Abstract
This study evaluated six adeno-associated viral (AAV) vectors expressing green fluorescent protein (GFP) from the liver-specific thyroid hormone–binding globulin (TBG) promoter made with novel capsids in canine liver-directed gene transfer. Studies in 1.5-month-old dogs, which were administered vector through a peripheral vein, showed that AAV8 capsid vectors had the most favorable performance profiles. Interestingly, the absolute levels of hepatocyte transduction achieved with AAV8 were lower in dogs compared with what had been achieved in mice and nonhuman primates. Additional studies were performed with AAV8 delivered into the hepatic artery in adult dogs, with higher doses of vector used to assess potential dose-limiting toxicities. These studies showed good transduction on day 7 in one dog that apparently was lost by day 28 in another dog through the generation of GFP-specific T cells. Each adult dog was carefully monitored for any hemodynamic changes associated with vector infusion. Both animals demonstrated mild to moderate hypotension and bradycardia, which appeared to be anesthesia-related, making it difficult to evaluate contributions of the vector.
In this study, six clinically promising AAV serotypes were evaluated in dogs, using the same liver-specific promoter (TBG) and dose (3 × 1012 GC/kg). AAV8 emerged as the best performing serotype in liver, as observed previously in mice and nonhuman primates. However, AAV8 transgene expression levels were lower and less persistent in dogs than in other animal models.
Introduction
The liver has been widely used for the expression of therapeutic transgenes, both because of the accessibility of hepatocytes via the blood stream through a fenestrated epithelium and because of its importance for many metabolic functions and the production of plasma proteins. Adeno-associated virus (AAV)-based vectors are promising tools for liver-directed gene transfer, and a number of different serotypes have been evaluated for their potential to efficiently transduce hepatocytes. Most of these experiments have been performed in mice, and indeed high and also stable levels of transgene expression were achieved in these animals (Vandendriessche et al., 2007; Cunningham et al., 2009; Wang et al., 2010b). However, the mouse appears not to be an optimal animal model in that it does not reflect some of the challenges likely to be encountered in clinical gene therapy trials. Mice lack preexisting neutralizing antibodies (NAbs) against AAV serotypes derived from monkeys or humans such as AAV8, which may confound expression data in nonhuman primates (NHPs) and other animal models. More importantly, gene transfer experiments with AAV carried out in NHPs demonstrated the potential development of T cell responses against the transgene, which is not the case in studies performed in mice. Using self-complementary AAV7 expressing green fluorescent protein (GFP) from a CB (cytomegalovirus-enhanced chicken β-actin) promoter, the T cell response against the transgene eliminated GFP expression 5 weeks after vector administration (Gao et al., 2009). Similar experiments in NHPs with AAV serotypes expressing GFP from the liver-specific TBG (thyroid hormone-binding globulin) promoter demonstrated a lower likelihood of developing transgene-specific T cells, and the presence of those T cells did not necessarily eliminate GFP expression (Wang et al., 2010a). An additional complication in humans could be the development of T cells against the capsid as observed in a clinical trial with AAV2 expressing factor IX (Manno et al., 2006; Mingozzi et al., 2007).
Although NHPs are the preferable model organism to test therapeutic gene transfer to allow comparisons with humans, there are a very limited number of NHP disease models available. Canine models of several human genetic diseases are available and provide unique opportunities for evaluating in vivo gene therapy (High, 2005; Casal and Haskins, 2006; Haskins, 2009).
We previously tested several AAV serotypes as clinical candidates for liver-directed gene transfer in mice (AAV8, AAV7, AAV2, AAV6.2, AAVrh.64R1, AAVhu.37, AAVrh.8, AAVrh.32.33) and subsequently in NHPs (AAV8, AAVhu.37, AAVrh.8) (Wang et al., 2010a,b). These vectors belong to a larger group of AAV serotypes that have been isolated from human and NHP tissues (Gao et al., 2002, 2004; Vandenberghe et al., 2009). In the monkey experiments, the vectors were administered intravenously at a dose of 3 × 1012 genome copies (GC)/kg and expressed GFP from the liver-specific TBG promoter. On the basis of these studies, AAV8 emerged as the best performing candidate for clinical gene therapy trials.
