Abstract
Vascular gene therapy could potentially complement or replace current therapies for human atherosclerosis, while avoiding their side effects. However, development of vascular gene therapy is limited by lack of a useful vector. Helper-dependent adenovirus (HDAd) shows promise to overcome this barrier because, unlike first-generation adenovirus, HDAd achieves durable transgene expression in the artery wall with minimal inflammation. To begin to test whether HDAd, delivered to the artery wall, can limit atherosclerosis we constructed HDAd that expresses rabbit interleukin (IL)-10, a potent atheroprotective cytokine, and tested its activity in a rabbit model of early carotid atherogenesis. HDAd expressed immunoreactive, active IL-10 in vitro. In contrast to other HDAd-expressed transgenes, IL-10 expression from HDAd increased significantly between 3 days and 2 weeks after infusion and remained stable for at least 8 weeks. Rising, persistent IL-10 expression was associated with relative persistence of HDAdIL-10 genomes 4 weeks after infusion, compared with HDAdNull genomes. Surprisingly, IL-10 expression had no significant effects on atherosclerotic lesion size, macrophage content, or expression of either adhesion molecules or atherogenic cytokines. These results might be due to inadequate protein expression in vivo or lack of suitability of this rabbit model to reveal IL-10 therapeutic effects. IL-10 remains a promising agent for vascular gene therapy and HDAd remains a promising vector; however, proof of efficacy of HDAdIL-10 is elusive. Future preclinical studies will be aimed at increasing IL-10 expression levels and improving the sensitivity of this animal model to detect atheroprotective effects.
Du and colleagues examine the efficacy of delivering interleukin (IL)-10 via a helper-dependent adenovirus (HDAd) in a rabbit model of early carotid atherogenesis. The authors report that this treatment has no significant effects on atherosclerotic lesion size, macrophage content, or expression of either adhesion molecules or atherogenic cytokines.
Introduction
Atherosclerosis is a chronic inflammatory disease of the blood vessel wall caused by growth of plaques made up primarily of lipid and inflammatory cells (Lusis, 2000). Clinical manifestations of atherosclerosis include heart attacks, strokes, and lower limb amputations, all caused by obstruction of blood vessels by atherosclerotic plaques or superimposed thrombosis. Despite major advances in medical and surgical therapies, atherosclerosis remains a leading cause of death in Western societies and a growing cause of death in the developing world (Gersh et al., 2010; Lloyd-Jones et al., 2010). New, cost-effective approaches are needed for atherosclerosis prevention and treatment.
For several reasons, gene therapy is an attractive approach for preventing and treating atherosclerosis. First, much is known about the molecular pathogenesis of atherosclerosis, allowing rational selection of therapeutic genes (Lusis, 2000; Rissanen and Ylä-Herttuala, 2007). Second, gene therapy can potentially provide stable, lifelong treatment (Aiuti et al., 2009), suitable for a lifelong disease such as atherosclerosis. Third, gene therapy for atherosclerosis would likely be cost-effective. A single dose of a gene therapy vector that durably treats or prevents atherosclerosis would potentially cost far less than the current therapeutic approach of lifelong multidrug therapy with occasional superimposed percutaneous coronary intervention and bypass surgery. Fourth, gene therapy for atherosclerosis that is delivered directly to the blood vessel wall could potentially treat and prevent atherosclerosis without any systemic side effects. The potential for gene therapy to deliver strictly local therapy contrasts with current atheroprotective drug therapies that, despite their general efficacy, are delivered systemically and are often associated with bothersome side effects (Hippisley-Cox and Coupland, 2010).
We and others have begun to develop blood vessel-targeted atheroprotective gene therapy, using animal models of atherosclerosis. Several of these experiments, performed primarily with first-generation adenoviral (FGAd) vectors, show atheroprotective effects (Kawashiri Ma and Rader, 2000; Ylä-Herttuala and Martin, 2000). However, these therapeutic effects have typically been of only brief duration because expression from FGAd in the artery wall persists in vivo for only about 2 weeks (Gruchala et al., 2004). In addition, infusion of FGAd provokes a local inflammatory response in the artery wall, and enhanced vascular inflammation would likely accelerate atherosclerosis (Newman et al., 1995; Gruchala et al., 2004). The advent of third-generation or “helper-dependent” adenoviral (HDAd) vectors (Chen et al., 1996; Parks et al., 1996) provides a potential solution to both of these problems because HDAd can express a transgene for at least 8 weeks (the latest time point tested) in the artery wall and for well over 2 years in other tissues (Wen et al., 2004; Brunetti-Pierri et al., 2009). Moreover, in contrast to FGAd, infusion of HDAd to the artery wall produces only mild inflammation (Wen et al., 2004).
To begin to test the ability of HDAd to deliver durable, yet local atheroprotective vascular gene therapy, we constructed HDAd vectors that express rabbit interleukin (IL)-10. IL-10 is a potent antiinflammatory protein (de Vries, 1995) that has been termed an “immunologic scalpel for atherosclerosis” (Terkeltaub, 1999). IL-10 is atheroprotective when delivered to mice either systemically by germ line or somatic gene transfer (Mallat et al., 1999; Pinderski Oslund et al., 1999; Von Der Thusen et al., 2001; Pinderski et al., 2002; Namiki et al., 2004; Potteaux et al., 2004; Yoshioka et al., 2004; Liu et al., 2006), or locally by first-generation adenovirus (Von Der Thusen et al., 2001). To move beyond murine studies and closer to clinical application, here we tested the efficacy of HDAd IL-10 in a large animal (rabbit) model of early carotid artery atherogenesis (Schneider et al., 2000; Falkenberg et al., 2002).
Materials and Methods
Adenoviral vectors and cell culture
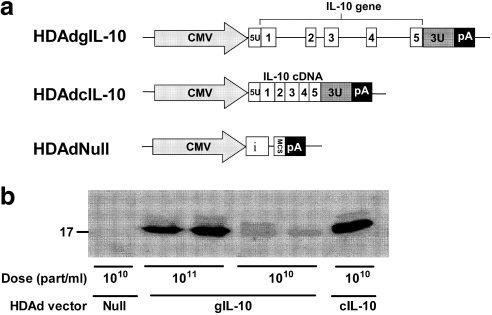
Three HDAds were used in this study: HDAdcIL-10, HDAdgIL-10, and HDAdNull (Fig. 1a). HDAdcIL-10 and HDAdgIL-10 express, respectively, a rabbit IL-10 cDNA (a kind gift from H. Perkins, Australian National University, Canberra, ACT, Australia) (Perkins et al., 2000) and a rabbit genomic IL-10 clone generated in our laboratory (GenBank accession no. DQ437508). In both vectors IL-10 expression is driven by the cytomegalovirus immediate-early promoter. HDAdNull contains the same backbone expression cassette as the two IL-10 vectors, includes a synthetic intron, but lacks any IL-10 sequences. Cloning of the rabbit IL-10 gene and construction of the IL-10 and HDAdNull vectors are described elsewhere (Dronadula et al., 2011). Vectors were amplified and propagated in 293 Cre4 cells (Microbix Biosystems, Toronto, ON, Canada) (Chen et al., 1996) coinfected with helper virus (H14) (Parks et al., 1996; Sandig et al., 2000), and purified by cesium chloride density-gradient ultracentrifugation. Vector concentrations, measured by spectrophotometry (Mittereder et al., 1996), were approximately 2–4 × 1012 particles/ml. All preparations had less than 1 E1A-containing genome in 106 vector genomes, and helper virus contamination was less than 1% as measured by quantitative real-time PCR (for primers and probes, see Supplementary Table S1; supplementary data are available online at www.liebertonline.com/hum). Before use in vivo, both IL-10 vectors were tested for their ability to express IL-10 mRNA after in vitro transduction of bovine aortic endothelial cells (BAECs; Cell Applications, San Diego, CA) (passage 7 or 8).
