulate smoothed cell rate to control proportional muscle stimulation. FES was delivered to muscles EDC & ED4,5 at 50/s, with current proportional to cell rate above a stimulation threshold (0.4 mA/pps × [cell rate – 16 pps]; ≤ 10 mA). (C) Histograms of cell rates while acquiring the extensor and center targets, illustrating cell activity used to successfully control FES. Shading indicates target hold period and horizontal line denotes baseline cell rate.
In previous biofeedback studies monkeys rapidly learned to control the discharge rates of newly isolated neurons in motor cortex to obtain rewards14,15. We used a similar operant conditioning paradigm for single neurons in hand and wrist area of motor cortex of two monkeys (see methods and supplementary information). We tested volitional control of cell activity by displaying smoothed discharge rate as cursor position on a monitor and rewarding the monkeys for maintaining activity within randomly presented high- or low-rate targets. The directional tuning of most cells was also characterized in an isometric 2-dimensional wrist tracking task. However, our experiment employed all sufficiently well-isolated cells encountered, with no selection bias for possible association to movement or directional tuning.
Monkeys demonstrated volitional control of the discharge rates of nearly all cells tested within the first 10-minute practice session. Although cell activity controlled the cursor directly, monkeys often continued to produce wrist torques during these initial sessions (Figure S1). We then blocked peripheral nerves innervating the wrist muscles with a local anesthetic (see methods). Despite loss of motor function and sensory feedback from the innervated forearm, monkeys continued to control the cursor with cell activity for 45 of 46 cells after the nerve block. Figure S1 shows the loss of flexor and extensor torques following injections of local anesthetic, while the monkey continued to volitionally control the cell activity. The nerve block was confirmed by the monkey’s inability to perform the 2-dimensional torque tracking task.
We then converted cell activity into proportional stimuli delivered to paralyzed muscles. The cursor was now controlled by wrist torque, and the monkey was rewarded for maintaining FES-evoked torque within peripheral and center (i.e., zero-torque) targets for 0.5 – 1.0 s. To allow the monkey to grade contraction force, stimulation current was made linearly proportional to cell rate when the cell discharged above a threshold.
The example in Figure 1B shows a monkey modulating cell activity to control FES and generate appropriate torques via paralyzed wrist extensor muscles. The monkey learned to increase cell activity to activate the stimulator and acquire the extensor targets, and to maintain activity below the stimulation threshold to relax the muscle and acquire the center targets. Both monkeys were able to control muscle FES during nerve block and acquire torque targets with 44 of the 45 cells tested (5 cells from monkey I and 39 from monkey L).
For each cell the monkeys’ control improved with practice, as evidenced by more rapid acquisition of targets and fewer errors. Monkeys began using cell activity to control the stimulator almost immediately, and improved substantially during the relatively brief practice sessions with each cell (mean duration 66 min). To quantify improvement we compared performance during the initial two minutes of practice and during the two-minute period with the highest performance, typically just before task difficulty was increased to probe the limits of FES control. The rate of target acquisition with FES control was over three times greater during peak performance (14.1 ± 5.3 torque targets acquired /minute; mean ± SD) compared to the beginning of practice (4.0 ± 4.3 targets/min; p < 0.001; Figure S2). Peak target acquisition rates during brain-controlled FES were similar to those seen when cell activity controlled the cursor directly before nerve block (13.2 ± 5.5 targets/min; p = 0.66).
With continued practice monkeys also learned to control the torque more precisely with cell activity, making fewer target acquisition errors and more often acquiring targets on the first attempt. A target acquisition error was defined as triggering the stimulator to acquire the peripheral target when the center target was displayed. Monkeys made target errors on only 0.8 ± 5.1% of targets during peak performance for each cell compared to 20.7 ± 28.9% of targets at the beginning of practice (p < 0.001; Figure S3). They also made 81% fewer failed attempts to acquire the target during peak performance (0.10 ± 0.31 failed attempts per target) compared to the beginning of practice (0.52 ± 0.93; p <0.001).
