Abstract
The large amounts of recombinant adeno-associated virus (rAAV) vector needed for clinical trials and eventual commercialization require robust, economical, reproducible, and scalable production processes compatible with current good manufacturing practice. rAAV produced using baculovirus and insect cells satisfies these conditions; however, recovering rAAV particles from 200-liter bioreactors is more complicated than bench-scale vector preparations. Using a variety of processing media, we developed a reliable and routine downstream procedure for rAAV production that is scalable from 0.02- to 200-liter cultures. To facilitate the upstream process, we adapted the titerless infected-cell preservation and scale-up process for rAAV production. Single-use aliquots of cryopreserved baculovirus-infected insect cells (BIIC) are thawed and added to the suspension culture to achieve the desired ratio of BIIC to rAAV-producer cells. By using conditions established with small-scale cultures, rAAV was produced in larger volume cultures. Strikingly consistent rAAV yields were attained in cultures ranging from 10 liters to 200 liters. Based on the final yield, each cell produced 18,000 ± 6,800 particles of purified rAAV in 10-, 20-, 100-, and 200-liter cultures. Thus, with an average cell density of 4.32 × 106 cells/ml, ≥1016 purified rAAV particles are produced from 100 to 200 liters. The downstream process resulted in about 20% recovery estimated from comparing the quantities of capsid protein antigen in the crude bioreactor material and in the final, purified product. The ease and reproducibility of rAAV production in 200-liter bioreactors suggest that the limit has not been reached, and 500-liter productions are planned.
Production of recombinant AAV in Sf9 insect cells infected with baculovirus is an established, economical, and scalable process. However, under serum-free clinical manufacturing conditions, cryopreserved baculovirus loses its ability to infect new batches of producer cells over time. This study by Cecchini and colleagues reports that cryopreserving insect cells infected with baculovirus provides a viable alternative for obtaining consistent levels of rAAV from any size culture.
Introduction
Producing recombinant adeno-associated virus (rAAV) vector in lepidopteran Spodoptera fugiperda (Sf9) cells by using recombinant Autographa californica multicapsid nuclear polyhedrosis virus (AcMNPV or baculovirus) has been established as an economical and scalable process (Urabe et al., 2002; Negrete and Kotin, 2007; Negrete et al., 2007a; for review, see Virag et al., 2009). Vector produced from the Sf9 cell–baculovirus system has been used in large-animal preclinical studies and, importantly, in clinical trials. The first marketing approval application for a rAAV product, alipogene tiparvovec (AMT-011, Glybera; Amsterdam Molecular Therapeutics, Amsterdam, The Netherlands), is based on the Sf9 cell–baculovirus production platform. Thus, the production platform has proven robust and compliant with current good manufacturing practice (cGMP).
From the manufacturing perspective, a major advantage of using Sf9 cells and baculovirus for producing rAAV is the volumetrically scalable format of suspension cell culture rather than a two-dimensionally scalable format for adherent cells. Downstream processing of suspension cells is also easily scaled up, involving fluid handling rather than manipulation of roller bottles or multilayer cell cubes. The cost of production and the time involved, including both personnel and consumables, are substantially less than those for producing vector in transiently transfected mammalian cells. Vector produced in Sf9 cells appears indistinguishable from rAAV produced in mammalian cells, and insect cells generate high rAAV yields per cell, and with cell density of ≤6 × 109 suspension cells per liter, the yield of purified vector approaches 1014 per liter.
Baculovirus stocks retain infectivity after long-term storage at 4°C in the cell culture supernatant in which the virus was amplified, or with serum supplementation if the virus was amplified in serum-free medium. Loss of infectious activity has been attributed to photoinactivation, which can be avoided by excluding light (Jarvis and Garcia, 1994). Fetal calf serum in the media complicates the cGMP process necessary for clinical applications. Several commercially available serum-free medium formulations support Sf9 cell growth and rAAV production. However, baculovirus infectivity decreased approximately 30-fold in less than 1 year in serum-free media (Jorio et al., 2006). Alternatively, baculovirus that has been concentrated with diafiltration into a cryopreservative medium remains infectious after frozen storage (Jorio et al., 2006). Regardless, minimizing and simplifying the manipulations required for concentrating and preserving the baculovirus would facilitate the reproducibility and reliability of the process.
To provide baculovirus sufficient for large-volume insect cell cultures, e.g., ≥100 L, in conveniently stored, single-use aliquots that remain infectious, we examined whether cryopreserved, baculovirus-infected insect cells (BIIC) are suitable for rAAV production. This process, previously described for recombinant protein production, is referred to as “titerless infected-cell preservation and scale-up” (TIPS) (Wasilko and Lee, 2006; Wasilko et al., 2009). The BIIC aliquots are cryopreserved late in infection, but before viability diminishes. After thawing, the remaining viable cells continue producing baculovirus, serving as a reservoir for virus to infect the rAAV producer cells in the bioreactor. Importantly, the duration that the cells remain frozen in liquid nitrogen does not further affect viability or rAAV production. The conditions, optimized using a matrix of different BIIC:producer cells, enabled us to prepare BIIC in single-use aliquots, in principle, for any production volume. The BIIC:producer cell ratio is consistent between preparations for a particular baculovirus. Once established, these conditions do not have to be re-derived. In addition, cell number determination is more accurate and reliable than baculovirus infectious titer determination, resulting in more uniform rAAV production.
Although product recovery was estimated to be only about 20% efficient overall, we are hopeful that modifying the downstream process will improve the efficiency. Even a modest increase of recovered vector, e.g., 10%, represents a substantial quantity of vector from the large-scale bioreactor productions.
Materials and Methods
Cell culture and BIIC production
Spodoptera frugiperda (Sf9) cells were propagated in serum-free medium (SFX; HyClone, Logan, UT) at 27–28°C in appropriately sized Ehrlenmeyer polycarbonate flask with vent cap (Corning, Inc., NY). Cells were maintained in suspension in an orbital shaking incubator at 130 rpm (50 mm throw) (Multitron, Infors HT, Bottmingen, Switzerland) and expanded by dilution with fresh, serum-free medium when cells reached 4 × 106/ml. BIIC were prepared by adding cell-free baculovirus to the Sf9 suspension culture. By using cell diameter as an indicator, the baculovirus-infected cells were collected at regular postinfection time points and cryopreserved for further analysis.