FIG. 1.
Helper-dependent adenoviral (HDAd) vectors express interleukin (IL)-10 protein in vitro. (a) Expression cassette structure. CMV, cytomegalovirus promoter from pCI plasmid; i, β-globin/IgG chimeric intron from pCI; MCS, multiple cloning site; pA, simian virus 40 poly(A) signal; 5U, 5′-untranslated region of the rabbit IL-10 gene; blocks 1, 2, 3, 4, and 5, rabbit IL-10 exons; 3U, 3′-untranslated region of the rabbit IL-10 gene. Drawing is not to scale. (b) HEK 293 cells were transduced with HDAdNull, HDAdcIL-10, or HDAdgIL-10, at the viral doses indicated (particles/ml). Conditioned medium was collected and analyzed by Western blotting for IL-10. Each lane is from cells in a separate well. Size marker is presented as kilodaltons (kDa).
Animals and gene transfer surgery
Experiments were performed with specific-pathogen-free adult male New Zealand White rabbits (3.0–4.0 kg; Western Oregon Rabbit Company, Philomath, OR). All animal protocols were approved by the Office of Animal Welfare of the University of Washington (Seattle, WA). In most experiments rabbits were fed chow (100 g/day) containing 0.25% cholesterol and 3% soybean oil (Dyets, Bethlehem, PA). Plasma cholesterol was measured 2, 3, and 4 weeks after beginning the high-fat diet and at the time of harvest (Abbott Spectrum; Abbott Laboratories, Abbott Park, IL). On the basis of their cholesterol levels at 4 weeks, rabbits were assigned to receive infusions of either HDAdNull or HDAdgIL-10 in such a manner that the mean cholesterol values of the HDAdNull and HDAdgIL-10 groups in each experiment were similar at the time of surgery (Supplementary Table S2). Bilateral common carotid artery gene transfer was performed as described (Schneider et al., 2000), with vectors infused at 2 × 1011 particles/ml. This approach yields gene transfer almost exclusively to luminal endothelium (Schulick et al., 1995). Vectors used in the in vivo study were from a single preparation each of HDAdgIL-10 and HDAdNull. We used HDAdgIL-10 instead of HDAdcIL-10 in vivo because preliminary data generated in chow-fed rabbits suggested that HDAdgIL-10 expressed more IL-10 mRNA in vivo than did HDAdcIL-10 (Dronadula et al., 2011).
Western analysis
IL-10 was detected by Western analysis of medium conditioned by transduced 293 Cre4 cells (Chen et al., 1996), BAECs, or by explanted, in vivo-transduced rabbit carotid arteries. Cells were transduced by incubation with HDAd at 1 × 1010 or 1 × 1011 particles/ml for 6 hr. Medium was then changed to Dulbecco's modified Eagle's medium (DMEM), collected after 24 hr, and frozen at −80°C. Carotid artery gene transfer was performed with HDAdgIL-10 or HDAdNull, as described previously, and arteries were removed 14 days after vector infusion. Two segments were cut from each artery and placed in culture, and conditioned medium was collected as described further below. To optimize detection of IL-10 protein, samples of conditioned medium from several arteries were pooled and concentrated with centrifugal filter units (3K NMWL [nominal molecular weight limit]; Millipore, Billerica, MA) before analysis. Conditioned medium samples (50 μl) were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) (0.1% SDS, 15% polyacrylamide), and proteins were transferred to an Immobilon-P membrane (Millipore). After blocking membranes with 10 mM Tris-HCl buffer, pH 8.0, containing 150 mM sodium chloride, 0.1% Tween 20, and 5% (w/v) nonfat dry milk, IL-10 protein was detected by incubation with goat anti-human IL-10 antibodies (R&D Systems, Minneapolis, MN) followed by peroxidase-conjugated donkey anti-goat secondary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) and detection of bound antibody by chemiluminescence (Immun-Star WesternC kit; Bio-Rad Laboratories, Hercules, CA).
IL-10 functional assay
We generated IL-10-containing and control medium by transducing 293 Cre4 cells with one of the HDAdIL-10 vectors or with HDAdNull (1 × 1010 particles/ml). To permit replication of the HDAd genomes and potentially generate higher concentrations of IL-10 protein, helper virus was added at a multiplicity of infection of 100. The 293 Cre4 cells were then incubated in DMEM with 10% fetal bovine serum (FBS) for 6 hr, infection medium was removed, cells were washed twice with DMEM without FBS, and DMEM was added. Conditioned medium was collected after 24 hr, concentrated 12-fold (to a final volume of 1 ml) with the centrifugal filter units described previously, and stored at −80°C. The presence of IL-10 in the medium from HDAdIL-10-transduced cells and the absence of IL-10 in medium from HDAdNull-transduced cells was confirmed by Western blotting.
Peripheral blood mononuclear cells (PBMCs) were isolated from unoperated rabbits by ear vein phlebotomy, centrifugation of blood for 15 min at 2000 × g, removal of plasma, and aspiration of the buffy coat layer. PBMCs were pelleted at 600 × g and 1 ml of room-temperature red blood cell (RBC) lysis buffer (155 mM NH4Cl, 10 mM KHCO3, 1 mM EDTA) was added to the cells for 2 min. One milliliter of ice-cold PBS with heparin (5 U/ml) and 2% FBS was then added to the cells, followed by centrifugation at 600 × g for 5 min at 4°C. This process (RBC lysis followed by addition of ice-cold PBS) was repeated and the PBMCs were then resuspended in RPMI 1640 with 10% FBS and 1% penicillin–streptomycin. PBMCs from three to six animals were pooled, and 1.2 × 106 cells per well were incubated overnight with 1 ml of medium in a 6-well tissue-culture plate at 37°C. Cytokine expression was induced by addition of lipopolysaccharide (LPS, 200 ng/ml) (L4391; Sigma-Aldrich, St. Louis, MO) followed immediately by addition of 200 μl of IL-10-containing or control conditioned medium. After 5 hr, cells were lysed and RNA was extracted with an RNeasy mini kit (Qiagen, Valencia, CA).