To test whether FES could also be controlled by decreases in cell activity, we set stimulation current to be inversely proportional to cell rate below a threshold for 11 cells. Monkey L learned to control stimulation with this inverse relation just as well as with a positive relation between cell rate and stimulus current (38 cells, some tested in both groups; p > 0.46), acquiring 13.4 ± 3.9 targets per minute and making no errors during peak performance.
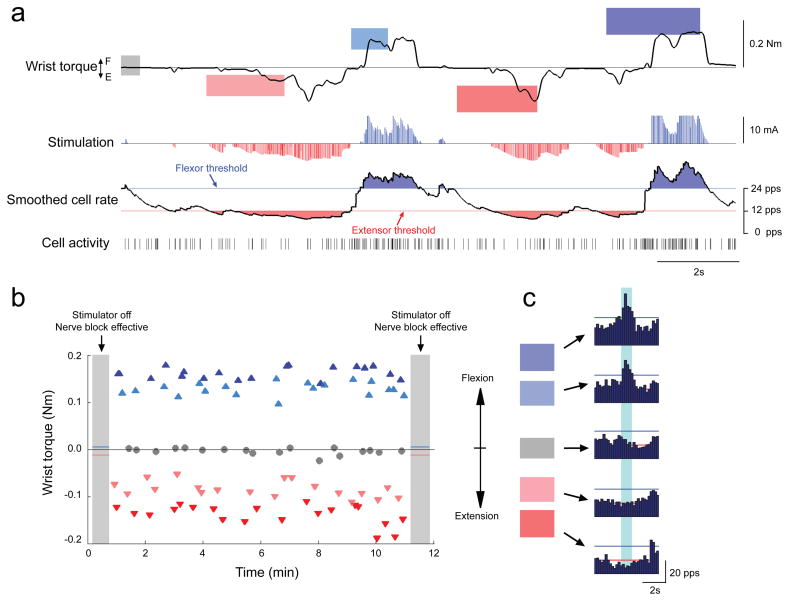
The activity of a single cell could also be used to control stimulation of antagonist muscle groups and restore bi-directional movements. Figure 2 shows an example of one cell that controlled stimulation of flexor muscles with high discharge rates and extensor muscles with low rates. The monkey learned to control cell activity and grade contraction force to rapidly satisfy targets at five different torque levels. The nerve blocks remained very effective, as evidenced by negligible torques produced in either direction when the stimulators were turned off during target presentation (Figure 2B). Seven cells tested with such bi-directional control performed similarly to cells that controlled only one muscle group, although target acquisition rates were marginally slower (9.8 ± 3.7 targets/min; p = 0.06).
Figure 2. Brain-controlled FES of multiple muscles restores graded torque in two directions.
(A) The monkey acquired targets at five levels of flexion-extension (F-E) torque using the activity of a single cell to grade FES delivered to both flexor (FCU) and extensor (ECU & ED4,5) muscles. Flexor FES was proportional to rate above a threshold (0.8 mA/pps × [cell rate – 24 pps]; ≤ 10 mA); extensor FES was inversely proportional to cell rate below a second threshold (0.6mA/pps × [12 pps – cell rate]; ≤ 10 mA). (B) Average torques produced to satisfy the five targets during 12 min of practice. With the stimulator off (shaded periods), the monkey could not produce torques greater than 10% of magnitudes used to acquire the targets (blue and red lines), confirming the efficacy of nerve block. (C) Histograms of cell rate used to acquire five target levels (colored boxes at left). Horizontal lines indicate FES thresholds for flexor (blue) and extensor (red) stimulation.
