Abstract
This review focusses on cross-modal plasticity resulting from visual deprivation. This is viewed against the background of task-specific visual cortical recruitment that is routine during tactile tasks in the sighted and that may depend in part on visual imagery. Superior tactile perceptual performance in the blind may be practice-related, although there are unresolved questions regarding the effects of Braille-reading experience and the age of onset of blindness. While visual cortical areas are clearly more involved in tactile microspatial processing in the blind than in the sighted, it still remains unclear how to reconcile these tactile processes with the growing literature implicating visual cortical activity in a wide range of cognitive tasks in the blind, including those involving language, or with studies of short-term, reversible visual deprivation in the normally sighted that reveal plastic changes even over periods of hours or days.
In this paper, we review studies of cross-modal plasticity resulting from visual deprivation, focussing on whether neural processing of tactile inputs differs from that in the sighted, and whether perceptual capabilities are correspondingly different. Comparable studies focussing on auditory perception and its neural processing have been reviewed recently by others (Collignon et al., 2009). We attempt to distinguish neuroplasticity in sensory domains from that in cognitive-linguistic domains: this distinction has not always been made clearly. Interpreting cross-modal plasticity in visual cortex of the blind is complicated by the plethora of findings, accumulated over the last decade or so, that have established the existence of task-specific visual cortical recruitment during many tactile tasks in the sighted – this literature is first reviewed briefly to provide a backdrop against which the findings from studies of visual deprivation should be considered.
Visual cortical involvement in tactile perception in the sighted
The first report of visual cortical activity during tactile perception in normally sighted individuals was a positron emission tomographic (PET) scanning study from our laboratory (Sathian et al., 1997). This study employed discrimination of grating orientation, which involves applying gratings of alternating ridges and grooves to the pad of the immobilized finger, and asking subjects to report whether the grating is oriented along or across the long axis of the finger (Van Boven and Johnson, 1994). Contrasting this task with a control task requiring discrimination of grating groove width yielded activation at a parieto-occipital focus previously shown to be active during visual discrimination of grating orientation (Sergent et al., 1992) and probably located in human visual area V6 (Pitzalis et al., 2006). We then used transcranial magnetic stimulation (TMS) to show that disrupting processing at this focus impaired tactile discrimination of grating orientation (but not of grating groove width), thereby demonstrating functional involvement of this area in tactile perception (Zangaladze et al., 1999). Based on these studies, we proposed that visual cortical involvement occurs for “macrogeometric” or large-scale tactile tasks such as grating orientation, but not for “microgeometric” tasks of the order of a few mm. Similarly, a TMS study (Merabet et al., 2004) using dot patterns presented to the finger suggested visual cortical involvement during tactile judgments of inter-dot distance but not roughness. However, a complementary functional magnetic resonance imaging (fMRI) study (Merabet et al., 2007) failed to find correspondingly task-selective visual cortical activation, so that the interpretation of these findings with dot patterns remains uncertain. Others reported that an area involved in perception of visual motion, the MT complex, is also recruited during perception of tactile motion (Hagen et al., 2002; Blake et al., 2004). Moreover, the tactually perceived direction of motion of a rotating globe can influence its visually perceived direction when the latter is ambiguous (Blake et al., 2004; James & Blake, 2004). This suggests that both visual and tactile motion engage a common representation.
The lateral occipital complex (LOC) is a region in the ventral visual pathway that is object-selective (Malach et al., 1995), and is regarded as the human homolog of macaque inferotemporal cortex (Grill-Spector et al., 1998). A number of fMRI studies have shown that the LOC is also recruited during haptic object perception (Amedi et al., 2001, 2002; James et al., 2002; Zhang et al., 2004; Stilla & Sathian, 2008). However, its lack of activation by object-specific sounds, except when people are trained to perceive object shape using “soundscapes” derived by transformation of visual input according to a specific algorithm, suggests that the LOC is specialized for coding object geometry (Amedi et al., 2002, 2007). Further, the LOC seems particularly concerned with macrogeometric object perception, rather than microgeometric perception, since it is not active during gap detection (Stoesz et al., 2003), and is only minimally active during microspatial discrimination of the direction of offset of the central dot in a linear 3-dot array (Stilla et al., 2007). The notion of a common neural representation for visual and haptic shape is suggested by the observations that both visual and haptic object perception engage the LOC, and is further supported by many lines of evidence, including psychophysical (Easton et al., 1997a,b; Reales and Ballesteros, 1999) and fMRI (Amedi et al., 2001; James et al., 2002) demonstrations of cross-modal visuo-haptic priming, correlations across subjects in the magnitudes of visually- and haptically-evoked activation in the right LOC (Stilla & Sathian, 2008), and case reports of patients with damage to the LOC resulting in both visual and haptic agnosia (Feinberg et al., 1986; James et al., 2006). We have recently shown that cross-modal (visuo-haptic) object recognition is view-independent, in contrast to within-modal object recognition which is view-dependent in both vision and touch (Lacey et al., 2007). This implies that the underlying modality-independent representation of object shape is also view-independent, whereas the modality-specific representations are view-dependent. Moreover, when view-independence is achieved unimodally in either vision or touch by training, such view-independence transfers automatically to the other modality without further training (Lacey et al., submitted).
The mechanisms underlying such cross-modal recruitment of visual cortex during tactile perception remain uncertain. One possibility is that visual imagery is responsible. Consistent with this, macrogeometric tasks preferentially involve visual cortical processing (as discussed earlier), and are also more associated with visual imagery (Sathian and Zangaladze, 2001). It is relevant that the LOC is active during visual imagery of object shape in sighted subjects (De Volder et al., 2001). Further, the magnitude of shape-selective activity in the right LOC is predicted by ratings of the vividness of visual imagery (Zhang et al., 2004). However, some have argued against visual imagery as the basis for LOC recruitment during haptic shape perception, favoring instead the idea that such recruitment reflects engagement of a multisensory shape representation (Amedi et al., 2001, 2002; James et al., 2002). Indeed, as reviewed above, there is substantial evidence to support the idea that vision and touch share a common representation of shape. The imagery explanation would imply that haptic activation of the LOC is achieved via top-down connections from prefrontal and parietal cortex, whereas the multisensory account would require bottom-up projections from somatosensory to visual cortex. Recent work using event-related potentials (ERPs) provides support for bottom-up somatosensory input into the LOC during discrimination of shapes applied to the fingerpad, with the appearance of shape-selective activity in the LOC as early as 150 ms following the onset of tactile stimulation (Lucan et al., 2008). It is worth noting that finding similar tactually-evoked activation patterns in visual cortex of the sighted and congenitally blind does not necessarily rule out a role for visual imagery in the sighted, as some have argued (e.g. Pietrini et al., 2004) – cross-modal plasticity following visual deprivation results in substantially different functional organization of many visual cortical areas, as will be reviewed later in this paper. Indeed, effective connectivity analysis of fMRI data acquired during haptic perception offers evidence for the existence of both bottom-up and top-down connections (Peltier et al., 2007; Deshpande et al, 2008; Stilla et al., 2008a). Further, the role of visual imagery during haptic object perception appears to depend on object familiarity, being strong for familiar objects drawn from a variety of categories, but less important for unfamiliar objects that are categorically similar (Stilla et al., 2008a).