Harvest of carotid arteries, peripheral organs, and blood
Carotid arteries were harvested 3, 14, 28, and 56 days postoperatively. To measure the consistency of gene transfer along an artery, a group of arteries was harvested 3 days after gene transfer and cut into four segments, and each segment was analyzed for IL-10 mRNA (Supplementary Fig. S1a). All other arteries were cut into seven pieces, to be used for molecular and histological analyses (Supplementary Fig. S1b). Of these seven segments, four evenly spaced segments were embedded in O.C.T. compound for frozen sectioning; two segments were placed in culture to generate conditioned medium followed by extraction and measurement of vector DNA and one segment was used for RNA analyses. Conditioned medium was generated by placement of segments in ice-cold DMEM, trimming their periadventitial tissue, opening them longitudinally, and incubating them at 37°C for 1 hr followed by vigorous rinsing twice to remove residual plasma proteins. Segments were then placed in DMEM at 37°C for 24 hr, conditioned medium was collected and frozen at −80°C, and the segments were frozen for later DNA extraction. We also harvested approximately 2 g of spleen and liver during the 3-day artery harvests. Tissues were snap-frozen in liquid nitrogen and RNA was extracted later as described below for the arteries. Venous blood was collected preoperatively and at the time of artery harvest for measurement of plasma cholesterol, complete blood counts, and serum chemistries (the latter two tests done by Phoenix Central Laboratories, Everett, WA).
Quantification of IL-10 mRNA and vector DNA
RNA was extracted with an RNeasy mini kit (Qiagen). IL-10 mRNA was measured by quantitative reverse-transcriptase (qRT)-mediated PCR, using the ΔΔCt method (Schmittgen and Livak, 2008) with RNA from a single HDAdNull-infused rabbit carotid as the “calibrator.” IL-10 mRNA values were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA, measured in the same samples. DNA was extracted from two segments per carotid artery with the DNeasy kit (Qiagen), and quantified by spectrophotometry (NanoDrop Products, Wilmington, DE). Vector copy numbers were measured in 100 ng of total DNA extracted from two segments per artery and then combined, using quantitative real-time PCR (qPCR) amplification of a sequence in the cytomegalovirus (CMV) promoter and reference to a standard curve generated with a CMV promoter-containing plasmid. Primers (Bioneer, Alameda, CA) and probes (Life Technologies [Carlsbad, CA] or Integrated DNA Technologies [Coralville, IA]) for measuring IL-10, GAPDH, and CMV sequences are listed in Supplementary Table S1.
Histochemical and immunohistochemical staining
O.C.T. compound blocks containing four evenly spaced artery segments were cut into 6-μm-thick sections. To maximize the likelihood that intact sections from each of the four segments would be available for analysis, two sets of step sections from each block, 180 μm apart, were placed on a single slide. Serial sections taken at each step were placed on separate slides and stained with hematoxylin and eosin (H&E) or Verhoeff–Van Gieson (VVG) stains, as well as with antibodies that detect macrophages (RAM-11, 1:50 dilution; Dako, Carpinteria, CA), vascular cell adhesion molecule (VCAM)-1, and intercellular adhesion molecule (ICAM)-1 (Rb1/9 and Rb2/3, both at 1:50 dilution; from M. Cybulsky, University of Toronto, Toronto, ON, Canada). The specificity of the primary antibody binding was tested by substituting an isotype-matched antibody (for RAM-11) (14-4714; eBioscience, San Diego, CA) or by omitting the primary antibody (for VCAM-1 and ICAM-1).
Analysis of histological sections
Digital images of sections were obtained with the Leica DFC295 system (Leica Microsystems, Wetzlar, Germany) and analyzed by observers blinded to the identity of the specimens. Some of the analyses were performed with an image analysis program (Image-Pro Plus; Media Cybernetics, Bethesda, MD). Intimal and medial areas were calculated on the basis of planimetry of the luminal surface, internal elastic lamina, and external elastic lamina. Intimal area staining positive for RAM-11 was measured with the image analysis program and color thresholding, and the percentage area of RAM-11-stained area was calculated by dividing the RAM-11-positive area by the total intimal area measured on the same slide. The extent of intimal ICAM-1 and VCAM-1 staining per section was scored according to a semiquantitative scale (Newman et al., 1995). Four sections per vessel (one section from each of the four embedded segments) were analyzed for each end point and the mean of these four values was used as a single value for each artery. These mean values (one per artery) were used for group comparisons.
Cytokine expression
Levels of mRNAs encoding tumor necrosis factor (TNF)-α, monocyte chemotactic protein (MCP)-1, IL-6, IL-1β, and interferon (IFN)-γ were measured by qRT-PCR, using the ΔΔCt method. For each assay, the sample with the lowest Ct value was used as the calibrator, and values for cytokine mRNA were normalized to GAPDH mRNA measured in the same samples. Primers and probes are listed in Supplementary Table S1.
Measurement of serum anti-adenoviral antibodies
Serum was collected from rabbits preoperatively and at the time of vessel harvest (both 14 and 28 days postoperatively) and frozen until use. Antibodies to human adenovirus type 5 were detected as described (Wen et al., 2000), using serum samples diluted 1:500 and wells coated with an adenovirus type 5-based vector.
Statistical analyses
Results are reported as means ± SEM or as medians and interquartile ranges for data not normally distributed. Normally distributed data with equal group variances were compared by unpaired t test. Except as noted, other data were compared by Mann–Whitney rank-sum test. Comparisons between multiple groups were made by one-way analysis of variance (ANOVA). These tests and the sample size calculations were performed with the SigmaStat software package (Systat Software, San Jose, CA).
Results
HDAdgIL-10 produces biologically active IL-10
Immunoreactive IL-10 was detected by Western analysis of medium conditioned by cells transduced with either HDAdgIL-10 or HDAdcIL-10, but not in medium conditioned by cells transduced with HDAdNull (Fig. 1b). Concentrated conditioned media from both HDAdgIL-10- and HDAdcIL-10-transduced cells were used for the IL-10 activity assay.
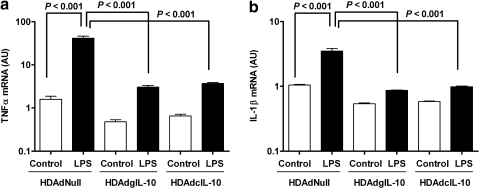
Treatment of rabbit PBMCs with LPS in the presence of conditioned medium from HDAdNull-transduced 293 Cre4 cells increased TNF-α and IL-1β mRNAs (30- and 3-fold, respectively; p < 0.001 for both; Fig. 2). Exposure of PBMCs to vector-expressed IL-10 (in medium conditioned by 293 Cre4 cells transduced with either HDAdgIL-10 or HDAdcIL-10) substantially reduced both baseline and LPS-stimulated expression of both cytokines (≥95% reduction of LPS-stimulated cytokine expression; p ≤ 0.001 for both; Fig. 2). This assay was performed two more times with the more abundant HDAdcIL-10-generated protein (Fig. 1b), and yielded similar results (Supplementary Fig. S2).
FIG. 2.
Activity of vector-expressed IL-10. Rabbit peripheral blood mononuclear cells were treated with conditioned medium generated by transduction of 293 Cre4 cells with HDAdNull, HDAdgIL-10, or HDAdcIL-10. Aliquots of cells in all three groups were treated with LPS. After 5 hr, RNA was extracted and the expression of (a) TNF-α and (b) IL-1β was measured by qRT-PCR. Columns represent means of n = 3 separate wells of treated cells per group, with normal distribution assumed based on extrapolation from a larger experiment (Supplementary Fig. S2).