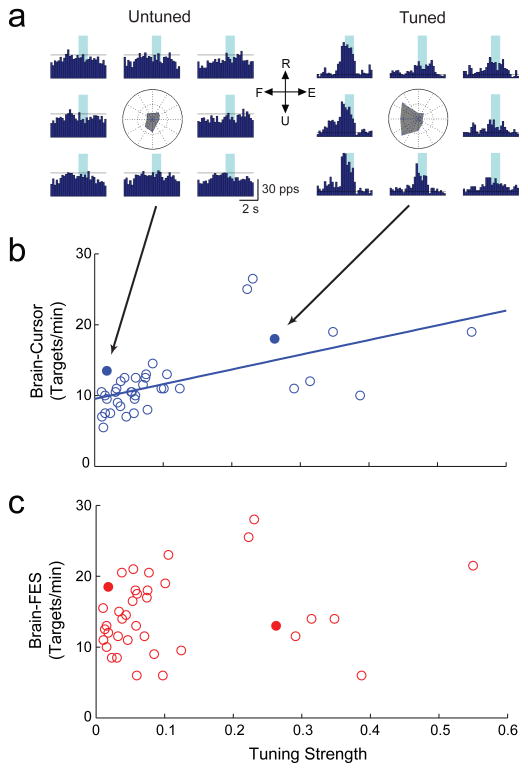
The assumptions underlying common neural decoding schemes would predict that monkeys should be able to control FES torque better with cells that are strongly related to wrist movements than with unrelated cells. To investigate this, we documented cell activity during a 2-dimensional wrist tracking task before the nerve block, and calculated the directional tuning for each cell (Figure 3A). The magnitude of directional tuning did correlate significantly with the monkeys’ ability to bring the cursor into the optimally placed targets with cell activity during the initial 10-minute practice period (r2 = 0.33, p < 0.001; Figure 3B). However, cell tuning was not a good predictor of the peak target acquisition rates during subsequent brain-controlled FES (r2 = 0.03, p = 0.33; Figure 3C). For example, with the untuned cell on the left in Figure 3A the monkey acquired 18.5 targets per minute. The tuned (n = 9) and untuned (n = 29) cells showed no difference in any measure of FES control (target acquisition rates, errors, or failed attempts; p > 0.51).
Figure 3. Cell directional tuning is unrelated to FES control.
(A) Responses of an untuned and strongly tuned cell (solid symbols in B & C). The surrounding peri-event histograms show cell activity while acquiring each of eight peripheral torque targets in the flexion-extension (F-E) and radial-ulnar (R-U) plane during the un-paralyzed tracking task (horizontal lines denote baseline cell rates). The radial plot at center summarizes cell activity while matching each peripheral target (shading). Maximum target acquisition rates during direct brain control of cursor (B) and brain-controlled FES (C) plotted as a function of directional tuning strength for cells recorded during the torque-tracking task (n = 38). Performance controlling a cursor directly with cell activity was significantly correlated with cell tuning (B; r2 = 0.33, p < 0.001). Subsequent brain-controlled FES performance was uncorrelated with cell tuning (C; r2 = 0.03, p = 0.33).
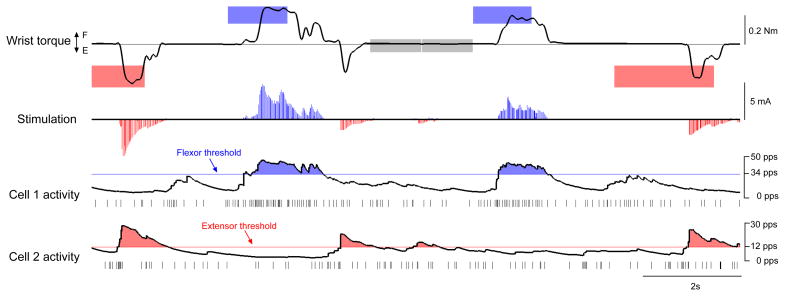
Extending the strategy of direct neural control to more complex movements will require additional control signals. As a first step toward this goal, we tested a monkey’s ability to simultaneously control two cell-muscle pairs. Figure 4 shows monkey L using high discharge rates of one cell to control FES of flexor muscles and high rates of a second cell to control extensor muscles. The monkey learned to independently modulate the activity of five cell pairs in order to control antagonist muscles and rapidly acquire bidirectional torque targets at rates similar to single cells (11.6 ± 3.8 targets/min, p = 0.32).