Cells were recovered by low-speed centrifugation (300 g, 10 min) and resuspended in either cryopreservation medium I [90% HyClone SFX (serum-free insect cell culture medium), 10% dimethyl sulfoxide (DMSO), and bovine serum albumin (10 mg/ml)] or cryopreservation medium II a 1:1 mixture of conditioned serum-free cell culture medium and freezing medium (86% serum-free medium, 0.3 M trehalose, and 7% DMSO). One-milliliter aliquots containing 2 × 107 cells were dispensed into cryotubes and placed in a freezing cassette that controlled the rate of cooling (–1°C/min) to a final temperature of −80°C. Cryotubes were placed in the vapor phase of a liquid nitrogen freezer for long-term storage.
Large-scale rAAV production
Cell growth
Three cell culture configurations were used, depending on the culture volume: (1) <30 L, rocking platform bioreactor (WAVE Bioreactor System 20/50; GE Healthcare Life Sciences, Piscataway, NJ); (2) 100 L, single-use, stirred-tank bioreactor with pitched blade impeller (100 L S.U.B.; HyClone/Thermo Scientific, Logan, UT); (3) 200 L, single-use, stirred-tank bioreactor with a paddle-drive agitator (Integrity PadReactor System; ATMI Life Sciences, Minneapolis, MN).
For each configuration, the bioreactor was batched with the minimum working volume of serum-free medium (SFX-Insect, HyClone/Thermo Scientific) and then inoculated with Sf9 cells to obtain a final density of approximately 0.75 × 106/ml. The cells were expanded in situ until the maximum working volume was attained. The rocking platform bioreactor maintained temperature, and 30% O2 in air mixture was provided in the headspace. The liquid and gas phases each occupied about 50% of the total inflated bag volume. Neither pH nor dissolved oxygen (dO) was controlled or monitored in this configuration. For both stirred-tank bioreactors, the pH was monitored, but not controlled because the cell culture medium provided sufficient buffering capacity (Aucoin et al., 2010). The 30% dO level in the culture medium was maintained with mass-flow controlled air and oxygen mixture delivered through a sparger. A circulating chiller (Integral T 1200 or Integral T 4600; LAUDA-Brinkmann, LP, Delran, NJ) connected to the water-jacketed vessels maintained constant temperature. A Finesse TruViu (Finesse Solutions, San Jose, CA) controller maintained the set points for temperature, dO, and agitation, whereas pH and oxygen consumption values were logged. During the 200-L bioreactor runs, the cell density was monitored continuously and correlated with off-line cell viability and density counting. Cell density, viability, and diameter were measured at least daily using trypan blue dye exclusion and an automated cell counter (Cellometer T4; Nexelcom Bioscience, LLC, Lawrence, MA).
Downstream processing
Both intra- and extracellular rAAV particles were recovered by processing the entire bioreactor contents. A dual-piston mechanical cell disrupter (deBEE 1000; BEE International, South Easton, MA) operating at 15,000 psi and a 1-mm orifice reaction chamber with a flow rate of approximately 0.9 L/min solubilized the biomass and effectively lysed the cells with a single pass. Following homogenization, the lysate was returned to the bioreactor for nuclease treatment (TurboNuclease; Acelagen, Inc., San Diego, CA) to reduce viscosity and degrade RNA, genomic DNA, baculovirus DNA, and unencapsidated vector DNA (5 U/ml at 37°C for approximately 2 hr).
Following nuclease treatment, solid NaCl was added to the bioreactor to a final concentration of 0.4 M. Depth filtration was used for clarification (Sartopure GF + Maxicaps, 1.2 μm; Sartorius Stedim North America, Bohemia, NY) and then microfiltration using a two-stage 0.8-μm and 0.2-μm capsule filter (Sartopore 2XLG). For each 100 L processed, a 30-inch capsule depth filter (1.2 m2) and 10-inch capsule microfilter (0.8 m2) were used. Proportionally sized filters were used for smaller process volumes. The filtrate was collected in a sterile bioprocessing bag and either processed immediately (see below) or stored overnight at 4°C.
Immunoaffinity (IA) chromatography
IA chromatography was used for the primary purification and concentration steps. The IA medium, AVB-Sepharose (GE Life Sciences Healthcare) specifically binds several AAV serotypes, including serotypes 1, 2, 6, and 8. The filtered cell lysate was applied to a 20-cm diameter column (BPG 200/500; GE Lifesciences) with approximately 6-cm bed height at about 50% of the recommended maximum linear velocity of 150 cm/hr corresponding to a flow rate of 417 ml/min. The column was washed with phosphate-buffered saline (PBS) until the ultraviolet (UV) absorbance curve returned to baseline and stabilized. The adsorbed rAAV particles were eluted in acidic medium (50 mM sodium citrate adjusted with HCl to pH 3.0), and the column eluate was adjusted immediately with 1/10 volume of 1 M Tris-Cl (pH 8.0).
The neutralized eluate was concentrated and diafiltrated against PBS using tangential flow filtration (100 kDa NMWCO). Gel filtration chromatography as an optional step was performed when polishing and additional purification were required. The concentrated vector solution was applied (50–70 ml) to a 10-cm-diameter column with Superdex 200 medium (2-L bed volume). The rAAV particles eluted in the void fraction and these fractions were pooled, concentrated, and diafiltrated using TFF. The final vector product was sterile-filtered (0.22-μm pore) and dispensed into sterile cryogenic storage vials.