IL-10 mRNA is expressed evenly along the length of transduced carotid arteries
Because for our in vivo studies we planned to collect data from separate segments harvested along the length of each transduced artery, we tested the underlying hypothesis that vector-mediated transgene expression was equivalent along the length of each transduced artery. Mean IL-10 mRNA expression did not vary significantly along the length of arteries of chow-fed rabbits infused with HDAdgIL-10 (see Supplementary Fig. S3; p = 0.4 by one-way ANOVA).
Selection of dose of HDAdgIL-10 for atheroprotection studies
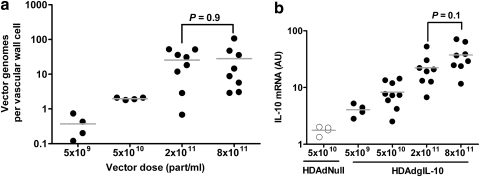
Preliminary data, generated for a separate study focused on expression cassette development, showed that although HDAdcIL-10 expressed more IL-10 than HDAdgIL-10 in BAECs (as in Fig. 1b), HDAdgIL-10 tended to express more IL-10 than HDAdcIL-10 in vivo in carotid arteries of chow-fed rabbits (Dronadula et al., 2011). We repeated a small expression study in fat-fed rabbits, and found similar expression from HDAdcIL-10 and HDAdgIL-10 (Supplementary Fig. S4). Therefore we used HDAdgIL-10 for in vivo experiments that tested the efficacy of IL-10 for atheroprotective gene therapy. To identify an optimal dose of HDAdgIL-10, we infused HDAdgIL-10 into carotids of chow-fed rabbits at concentrations ranging from 5 × 109 to 8 × 1011 particles/ml and harvested arteries 3 days later. Increasing vector concentration from 5 × 109 to 2 × 1011 particles/ml yielded steadily higher amounts of vector DNA (p = 0.002 by one-way ANOVA), with no further increase in vector DNA in arteries infused with 8 × 1011 particles/ml (Fig. 3a). IL-10 mRNA was detectable in arteries infused at 5 × 109 particles/ml, increased steadily as the vector dose was increased to 8 × 1011 particles/ml (p < 0.001 by one-way ANOVA), but did not differ significantly between 2 × 1011 and 8 × 1011 particles/ml (Fig. 3b). We therefore selected 2 × 1011 particles/ml for the remainder of the studies.
FIG. 3.
Identification of an optimal in vivo dose for HDAdgIL-10. (a) HDAdgIL-10 was infused at the indicated concentrations and arteries were harvested 3 days later. Vector genomes (measured by qPCR) are expressed as genomes per vascular wall cell, calculated on the basis of total DNA in the extracts. (b) RNA was extracted from the arteries in (a), from five additional HDAdgIL-10-infused arteries from a separate study (5 × 1010 particles/ml; Supplementary Fig. S3), and from control arteries infused with HDAdNull. IL-10 mRNA was measured by qRT-PCR and normalized to GAPDH mRNA in the same extracts. Data points represent individual arteries; bars indicate group means.
Rabbit plasma cholesterol, blood counts, and serum chemistries
Plasma cholesterol levels did not differ significantly at any time point between groups of cholesterol-fed rabbits that received infusions of either HDAdgIL-10 or HDAdNull (Supplementary Table S2). Complete blood counts performed 14 and 56 days after vector infusion revealed occasional borderline low hematocrits in rabbits receiving either vector, with no other abnormalities and no differences between the two groups (Supplementary Table S3). Serum chemistries measured 14 days after vector infusion showed no significant abnormalities in rabbits receiving either of the vectors and no differences between the two groups (Supplementary Table S3).
IL-10 mRNA and HDAd vector DNA persist for 8 weeks in vivo in arteries of cholesterol-fed rabbits
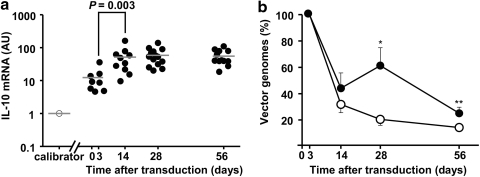
IL-10 mRNA was increased in HDAdgIL-10 versus HDAdNull arteries harvested 3, 14, 28, and 56 days after vector infusion (4- to 17-fold; p ≤ 0.001; Supplementary Fig. S5). Surprisingly, the relative increase in IL-10 mRNA in HDAdgIL-10 versus HDAdNull arteries was larger at 14–56 days than at 3 days after gene transfer, with the largest relative increases at 28 and 56 days (11- to 17-fold). To more directly test whether vector-mediated IL-10 expression increased over time, we measured IL-10 mRNA simultaneously in extracts from all of the HDAdgIL-10 arteries harvested at the four time points. An extract from a single HDAdNull artery was used as the “calibrator” for ΔΔCt calculations. These measurements confirmed that IL-10 mRNA increased significantly (4-fold; p = 0.003) between 3 and 14 days, and then remained stable through 56 days (Fig. 4a).
FIG. 4.
Persistence of IL-10 expression and vector genomes after HDAd infusion. (a) Arteries were infused with HDAdgIL-10 (solid circles) or HDAdNull (open circles) and harvested at the indicated times. IL-10 mRNA was measured in all samples simultaneously by qRT-PCR and normalized to GAPDH mRNA in the same extracts. RNA from a single HDAdNull-infused artery harvested on day 3 served as the “calibrator” for ΔΔCt calculations (Schmittgen and Livak, 2008). Data points represent individual arteries; bars indicate group means. (b) Arteries were infused with either HDAdNull (open circles) or HDAdgIL-10 (closed circles) and harvested at the indicated times. Vector DNA was measured by qPCR and expressed as a percentage of vector DNA measured in arteries infused with the same vector and harvested on day 3 (set at 100% for both groups). Data represent means ± SEM, with n = 8–14 arteries per group. *p = 0.01; **p = 0.07 versus HDAdNull.
To begin to investigate mechanisms of persistent IL-10 expression in HDAdgIL-10 arteries, we measured vector copy number in DNA extracted from HDAdgIL-10 and HDAdNull arteries. DNA of both vectors declined substantially (60–70%) between 3 and 14 days, with relative persistence/slower declines from 14 to 56 days (Fig. 4b). Vector DNA was relatively more persistent in HDAdgIL-10 than in HDAdNull arteries at both 28 days (3-fold; p = 0.01) and 56 days (2-fold; p = 0.07). One-way ANOVA revealed a significant time-related decline from 3 to 56 days in HDAdNull arteries (p < 0.001) but only a trend in HDAdgIL-10 arteries (p = 0.1).