In the present report, we evaluated six of these AAV serotypes in dogs, including the three serotypes that had been tested in monkeys, using the same promoter (TBG) and dose (3 × 1012 GC/kg) as was done in NHPs. These serotypes were selected as initial clinical candidates for further detailed analysis because of their superior liver transduction efficiency compared with other members in the same clade in our pilot studies. More specifically, dogs at 1.5 months of age were injected via the cephalic vein with AAV8, AAV2, AAV6.2, AAVhu.37, AAVrh.64R1, and AAVrh.8 and GFP expression in liver was examined after 7 days and, in the case of AAV2 and AAV8, also after 4 weeks. Two additional AAV2-injected animals were evaluated after 12 days. We further examined liver toxicity by serum chemistry, development of T cells against transgene and capsid, and distribution of vector genomes in liver and spleen. In addition, AAV8 was infused into two adult dogs via the hepatic artery at higher doses (1.82 × 1013 and 7.9 × 1012 GC/kg) and vital signs and other clinical data were recorded during and after the administration. The livers of these two animals were examined on days 7 and 35, respectively.
Materials and Methods
Vectors
AAV vectors (serotypes 8, 2, 6.2, hu.37, rh.64R1, and rh.8) expressing enhanced GFP (EGFP) were produced and purified by the Penn Vector Core at the University of Pennsylvania (Philadelphia, PA) as described previously (Gao et al., 2006; Wang et al., 2010a). All vectors contained the liver-specific TBG promoter downstream of two copies of the α1-microglobulin/bikunin enhancer sequence (Ill et al., 1997, Gao et al., 2006).
Animals
The outbred dogs used in this study were mixed breeds, with most animals based on a German shepherd background mixed with beagle, springer spaniel, corgi, plott hound, Newfoundland, and Irish setter. They were maintained at the School of Veterinary Medicine at the University of Pennsylvania under National Institutes of Health and U.S. Department of Agriculture guidelines for the care and use of animals in research. The study was performed according to a protocol approved by the Office of Environmental Health and Radiation Safety, the Institutional Biosafety Committee, and the Institutional Animal Care and Use Committee of the University of Pennsylvania. For the experiments performed in young dogs, animals were injected at 1.5 months of age via the cephalic vein with vector (3 × 1012 GC/kg) diluted in 4 ml of phosphate-buffered saline (PBS). Dogs were sacrificed by intravenous injection of sodium phenobarbital (80 mg/kg) at 7, 12, or 28 days and the liver and spleen were removed.
The two adult dogs received vector (AAV8.TBG.EGFP) by infusion into the hepatic artery with 1.82 × 1013 GC/kg in 8.6 ml of PBS (S473) or 7.9 × 1012 GC/kg in 8.7 ml of PBS (S468). Before injection, the vector solution was mixed with 3 ml of contrast agent (Omnipaque; GE Healthcare Life Sciences, Piscataway, NJ) for fluoroscopy. In both animals, propofol was intravenously administered to induce anesthesia, which then was maintained with isoflurane. The right femoral artery was exposed and a no. 5 French introducer was advanced into the artery. An injection catheter was then introduced into the hepatic artery under fluoroscopic guidance for vector delivery. Propofol was administered intravenously so that respiration could be temporarily suppressed for better visualization of the catheterization procedure. Both dogs received the nonsteroidal antiinflammatory drug carprofen (2.2 mg/kg) for 3 days after the procedure. More details of the anesthesia protocol and vector administration are described in Results and summarized in Figs. 9 and 10.
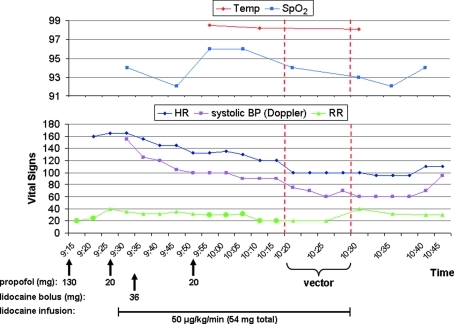
FIG. 9.