Superior tactile perception in the blind
It is commonly believed that blindness is associated with superior tactile perception. However, empirical support for this idea is mixed. Thus, haptic perception of three-dimensional shape has been variously reported to be better in the blind (Heller, 1989a), unaffected by visual status (Morrongiello et al., 1994), or better in the sighted (Bailes and Lambert, 1986; Lederman et al., 1990). Blind Braille readers have been found to be superior to their sighted counterparts on a number of tactile tasks involving two-dimensional stimuli presented to a fingerpad, including perception of Braille-like patterns (Foulke and Warm, 1967), detecting gaps in or judging the orientation of bar stimuli (Stevens et al., 1996), discrimination of grating orientation (Van Boven et al., 2000; Goldreich and Kanics, 2003), and detecting whether or not the central dot in a linear 3-dot array had a spatial offset (Grant et al., 2000). On other tasks, however, the blind did not differ significantly from the sighted – discriminating bar length (Stevens et al., 1996) or textures (Grant et al., 2000; Heller, 1989b). In a recent study from our laboratory (Stilla et al., 2008b), acuity thresholds of blind individuals were comparable to those of the sighted on a purely spatial task requiring discrimination of the direction of offset of the central dot in a 3-dot array. All the other tasks studied previously could potentially have been solved using intensive cues (see Stilla et al., 2008b), including the grating orientation task, which may be susceptible to anisotropies on the fingerpad evidenced both psychophysically and neurophysiologically (Wheat and Goodwin, 2000).
The superiority of the blind on some, but not other tactile tasks may be practice-related. Practiced sighted subjects can match the blind on the Optacon (Craig, 1988), a vibrotactile reading aid for the blind, and on the dot-offset discrimination task (Grant et al., 2000), and can also achieve the performance of the deaf-blind in decoding speech tactually (Reed et al., 1978, 1982). Interestingly, blind subjects showed no improvement in performance on dot-offset discrimination over a few days of practice, whereas the sighted did (Grant et al., 2000), suggesting that the blind were already operating at the limits of the system. Although the dot patterns used in this task and certain other tasks mentioned above resemble Braille patterns, Braille reading itself is rather different from these discriminative tasks. In fact, the effect of specific Braille-reading practice on performance of various tactile acuity tasks is unclear: one study reported lower grating orientation discrimination thresholds on Braille-reading fingers (Van Boven et al., 2000), another study found that Braille-reading ability was unrelated to grating orientation discrimination performance in the blind (Goldreich and Kanics, 2003), and our group did not find differences on dot-offset tasks between the Braille-reading hand and its counterpart (Grant et al., 2000; Stilla et al., 2008b). There is also no definite evidence that visual deprivation during a critical period in early life, compared to that in later life, results in enhanced tactile capabilities, since performance does not appear to differ significantly between the early and late blind (Grant et al., 2000; Goldreich and Kanics, 2003; Stilla et al., 2008b). Thus, the precise nature of superior tactile perception in the blind remains unresolved in many respects.
Neocortical plasticity following visual deprivation
Following neonatal visual deprivation, rodents show alterations in the whisker barrel representations in somatosensory cortex (Rauschecker et al., 1992; Toldi et al., 1994a). In blind human Braille readers, the cortical sensory representation of the Braille-reading finger is expanded (Pascual-Leone and Torres, 1993), while those who read Braille with multiple fingers in concert mislocalize touch on the reading fingers and exhibit correspondingly disordered cortical somatotopy (Sterr et al., 1998). Paralleling these somatosensory cortical changes, neonatal visual deprivation also triggers somatosensory responsiveness in extrastriate visual cortex of rats (Toldi et al., 1994b) and monkeys (Hyvärinen et al., 1981), and occipital cortical areas were found in a PET study to be more metabolically active in early blind individuals than in the late blind or sighted (Veraart et al., 1990).
Medial occipital cortex, including the anatomic region corresponding to primary visual cortex (V1), was shown by PET scanning to be active in the blind, relative to a rest control, during reading Braille to discriminate words from non-words (Sadato et al., 1996). Less intense activation was found in this study when blind subjects performed various other non-Braille discrimination tasks that employed stimuli constructed from Braille fields, but not when subjects simply swept the finger over a Braille field, whereas sighted subjects showed decreased activation of these visual cortical areas by the non-Braille discrimination tasks. The same non-Braille discriminative tasks activated ventral occipital cortex but deactivated the region of the second somatosensory cortex (S2) in the blind, with the reverse pattern in sighted subjects (Sadato et al., 1998). That the observed effects required active finger movement was excluded by an fMRI study involving discrimination of Braille characters presented to the passive finger (Sadato et al., 2002). Activation of medial occipital cortex by Braille reading (relative to rest) occurs in early blind subjects (Cohen et al., 1999; Sadato et al., 2002), whereas the late blind and sighted deactivate these regions (Sadato et al., 2002). In blind subjects, TMS over medial occipital cortex impaired the ability to tactually identify Braille or Roman letters and distorted subjective percepts of the stimuli, whereas in sighted subjects Roman letter identification was unaffected by occipital TMS, but more affected by TMS over contralateral sensorimotor cortex than in the blind (Cohen et al., 1997). Together with the remarkable findings in an early blind person who developed alexia for Braille with otherwise normal somatosensory perception after a bilateral infarct of occipital cortex (Hamilton et al., 2000), these TMS findings demonstrate functional involvement of visual cortex in Braille reading. Like the corresponding activation studies cited above, the TMS effects were found in early blind but not late blind subjects (Cohen et al., 1999), pointing to cross-modal plasticity during the critical period of visual system development.
Is visual cortical recruitment in the blind driven by linguistic or sensory processing?
Braille reading involves not only tactile sensory processing but also higher-level cognitive, especially linguistic, operations. In the imaging studies cited in the preceding paragraph, the control condition used was a rest state, without sensory or cognitive demands. These studies thus left open whether sensory, linguistic or other cognitive processes account for the observed differences between blind and sighted populations in visual cortical recruitment. In fact, a PET study of Braille reading controlling for linguistic processes using an auditory word control found that the late blind, but not the early blind, recruited activity in presumptive V1 (Büchel et al., 1998a). This suggested that visual cortical recruitment might arise from linguistic processing in the early blind, an idea that is supported by a number of studies: A PET study found selective activation for words compared to non-words in a left occipito-temporal region, whether during visual presentations to sighted subjects or Braille presentations to blind subjects (Büchel et al., 1998b). In a pair of fMRI studies, covert verb generation in response to Braille nouns (Burton et al., 2002a) or heard nouns (Burton et al., 2002b) was shown to yield essentially identical activations in occipital and occipito-temporal visual cortical areas, including V1. TMS over medial occipital cortex is, moreover, disruptive to verb generation performance in the congenitally blind (Amedi et al., 2004). Visual cortical activation in the early blind is preferentially recruited by semantic relative to phonological processing (Burton et al., 2003), and the magnitude of fMRI activation scales with both semantic and syntactic complexity (Röder et al., 2002). The congenitally blind were reported to recruit occipital cortex even during a verbal memory task lacking sensory stimulation, with a preference for posterior occipital regions, including V1 whose activation magnitude correlated with verbal memory performance (Amedi et al., 2003). It is abundantly clear from this set of studies that visual cortex is recruited for language processing in the blind, but not whether it is also involved in other non-visual sensory functions.