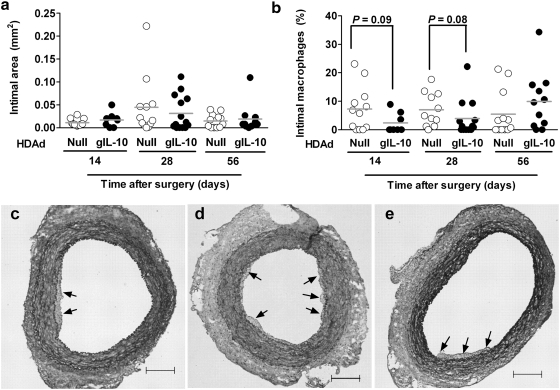
HDAdgIL-10 does not significantly alter intimal growth or macrophage accumulation in arteries of cholesterol-fed rabbits
In contrast to results generated in similar studies performed with FGAd (Schneider et al., 2000; Falkenberg et al., 2002), only minimal intimal growth was present in arteries infused with either HDAdNull or HDAdgIL-10 and harvested 14 days after vector infusion. Intimal areas of sections from HDAdNull arteries were 0.01 ± 0.002 mm2 versus 0.017 ± 0.006 mm2 for HDAdgIL-10 arteries (p = 0.6; Fig. 5a and c). Intimas of 28-day arteries were larger, on average, than intimas of 14-day arteries, but most arteries had virtually no intimal lesions and there was no significant difference between these two groups (0.045 ± 0.02 vs. 0.031 ± 0.01 mm2 for HDAdNull vs. HDAdgIL-10 arteries; p = 0.6; Fig. 5a and d). Intimal growth in 56-day arteries was similar to intimal growth in 14-day arteries, and therefore less than in 28-day arteries (0.015 ± 0.004 vs. 0.019 ± 0.009 for HDAdNull vs. HDAdgIL-10 arteries; p = 1.0; Fig. 5a and e). Intimal lesions at all three time points had a fairly low percentage of area that stained for a macrophage marker (generally < 10–15% of lesion area; Fig. 5b). At 14 and 28 days, the percentage of lesion area occupied by macrophages was lower in HDAdgIL-10 than in HDAdNull arteries (2.3 ± 1.2 vs. 7.2 ± 2.2% and 3.9 ± 1.8 vs. 7.0 ± 1.8%, respectively); however, these differences were of only borderline significance (p = 0.09 and 0.08, respectively; Fig. 5b).
FIG. 5.
Intimal area and macrophage content in arteries infused with HDAdNull or HDAdgIL-10. Carotid arteries of cholesterol-fed rabbits were removed at the indicated time points after vector infusion, sectioned, and stained. (a) Mean intimal areas. (b) Mean percentages of intimal area occupied by macrophages. (c–e) Representative sections of HDAdNull-infused arteries harvested at 14, 28, and 56 days, respectively. Arrows indicate intimal lesions. Total intimal areas were as follows: (c) 0.02 mm2; (d) 0.038 mm2; (e) 0.032 mm2. Data points in (a) and (b) are from individual arteries and are means of areas measured on four step sections per artery; bars represent group means. Sections in (c)–(e): Verhoeff–Van Gieson stain; size bars, 200 μm.
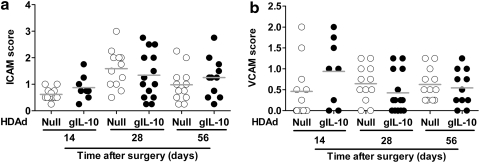
HDAdgIL-10 does not significantly alter adhesion molecule or cytokine expression in arteries of cholesterol-fed rabbits
Intimal expression of ICAM-1 and VCAM-1 was relatively low in both HDAdgIL-10 and HDAdNull arteries at all time points (Fig. 6a and b). Median ICAM-1 expression scores were ≤1.6 and VCAM-1 expression scores were <1.0 in all groups, with a score of 1.0 corresponding to “rare positive cells or staining clearly visible at low power” (Newman et al., 1995). Many sections did not show any ICAM-1 or VCAM-1 expression (score, 0). The ICAM-1 and VCAM-1 scores did not differ significantly between HDAdgIL-10 and HDAdNull arteries at any time point (p ≥ 0.15 for all comparisons). Because vector-expressed IL-10 can substantially blunt induction of cytokine expression in vitro (Fig. 2), we also measured mRNAs encoding several atherogenic cytokines in arteries infused with HDAdgIL-10 or HDAdNull and harvested 3–56 days later. Expression of IL-10 had no significant effect on expression of mRNA for TNF-α, MCP-1, IL-6, IL-1β, or IFN-γ at any time point (Supplementary Fig. S6; p ≥ 0.06 for all comparisons).
FIG. 6.
HDAdgIL-10 does not alter expression of adhesion molecules (ICAM-1 and VCAM-1) in rabbit carotid artery intima. Carotid arteries were transduced with HDAdNull or HDAdgIL-10 and harvested 14, 28, or 56 days later. (a and b) Sections of arteries were stained, and intimal ICAM-1 and VCAM-1 staining intensity was scored, using semiquantitative immunohistochemistry. Data points represent individual arteries; bars indicate group means.
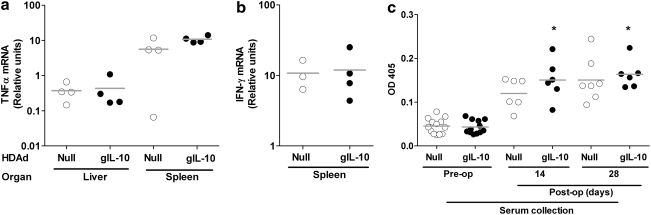
HDAdgIL-10 infusion does not have systemic immunosuppressive effects
When expressed systemically, IL-10 can have immunosuppressive effects including reduction of cytokine expression in liver and spleen (Von Der Thusen et al., 2001; Namiki et al., 2004) and promotion of tolerance to exogenous antigens (Akdis and Blaser, 2001). The stability over time of IL-10 expression in transduced carotids (Fig. 4a) and the relative persistence of HDAdgIL-10 versus HDAdNull vector DNA in the artery wall (Fig. 4b) both suggested that vector-expressed IL-10 might suppress immune responses to the HDAdgIL-10 vector. However, neither splenic mRNAs for TNF-α and IFN-γ nor hepatic mRNA for IFN-γ were reduced after bilateral carotid infusion of HDAdgIL-10 versus HDAdNull (Fig. 7a and b). Moreover, anti-adenoviral capsid antibodies were elevated to similar levels in serum of rabbits infused with either HDAdgIL-10 or HDAdNull (Fig. 7c).
FIG. 7.
No evidence of systemic immunosuppression in rabbits expressing IL-10 in carotid arteries. (a and b) Liver and spleen RNA were harvested 3 days after carotid artery infusion with HDAdNull or HDAdgIL-10. IL-1β and IFN-γ mRNA were measured by qRT-PCR. Expression of IFN-γ in the liver (data not shown) was at or below the limit of detection in all samples. (c) Serum was collected from rabbits before arterial transduction with HDAdNull or HDAdgIL-10 (preoperative) and then again at harvest (14 or 28 days postoperative). Antibodies to human adenovirus type 5 were measured by ELISA, using a substrate that absorbs light at 405 nm (OD405). Data points represent individual rabbits; bars indicate group means. *p ≥ 0.2 for comparison of these HDAdgIL-10 groups with the HDAdNull control groups at the same time point.