Figure 4. Two neurons control FES.
Monkey L simultaneously modulated activity of two neurons, each controlling proportional stimulation of a different muscle group when above threshold. L acquired randomly presented flexor (blue), extensor (red) and center (grey) targets by using Cell 1 to stimulate a flexor muscle (FCU; 0.2 mA/pps ×[cell rate–34 pps]) and Cell 2 to stimulate extensor muscles (ECU & ED4,5; 0.4 mA/pps × [cell rate–12 pps]).
These findings have several implications for future approaches to neuroprosthetic control. In contrast to the conventional strategy of deriving control signals from the combined activity of a neural population4–6,8–10, it may prove efficacious to maintain separate signal pathways from cells to muscles. Using direct channels from single cells to specific muscles may provide the brain with more distinguishable outcomes of the cell activity16 and allow innate motor learning mechanisms to help optimize control of the new connections. The brain’s ability to adapt to novel but consistent sensorimotor contingencies has been amply documented17,18, and motor cortex can adapt rapidly to learn new motor skills19,20. Motor circuitry can compensate for drastic changes in connectivity, such as surgically cross-connected nerves controlling wrist flexor and extensor muscles21, or targeted reinnervation for control of prosthetic limbs22.
Our finding that monkeys could learn to use virtually any motor cortex cell to control muscle stimulation, regardless of the cell’s original relation to wrist movement (Figure 3C), suggests another advantage of directly tapping single cell activity. Strategies based on decoding the activity of neural ensembles to obtain movement parameters or muscle activity depend on finding cells that modulate sufficiently with the output variables during actual or imagined movements4–6,8–10. Instead, arbitrary cells available on recording arrays could be brought under volitional control using biofeedback, substantially expanding the source of control signals for brain-machine interfaces. This and previous biofeedback studies14,15 have shown that even cells with no discernable relation to muscles can be volitionally modulated after brief practice sessions. Issues concerning the use of individual cells and neural populations for prosthetic control are further discussed in the supplement.
The degree of FES control demonstrated here was limited by the relatively brief training time provided by the transient nerve block. Implanted electronic circuitry will enable adaptive learning over much longer times and under more varied conditions1. For example, the autonomous ‘Neurochip’ system can discriminate single cell activity and deliver stimulation through days of free behaviour23,24. In several preliminary FES sessions, we confirmed that this system would allow a monkey to trigger stimulation of a paralyzed muscle with cell activity and acquire torque targets (Figure S4). Such autonomous low-power circuits could permit subjects to practice continuously with an artificial connection from brain to muscles or the spinal cord25–27, without requiring complex decoding algorithms or robotic arms. Further development of such direct-control strategies may lead to implantable devices that could help restore volitional movements to individuals living with paralysis.
Methods summary
See complete methods and supplementary information for additional methods.
Subjects
Two male Macaca nemestrina monkeys participated in the experiments (4–5 years old, weight 4.5–6.5 kg). All procedures were approved by the University of Washington Institutional Animal Care and Use Committee.
Recording and paralysis
Activity of single motor cortex cells was recorded using either acute (Monkey I & L) or chronic (Monkey L) electrodes. Each session began by quantifying the cell’s responses during an isometric, eight-target wrist torque tracking task. Volitional control of cell activity was confirmed by operantly rewarding acquisition of targets with a cursor controlled by cell rates. Wrist muscles were then paralyzed by injecting anesthetic (3% chloroprocaine or 2% lidocaine, each with 1:100,000 epinephrine) into catheters or cuffs surrounding the median, ulnar and/or radial nerves.