We turn our focus now to the nature of visual cortical involvement in somatosensory processes. Numerous studies employing diverse tactile tasks in the absence of Braille reported that visual cortical activity was greater in blind than sighted individuals. The tasks used included vibrotactile frequency discrimination (Burton et al., 2004), tactile motion tasks (Goyal et al., 2006; Ricciardi et al., 2007), orientation dsicrimination of electrotactile stimuli to the tongue (Ptito, Moesgard, Gjedde & Kupers, 2005) and haptic object perception (Pietrini et al., 2004). However, all these studies were limited by the use of rest controls, so that it is impossible to know whether the observed visual cortical activations were due to sensory, linguistic or other cognitive processes. A recent study (Ptito et al., 2008) reported induction of tactile paresthesias in some early blind subjects during TMS over occipital cortex, but the significance of this finding for functional tactile perception is unclear.
To address whether somatosensory processing itself can recruit visual cortical activity, we conducted fMRI studies using the 3-dot task – subjects were required to discriminate the direction of offset of the central dot. This task, as pointed out above, is a purely spatial one and cannot be solved using non-spatial cues. We focussed on activity that was specific for tactile spatial performance, using a temporal control task where the stimulus was an array without spatial offset. In sighted individuals, the spatial task evoked only minimal LOC activity, and no activity elsewhere in visual cortex (Stilla et al., 2007). Spatially-selective activation in the sighted was mainly in a distributed frontoparietal network, with the magnitude of activation in right posteromedial parietal cortex predicting tactile acuity in individual subjects (tactile tasks were presented to the right hand). This region corresponds to the part of the intraparietal sulcus (IPS) that has been termed CIP (caudal intraparietal area), based on the study of Shikata et al. (2008). Effective connectivity analysis revealed that the strength of inputs into the CIP region was significantly greater for sighted subjects with better acuity, compared to those with poorer acuity (Stilla et al., 2007). Furthermore, in subjects with better acuity, the paths whose weights predicted acuity converged from left primary somatosensory cortex (S1) and the right frontal eye field (FEF) into the right CIP. These paths were selective for the spatial task, and their weights also predicted the level of right CIP activity. We proposed (Stilla et al., 2007) that optimal performance of this task in the sighted depends on interaction between somatosensory cortical inputs and a top-down control signal, possibly attentional in nature, in CIP.
A study was conducted in blind individuals (Stilla et al., 2008b), using exactly the same experimental and control tasks as the study of Stilla et al. (2007) in the sighted. Comparison of the findings of these studies showed that many aspects of the activity and effective connectivity during tactile microspatial discrimination differed between the blind and sighted, with some of the key differences being in visual cortex. Both hands were studied in the blind subjects, but differences in activation resulting from stimulation of the two hands were minor. The spatially-selective network in blind subjects included some frontoparietal regions also similarly selective in the sighted, and visual cortical areas that showed little to no activity in the sighted. These included the right LOC, bilateral foci in the fusiform gyrus (FG), and a region on the right corresponding approximately to the retinotopically mapped region known as V3A. Thus, this study (Stilla et al., 2008b) establishes clearly that tactile microspatial processing specifically engages parts of visual cortex in the blind, whereas the same task is associated with little to no visual cortical activity in the sighted (Stilla et al., 2007). Further, visual cortical regions were significantly involved in connectivity in the blind, interacting with one another as well as with somatosensory cortex (Stilla et al., 2008b).
Acuity thresholds were predicted in the blind by activation magnitudes in left S1 and bilateral visual cortex (Stilla et al., 2008b). Some of the visual cortical foci were in what is normally retinotopic visual cortex: in right V1/V2 and left V3A; whereas others lay more inferiorly in what is normally object-selective cortex: in the right LOC, left posterior fusiform gyrus (FG) and left inferotemporal cortex (ITC). Most of these foci showed some degree of spatial selectivity. Acuity thresholds were also predicted by the weights of projections from the left FEF to the left CIP, and from left S1 to the right LOC. Together with the differing locations of regions whose activation magnitude predicted acuity threshold, these findings indicate that optimal tactile spatial discrimination engages a rather different network in the blind compared to the sighted. In contrast to the sighted (see above), the blind seem to depend on a more distributed network involving left somatosensory cortex and a complex of visual cortical areas in both hemispheres, including some areas that in the sighted are known to be retinotopically mapped, and others that are object-selective. The interaction between somatosensory and visual cortex appears to be pivotal, since one of the paths predicting acuity linked left S1 and right LOC, both of which had activation magnitudes that also predicted acuity (Stilla et al., 2008b).
A widely distributed set of somatosensory, visual, posterior parietal and frontal cortical regions exhibited greater spatial selectivity in early blind compared to late blind participants (Stilla et al., 2008b). Among these regions, activation magnitudes in right S1, right FEF and right CIP, along with the weight of a path from the right LOC to right medial occipital cortex (this region corresponds to V1 and the dorsal part of V2) predicted the age of complete blindness. Thus, the timing of visual deprivation is an important factor in determining the extent of tactually-evoked activity in somatosensory cortex and frontoparietal regions (it is interesting that the right FEF and right CIP both featured prominently in the acuity-correlated circuitry in the sighted), as well as the strength of tactually-related effective connectivity between visual cortical regions. Presumably, this reflects interaction between visual deprivation and critical periods of developmental maturation of the visual system. As in the case of perceptual performance, the impact on neocortical reorganization of other factors such as the severity of visual impairment, duration of blindness and amount of Braille-reading experience, remain to be resolved.
Despite these findings (Stilla et al., 2008b) demonstrating more visual cortical involvement in tactile microspatial processing in the blind than in the sighted, it still remains unclear how to reconcile the somatosensory function of visual cortex in the blind with the growing literature implicating visual cortical activity in a wide range of cognitive tasks in the blind. Such tasks include those that involve linguistic processing, as reviewed above, as well as a variety of tasks where attention appears to be the primary demand (Alho et al., 1993; Garg et al., 2007; Kujala et al., 1992, 1995, 2005; Röder et al., 1996; Stevens et al., 2007; Weaver & Stevens, 2007). Moreover, auditory processing also recruits visual cortex in the blind (Collignon et al., 2009). The extent to which these different types of processing engage distinct areas of visual cortex remains to be ascertained. The report of Amedi et al. (2003) suggests that there may be functional specialization within visual cortex in the blind, since Braille reading recruited the LOC while verbal memory recruited the region of V1. This idea merits further investigation.