Vector characterization
The total particle concentration was assessed by protein concentration assays, and the filled particle number, i.e., particles that contain vector genomes (vg), was determined using two independent methods: (1) quantitative PCR (qPCR) using vector-specific primers and (2) directly determining the amount of DNA extracted from the particles using SYBR Gold nucleic acid dye. After dialyzing the vector samples against PBS with 2 mM MgCl2, aliquots (70 μl) were treated with proteinase K (40 μg) (Invitrogen Corp., Carlsbad, CA) and incubated for 30 min at 56°C. The proteinase K was heat inactivated (95°C for 5 min) and the samples were slowly equilibriated to ambient temperature to allow time for annealing the complementary single-stranded vector genomes. Using a black bottom 96 wells plate, samples were diluted and added to a final volume of 200 μl of SYBR Gold solution (3 μl of SYBR Gold [Invitrogen Corp.], in 30 ml of fluorescent buffer—10 mM HEPES and 1 mM EDTA, pH 8.0). The fluorescent signal, proportional to the amount of DNA, was read in a black bottom, 96 well plate (Costar, Corning Corp., Corning, NY) and fluorescent plate reader (485 nm/535 nm, 1 s) (VICTOR 2, Perkin Elmer, Waltham, MA). DNA reference standards were produced using dilutions of a commercially available DNA ladder (Fermantas, Glen Burnie, MD).
Real-time PCR was used for quantitative analysis of encapsidated vector genomes using vector specific forward and reverse primers. The primers used for U7smOPT genome were: forward 5′-ACAGCTCCTATGTTGTTATC and reverse 5′-CACTTCCGCAAACATTAACC, amplified a 241 bp product, using the following 3–step reactions conditions initial denaturation −95°C (10 min), 40 cycles of: 95°C (15 sec), 52°C (30 sec), 72°C (30 sec).
The same primers were used for qPCR analyses of both the alkaline phosphatase and GFP vectors: Forward 5′-TGGTGATGCGGTTTTGGCAG, and reverse 5′-AATGGGGCGGAGTTGTTACGA, producing a fragment of 153bp of the CMV promoter region. PCR copy number standards were prepared from the respective plasmids. All qPCR reactions were performed with a real-time, thermocyler (ICycler, BioRad, Hercules, CA) using SYBR green PCR master mix (RT2 SYBR Green/Fluorescein Mastermix, SABiosciences, Frederick, MD). Empty and filled particles were separated using cesium chloride isopycnic gradients. Fractions of the gradients were analyzed for capsid proteins (western blots) and DNA content (qPCR) using vector genome–specific primers.
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and western blot analyses
Following fractionation using SDS-PAGE, proteins were electrophoretically transferred to either polyvinylidene fluoride (PVDF) or nitrocellulose membranes. Silver staining and western blotting were performed as previously described (Smith et al., 2009). In brief, membranes were incubated for 30 min at room temperature in blocking solution [0.5% fat-free milk powder diluted in PBS with Tween 20 (PBS-T) buffer]. Rabbit polyclonal antiserum raised against recombinant VP1 from AAV5 was used at a final 1:2,000 dilution. After 1-hr incubation at room temperature, the solution was removed and the membrane washed in PBS-T buffer (3 × 15 min at room temperature). The secondary antibody, anti-rabbit IgG antibody produced in goat, conjugated with horseradish peroxidase (HRP) (Sigma-Aldrich, St. Louis, MO) was diluted 1:2,000 and added to the solution. Following 1-hr incubation, the solution was removed and the membrane washed 3 × 15 min with PBS-T at room temperature. Light is emitted from the HRP-catalyzed reaction with SuperSignal West Dura Extended Duration Substrate (Thermo Scientific Pierce Protein Research Products, Rockford, IL). Images were captured using a cooled CCD camera and quantitatively analyzed using the software package provided in the G:BOX Chemi system (Syngene USA, Frederick, MD).
Transmission electron microscopy (TEM) of AAV particles
TEM methods were performed as previously described (Virag et al., 2009). In brief, AAV-containing solutions were applied to Formvar and carbon-coated 300-mesh copper grids (Electron Microscopy Sciences, Hatfield, PA) for 1 min, allowing the particles time to adsorb. After adsorption, grids were washed with distilled water and stained with four droplets of 2% ammonium molybdate. Excess stain was wicked away, and the grids were air-dried. The grids were then examined using either a JEM 1200EX II or a JEM 1400 transmission electron microscope (JEOL, Tokyo, Japan).
Results
There are two components to the TIPS process: the first part involves determining the optimal time point for harvesting the BIIC, and the second part determines the ratios of BIIC to Sf9 cells for producing rAAV. The time point for harvesting the BIIC was developed using small-scale, shake flasks (0.02 L). Initially, low-passage Sf9 cell cultures were infected with Bac-GFP [containing the green fluorescent protein (GFP) transcription cassette regulated with the AcMNPV p10 late promoter (Urabe et al., 2002)], and a time course of infection was established by measuring cell viability, diameter, and density, and green fluorescence. Uninfected cells are approximately 14 μm in diameter; within 24 hr post infection, cell diameter increased to 17.4 μm, and at 48 hr post infection, the diameter increased to 19 μm, with cell viability remaining at 90%. Cells were harvested at 48 hr post infection, because the cell viability diminished beyond 48 hr.
The yield of rAAV produced in Sf9 cells depends on the cell density at the time of infection (Negrete et al., 2007b). In cultures with cell densities up to about 5 × 106 cells/ml, the rAAV produced per cell is independent of density; the medium can support the cell metabolism and the rAAV produced per cell is unaffected. Beyond 5 × 106 cells/ml, the specific yield of rAAV diminishes and the increased biomass compromises the downstream processing. Therefore, the ratios of BIIC to Sf9 cells were determined over a 4-log range of BIIC:producer cell ratios, i.e., 10–2, 10–3, 10–4, and 10–5. Cells were monitored for 3 days post-BIIC inoculation for cell density, viability, and diameter.