Discussion
We used a rabbit model of early atherogenesis to test whether local expression of IL-10 from HDAd could prevent atherosclerotic lesion formation and vascular inflammation. Our major findings were as follows: (1) The HDAdIL-10 vectors (cDNA and genomic) both express immunoreactive, active rabbit IL-10; (2) infusion of HDAd results in stable IL-10 expression in the carotid artery wall, with no initial decline in gene expression and stable expression for at least 8 weeks; and (3) atherosclerotic lesion formation was limited and expression of IL-10 had no significant effects on lesion size, macrophage content, and adhesion molecule or atherogenic cytokine expression.
IL-10 is an extremely promising agent for atheroprotective gene therapy. Numerous studies in atherosclerosis-prone mice reveal that deletion of the IL-10 gene accelerates atherosclerosis and that systemic IL-10 overexpression limits plaque growth (Mallat et al., 1999; Pinderski Oslund et al., 1999; Von Der Thusen et al., 2001; Pinderski et al., 2002; Namiki et al., 2004; Potteaux et al., 2004; Yoshioka et al., 2004; Liu et al., 2006). Human studies also support a salutary role for systemic IL-10 levels in coronary artery disease. Serum IL-10 levels are lower in patients with unstable versus stable angina (Smith et al., 2001) and elevated serum IL-10 is associated with improved endothelial function in patients with coronary artery disease (Fichtlscherer et al., 2004).
Expression of transgenes in vascular endothelial cells is a potent means of altering the biology and structure of the underlying vascular wall (Schulick et al., 1998; Schneider et al., 2000). Therefore, expression of therapeutic transgenes in endothelium is a promising approach for atheroprotective gene therapy. The ability of IL-10 to exert atheroprotective effects directly on the artery wall is less well established. Most of the murine studies cited previously involve manipulation of IL-10 expression in nonvascular cells (including T cells, macrophages, splenocytes, skeletal muscle, and hepatocytes), leaving open the question of whether atheroprotection by IL-10 is mediated outside the artery wall, for example, via effects on systemic immunity (Pinderski et al., 2002; Namiki et al., 2004; Potteaux et al., 2004) or even on plasma cholesterol (Von Der Thusen et al., 2001; Yoshioka et al., 2004). However, one study showed that IL-10 expressed from a locally infused first-generation adenoviral vector was almost as effective at limiting mouse carotid atherosclerosis as was systemic IL-10 overexpression (Von Der Thusen et al., 2001) and one report showed that macrophage-specific overexpression of IL-10 significantly decreased plaque volume without altering plasma IL-10 or cholesterol levels (Han et al., 2010). These two studies suggest that IL-10 can retard atherosclerosis via direct actions on the artery wall, supporting our strategy of expressing IL-10 in endothelium.
The extensive data demonstrating atheroprotective effects of IL-10, including effects on the artery wall, along with our discovery that HDAd (unlike first-generation Ad) could express a transgene in the artery wall for at least 8 weeks (Wen et al., 2004), prompted us to test whether HDAdIL-10 could suppress carotid atherogenesis. Because HDAd expresses transgenes for years in both rodents and primates (Brunetti-Pierri et al., 2009), HDAd-IL-10 expression from the artery wall might eventually be developed as an atheroprotective human gene therapy that durably prevents lesion growth without any dangerous systemic immunosuppressive effects. Efficacy in an informative large animal (rabbit) model of early lesion formation (Schneider et al., 2000; Falkenberg et al., 2002) would represent an important step toward clinical application. If effective in a large animal model, IL-10 could later be tested as a local gene therapy for human vein graft disease (Bhardwaj et al., 2008) or, after incorporation into a vasculature-targeted vector (White et al., 2008; Chen et al., 2009), as a systemically delivered atheroprotective gene therapy.
Unfortunately, HDAdgIL-10 did not significantly suppress atherogenesis and had no significant effects on other end points including adhesion molecule and cytokine expression. We considered several possible explanations for these results. First, the level of IL-10 expression in vivo may be inadequate. This possibility is difficult to exclude because neither published study that shows atheroprotection by local IL-10 expression includes quantitation of IL-10 protein (Von Der Thusen et al., 2001; Han et al., 2010), and—because rabbit IL-10 is not detected by commercially available ELISAs for mouse and human IL-10 (data not shown and communications from manufacturers)—we do not have a sensitive method of measuring IL-10 secretion from transduced arteries. Only by pooling and concentrating medium from several arteries were we able to detect immunoreactive IL-10 secreted by HDAdgIL-10-transduced arteries (Supplementary Fig. S7), suggesting that IL-10 protein expression in vivo is relatively low. The question of whether higher levels of IL-10 expression would be effective will be answered only by construction and testing of higher-expressing HDAds (Dronadula et al., 2011).
Another possible explanation for our negative results is that this rabbit model is an insensitive experimental setting in which to detect atheroprotection by IL-10. Virtually all work showing atheroprotection by IL-10 has been done in mice, and IL-10 might not be atheroprotective in rabbits. A study in hyperlipidemic rabbits, however, showed that infusion of human IL-10 suppressed both intimal growth and intimal macrophage accumulation (Feldman et al., 2000), and rabbit leukocytes are clearly reactive to IL-10 (Fig. 2), so this seems unlikely. Alternatively, atherosclerotic lesions in this model may be at a stage at which IL-10 has no net effect. Early lesion growth in this model may be driven primarily by extracellular lipid accumulation and adenovirus-induced inflammation (Schneider et al., 2000); processes that may be relatively insensitive to IL-10. Moreover, IL-10 appears to exert atheroprotective effects largely via actions that increase both lipid uptake and efflux (Halvorsen et al., 2005; Han et al., 2009). It is possible that IL-10-mediated lipid uptake could predominate during early lesion formation, and increased lipid uptake alone would likely not affect lesion size. Last, lesions in this model might be too small to easily reveal an atheroprotective effect of IL-10. On the basis of the result obtained here with HDAdNull (Fig. 5), a large number of rabbits would be required to detect or exclude a 50% decrease in lesion size at 4 weeks (>100 arteries per group with α = 0.05 and β = 0.8). The small lesion size was surprising to us, because lesions in arteries infused with a first-generation AdNull are consistently larger, permitting us to detect significant (50–100%) increases in lesion area with n = 5–18 arteries per group (Schneider et al., 2000; Falkenberg et al., 2002). Minimal lesion growth in arteries of cholesterol-fed rabbits infused with HDAdNull bodes well for the use of HDAd for vascular gene therapy; however, the lack of lesion growth also lessens the utility of this animal model to detect atheroprotective versus atherogenic effects of HDAd-expressed transgenes. We are currently developing methods, including coinfusion of FGAd, periarterial collar placement (Booth et al., 1989), and use of a higher concentration of dietary cholesterol (Kaul et al., 1992), to accelerate lesion growth after HDAd infusion, with a goal of easier detection of therapeutic transgene effects.