Effect of short-term visual deprivation
A few studies have explored the consequences of short-term visual deprivation of normally sighted subjects. Blindfolding subjects for 90 minutes resulted in reversible improvement of discrimination of grating orientation (Facchini and Aglioti, 2003), of similar magnitude to that reported in the blind. The excitability of visual cortex is enhanced by blindfolding over a similar time frame, as demonstrated by TMS and fMRI (Boroojerdi et al., 2000). Intriguingly, such short-term blindfolding actually led to deactivations during tactile form discrimination as well as gap detection in visual cortical regions approximately corresponding to V3A and V7, along with task-specific increases in activation for the form relative to the gap task along the IPS and in frontotemporal cortex (Weisser et al., 2005). Blindfolding for five days improved the ability to discriminate Braille characters (Kauffman et al., 2002; Merabet et al., 2008), without any changes on grating orientation discrimination or von Frey thresholds (Merabet et al., 2008). Correspondingly, neuroimaging revealed the appearance of responsiveness to tactile stimulation (compared to a rest control) in occipital cortex, and TMS became able to disrupt Braille reading performance. These findings mimic those reported in the blind, were absent in control sighted subjects who were not visually deprived, and reverted to normal within 24 hours after removing the blindfold (Merabet et al., 2008). They suggest that cross-modal activation of visual cortex does not necessarily require the formation of new connections, but could work through unmasking of pre-existing connectivity. However, at the present juncture, it is not yet possible to integrate the effects of blindfolding for a few days (Merabet et al., 2008) with those of blindfolding for just two hours (Facchini and Aglioti, 2003; Weisser et al., 2005), in part because of the variety of tasks used. Further research is necessary to address the temporal evolution of cross-modal plasticity following visual deprivation.
Conclusions
Normally sighted subjects regularly show task-specific recruitment of visual cortex during tactile perception, and greater involvement of early visual cortical areas following a few days of reversible visual deprivation. The blind appear to be better than the sighted on certain tactile tasks, perhaps as a result of their specific tactile experience, but many of these tasks potentially involve non-spatial cues, and the impact of early versus late blindness, and of Braille-reading experience, remain uncertain. It is now clear that visual cortical areas are recruited during tactile spatial perception in the blind, as well as during a variety of other tasks including linguistic ones. Future research should focus on delineating the extent of functional specialization within visual cortex in the blind and relating such long-term cross-modal plasticity to its temporal evolution, its perceptual consequences and the influence of modulatory factors such as specific types of sensorimotor experience.
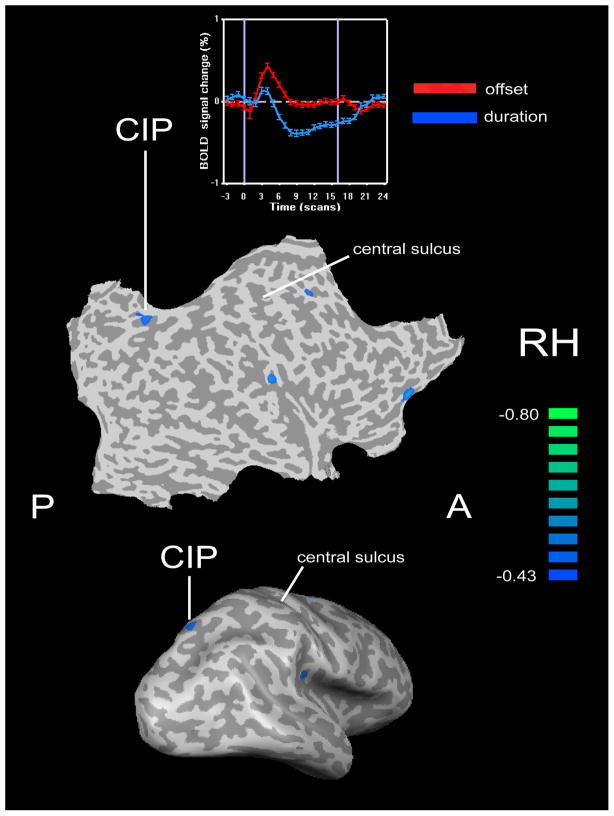
Figure 1.
Lateral view of inflated representation (bottom) and flat map (middle) of the right hemisphere (RH) showing location of CIP activity in normally sighted subjects that was selective for tactile microspatial (offset) discrimination, relative to tactile temporal (stimulus duration) discrimination, and whose activation magnitude in the offset task (relative to rest) predicted tactile acuity threshold. Scale represents correlation coefficient (r) of this predictive relationship. The negative correlation indicates that greater activation magnitudes were associated with lower thresholds (better acuity). Time-courses of blood oxygenation level-dependent (BOLD) signal at the CIP focus in the spatial and temporal tasks (top) demonstrate spatial selectivity. Time axis is in terms of the number of scans (each scan comprised 1.5 s). Based on data in Stilla et al. (2007). A: anterior; P: posterior.
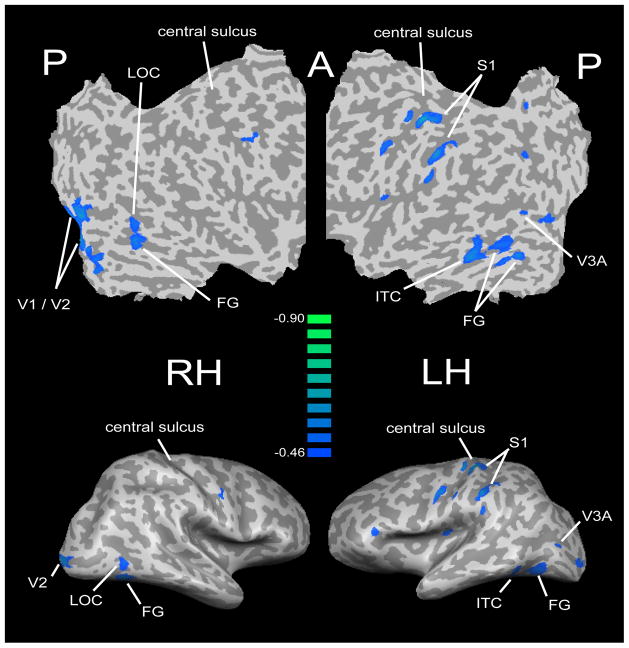
Figure 2.
Flat maps (top) and lateral views of inflated representations (bottom) of the right and left hemisphere (RH, LH) showing regions whose activation magnitude in the offset task (relative to rest) predicted tactile acuity threshold in a group of blind subjects including early and late blind. Scale represents correlation coefficient (r) of this predictive relationship. Modified from Stilla et al. (2008b). Abbreviations in Figures 1&2 refer to areas discussed in the text.