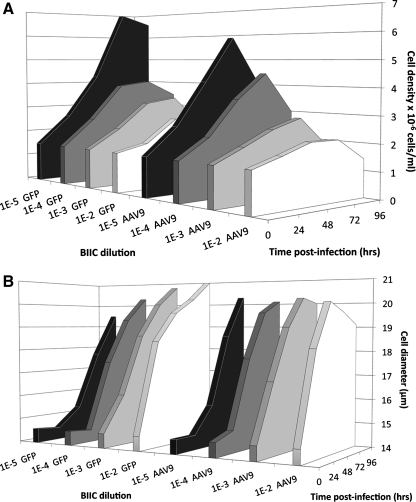
The effect of baculovirus infection on cell growth was evaluated by measuring cell density after inoculation with BIIC (Fig. 1A). The density of cells in cultures inoculated with the lowest BIIC dilution did not double (10–2), and at the highest dilution (10–5) the cells did not arrest. However, cultures inoculated with BIIC dilutions of either 10–3 or 10–4 continued to grow for 72 hr. Cell-diameter increase correlates with baculovirus infection, thereby providing another measure of the infection rate. The lag time in cell-diameter increase occurred with the two highest BIIC dilutions, indicating that the infection (Fig. 1B) was delayed, whereas the cell density continued to increase geometrically (Fig. 1A). By 72 hr post inoculation, the average cell diameter had reached similar values regardless of the amount of BIIC added. Based on these results, 10–4 BIIC:Sf9 producer cell ratio caused cell-cycle arrest at the optimum cell density and was used for the large-scale productions.
FIG. 1.
Determining the dilution of cryopreserved BIIC required to achieve the optimal infection rate of the rAAV Sf9-producing cells. The infection rates of Sf9 cells were determined measuring the cell culture densities (A) and cell diameters (B) as a function of the dilution of BIIC added over time. In a typical experiment, the indicated dilutions of BIIC were added to Sf9 cells (1.5 × 106 cells/ml in 20 ml). Cell density continued at similar rates at the two highest dilutions for 48 hr (A), and the 24-hr lag phase in cell diameter increase (B) occurred also at the two highest BIIC dilutions. Therefore, the criteria for establishing the optimal BIIC dilutions for rAAV production were based on using the least amounts of BIIC that produced sufficient baculovirus to arrest cell growth at ≤5 × 106 cells/ml.
TIPS for larger volume rAAV production
Based on the findings described above, rAAV was produced in larger volume cultures using BIIC. A series of production runs in volumes ranging from 10 to approximately 200 L was performed (summarized in Table 1). Initially, rAAV was produced in the rocking platform bioreactor in a set of five runs: two 10-L, two 20-L, and one 27-L culture volume. Changes in cell viability and cell diameter were relatively uniform among the five production runs. The average yield of purified rAAV for the rocking platform productions was 15,400 (±7,050) vg/cell, with specific yields ranging between 7.3 × 103 to 24.1 × 103 rAAV particles/cell. The one relatively poor production resulted from the addition of suboptimal amounts of BIIC.
Table 1.
Large-Scale Production Results
| AAV serotype | Genotype | Prod. Volume (l) | Total Yield | Yield/l | Cells/ml | Yield/cell |
|---|---|---|---|---|---|---|
| 9 | GFP | 10 | 8 × 1014 | 8 × 1013 | 3.87 × 106 | 2.07 × 104 |
| 9 | U7smOPT | 10 | 7.8 × 1014 | 7.8 × 1013 | 3.23 × 106 | 2.41 × 104 |
| 6 | PLS | 20 | 5 × 1014 | 2.5 × 1013 | 3.44 × 106 | 7.27 × 103 |
| 8 | GFP | 20 | 1 × 1015 | 5 × 1013 | 3.38 × 106 | 1.48 × 104 |
| 6 | GFP | 27 | 1.2 × 1015 | 4.44 × 1013 | 4.38 × 106 | 1.01 × 104 |
| 6 | U7smOPT | 100 | 8 × 1015 | 8 × 1013 | 3.59 × 106 | 2.23 × 104 |
| 6 | U7smOPT | 100 | 8 × 1015 | 8 × 1013 | 3.85 × 106 | 2.08 × 104 |
| 6 | U7smOPT | 100 | 1.5 × 1016 | 1.5 × 1014 | 5.52 × 106 | 2.72 × 104 |
| 6 | U7smOPT | 100 | 4 × 1015 | 4 × 1013 | 4.18 × 106 | 9.57 × 103 |
| 6 | U7smOPT | 193 | 1.59 × 1016 | 8.24 × 1013 | 4.32 × 106 | 1.91 × 104 |
| 6 | U7smOPT | 194 | 1.63 × 1016 | 8.4 × 1013 | 4.5 × 106 | 1.87 × 104 |
| Sums/averages | 874 | 7.15 × 1016 | 6.49 × 1013 | 1.77 × 104 | ||
| ±3.95 × 1013 | ±6,435 |
Stirred-tank bioreactors
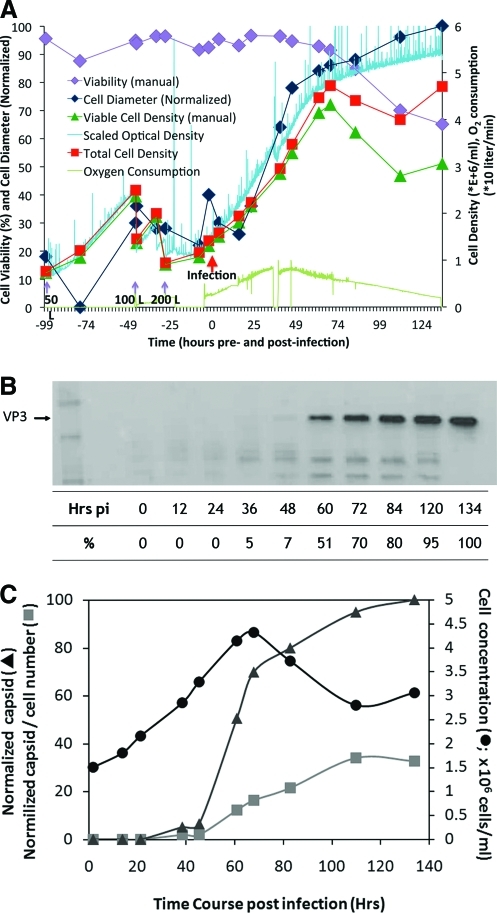
Based on the conditions established in the small- and mid-scale production runs, the process was transferred to100-L and 200-L stirred-tank, single-use bioreactors. The procedure for 100-L and 200-L single-use bioreactors was similar, starting with the minimum working volume of medium for both bioreactors, which coincidentally was 40 L. After temperature and dO were equilibrated, 3 × 1010 Sf9 cells were added to achieve the initial cell density of 7.5 × 105/ml. The cells were expanded by repetitive twofold dilutions with fresh medium while maintaining the density between 1 and 2 × 106 cells/ml. Once the final working volume was reached and the cell density reached 1.6 to 1.8 × 106 cells/ml, the BIIC aliquots were thawed and added to the culture (t = 0). The cells continued to grow and cell density to increase. As the thawed BIIC revived, the two baculoviruses were released into the medium. Measuring the increase in cell diameter indicates the infection rate. Figure 2A shows typical culture values for the 200-L production. The cell growth rate was logarithmic during the expansion and continued until approximately t = 62 hr post infection, when the baculovirus infection caused cell-cycle arrest, indicating that the majority of the cells were infected with at least one baculovirus. Shortly thereafter, the slope of the cell viability curve became negative. The cell viability at harvest depended on the vector: Sf9 cells producing rAAV-cU7 remained at higher viability levels than cells producing rAAV-GFP vectors. This difference was serotype-independent and may be due to the expressed transgene affecting baculovirus–cell interactions. Capsid proteins first appeared about 36 hr post infection and continued increasing until harvesting at 134 hr post infection (Fig. 2B). The rAAV produced reflects the cell viability and cell density; however, specific production, i.e., the rAAV yield per cell, leveled off after 120 hr post infection (Fig. 2C).