Vital signs of dog S473 (18 kg) around the time of vector administration. Temp, temperature (°F); SpO2, percent oxygen saturation measured by pulse oximetry; HR, heart rate (bpm); BP, systolic blood pressure measured by Doppler ultrasound (mmHg); RR, respiratory rate (rpm). Circles in the respiratory rate plot indicate mechanical ventilation. The time scale on the x axis between 10:20 and 10:40 is doubled to accommodate additional data points. Times of propofol boluses, lidocaine administration, and vector infusion are shown.
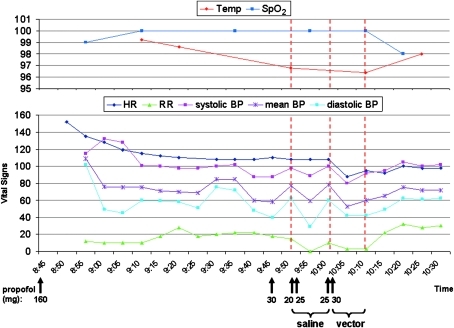
FIG. 10.
Vital signs of dog S468 (20 kg) around the time of vector administration. Temp, temperature (°F); SpO2, percent oxygen saturation measured by pulse oximetry; HR, heart rate (bpm); BP, blood pressure (systolic, mean, and diastolic BP measured with an arterial line, mmHg); RR, respiratory rate (rpm). Times of propofol boluses, saline, and vector infusion are shown.
Analysis of GFP expression
Liver samples were fixed overnight in formalin, washed in PBS for 30 min, and frozen in O.C.T. compound (Sakura Finetek USA, Torrance, CA) for cryosectioning. For quantification of GFP expression, 10 random images were taken from sections of different lobes at identical microscope and camera settings. GFP expression was quantified both for the percentage of area occupied by GFP-positive cells in liver sections as well as for the intensity of GFP fluorescence in tissue sections as described previously (Wang et al., 2010a). Briefly, the percentage of GFP-positive liver area was obtained by “thresholding” the images, that is, selecting the area considered GFP-positive based on comparison with liver sections from untreated dogs, and calculating the average percentage of this area. GFP intensity was determined by calculating the sum of the intensity values of all pixels of each image minus the background values as measured from untreated control livers. The average value from 10 images is shown for both measurements in Fig. 2. Values of p were calculated by Student t test. ImageJ software (Rasband, 1997–2006; National Institutes of Health, Bethesda, MD; http://rsb.info.nih.gov/ij/) was used for all of the described quantification procedures.
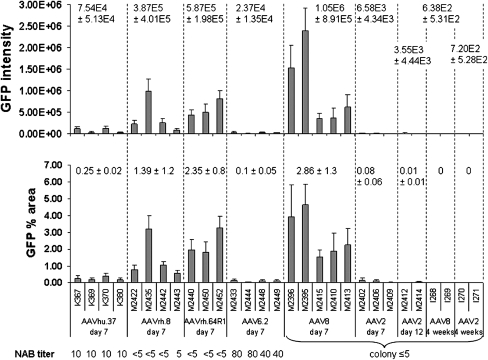
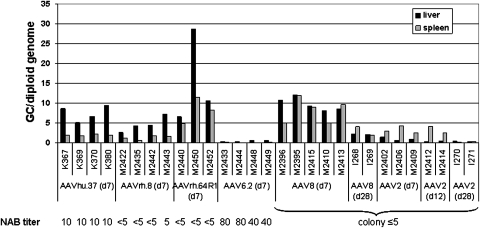
FIG. 2.
GFP intensity (top) and percentage of GFP-positive area (bottom) of liver sections as a quantitative measure of transgene expression. GFP intensity was calculated as the sum of brightness values of each image minus the background value. For both measurements, 10 random images of sections from different parts of the liver were taken for each animal and the mean ± SD is shown. The average values for each group are indicated above the columns. Neutralizing antibody (NAb) titers (shown as reciprocal of serum dilution) are for the corresponding vector at the time before injection for each animal. For AAV2 and AAV8, the individual titers are not known, but testing of 21 dogs from the same colony revealed titers below 1:5 and in one case equaling 1:5.