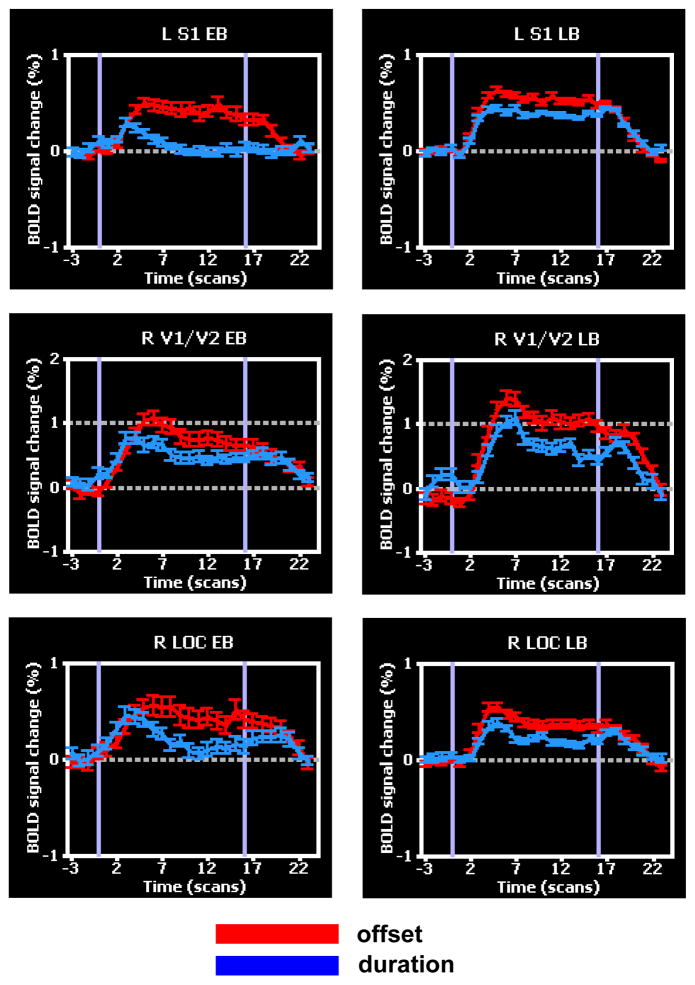
Figure 3.
Time-courses of BOLD signal at selected foci from Figure 2, in the spatial (offset) and temporal (stimulus duration) tasks. These foci were spatially selective in both early blind (EB) and late blind (LB). Modified from Stilla et al. (2008b).
Acknowledgments
Support to KS over the years from the NEI, NINDS, NSF and the VA is gratefully acknowledged. The authors thank past and present colleagues for their invaluable contributions.
References
- Alho K, Kujala T, Paavilainen P, Summala H, Näätänen R. Auditory processing in visual brain areas of the early blind: evidence from event-related potentials. EEG Clin Neurophysiol. 1993;86:418–427. doi: 10.1016/0013-4694(93)90137-k. [DOI] [PubMed] [Google Scholar]
- Amedi A, Floel A, Knecht S, Zohary E, Cohen LG. Transcranial magnetic stimulation of the occipital pole interferes with verbal processing in blind subjects. Nature Neurosci. 2004;7:1266–70. doi: 10.1038/nn1328. [DOI] [PubMed] [Google Scholar]
- Amedi A, Jacobson G, Hendler T, Malach R, Zohary E. Convergence of visual and tactile shape processing in the human lateral occipital complex. Cereb Cortex. 2002;12:1202–1212. doi: 10.1093/cercor/12.11.1202. [DOI] [PubMed] [Google Scholar]
- Amedi A, Malach R, Hendler T, Peled S, Zohary E. Visuo-haptic object-related activation in the ventral visual pathway. Nature Neurosci. 2001;4:324–330. doi: 10.1038/85201. [DOI] [PubMed] [Google Scholar]
- Amedi A, Raz N, Pianka P, Malach R, Zohary E. Early ‘visual’ cortex activation correlates with superior verbal memory performance in the blind. Nature Neurosci. 2003;6:758–766. doi: 10.1038/nn1072. [DOI] [PubMed] [Google Scholar]
- Amedi A, Stern WM, Camprodon JA, Bermpohl F, Merabet L, Rotman S, Hemond C, Meijer P, Pascual-Leone A. Shape conveyed by visual-to-auditory substitution activates the lateral occipital complex. Nature Neurosci. 2007;10:687–689. doi: 10.1038/nn1912. [DOI] [PubMed] [Google Scholar]
- Bailes SM, Lambert RM. Cognitive aspects of haptic form recognition by blind and sighted subjects. Brit J Psychol. 1986;77:451–458. doi: 10.1111/j.2044-8295.1986.tb02210.x. [DOI] [PubMed] [Google Scholar]
- Blake R, Sobel KV, James TW. Neural synergy between kinetic vision and touch. Psychol Sci. 2004;15:397–402. doi: 10.1111/j.0956-7976.2004.00691.x. [DOI] [PubMed] [Google Scholar]
- Boroojerdi B, Bushara KO, Corwell B, Immisch I, Battaglia F, Muellbacher W, Cohen LG. Enhanced excitability of the human visual cortex induced by short-term light deprivation. Cereb Cortex. 2000;10:529–534. doi: 10.1093/cercor/10.5.529. [DOI] [PubMed] [Google Scholar]
- Büchel C, Price C, Frackowiak RSJ, Friston K. Different activation patterns in the visual cortex of late and congenitally blind subjects. Brain. 1998a;121:409–419. doi: 10.1093/brain/121.3.409. [DOI] [PubMed] [Google Scholar]
- Büchel C, Price C, Friston K. A multimodal language region in the ventral visual pathway. Nature. 1998b;394:274–277. doi: 10.1038/28389. [DOI] [PubMed] [Google Scholar]
- Burton H, Diamond JB, McDermott KB. Dissociating cortical regions activated by semantic and phonological tasks: a fMRI study in blind and sighted people. J Neurophysiol. 2003;90:1965–1982. doi: 10.1152/jn.00279.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton H, Sinclair RJ, McLaren DG. Cortical activity to vibrotactile stimulation: an fMRI study in blind and sighted individuals. Hum Brain Mapp. 2004;23:210–228. doi: 10.1002/hbm.20064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton H, Snyder AZ, Conturo TE, Akbudak E, Ollinger JM, Raichle ME. Adaptive changes in early and late blind: a fMRI study of Braille reading. J Neurophysiol. 2002a;87:589–607. doi: 10.1152/jn.00285.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton H, Snyder AZ, Diamond JB, Raichle ME. Adaptive changes in early and late blind: a fMRI study of verb generation to heard nouns. J Neurophysiol. 2002b;88:3359–3371. doi: 10.1152/jn.00129.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen LG, Celnik P, Pascual-Leone A, Corwell B, Faiz L, Dambrosia J, Honda M, Sadato N, Gerloff C, Catala MD, Hallett M. Functional relevance of cross-modal plasticity in blind humans. Nature. 1997;389:180–183. doi: 10.1038/38278. [DOI] [PubMed] [Google Scholar]
- Cohen LG, Weeks RA, Sadato N, Celnik P, Ishii K, Hallett M. Period of susceptibility for cross-modal plasticity in the blind. Ann Neurol. 1999;45:451–460. doi: 10.1002/1531-8249(199904)45:4<451::aid-ana6>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Collignon O, Voss P, Lassonde M, Lepore F. Cross-modal plasticity for the spatial processing of sounds in visually deprived subjects. Exp Brain Res. 2009;192:343–358. doi: 10.1007/s00221-008-1553-z. [DOI] [PubMed] [Google Scholar]
- Craig JC. The role of experience in tactual pattern perception: a preliminary report. Int J Rehabil Res. 1988;11:167–183. [Google Scholar]
- Deshpande G, Hu X, Stilla R, Sathian K. Effective connectivity during haptic perception: a study using Granger causality analysis of functional magnetic resonance imaging data. Neuro Image. 2008;40:1807–1814. doi: 10.1016/j.neuroimage.2008.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Volder AG, Toyama H, Kimura Y, Kiyosawa M, Nakano H, Vanlierde A, Wanet-Defalque MC, Mishina M, Oda K, Ishiwata K, Senda M. Auditory triggered mental imagery of shape involves visual association areas in early blind humans. Neuro Image. 2001;14:129–139. doi: 10.1006/nimg.2001.0782. [DOI] [PubMed] [Google Scholar]