Brain-controlled FES
Cell activity controlled the intensity of stimuli delivered via bipolar electrodes implanted in one or more paralyzed wrist muscles. When cell rate (smoothed over 0.5 s sliding window) crossed a threshold, biphasic constant-current stimuli (cathode-leading; 0.75–1.0 ms pulse width) were delivered at 50/s. For most cells, stimulus current was made proportional to cell rate above a threshold to allow the monkey to grade contraction force (e.g., stimulus current = 0.1 mA × [cell rate –threshold]; to a maximum of 10 mA). Some cells controlled stimulation in inverse proportion to cell rate below a threshold.
Analysis
Strength of directional tuning was calculated for cells during the initial torque tracking task using the vector method28. Peak performance was quantified by the maximum number of targets acquired during a two-minute period. Peak performance was compared among conditions and to performance during the initial two minutes of practice using the nonparametric ranksum test. Regression analysis determined correlations between directional tuning and peak performance during brain control of a cursor or FES.
Methods
Cortical recording
Sterile surgeries were performed with isoflurane anesthesia (1–1.5% in 50:50 02:N20). All surgeries were followed by a program of analgesics (buprenorphine 0.15 mg/kg IM and ketoprofen 5mg/kg PO) and antibiotics (cephalexin 25 mg/kg PO). Each animal was implanted with a cranial recording chamber over left hand and wrist area of motor cortex at stereotaxic coordinates A: 13mm, L: 18mm to permit cortical recordings29,30. To obtain longer duration cell recordings, monkey L was re-implanted with a chronic electrode array over left motor cortex. The array of 12 independently movable microwires is fully described elsewhere31. Briefly, 50 μm tungsten wires were threaded through individual polyamide guide-tubes in a 2 × 6 array that was anchored to the skull. This array provided stable recordings from the same isolated cell for the duration of an experimental session, and across multiple days for ten cells24,31,32.
Nerve block implant
Reversible paralysis of the right wrist was achieved with one of two nerve block methods. First, catheters were implanted in the brachial plexus, near cords giving rise to the radial, ulnar and median nerves. Epidural catheters (19 Ga., Arrow International) were inserted into the epineurium surrounding each nerve and anchored in place with cyanoacrylate. Second, nerve cuffs with catheters33 were implanted around the median and ulnar nerves in the upper arm. Catheters terminating in the lumen of each Silastic cuff (4 mm inner diameter, 30 mm long) permitted the nerves to be bathed in anesthetic. Nerves were identified by electrical stimulation, and catheters were tunneled subcutaneously to exit the skin on the upper back and sealed with an injection port. Thirty-one cells controlled FES during nerve blocks induced by the catheter method, and the remaining 13 cells during blocks induced by cuffs.
Experimental paradigm
The monkey sat with his right elbow and hand immobilized by padded splints while a transducer measured the flexion-extension (F-E) and radial-ulnar (R-U) torques produced about the wrist (see Figure 1A). To receive an applesauce reward, the monkey maintained wrist torque within a center target (zero torque) followed by one of eight peripheral targets specifying different combinations of F-E and R-U torque (average magnitude 0.13 ± 0.01 nM). Isolated cell activity was discriminated online using template-matching software (Alpha Omega MSD). Subsequently, cell activity controlled cursor movement in one dimension. Inter-spike intervals were averaged over a 0.5 s sliding window to create a continuous signal for cursor position (and later FES control). If the cell was directionally tuned, targets were aligned with its preferred direction. For untuned cells or cells without tuning information (i.e., cells isolated after nerve block began), either the left or right screen position was arbitrarily chosen to represent high discharge rates for visual feedback. Monkeys practiced cell control for 10 minutes, maintaining discharge rate within each target for 0.5–1.0 s to receive a reward.
Nerve block
We blocked nerves leading to wrist muscles with local anesthetic to create temporary motor paralysis. Block onset typically occurred after 5–60 minutes, depending on anesthetic and block method. During this time the monkeys continued to perform the cell-controlled target tracking task. Additional doses were given regularly to maintain paralysis during FES control.