NAb assays
NAb assays were performed as described (Calcedo et al., 2009) by incubating the corresponding serotype of AAV.CMV.LacZ with 2-fold dilutions of heat-inactivated dog serum and determining β-galactosidase expression in Huh7 cells. The highest serum dilution inhibiting LacZ expression by at least 50% corresponds to the reported NAb titer.
Vector biodistribution and RNA analysis
DNA was extracted from tissues, using a QIAamp DNA mini kit (Qiagen, Valencia, CA). For liver, samples were taken from different lobes of each animal. Detection and quantification of vector genomes in extracted DNA were performed by real-time PCR as described previously (Bell et al., 2005). Vector genome copies were calculated per diploid genome, assuming a DNA content of 6.6 pg per cell nucleus.
GFP RNA in liver and spleen was quantified by real-time reverse transcriptase-PCR as described (Livak and Schmittgen, 2001; Gao et al., 2009), using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) RNA as reference.
Lymphocyte isolation and interferon-γ enzyme-linked immunospot assay
Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood collected in EDTA-containing Vacutainer tubes after Ficoll density-gradient centrifugation. At the time of necropsy, lymphocytes were also isolated from liver by Percoll density-gradient centrifugation and from spleen by grinding through 40-μm (pore size) strainers (BD Biosciences, San Jose, CA). Enzyme-linked immunospot (ELISpot) assays to detect canine interferon (IFN)-γ were performed with the canine IFN-γ ELISpot development module and ELISpot blue color module (R&D Systems, Minneapolis, MN), in accordance with the manufacturer's instructions. T cells were stimulated with a GFP peptide library or peptide pools for AAV2 and AAV8 viral protein-1 (VP1) as described (Wang et al., 2007).
Results
Liver-directed gene transfer with AAV serotypes in young dogs (1.5 months)
Levels of GFP expression
Transgene expression from six AAV serotypes was evaluated in liver of 1.5-month-old dogs 7 days after vector administration. Dogs received, via the cephalic vein, AAV serotype 2, 8, 6.2, hu.37, rh.64R1, or rh.8 (n = 3–5 dogs per serotype) expressing GFP from the liver-specific TBG promoter. Seven days later we examined the livers and quantified sections both for GFP intensity and the percentage of GFP-positive liver area (Figs. 1 and 2). Both measurements were correlated: in general, the more transduced hepatocytes that were present, the higher the value for overall GFP intensity. This implied that there was no abnormal transduction pattern among the tested serotypes, that is, there were no liver sections with either many weakly transduced hepatocytes, or few strongly transduced hepatocytes (Fig. 1). GFP expression was restricted to hepatocytes; no other cell types in the liver such as Kupffer cells or endothelial cells were found to be GFP-positive. We further examined the spleens of all dogs that were found to be negative for GFP fluorescence (data not shown).
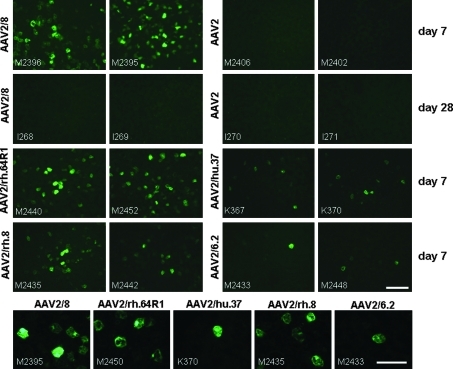
FIG. 1.
Green fluorescent protein (GFP) expression in dog liver 7 or 28 days after gene transfer via the cephalic vein with the indicated AAV serotype at a dose of 3 × 1012 genome copies (GC)/kg. Four animals per group were analyzed except for the day 28 time point, which included only two dogs per group. For the day 7 animals, only the two livers with the strongest GFP expression are shown. Bottom: Liver images at higher magnification, demonstrating hepatocyte transduction. Scale bars: 100 μm (low magnification) and 50 μm (high magnification).