- Easton RD, Greene AJ, Srinivas K. Transfer between vision and haptics: memory for 2-D patterns and 3-D objects. Psychonomic Bull Rev. 1997a;4:403–410. [Google Scholar]
- Easton RD, Srinivas K, Greene AJ. Do vision and haptics share common representations? Implicit and explicit memory within and between modalities. J Exp Psychol: Learn Mem Cogn. 1997b;23:153–163. doi: 10.1037//0278-7393.23.1.153. [DOI] [PubMed] [Google Scholar]
- Facchini S, Aglioti SM. Short term light deprivation increases tactile spatial acuity in humans. Neurology. 2003;60:1998–1999. doi: 10.1212/01.wnl.0000068026.15208.d0. [DOI] [PubMed] [Google Scholar]
- Feinberg TE, Rothi LJ, Heilman KM. Multimodal agnosia after unilateral left hemisphere lesion. Neurology. 1986;36:864–867. doi: 10.1212/wnl.36.6.864. [DOI] [PubMed] [Google Scholar]
- Foulke E, Warm JS. Effects of complexity and redundancy on the tactual recognition of metric figures. Percept Mot Skills. 1967;25:177–187. doi: 10.2466/pms.1967.25.1.177. [DOI] [PubMed] [Google Scholar]
- Garg A, Schwartz D, Stevens AA. Orienting auditory spatial attention engages frontal eye fields and medial occipital cortex in congenitally blind humans. Neuropsychologia. 2007;45:2307–2321. doi: 10.1016/j.neuropsychologia.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldreich D, Kanics IM. Tactile acuity is enhanced in blindness. J Neurosci. 2003;23:3439–3445. doi: 10.1523/JNEUROSCI.23-08-03439.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal MS, Hansen PJ, Blakemore CB. Tactile perception recruits functionally related visual areas in the late blind. Neuro Report. 2006;17:1381–1384. doi: 10.1097/01.wnr.0000227990.23046.fe. [DOI] [PubMed] [Google Scholar]
- Grant AC, Thiagarajah MC, Sathian K. Tactile perception in blind Braille readers: A psychophysical study of acuity and hyperacuity using gratings and dot patterns. Percept Psychophys. 2000;62:301–312. doi: 10.3758/bf03205550. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Kushnir T, Hendler T, Edelman S, Itzchak Y, Malach R. A sequence of object-processing stages revealed by fMRI in the human occipital lobe. Hum Brain Mapp. 1998;6:316–328. doi: 10.1002/(SICI)1097-0193(1998)6:4<316::AID-HBM9>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen MC, Franzen O, McGlone F, Essick G, Dancer C, Pardo JV. Tactile motion activates the human middle temporal/V5 (MT/V5) complex. Eur J Neurosci. 2002;16:957–964. doi: 10.1046/j.1460-9568.2002.02139.x. [DOI] [PubMed] [Google Scholar]
- Hamilton R, Keenan JP, Catala M, Pascual-Leone A. Alexia for Braille following bilateral occipital stroke in an early blind woman. Neuro Report. 2000;11:237–240. doi: 10.1097/00001756-200002070-00003. [DOI] [PubMed] [Google Scholar]
- Heller MA. Picture and pattern perception in the sighted and the blind: the advantage of the late blind. Perception. 1989a;18:379–389. doi: 10.1068/p180379. [DOI] [PubMed] [Google Scholar]
- Heller MA. Texture perception in sighted and blind observers. Percept Psychophys. 1989b;45:49–54. doi: 10.3758/bf03208032. [DOI] [PubMed] [Google Scholar]
- Hyvärinen J, Carlson S, Hyvärinen L. Early visual deprivation alters modality of neuronal responses in area 19 of monkey cortex. Neurosci Lett. 1981;26:239–243. doi: 10.1016/0304-3940(81)90139-7. [DOI] [PubMed] [Google Scholar]
- James TW, Blake R. Perceiving object motion using vision and touch. Cognit Affect Behav Neurosci. 2004;4:201–207. doi: 10.3758/cabn.4.2.201. [DOI] [PubMed] [Google Scholar]
- James TW, Humphrey GK, Gati JS, Servos P, Menon RS, Goodale MA. Haptic study of three-dimensional objects activates extrastriate visual areas. Neuropsychologia. 2002;40:1706–1714. doi: 10.1016/s0028-3932(02)00017-9. [DOI] [PubMed] [Google Scholar]
- James TW, James KH, Humphrey GK, Goodale MA. Do visual and tactile object representations share the same neural substrate? In: Heller MA, Ballesteros S, editors. Touch and Blindness: Psychology and Neuroscience. Mahwah NJ: Lawrence Erlbaum Associates; 2006. pp. 139–155. [Google Scholar]
- Kauffman T, Theoret H, Pascual-Leone A. Braille character discrimination in blindfolded human subjects. Neuro Report. 2002;13:571–574. doi: 10.1097/00001756-200204160-00007. [DOI] [PubMed] [Google Scholar]
- Kujala T, Alho K, Paavilainen P, Summala H, Näätänen R. Neural plasticity in processing of sound location by the early blind: an event-related potential study. EEG Clin Neurophysiol. 1992;84:469–472. doi: 10.1016/0168-5597(92)90034-9. [DOI] [PubMed] [Google Scholar]
- Kujala T, Huotilainen M, Sinkkonen J, Ahonen AI, Alho K, Hämäläinen MS, Ilmoniemi RJ, Kajola M, Knuutila JET, Lavikainen J, Salonen O, Simola J, Standertskjöld-Nordenstam C-G, Tiitinen H, Tissari SO, Näätänen R. Visual cortex activation in blind humans during sound discrimination. Neurosci Lett. 1995;183:143–146. doi: 10.1016/0304-3940(94)11135-6. [DOI] [PubMed] [Google Scholar]
- Kujala T, Palva MJ, Salonen O, Alku P, Huotilainen M, Järvinen A, Näätänen R. The role of blind humans’ visual cortex in auditory change detection. Neurosci Lett. 2005;379:127–131. doi: 10.1016/j.neulet.2004.12.070. [DOI] [PubMed] [Google Scholar]