In contrast to the atherosclerosis data, our findings of persistent expression of IL-10 and, in particular, the lack of an early decline in transgene expression from HDAdgIL-10 (Fig. 4a) are exciting and promising results. Persistent expression of IL-10 differs from results we obtained with two other HDAd-expressed transgenes in this model (urokinase and apoA-I), the expression of which declined significantly and substantially (50–70%) between 3 and 28 days (Wen et al., 2004; and data not shown). The mechanism through which IL-10 expression persists compared with these other transgenes is unclear. Our data suggest that IL-10 expression increases HDAd genome persistence (Fig. 4b), which would in turn increase IL-10 expression. However, genome persistence does not seem to be the only explanation for increased IL-10 expression because HDAdgIL-10 genomes are no longer significantly increased at 56 days, yet IL-10 expression remains stable (Fig. 4). Systemic immunosuppression by IL-10, if present, could explain increased vector persistence, but we found no evidence of a diminished systemic adaptive immune response to adenovirus in rabbits infused with HDAdgIL-10. Transcription from HDAd also appears to be regulated by the innate immune system, via intracellular signals initiated by ligands to Toll-like receptor-2 (Suzuki et al., 2010). Local expression of IL-10 in the artery wall could antagonize these signals, thereby increasing IL-10 expression. Future work will be aimed at identifying these pathways and investigating their modulation by IL-10.
In summary, HDAdgIL-10 expresses IL-10 stably in the artery wall but does not significantly affect atherogenesis in this rabbit model. Achievement of higher expression levels and modification of the animal model to yield larger lesions are the next steps toward realizing the substantial promise of IL-10 as a local atheroprotective gene therapy.
Supplementary Material
Acknowledgments
This work was supported by a grant from the National Heart, Lung, and Blood Institute (HL076226; to D.A.D.) and by the John L. Locke, Jr. Charitable Trust. Dr. Tanaka was supported in part by a grant from Osaka City University. The authors thank Harvey Perkins for supplying the rabbit IL-10 cDNA; AdVec for permission to use the HDAd reagents; Dan Minter, Beau Green, Jessica Chang, and Denise Zhou for technical assistance; and Margo Weiss for administrative assistance.
Author Disclosure Statement
No competing financial interests exist.
References
- Aiuti A. Cattaneo F. Galimberti S., et al. Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N. Engl. J. Med. 2009;360:447–458. doi: 10.1056/NEJMoa0805817. [DOI] [PubMed] [Google Scholar]
- Akdis C.A. Blaser K. Mechanisms of interleukin-10-mediated immune suppression. Immunology. 2001;103:131–136. doi: 10.1046/j.1365-2567.2001.01235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj S. Roy H. Ylä-Herttuala S. Gene therapy to prevent occlusion of venous bypass grafts. Expert Rev. Cardiovasc Ther. 2008;6:641–652. doi: 10.1586/14779072.6.5.641. [DOI] [PubMed] [Google Scholar]
- Booth R.F.G. Martin J.F. Honey A.C., et al. Rapid development of atherosclerotic lesions in the rabbit carotid artery induced by perivascular manipulation. Atherosclerosis. 1989;76:257–268. doi: 10.1016/0021-9150(89)90109-3. [DOI] [PubMed] [Google Scholar]
- Brunetti-Pierri N. Stapleton G.E. Law M., et al. Efficient, long-term hepatic gene transfer using clinically relevant HDAd doses by balloon occlusion catheter delivery in nonhuman primates. Mol. Ther. 2009;17:327–333. doi: 10.1038/mt.2008.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. Anton M. Graham F.L. Production and characterization of human 293 cell lines expressing the site-specific recombinase Cre. Somat. Cell Mol. Genet. 1996;22:477–488. doi: 10.1007/BF02369439. [DOI] [PubMed] [Google Scholar]
- Chen Y.H. Chang M. Davidson B.L. Molecular signatures of disease brain endothelia provide new sites for CNS-directed enzyme therapy. Nat. Med. 2009;15:1215–1218. doi: 10.1038/nm.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries J.E. Immunosuppressive and anti-inflammatory properties of interleukin 10. Ann. Med. 1995;27:537–541. doi: 10.3109/07853899509002465. [DOI] [PubMed] [Google Scholar]
- Dronadula N. Du L. Flynn R., et al. Construction of a novel expression cassette for increasing transgene expression in vivo in endothelial cells of large blood vessels. Gene Ther. 2011 doi: 10.1038/gt.2010.173. [Epub ahead of print]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenberg M. Tom C. Deyoung M.B., et al. Increased expression of urokinase during atherosclerotic lesion development causes arterial constriction and lumen loss, and accelerates lesion growth. Proc. Natl. Acad. Sci. U.S.A. 2002;99:10665–10670. doi: 10.1073/pnas.162236599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman L.J. Aguirre L. Ziol M., et al. Interleukin-10 inhibits intimal hyperplasia after angioplasty or stent implantation in hypercholesterolemic rabbits. Circulation. 2000;101:908–916. doi: 10.1161/01.cir.101.8.908. [DOI] [PubMed] [Google Scholar]
- Fichtlscherer S. Breuer S. Heeschen C., et al. Interleukin-10 serum levels and systemic endothelial vasoreactivity in patients with coronary artery disease. J. Am. Coll. Cardiol. 2004;44:44–49. doi: 10.1016/j.jacc.2004.02.054. [DOI] [PubMed] [Google Scholar]
- Gersh B.J. Sliwa K. Mayosi B.M. Yusuf S. Novel therapeutic concepts: The epidemic of cardiovascular disease in the developing world: Global implications. Eur. Heart J. 2010;31:642–648. doi: 10.1093/eurheartj/ehq030. [DOI] [PubMed] [Google Scholar]
- Gruchala M. Bhardwaj S. Pajusola K., et al. Gene transfer into rabbit arteries with adeno-associated virus and adenovirus vectors. J. Gene Med. 2004;6:545–554. doi: 10.1002/jgm.535. [DOI] [PubMed] [Google Scholar]
- Halvorsen B. Waehre T. Scholz H., et al. Interleukin-10 enhances the oxidized LDL-induced foam cell formation of macrophages by antiapoptotic mechanisms. J. Lipid Res. 2005;46:211–219. doi: 10.1194/jlr.M400324-JLR200. [DOI] [PubMed] [Google Scholar]
- Han X. Kitamoto S. Lian Q. Boisvert W.A. Interleukin-10 facilitates both cholesterol uptake and efflux in macrophages. J. Biol. Chem. 2009;284:32950–32958. doi: 10.1074/jbc.M109.040899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X. Kitamoto S. Wang H. Boisvert W.A. Interleukin-10 overexpression in macrophages suppresses atherosclerosis in hyperlipidemic mice. FASEB J. 2010;24:2869–2880. doi: 10.1096/fj.09-148155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippisley-Cox J. Coupland C. Unintended effects of statins in men and women in England and Wales: Population based cohort study using the QResearch database. BMJ. 2010;340:c2197. doi: 10.1136/bmj.c2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul S. Padgett R.C. Waack B.J., et al. Effect of atherosclerosis on responses of the perfused rabbit carotid artery to human platelets. Arterioscler. Thromb. 1992;12:1206–1213. doi: 10.1161/01.atv.12.10.1206. [DOI] [PubMed] [Google Scholar]