AAV8 showed the highest levels of GFP expression, followed by AAVrh.64R1 and AAVrh.8 (Figs. 1 and 2). However, GFP expression was relatively low, especially when compared with previous data on NHP livers from animals that had received the same dose of vector (Wang et al., 2010a), and even more so in mice that had received a comparable dose of 1 × 1011 GC per animal (Wang et al., 2010b). However, in the mouse study, a CB promoter was used instead of the TBG promoter. In the dogs, the average transduced liver area for AAV8 was 2.86 ± 1.3%. The average transduced areas for AAVrh.64R1 and AAVrh.8 were 2.35 ± 0.8 and 1.39 ± 1.2%, respectively. Even lower GFP expression levels were observed for AAVhu.37 (0.25 ± 0.02%) and AAV6.2 (0.10 ± 0.05%), well below 1% of GFP-positive liver area (Figs. 1 and 2). AAV2 also failed to produce any appreciable GFP expression in the livers except for a few transduced hepatocytes that were occasionally found. Compared with AAV8, these vectors achieved 82.2% (AAVrh.64R1), 48.6% (AAVrh.8), 8.7% (AAVhu.37), 3.5% (AAV6.2), and 2.8% (AAV2) of the AAV8 area transduction value. The GFP intensity values showed a higher degree of variability than the area values within the AAV8 group on day 7. Values of p were determined between the AAV8 group and each of the other groups examined on day 7. There was no statistically significant difference (p ≤ 0.05) between the GFP intensity of the AAV8 group compared with AAVhu.37 (p = 0.068), AAVrh.8 (p = 0.214), AAVrh.64R1 (p = 0.423), AAV6.2 (p = 0.058), and AAV2 (p = 0.097). However, the percentages of transduced liver area were significantly different from AAV8 with AAVhu.37 (p = 0.007), AAV6.2 (p = 0.005), and AAV2 (p = 0.014) but not with AAVrh.8 (p = 0.136) and AAVrh.64R1 (p = 0.586).
The livers of two additional animals that received AAV2 were analyzed after 12 days, the rationale being that AAV2 may need a longer time to express the transgene. After 12 days GFP expression was not detected except for a few individual GFP-positive hepatocytes that could occasionally be found (Fig. 2).
We further analyzed liver and spleen from selected animals for the presence of GFP transcripts, to see whether GFP RNA is present in organs that did not show GFP fluorescence (Table 1). As observed previously in our NHP study (Wang et al., 2010a), low levels of GFP RNA (relative to control spleen from an untreated dog) are detectable in spleen from all animals tested despite the presence of relatively high copy numbers of vector genomes in some of the spleens (compare with Fig. 3). For example, animal M2395 had about the same amount of vector genome copies in liver as in spleen, but the GFP RNA level was more than 400-fold higher in liver than in spleen. The overall low levels of GFP RNA indicate that the TBG promoter was not active in this organ and is indeed liver-specific. Also, those livers where no GFP had been detected by microscopy showed similar low values for GFP transcripts, in contrast to liver from dogs treated with AAV8 and analyzed after 7 days (Table 1). The GFP fluorescence data therefore correlate well with the GFP RNA values.
Table 1.
Levels of Green Fluorescent Protein (GFP) Transcripts in Liver and Spleen in the Absence of Detectable GFP Fluorescence
| Group | Animal ID | Relative GFP RNA levels |
|---|---|---|
| Liver | ||
| AAV2, day 7 | M2402 | 4.53 × 101 |
| M2406 | 1.60 × 101 | |
| AAV2, day 12 | M2412 | 2.83 × 101 |
| M2414 | 1.58 × 101 | |
| AAV8, 4 weeks | I268 | 1.55 × 102 |
| I269 | 7.60 × 101 | |
| AAV8, day 7 | M2396 | 5.85 × 104 |
| M2395 | 3.00 × 104 | |
| untreated control | I276 | 1 × 100 |
| Spleen | ||
| AAV2, day 7 | M2402 | 6.72 × 100 |
| M2406 | 1.28 × 101 | |
| AAV8, day 7 | M2396 | 1.05 × 102 |
| M2395 | 7.14 × 101 | |
| AAVrh.64R1, day 7 | M2440 | 1.16 × 102 |
| M2452 | 1.03 × 102 | |
| AAVrh.8, day 7 | M2435 | 1.54 × 100 |
| M2442 | 6.86 × 100 | |
| AAVhu.37 | K367 | 2.35 × 101 |
| K370 | 6.34 × 100 | |
| AAV6.2 | M2433 | 1.21 × 10−1 |
| M2448 | 1.24 × 100 | |
| untreated control | I276 | 1 × 100 |
Shown are relative copy numbers of GFP RNA per 50 ng of total RNA normalized for GAPDH RNA levels and values from corresponding control tissues from an untreated dog (values from control liver and spleen = 1). Liver GFP RNA values are shown for livers with no visible GFP fluorescence (AAV8 at 4 weeks and AAV2 on days 7 and 12) compared with livers with the highest GFP expression (AAV8 on day 7). Relative copy numbers of spleen GFP RNA are shown for the two dogs from each group with the strongest liver GFP fluorescence on day 7. This also includes the spleen with the highest copy number of vector DNA for each group. No GFP fluorescence was detectable in the spleens of any of the animals.