FIG. 2.
Cell culture growth and rAAV capsid production in 200-L single-use, stirred-tank bioreactors. (A) The minimum working volume (40 L) of serum-free medium was added to the bioreactor and inoculated with 3 × 1010 Sf9 cells. During exponential growth, the cell density doubled from 0.75 × 106/ml to 1.5 × 106/ml in approximately 15 hr (green line and green triangles). Additional medium was added to a total final volume of 200 L. Once the cell density reached 1. 5 × 106/ml, aliquots of BIIC (10 ml of each BIIC) were thawed and added to the bioreactor. Exponential cell growth continued for approximately 74 hr post infection. During the log-phase growth period, cell viability remained >90% (purple line and purple squares). Beyond 74 hr post infection, the cell viability and total cell density declined with a corresponding decrease in oxygen consumption (lower green curve). (B) Western blot detection of the AAV6 capsid proteins. Samples were removed from the bioreactor at the indicated times post infection, and aliquots were fractionated by SDS-PAGE and transferred to PVDF membranes for western blot analysis. The arrow indicates the position of VP3, the most abundant capsid protein, and the first measurable AAV6 capsid proteins were detected in the 48-hr postinfection sample. The values on the bottom represent the relative capsid production normalized to the highest enhanced chemiluminescence signal on the western blot (134 hr post infection). (C) Time course of specific rAAV production. The relative specific production of rAAV was calculated by western blot analysis for capsid amount (B) and viable cell number determined at the specified time points. The value obtained by dividing the normalized amount of capsid protein by viable cell number generated the normalized capsid/cell number values. The x-axis indicates hours after BIIC addition. The left y-axis is a relative scale, 0 to 100, related to either the amount of capsid protein present in the bioreactor or the amount of capsid protein produced per viable cell. The right y-axis indicates the viable cells per milliliter, in millions. Color images available online at www.liebertonline.com/hum
Downstream processing and production results
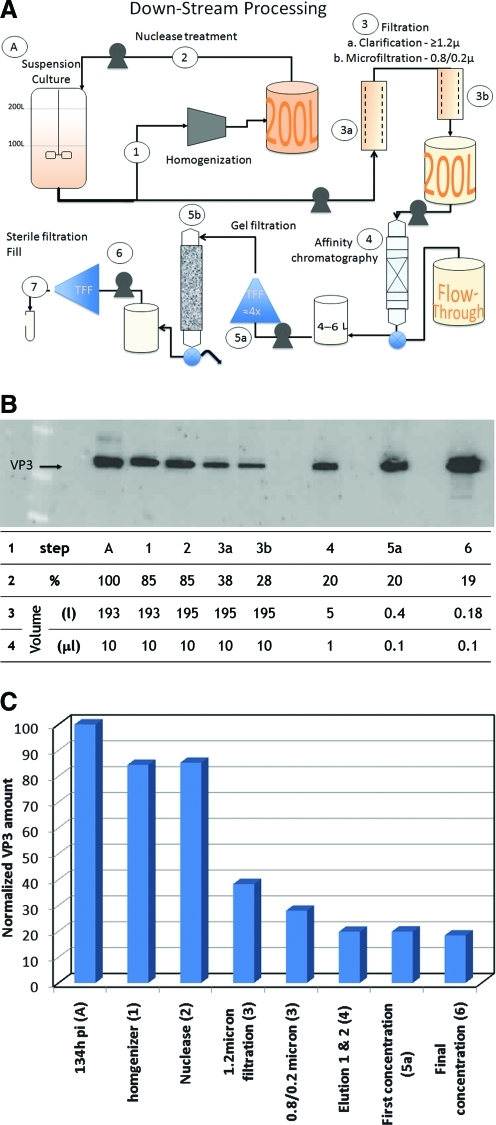
The downstream process flow diagram is represented schematically in Fig. 3A. Single-use components were used for most of the processing steps, except for the homogenizer and preparative chromatography systems, which are reusable and require cleaning and sanitization between productions. Following homogenization (Fig. 3A, step 1), the mixture of cell lysate and medium was returned to the bioreactor for nuclease treatment (Fig. 3A, step 2). Three filter membranes were used for clarification: a 1.2-μm depth filter, then a two-stage 0.8-μm/0.2-μm capsule filter (Fig. 3A, step 3). The vector recovery after each downstream processing step was analyzed by PAGE and western blots (Fig. 3B). The major vector loss occurs during the first filtration step (Fig. 3B and C, compare steps 2 and 3a). Approximately 55% of the vector capsid is retained, i.e., lost, during the initial clarification step. Although the vector recovered after each subsequent steps was more efficient (Fig. 3B and C), the cumulative recovery of steps 4, 5, and 6 was about 50%.
FIG. 3.