- Kawashiri Ma M. Rader D.J. Gene therapy for lipid disorders. Curr. Control Trials Cardiovasc. Med. 2000;1:120–127. doi: 10.1186/cvm-1-2-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. Li D. Chen J., et al. Inhibition of atherogenesis in LDLR knockout mice by systemic delivery of adeno-associated virus type 2-hIL-10. Atherosclerosis. 2006;188:19–27. doi: 10.1016/j.atherosclerosis.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Lloyd-Jones D. Adams R.J. Brown T.M., et al. Heart disease and stroke statistics—2010 update: A report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- Lusis A.J. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallat Z. Besnard S. Duriez M., et al. Protective role of interleukin-10 in atherosclerosis. Circ. Res. 1999;85:e17–e24. doi: 10.1161/01.res.85.8.e17. [DOI] [PubMed] [Google Scholar]
- Mittereder N. March K.L. Trapnell B.C. Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J. Virol. 1996;70:7498–7509. doi: 10.1128/jvi.70.11.7498-7509.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namiki M. Kawashima S. Yamashita T., et al. Intramuscular gene transfer of interleukin-10 cDNA reduces atherosclerosis in apolipoprotein E-knockout mice. Atherosclerosis. 2004;172:21–29. doi: 10.1016/j.atherosclerosis.2003.08.032. [DOI] [PubMed] [Google Scholar]
- Newman K.D. Dunn P.F. Owens J.W., et al. Adenovirus-mediated gene transfer into normal rabbit arteries results in prolonged vascular cell activation, inflammation, and neointimal hyperplasia. J. Clin. Invest. 1995;96:2955–2965. doi: 10.1172/JCI118367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks R.J. Chen L. Anton M., et al. A helper-dependent adenovirus vector system: Removal of helper virus by Cre-mediated excision of the viral packaging signal. Proc. Natl. Acad. Sci. U.S.A. 1996;93:13565–13570. doi: 10.1073/pnas.93.24.13565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins H.D. Van Leeuwen B.H. Hardy C.M. Kerr P.J. The complete cDNA sequences of IL-2, IL-4, IL-6 AND IL-10 from the European rabbit (Oryctolagus cuniculus) Cytokine. 2000;12:555–565. doi: 10.1006/cyto.1999.0658. [DOI] [PubMed] [Google Scholar]
- Pinderski L.J. Fischbein M.P. Subbanagounder G., et al. Overexpression of interleukin-10 by activated T lymphocytes inhibits atherosclerosis in LDL receptor-deficient mice by altering lymphocyte and macrophage phenotypes. Circ. Res. 2002;90:1064–1071. doi: 10.1161/01.res.0000018941.10726.fa. [DOI] [PubMed] [Google Scholar]
- Pinderski Oslund L.J. Hedrick C.C. Olvera T., et al. Interleukin-10 blocks atherosclerotic events in vitro and in vivo. Arterioscler. Thromb. Vasc. Biol. 1999;19:2847–2853. doi: 10.1161/01.atv.19.12.2847. [DOI] [PubMed] [Google Scholar]
- Potteaux S. Esposito B. Van Oostrom O., et al. Leukocyte-derived interleukin 10 is required for protection against atherosclerosis in low-density lipoprotein receptor knockout mice. Arterioscler. Thromb. Vasc. Biol. 2004;24:1474–1478. doi: 10.1161/01.ATV.0000134378.86443.cd. [DOI] [PubMed] [Google Scholar]
- Rissanen T.T. Ylä-Herttuala S. Current status of cardiovascular gene therapy. Mol. Ther. 2007;15:1233–1247. doi: 10.1038/sj.mt.6300175. [DOI] [PubMed] [Google Scholar]
- Sandig V. Youil R. Bett A.J., et al. Optimization of the helper-dependent adenovirus system for production and potency in vivo. Proc. Natl. Acad. Sci. U.S.A. 2000;97:1002–1007. doi: 10.1073/pnas.97.3.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen T.D. Livak K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Schneider D.B. Vassalli G. Wen S., et al. Expression of Fas ligand in arteries of hypercholesterolemic rabbits accelerates atherosclerotic lesion formation. Arterioscler. Thromb. Vasc. Biol. 2000;20:298–308. doi: 10.1161/01.atv.20.2.298. [DOI] [PubMed] [Google Scholar]
- Schulick A.H. Dong G. Newman K.D., et al. Endothelium-specific in vivo gene transfer. Circ. Res. 1995;77:475–485. doi: 10.1161/01.res.77.3.475. [DOI] [PubMed] [Google Scholar]
- Schulick A.H. Taylor A.J. Zuo W., et al. Overexpression of transforming growth factor β1 in arterial endothelium causes hyperplasia, apoptosis, and cartilaginous metaplasia. Proc. Natl. Acad. Sci. U.S.A. 1998;95:6983–6988. doi: 10.1073/pnas.95.12.6983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D.A. Irving S.D. Sheldon J., et al. Serum levels of the anti-inflammatory cytokine interleukin-10 are decreased in patients with unstable angina. Circulation. 2001;104:746–749. doi: 10.1161/hc3201.094973. [DOI] [PubMed] [Google Scholar]
- Suzuki M. Cerullo V. Bertin T.K., et al. MyD88-dependent silencing of transgene expression during the innate and adaptive immune response to helper-dependent adenovirus. Hum. Gene Ther. 2010;21:325–336. doi: 10.1089/hum.2009.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terkeltaub R.A. IL-10: An “immunologic scalpel” for atherosclerosis? Arterioscler. Thromb. Vasc. Biol. 1999;19:2823–2825. doi: 10.1161/01.atv.19.12.2823. [DOI] [PubMed] [Google Scholar]
- Von Der Thusen J.H. Kuiper J. Fekkes M.L., et al. Attenuation of atherogenesis by systemic and local adenovirus-mediated gene transfer of interleukin-10 in LDLr–/– mice. FASEB J. 2001;15:2730–2732. doi: 10.1096/fj.01-0483fje. [DOI] [PubMed] [Google Scholar]
- Wen S. Graf S. Massey P.G. Dichek D.A. Improved vascular gene transfer with a helper-dependent adenoviral vector. Circulation. 2004;110:1484–1491. doi: 10.1161/01.CIR.0000141574.78032.A9. [DOI] [PubMed] [Google Scholar]
- Wen S. Schneider D.B. Driscoll R.M., et al. Second-generation adenoviral vectors do not prevent rapid loss of transgene expression and vector DNA from the arterial wall. Arterioscler. Thromb. Vasc. Biol. 2000;20:1452–1458. doi: 10.1161/01.atv.20.6.1452. [DOI] [PubMed] [Google Scholar]
- White K. Buning H. Kritz A., et al. Engineering adeno-associated virus 2 vectors for targeted gene delivery to atherosclerotic lesions. Gene Ther. 2008;15:443–451. doi: 10.1038/sj.gt.3303077. [DOI] [PubMed] [Google Scholar]
- Ylä-Herttuala S. Martin J.F. Cardiovascular gene therapy. Lancet. 2000;355:213–222. doi: 10.1016/S0140-6736(99)04180-X. [DOI] [PubMed] [Google Scholar]
- Yoshioka T. Okada T. Maeda Y., et al. Adeno-associated virus vector-mediated interleukin-10 gene transfer inhibits atherosclerosis in apolipoprotein E-deficient mice. Gene Ther. 2004;11:1772–1779. doi: 10.1038/sj.gt.3302348. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.