Brain-controlled FES
After onset of paralysis and an average of 36 ± 22 minutes of cell-controlled target tracking, the cell activity was converted to stimuli delivered to one or more paralyzed muscles. Wrist torque controlled the position of the cursor, and targets were randomly displayed on the monitor in one dimension. Monkeys were required to maintain torque within each target for 0.5–1.0 s (mean 0.56 s) to receive a reward. Targets remained on the screen until satisfied, followed by presentation of the next target either immediately or after a 1.5 s reward period. Forty-two cells controlled stimulation current in proportion to cell rate, permitting the monkey to grade contraction force. Two of these cells also controlled stimulation via the autonomous ‘Neurochip’23,24 to deliver a 1s train of stimuli (2.5 mA, 50/s) when smoothed cell rate exceeded a threshold (Figure S4). Similarly, the first two cells in monkey I also triggered a 1s train of 50/s stimuli at 5 mA.
To confirm continued nerve block during the practice session, the stimulator was turned off after every 10 minutes of FES to assure that the monkey could not acquire the peripheral target through volitional muscle contractions. Figures 2B & S4 illustrate the torques produced with the stimulator active compared to periods when the stimulator was turned off for 30 s. With the stimulator off, the monkey repeatedly attempted to satisfy the target but produced ≤10% of the torque used to acquire the active target. For all such test periods with each cell the monkeys produced an average maximum of only 18.0 ± 21.3 % of the torque used to satisfy the targets.
Data sampling
Signals were digitized and stored to disk for offline analysis. Raw recording from motor cortex was band-passed from 1–10 kHz and sampled at 25 kHz, along with spike times from the online discrimination. Wrist torques (flexion-extension and radial-ulnar) were sampled at 5 kHz, and smoothed and down-sampled to 500 Hz during offline analysis. We also recorded behavioural parameters (target on screen, etc.), and muscle stimulation amplitude and timing (5 kHz).
Data Analysis
Task difficulty was increased incrementally by raising levels of torque targets and increasing hold times. This complicated the quantification of skill learning. Improvements were evident as higher performance levels prior to increments in task requirements (e.g., Figure S3), and these times were compared with performance at the beginning of a practice session. Specifically, the two-minute period with the peak performance was compared to the initial two minutes of practice (e.g., targets per minute). Control precision was measured by target errors and the number of failed attempts to reach a target. A target acquisition error was counted when the monkey activated the stimulator while the center target was on the screen, resulting in sufficient torque to satisfy the peripheral target had it been presented. Target errors are reported as the percentage of center targets presented. A failed attempt was counted whenever the monkey briefly acquired a peripheral torque target but did not satisfy the required hold time. A T-test was used to compare average torques during graded FES control. Otherwise, the non-parametric ranksum test was used for all comparisons as at least one data set for each remaining comparison failed Lilliefors test for normality. All reported values are means ± standard deviation (SD).
Supplementary Material
Acknowledgments
We thank L. Shupe for programming assistance, C. Kent and L. Miller for advice on nerve block, C. Kirby, A. Price & K. McElwain for animal care, and A. Jackson, Y. Nishimura & A. Richardson for comments on the manuscript. This work was supported by grants from the National Institutes of Health.
Footnotes
Author Contributions C.M. and E. F. conceived and designed the experiments, C. M. and S. P. performed the experiments, and C. M. and E. F. wrote the paper.
Author Information Authors declare no competing financial interests.