- Lacey S, Pappas M, Kreps A, Lee K, Sathian K. View-independence of visuo-haptic object representations. Submitted. doi: 10.1007/s00221-009-1856-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey S, Peters A, Sathian K. Cross-modal object recognition is viewpoint-independent. PLoS ONE. 2007;2:e890. doi: 10.1371/journal.pone.0000890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederman SJ, Klatzky RL, Chataway C, Summers CD. Visual mediation and the haptic recognition of two-dimensional pictures of common objects. Percept Psychophys. 1990;47:54–64. doi: 10.3758/bf03208164. [DOI] [PubMed] [Google Scholar]
- Lucan JN, Foxe JJ, Gomez-Ramirez M, Sathian K, Molholm S. The spatio-temporal dynamics of somatosensory shape discrimination. IMRF Abstracts 2008 [Google Scholar]
- Malach R, Reppas JB, Benson RR, Kwong KK, Jiang H, Kennedy WA, Ledden PJ, Brady TJ, Rosen BR, Tootell RB. Object-related activity revealed by functional magnetic resonance imaging in human occipital cortex. Proc Natl Acad Sci USA. 1995;92:8135–8139. doi: 10.1073/pnas.92.18.8135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merabet LB, Hamilton R, Schlaug G, Swisher JD, Kiriakopoulos ET, Pitskel NB, Kauffman T, Pascual-Leone A. Rapid and reversible recruitment of early visual cortex for touch. PLoS ONE. 2008;3(8):e3046. doi: 10.1371/journal.pone.0003046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merabet LB, Swisher JD, McMains SA, Halko MA, Amedi A, Pascual-Leone A, Somers DC. Combined activation and deactivation of visual cortex during tactile sensory processing. J Neurophysiol. 2007;97:1633–1641. doi: 10.1152/jn.00806.2006. [DOI] [PubMed] [Google Scholar]
- Merabet LB, Thut G, Murray B, Andrews J, Hsiao S, Pascual-Leone A. Feeling by sight or seeing by touch? Neuron. 2004;42:173–179. doi: 10.1016/s0896-6273(04)00147-3. [DOI] [PubMed] [Google Scholar]
- Morrongiello BA, Humphrey K, Timney B, Choi J, Rocca PT. Tactual object exploration and recognition in blind and sighted children. Perception. 1994;23:833–848. doi: 10.1068/p230833. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Torres F. Plasticity of the sensorimotor cortex representation of the reading finger in Braille readers. Brain. 1993;116:39–52. doi: 10.1093/brain/116.1.39. [DOI] [PubMed] [Google Scholar]
- Peltier S, Stilla R, Mariola E, LaConte S, Hu X, Sathian K. Activity and effective connectivity of parietal and occipital cortical regions during haptic shape perception. Neuropsychologia. 2007;45:476–483. doi: 10.1016/j.neuropsychologia.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Pietrini P, Furey ML, Ricciardi E, Gobbini MI, Wu W-HC, Cohen L, Guazzelli M, Haxby JV. Beyond sensory images: object-based representation in the human ventral pathway. Proc Natl Acad Sci USA. 2004;101:5658–5663. doi: 10.1073/pnas.0400707101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitzalis S, Galletti C, Huang R-S, Patria F, Committeri G, Galati G, Fattori P, Sereno MI. Wide-field retinotopy defines human cortical visual area V6. Journal of Neuroscience. 2006;26:7962–7973. doi: 10.1523/JNEUROSCI.0178-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptito M, Moesgaard SM, Gjedde A, Kupers R. Cross-modal plasticity revealed by electrotactile stimulation of the tongue in the congenitally blind. Brain. 2005;128:606–614. doi: 10.1093/brain/awh380. [DOI] [PubMed] [Google Scholar]
- Ptito M, Fumal A, Martens de Noordhout A, Schoenen J, Gjedde A, Kupers R. TMS of the occipital cortex induces tactile sensations in the fingers of blind Braille readers. Exp Brain Res. 2008;184:193–200. doi: 10.1007/s00221-007-1091-0. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP, Tian B, Korte M, Egert U. Crossmodal changes in the somatosensory vibrissa/barrel system of visually deprived animals. Proc Natl Acad Sci USA. 1992;89:5063–5067. doi: 10.1073/pnas.89.11.5063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reales JM, Ballesteros S. Implicit and explicit memory for visual and haptic objects: cross-modal priming depends on structural descriptions. J Exp Psychol: Learn Mem Cogn. 1999;25:644–663. [Google Scholar]
- Reed CM, Doherty MJ, Braida LD, Durlach NI. Analytic study of the Tadoma method: further experiments with inexperienced observers. J Speech Hearing Res. 1982;25:216–223. doi: 10.1044/jshr.2502.216. [DOI] [PubMed] [Google Scholar]
- Reed CM, Rubin SI, Braida LD, Durlach NI. Analytic study of the Tadoma method: discrimination ability of untrained observers. J Speech Hearing Res. 1978;21:625–637. doi: 10.1044/jshr.2104.625. [DOI] [PubMed] [Google Scholar]
- Ricciardi E, Vanello N, Sani L, Gentili C, Scilingo EP, Landini L, Guazzelli M, Bicchi A, Haxby JV, Pietrini P. The effect of visual experience on the development of functional architecture in hMT+ Cereb Cortex. 2007;17:2933–2939. doi: 10.1093/cercor/bhm018. [DOI] [PubMed] [Google Scholar]
- Röder B, Rösler F, Hennighausen E, Näcker F. Event-related potentials during auditory and somatosensory discrimination in sighted and blind subjects. Cogn Brain Res. 1996;4:77–93. [PubMed] [Google Scholar]
- Röder B, Stock O, Bien SNH, Rösler F. Speech processing activates visual cortex in congenitally blind humans. Eur J Neurosci. 2002;16:930–936. doi: 10.1046/j.1460-9568.2002.02147.x. [DOI] [PubMed] [Google Scholar]
- Sadato N, Okada T, Honda M, Yonekura Y. Critical period for cross-modal plasticity in blind humans: a functional MRI study. Neuro Image. 2002;16:389–400. doi: 10.1006/nimg.2002.1111. [DOI] [PubMed] [Google Scholar]
- Sadato N, Pascual-Leone A, Grafman J, Deiber M-P, Ibanez V, Hallett M. Neural networks for Braille reading by the blind. Brain. 1998;121:1213–1229. doi: 10.1093/brain/121.7.1213. [DOI] [PubMed] [Google Scholar]