Down-stream processing (DSP). (A) Process flow diagram. The upstream production component, A, represents cell growth, expansion, and infection. Subsequent steps are numbered 1 to 7. The details are described in the text. Except for the volumes where indicated, the steps used for processing 200 L are derived from smaller-scale cultures with adjustments made for filter area or capsule filter lengths. TFF, tangential flow filtration. Gel exclusion chromatography (step 5b) was an optional step depending on vector administration. Step 6, the final TTF concentration/diafiltration procedure, was performed prior to the final sterile filtration and vialing step (step 7). (B) Vector recovery following each DSP step. The amount of capsid antigen was determined by western blot at each sampling point analyzed quantitatively to determine efficiency of recovery during the DSP. The capsid proteins in the starting material (step A) represent 100% of the rAAV. Line 1: “step” refers to the process in the DSP corresponding to the diagram in (A). Line 2: “%” represents the percentage of vector particle recovered determined by using volume-adjusted samples to accommodate concentration or dilution steps in the process. Lines 3 and 4: “Volume” indicates either the process volume at each step (in liters) or the amount of the process volume applied to the PAGE for the western blot analysis (in microliters), respectively. Steps 1 through 3 were performed on the entire bioreactor volume and required no adjustments for dilution or concentration. The capsid level apparently decreased by 15% during the homogenization and nuclease treatment steps, but the single largest change occurred during the first filtration step (step 3a). The second capsule filtration resulted in lower vector loss (step 3b). IA chromatography caused relatively minor vector loss, approximately 8% overall. The IA eluted fraction was concentrated and diafiltrated using TFF without vector loss (step 6). The last step (step 7) represents the final sterile filtration and vialing of the product. (C) Histogram of rAAV recovered following each DSP step. The graph represents the values obtained from the western blot analysis described in (B). Color images available online at www.liebertonline.com/hum
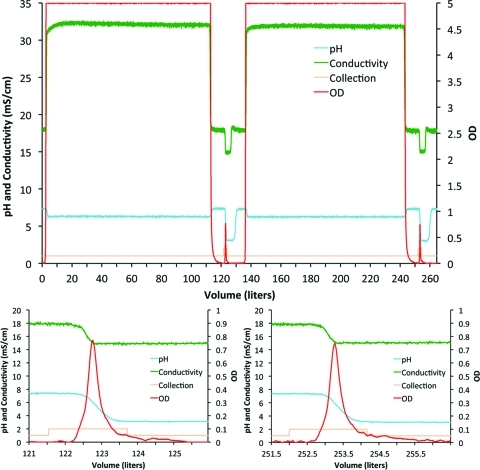
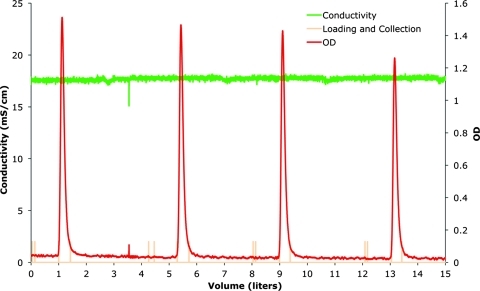
IA chromatography produced the greatest-fold purification in the downstream process (Fig. 3A, step 4). By using a chromatography skid specifically configured for this process, the entire bioreactor contents were pumped through a 20-cm-diameter by 6–7-cm-bed-height preparative column at 0.4 L/min. The UV (280 nm) absorbance, pH, and conductivity were monitored and recorded. Due to the relatively small column dimensions, approximately 100 L of treated culture were applied per bind–wash–elute cycle. After each cycle, the column was washed with PBS until the UV absorbance returned to baseline values, and then the rAAV was eluted using 0.05 M citrate (pH 3.0). The eluted vector appears as spikes in the full-scale chromatograph (Fig. 4, top panel) and as similar symmetric peaks in the enlarged chromatograph (Fig. 4, bottom panels).
FIG. 4.
IA chromatography profile (step 4 in Fig. 3). The entire bioreactor contents were applied to the preparative column. The top panel shows the full-scale UV (280 nm) absorbance profile of the flow-through and two elution peaks. Enlargements of both elution peaks are presented in the bottom panels. The particles began eluting at the onset of the pH change. The eluted fractions, 2.5 L from each load and elution, were pooled and concentrated into 0.4 L for subsequent processing. Color images available online at www.liebertonline.com/hum
A polishing step, when necessary, involved gel filtration using column chromatography with Superdex 200 (Fig. 3A, step 5b). Obtaining high-resolution fractionation limited the volume of vector solution applied to the column. Therefore, processing the entire quantity of vector required dividing the 0.4 L of concentrated vector solution into fourths and repeating the chromatography procedure for each aliquot. The chromatography profile (Fig. 5) demonstrates the reproducibility of this final purification step, and the vector DNA analysis demonstrated near-quantitative recovery from the gel filtration process (data not shown).
FIG. 5.
Gel filtration chromatography (step 5b in Fig. 3A) was used to remove any material that coeluted from the IA chromatography step. Concentrated vector from 100-L vector production was divided into 4 × 0.1 L for gel filtration chromatography (Superdex 200). The UV (280 nm) (red curve) profile is a series of four, symmetrical, sharp peaks that contain the rAAV particles eluting in the void fractions. The four fractions were pooled for subsequent concentration and diafiltration steps. Color images available online at www.liebertonline.com/hum
The solubility and recovery of rAAV were improved by a series of treatments: adding a surfactant did not improve vector recovery, whereas treating the lysed culture with nuclease and then adding NaCl (final concentration, 0.4 M) resulted in minor improvements in rAAV solubility (data not shown).
Table 1 shows the results of 10 large-scale rAAV production runs using BIIC at 10 L, 20 L, 100 L, and 200 L. The average rAAV yield per cell for all scales was 1.76 × 104 (±6.4 × 103) vg particles/cell. Vectors produced using rocking platform cultures averaged 1.54 × 104 (±6.30 × 103) vg particles/cell, and in stirred tank 100- and 200-L bioreactors, the yields were 1.98 × 104 (±6.49 × 103) vg particles/cell. Strikingly consistent production values were obtained in all formats and volumes, with the most reliable results obtained in the two 200-L productions yielding final purified rAAV of 1.89 × 104 (±282) vg particles/cell for a total of 3.22 × 1016. Overall, 7.15 × 1016 vg rAAV particles were obtained from 880 L of total culture volume (Table 1). The efficiency of vector recovery indicated that approximately 20% of the rAAV in the biomass was recovered in the final purified product.