Supplementary Information accompanies this manuscript
References
- 1.Jackson A, Moritz CT, Mavoori J, Lucas TH, Fetz EE. The Neurochip BCI: towards a neural prosthesis for upper limb function. IEEE Trans Neural Syst Rehabil Eng. 2006;14:187–90. doi: 10.1109/TNSRE.2006.875547. [DOI] [PubMed] [Google Scholar]
- 2.Lauer RT, Peckham PH, Kilgore KL. EEG-based control of a hand grasp neuroprosthesis. Neuroreport. 1999;10:1767–71. doi: 10.1097/00001756-199906030-00026. [DOI] [PubMed] [Google Scholar]
- 3.Fagg AH, et al. Biomimetic brain machine interfaces for the control of movement. J Neurosci. 2007;27:11842–6. doi: 10.1523/JNEUROSCI.3516-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carmena JM, et al. Learning to Control a Brain-Machine Interface for Reaching and Grasping by Primates. PLoS Biol. 2003;1:E42. doi: 10.1371/journal.pbio.0000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Velliste M, Perel S, Spalding MC, Whitford AS, Schwartz AB. Cortical control of a prosthetic arm for self-feeding. Nature. 2008 doi: 10.1038/nature06996. [DOI] [PubMed] [Google Scholar]
- 6.Hochberg LR, et al. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature. 2006;442:164–71. doi: 10.1038/nature04970. [DOI] [PubMed] [Google Scholar]
- 7.Kennedy PR, Bakay RA, Moore MM, Adams K, Goldwaithe J. Direct control of a computer from the human central nervous system. IEEE Trans Rehabil Eng. 2000;8:198–202. doi: 10.1109/86.847815. [DOI] [PubMed] [Google Scholar]
- 8.Musallam S, Corneil BD, Greger B, Scherberger H, Andersen RA. Cognitive control signals for neural prosthetics. Science. 2004;305:258–62. doi: 10.1126/science.1097938. [DOI] [PubMed] [Google Scholar]
- 9.Serruya MD, Hatsopoulos NG, Paninski L, Fellows MR, Donoghue JP. Instant neural control of a movement signal. Nature. 2002;416:141–2. doi: 10.1038/416141a. [DOI] [PubMed] [Google Scholar]
- 10.Taylor DM, Tillery SI, Schwartz AB. Direct cortical control of 3D neuroprosthetic devices. Science. 2002;296:1829–32. doi: 10.1126/science.1070291. [DOI] [PubMed] [Google Scholar]
- 11.Nannini N, Horch K. Muscle recruitment with intrafascicular electrodes. IEEE Trans Biomed Eng. 1991;38:769–76. doi: 10.1109/10.83589. [DOI] [PubMed] [Google Scholar]
- 12.Peckham PH, et al. An advanced neuroprosthesis for restoration of hand and upper arm control using an implantable controller. J Hand Surg [Am] 2002;27:265–76. doi: 10.1053/jhsu.2002.30919. [DOI] [PubMed] [Google Scholar]
- 13.Stein RB, Aoyagi Y, Mushahwar VK, Prochazka A. Limb movements generated by stimulating muscle, nerve and spinal cord. Arch Ital Biol. 2002;140:273–81. [PubMed] [Google Scholar]
- 14.Fetz EE, Baker MA. Operantly conditioned patterns on precentral unit activity and correlated responses in adjacent cells and contralateral muscles. J Neurophysiol. 1973;36:179–204. doi: 10.1152/jn.1973.36.2.179. [DOI] [PubMed] [Google Scholar]
- 15.Fetz EE, Finocchio DV. Correlations between activity of motor cortex cells and arm muscles during operantly conditioned response patterns. Exp Brain Res. 1975;23 :217–40. doi: 10.1007/BF00239736. [DOI] [PubMed] [Google Scholar]
- 16.Fetz EE. Volitional control of neural activity: implications for brain-computer interfaces. J Physiol. 2007;579:571–9. doi: 10.1113/jphysiol.2006.127142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shadmehr R, Mussa-Ivaldi FA. Adaptive representation of dynamics during learning of a motor task. J Neurosci. 1994;14:3208–24. doi: 10.1523/JNEUROSCI.14-05-03208.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thach WT. Correlation of neural discharge with pattern and force of muscular activity, joint position, and direction of intended next movement in motor cortex and cerebellum. J Neurophysiol. 1978;41:654–76. doi: 10.1152/jn.1978.41.3.654. [DOI] [PubMed] [Google Scholar]