- Sadato N, Pascual-Leone A, Grafman J, Ibanez V, Deiber M-P, Dold G, Hallett M. Activation of the primary visual cortex by Braille reading in blind subjects. Nature. 1996;380:526–528. doi: 10.1038/380526a0. [DOI] [PubMed] [Google Scholar]
- Sathian K, Zangaladze A. Feeling with the mind’s eye: the role of visual imagery in tactile perception. Optom Vis Sci. 2001;78:276–281. doi: 10.1097/00006324-200105000-00010. [DOI] [PubMed] [Google Scholar]
- Sathian K, Zangaladze A, Hoffman JM, Grafton ST. Feeling with the mind’s eye. Neuro Report. 1997;8:3877–3881. doi: 10.1097/00001756-199712220-00008. [DOI] [PubMed] [Google Scholar]
- Sergent J, Ohta S, MacDonald B. Functional neuroanatomy of face and object processing. A positron emission tomography study. Brain. 1992;115:15–36. doi: 10.1093/brain/115.1.15. [DOI] [PubMed] [Google Scholar]
- Shikata E, McNamara A, Sprenger A, Hamzei F, Glauche V, Büchel C, Binkofski F. Localization of human intraparietal areas AIP, CIP, and LIP using surface orientation and saccadic eye movement tasks. Hum Brain Mapp. 2008;29:411–421. doi: 10.1002/hbm.20396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterr A, Müller MM, Elbert T, Rockstroh B, Pantev C, Taub E. Perceptual correlates of changes in cortical represenataiton of fingers in blind multifinger Braille readers. J Neurosci. 1998;18:4417–4423. doi: 10.1523/JNEUROSCI.18-11-04417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens AA, Snodgrass M, Schwartz D, Weaver K. Preparatory activity in occipital cortex in early blind humans predicts auditory perceptual performance. J Neurosci. 2007;27:10734–10741. doi: 10.1523/JNEUROSCI.1669-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JC, Foulke E, Patterson MQ. Tactile acuity, aging and Braille reading in long-term blindness. J Exp Psychol: Applied. 1996;2:91–106. [Google Scholar]
- Stilla R, Deshpande G, LaConte S, Hu X, Sathian K. Posteromedial parietal cortical activity and inputs predict tactile spatial acuity. J Neurosci. 2007;27:11091–11102. doi: 10.1523/JNEUROSCI.1808-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stilla R, Sathian K. Selective visuo-haptic processing of shape and texture. Hum Brain Mapp. 2008;29:1123–1138. doi: 10.1002/hbm.20456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stilla R, Lacey S, Deshpande G, Hu X, Sathian K. Effective connectivity during haptic shape perception is modulated by object familiarity. Soc Neurosci Abstr. 2008a;189:19. doi: 10.1016/j.neuroimage.2009.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stilla R, Hanna R, Hu X, Mariola E, Deshpande G, Sathian K. Neural processing underlying tactile microspatial discrimination in the blind: a functional magnetic resonance imaging study. J Vision. 2008b;813(10):1–19. doi: 10.1167/8.10.13. http://journalofvision.org/8/10/13/ [DOI] [PMC free article] [PubMed]
- Stoesz M, Zhang M, Weisser VD, Prather SC, Mao H, Sathian K. Neural networks active during tactile form perception: common and differential activity during macrospatial and microspatial tasks. Int J Psychophysiol. 2003;50:41–49. doi: 10.1016/s0167-8760(03)00123-5. [DOI] [PubMed] [Google Scholar]
- Toldi J, Farkas T, Völgyi B. Neonatal enucleation induces cross-modal changes in the barrel cortex of rat. A behavioural and electrophysiological study. Neurosci Lett. 1994a;167:1–4. doi: 10.1016/0304-3940(94)91014-6. [DOI] [PubMed] [Google Scholar]
- Toldi J, Rojik I, Feher O. Neonatal monocular enucleation-induced cross-modal effects observed in the cortex of adult rat. Neuroscience. 1994b;62:105–114. doi: 10.1016/0306-4522(94)90318-2. [DOI] [PubMed] [Google Scholar]
- Van Boven RW, Hamilton RH, Kauffman T, Keenan JP, Pascual-Leone A. Tactile spatial resolution in blind Braille readers. Neurology. 2000;54:2230–2236. doi: 10.1212/wnl.54.12.2230. [DOI] [PubMed] [Google Scholar]
- Van Boven RW, Johnson KO. The limit of tactile spatial resolution in humans: grating orientation discrimination at the lip, tongue and finger. Neurology. 1994;44:2361–2366. doi: 10.1212/wnl.44.12.2361. [DOI] [PubMed] [Google Scholar]
- Veraart C, De Volder AG, Wanet-Defalque M-C, Bol A, Michel C, Goffinet AM. Glucose utilization in human visual cortex is abnormally elevated in blindness of early onset but decreased in blindness of late onset. Brain Res. 1990;510:115–121. doi: 10.1016/0006-8993(90)90735-t. [DOI] [PubMed] [Google Scholar]
- Weaver KE, Stevens AA. Attention and sensory interactions within the occipital cortex in the early blind: an fMRI study. J Cognit Neurosci. 2007;19:315–330. doi: 10.1162/jocn.2007.19.2.315. [DOI] [PubMed] [Google Scholar]
- Weisser V, Stilla R, Peltier S, Hu X, Sathian K. Short-term visual deprivation alters neural processing of tactile form. Exp Brain Res. 2005;166:572–582. doi: 10.1007/s00221-005-2397-4. [DOI] [PubMed] [Google Scholar]
- Wheat HW, Goodwin AW. Tactile discrimination of gaps by slowly adapting afferents: effects of population parameters and anisotropy in the fingerpad. J Neurophysiol. 2000;84:1430–1444. doi: 10.1152/jn.2000.84.3.1430. [DOI] [PubMed] [Google Scholar]
- Zangaladze A, Epstein CM, Grafton ST, Sathian K. Involvement of visual cortex in tactile discrimination of orientation. Nature. 1999;401:587–590. doi: 10.1038/44139. [DOI] [PubMed] [Google Scholar]
- Zhang M, Weisser VD, Stilla R, Prather SC, Sathian K. Multisensory cortical processing of object shape and its relation to mental imagery. Cognit Affect Behav Neurosci. 2004;4:251–259. doi: 10.3758/cabn.4.2.251. [DOI] [PubMed] [Google Scholar]