Vector characterization
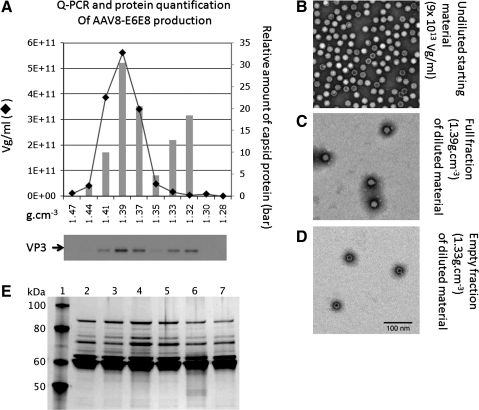
The protein components of the vector particles were analyzed using PAGE and western blots. The DNA content was assessed quantitatively using two techniques: qPCR and fluorescent dye binding to extracted virion DNA. Both methods provided similar results. The ratio of empty to filled capsid particles was estimated using cesium chloride isopycnic gradients. Following centrifugation, the gradients were fractionated and aliquots were analyzed for the presence of capsid proteins (PAGE/western blots) and for DNA (qPCR) (Fig. 6A). The signal from the VP3 western blot in each fraction was divided by the sum of VP3 signal in all fractions (Fig. 6A, lower panel). Capsid proteins were detected in two regions of the gradient: fractions with densities of 1.41, 1.39, and 1.37 g/cm3 and two fractions with densities of 1.33 and 1.32 g/cm3. The capsids in the three denser fractions represented 65% of the total capsids in the gradient (Fig. 6A) and, according to qPCR analysis, contain the vector genome. The morphology and empty/filled composition of the capsids were examined by negative staining and electron microscopy (EM). The EM image of unfractionated vector showed densely populated fields in the grid with apparently mostly filled particles, i.e., excluding the molybdate staining solution (Fig. 6B), whereas the EM images of particles in the 1.39 g/cm3 (Fig. 6C) and 1.32 g/cm3 (Fig. 6D) fractions showed filled particles and empty particles, respectively. Therefore, two independent analytical methods indicate that the majority of the capsids are filled. The products of seven different rAAV production runs are shown in Fig. 6E by silver-stained PAGE. An aliquot (0.5 μl) of the vector following IA chromatography and concentration and diafiltration using tangential flow filtration was processed.
FIG. 6.
Physical vector characterization. (A) Following CsCl isopycnic centrifugation, gradients were fractionated and assayed for the presence of capsid protein and vector DNA. The graph represents the DNA content of each fraction (solid line), and the columns indicate the relative amount of capsid protein in each fraction. The signal from each fraction was divided by total VP3 signal on the western blot (lower panel). The abscissa denotes the density of each fraction in g/cm3. The ordinates represent the DNA content, as vg/ml (left axis) and relative VP3 amount in each fraction (right axis). The buoyant density of empty particles is approximately 1.32 g/cm3 and that of filled particles is 1.39 g/cm3. Based on the distribution of vector DNA and protein across the gradient, approximately 65% of the capsids contain vector DNA. (B–D) TEM images of rAAV particles. Negatively stained rAAV particles from unfractionated material (B) and high- (C) and low-buoyant (D) density fractions are consistent with the ratio of full and empty particles described in (A). In the unfractionated, undiluted starting material (C), >50% of the particles appear full. (E) Silver-stained polyacrylamide gel of rAAV proteins following IA chromatography and TFF concentration/diafiltration. An aliquot (0.5 μl) of each vector was fractionated by SDS-PAGE, and proteins were visualized by silver staining. The rAAV6-AlkPhos production yielded 1.94 × 1016 vg from approximately 200 L. The samples and concentrations are listed. Lane 1, molecular mass standards; lane 2, rAAV6-U7smOPT (9 × 1013 vg/ml); lane 3, rAAV6-U7smOPT (5.77 × 1013 vg/ml); lane 4, rAAV6-AlkPhos (5.18 × 1013 vg/ml); lane 5, rAAV6-U7smOPT (7 × 1013 vg/ml); lane 6, rAAV6-U7smOPT (9 × 1013 vg/ml); lane 7, rAAV6-GFP (6.7 × 1013 vg/ml).
Discussion
Manufacturing rAAV gene therapeutics requires reproducible and reliable cGMP production processes. Eventual commercialization of rAAV biologics will not be realized without an economical production process that generates quantities of rAAV sufficient for supporting preclinical and clinical studies. For diseases treatable with locoregional administration of rAAV, e.g., subretinal injection for treating Leber's congenital amaurosis (Bennicelli et al., 2008; Maguire et al., 2008, 2009), therapeutic doses of rAAV are readily obtained from a relatively small number of rAAV producer cells. However, disseminated diseases requiring efficient transduction of a large mass of tissue throughout the body is especially daunting in many respects, for example, establishing route of administration to achieve clinically meaningful outcomes without inducing cytotoxicity or unmanageable immune responses toward the capsid proteins or the transgene products. Therefore, developing effective treatments of diseases such as Duchenne muscular dystrophy necessitates levels of rAAV production unattainable through conventional methods relying on transient transfection of adherent cells. The baculovirus–Sf9 suspension cultures provide a means for producing rAAV in amounts exceeding 1016 per run.
The TIPS process provides a solution to storing baculovirus for rAAV production (Wasilko and Lee, 2006; Wasilko et al., 2009). Concentrated BIIC are cryopreserved in single-use aliquots that release infectious baculovirus after thawing. By determining the optimal BIIC:Sf9 producer cell ratios for each BIIC stock, rAAV production remained constant and predictable. A typical 100-L rAAV production using the TIPS process required 10 ml of BIIC for each of the two baculoviruses used in the process. Thus, 1 L of cryopreserved BIIC, produced from 10 L of cell culture, can be used for 1,000 L of rAAV production. Based on our experience, the yield from 1,000 L would be ≥1017 rAAV particles.