- 19.Gandolfo F, Li C, Benda BJ, Schioppa CP, Bizzi E. Cortical correlates of learning in monkeys adapting to a new dynamical environment. Proc Natl Acad Sci U S A. 2000;97:2259–63. doi: 10.1073/pnas.040567097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nudo RJ, Milliken GW, Jenkins WM, Merzenich MM. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. J Neurosci. 1996;16:785–807. doi: 10.1523/JNEUROSCI.16-02-00785.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brinkman C, Porter R, Norman J. Plasticity of motor behavior in monkeys with crossed forelimb nerves. Science. 1983;220:438–40. doi: 10.1126/science.6836289. [DOI] [PubMed] [Google Scholar]
- 22.Kuiken TA, Dumanian GA, Lipschutz RD, Miller LA, Stubblefield KA. The use of targeted muscle reinnervation for improved myoelectric prosthesis control in a bilateral shoulder disarticulation amputee. Prosthet Orthot Int. 2004;28:245–53. doi: 10.3109/03093640409167756. [DOI] [PubMed] [Google Scholar]
- 23.Jackson A, Mavoori J, Fetz EE. Long-term motor cortex plasticity induced by an electronic neural implant. Nature. 2006;444:56–60. doi: 10.1038/nature05226. [DOI] [PubMed] [Google Scholar]
- 24.Mavoori J, Jackson A, Diorio C, Fetz E. An autonomous implantable computer for neural recording and stimulation in unrestrained primates. J Neurosci Methods. 2005;148:71–7. doi: 10.1016/j.jneumeth.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 25.Lemay MA, Grill WM. Modularity of motor output evoked by intraspinal microstimulation in cats. J Neurophysiol. 2004;91:502–14. doi: 10.1152/jn.00235.2003. [DOI] [PubMed] [Google Scholar]
- 26.Mushahwar VK, Gillard DM, Gauthier MJ, Prochazka A. Intraspinal micro stimulation generates locomotor-like and feedback-controlled movements. IEEE Trans Neural Syst Rehabil Eng. 2002;10:68–81. doi: 10.1109/TNSRE.2002.1021588. [DOI] [PubMed] [Google Scholar]
- 27.Tresch MC, Bizzi E. Responses to spinal microstimulation in the chronically spinalized rat and their relationship to spinal systems activated by low threshold cutaneous stimulation. Exp Brain Res. 1999;129:401–16. doi: 10.1007/s002210050908. [DOI] [PubMed] [Google Scholar]
- 28.Batschelet E. Circular Statistics in Biology. Academic Press; London: 1981. [Google Scholar]
- 29.Evarts EV. Relation of pyramidal tract activity to force exerted during voluntary movement. J Neurophysiol. 1968;31:14–27. doi: 10.1152/jn.1968.31.1.14. [DOI] [PubMed] [Google Scholar]
- 30.Woolsey CN, et al. Patterns of localization in precentral and "supplementary" motor areas and their relation to the concept of a premotor area. Res Publ Assoc Res Nerv Ment Dis. 1952;30:238–64. [PubMed] [Google Scholar]
- 31.Jackson A, Fetz EE. A compact moveable microwire array for long-term chronic unit recording in cerebral cortex of primates. J Neurophysiol. 2007 doi: 10.1152/jn.00569.2007. [DOI] [PubMed] [Google Scholar]
- 32.Jackson A, Mavoori J, Fetz EE. Correlations between the same motor cortex cells and arm muscles during a trained task, free behavior, and natural sleep in the macaque monkey. J Neurophysiol. 2007;97:360–74. doi: 10.1152/jn.00710.2006. [DOI] [PubMed] [Google Scholar]
- 33.Loeb GE, Hoffer JA. Activity of spindle afferents from cat anterior thigh muscles. II. Effects of fusimotor blockade. J Neurophysiol. 1985;54:565–77. doi: 10.1152/jn.1985.54.3.565. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.