The rAAV yields obtained using BIIC and the TIPS process in 100- and 200-L bioreactors have produced very consistent yields of purified rAAV: approximately 20,000 (±5,800) vg particles/cell from production runs totaling 800 L. The consistency between two 200-L bioreactor runs was more strikingly consistent: 18,900 (±282) vg particles/cell, which represents a 1.5% variation between the two runs.
Using conditions established for shake-flask cultures, larger volumes were tested for rAAV production. The downstream processing was adapted to the larger volumes and provided consistent results from 0.02 up to 200 L.
Approximately 45% of the vector was recovered from the initial clarification step, whereas subsequent filtration, chromatography, and diafiltration steps were about 50% efficient overall. Assuming that the capsid proteins detected in the crude lysate represent intact particles, we estimate that the overall yield is 18.5%. These conditions resulted in consistent rAAV yields of approximately 18 × 103 vg-containing particles per cell in the final formulation of product. Based on more than 10 production runs with a total volume of 874 L, the average yield of purified rAAV obtained was 6.49 × 1013 (±3.95 × 1013) vg/L. Using cesium chloride isopycnic gradients and other analytical methods, we determined that approximately two thirds of the capsids contained vector genomes.
Vector characterization analyses indicate that the particles are physically equivalent to mammalian cell-produced rAAV. In vitro and in vivo the vectors demonstrated biological activity, and these vector preparations have demonstrated efficacy in preclinical and clinical studies for lipoprotein lipase deficiency (Mingozzi et al., 2009).
Acknowledgments
Funding was provided by the Division of Intramural Research of the National Heart, Lung, and Blood Institute [National Institutes of Health (NIH)]. Additional funding was provided by the International Collaborative Effort (ICE) for Duchenne Muscular Dystrophy comprised of the French Duchenne Parent Project and the Association Monogasque contre les Myopathies. This research was conducted under a cooperative research and development agreement (CRADA) with Amsterdam Molecular Therapy. The authors are grateful to Richard H. Smith, Alejandro Negrete, and Lina Li for their assistance and insights related to this work. Thomas Wallach performed preliminary TIPS experiments, and Linda Yang and Yu Yang provided important technical support.
Author Disclosure Statement
Portions of the technology described in this report are covered by United States and European patents assigned to the Secretary of the Department of Health and Human Services. A fraction of the licensing fees and royalty payments made to the NIH is distributed to the inventors in accordance with U.S. Government and NIH policy.
References
- Aucoin M.G. Mena J.A. Kamen A.A. Bioprocessing of baculovirus vectors: a review. Curr. Gene Ther. 2010;10:174–186. doi: 10.2174/156652310791321288. [DOI] [PubMed] [Google Scholar]
- Bennicelli J. Wright J.F. Komaromy A., et al. Reversal of blindness in animal models of Leber congenital amaurosis using optimized AAV2-mediated gene transfer. Mol. Ther. 2008;16:458–465. doi: 10.1038/sj.mt.6300389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis D.L. Garcia A., Jr. Long-term stability of baculoviruses stored under various conditions. Biotechniques. 1994;16:508–513. [PubMed] [Google Scholar]
- Jorio H. Tran R. Kamen A. Stability of serum-free and purified baculovirus stocks under various storage conditions. Biotechnol. Prog. 2006;22:319–325. doi: 10.1021/bp050218v. [DOI] [PubMed] [Google Scholar]
- Maguire A.M. Simonelli F. Pierce E.A., et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N. Engl. J. Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire A.M. High K.A. Auricchio A., et al. Age-dependent effects of RPE65 gene therapy for Leber's congenital amaurosis: a phase 1 dose-escalation trial. Lancet. 2009;374:1597–1605. doi: 10.1016/S0140-6736(09)61836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingozzi F. Meulenberg J.J. Hui D.J., et al. AAV-1-mediated gene transfer to skeletal muscle in humans results in dose-dependent activation of capsid-specific T cells. Blood. 2009;114:2077–2086. doi: 10.1182/blood-2008-07-167510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negrete A. Kotin R.M. Production of recombinant adeno-associated vectors using two bioreactor configurations at different scales. J. Virol. Methods. 2007;145:155–161. doi: 10.1016/j.jviromet.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negrete A. Esteban G. Kotin R.M. Process optimization of large-scale production of recombinant adeno-associated vectors using dielectric spectroscopy. Appl. Microbiol. Biotechnol. 2007a;76:761–772. doi: 10.1007/s00253-007-1030-9. [DOI] [PubMed] [Google Scholar]
- Negrete A. Yang L.C. Mendez A.F., et al. Economized large-scale production of high yield of rAAV for gene therapy applications exploiting baculovirus expression system. J. Gene Med. 2007b;9:938–948. doi: 10.1002/jgm.1092. [DOI] [PubMed] [Google Scholar]
- Smith R.H. Levy J.R. Kotin R.M. A simplified baculovirus-AAV expression vector system coupled with one-step affinity purification yields high-titer rAAV stocks from insect cells. Mol. Ther. 2009;17:1888–1896. doi: 10.1038/mt.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urabe M. Ding C. Kotin R.M. Insect cells as a factory to produce adeno-associated virus type 2 vectors. Hum. Gene Ther. 2002;13:1935–1943. doi: 10.1089/10430340260355347. [DOI] [PubMed] [Google Scholar]
- Virag T. Cecchini S. Kotin R.M. Producing recombinant adeno-associated virus in foster cells: overcoming production limitations using a baculovirus-insect cell expression strategy. Hum. Gene Ther. 2009;20:807–817. doi: 10.1089/hum.2009.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasilko D.J. Lee S.E. Titerless: infected-cells preservation and scale-up. Bioprocess. J. 2006;5:29–32. [Google Scholar]
- Wasilko D.J. Lee S.E. Stutzman-Engwall K.J., et al. The titerless infected-cells preservation and scale-up (TIPS) method for large-scale production of NO-sensitive human soluble guanylate cyclase (sGC) from insect cells infected with recombinant baculovirus. Protein Expr. Purif. 2009;65:122–132. doi: 10.1016/j.pep.2009.01.002. [DOI] [PubMed] [Google Scholar]