FIG. 3.
Vector genome copies per diploid genome in liver and spleen on the indicated days after gene transfer. NAb titers (shown as the reciprocal of serum dilution) are for time before injection as in Fig. 2.
Prevalence and impact of neutralizing antibodies
The presence of preexisting antibodies is an important factor for gene therapy and impacts strongly on transgene expression. Therefore, the animals were prescreened for the presence of NAbs against the administered serotypes (see bottom legends in Figs. 2 and 3). No NAbs were detected against AAVrh.64R1 and AAVrh.8 in the animals treated with these serotypes, which all showed corresponding titers below 1:5. A screen of 21 animals from the dog colony for AAV2 and AAV8 revealed only one animal with a titer of 1:5 for AAV2; all other dogs had titers below 1:5 for both serotypes (the baseline samples from the AAV2 and AAV8 animals were not available). However, we found preexisting NAbs against AAV6.2 and AAVhu.37 in our dogs. The dogs that received AAV6.2 and AAVhu.37 (four animals each) had titers of preexisting NAbs against the corresponding serotype of 1:40–1:80 and 1:10, respectively (Figs. 2 and 3). Thus, the lower levels of GFP expression in dogs with AAV8, AAVrh.64R1, and AAVrh.8, compared with NHPs and mice, could not be explained by the presence of NAbs. Likewise, the almost complete absence of transgene expression from AAV2 appeared not to be related to the presence of interfering antibodies. The failure of transgene expression from AAV2 was not too surprising in the context of the relatively low performance of AAV8 in dogs, as AAV2 is known to have lower transduction levels in monkey and mouse liver compared with AAV8 (Lebherz et al., 2004; Sarkar et al., 2004; Thomas et al., 2004; Davidoff et al., 2005).
Vector genomes in liver and spleen
As expected, the number of genome copies for AAV6.2 administered in the presence of preexisting NAb titers equal to 1:40–1:80 averaged only 0.43 ± 0.2 GC per diploid genome, corresponding to the low GFP expression. AAV2 also had a low range of vector genomes in the liver, with an average of 1.02 ± 0.4 GC per diploid genome on day 7. The genomes of all other serotypes, however, could be found in a range of about 5–10 GC per diploid genome (Fig. 3). Dog M2450 was an exception with 28.7 GC per genome but only modest GFP expression (1.8% liver area). Animals treated with AAVhu.37 showed a discrepancy between vector genomes in the liver and GFP expression. The low levels of GFP expression in this group correlated with the presence of preexisting NAbs for AAVhu.37 (titer, 10), but were in contrast to relatively high numbers of vector genomes (average, 7.46 ± 1.9 GC per diploid genome). Whether this discrepancy was related to NAb is impossible to sort out because we did not evaluate this vector in animals that were NAb negative.
In previous studies with NHPs, those animals with preexisting NAbs against the AAV serotype administered (AAV8, AAVhu.37, AAVrh.8) tended to have an increased number of vector genome copies in spleen, with a spleen-to-liver ratio greater than 1 (Wang et al., 2010a). This was examined in the dogs. As mentioned, the highest preexisting NAbs observed in the dogs were those against AAV6.2. These animals had only low copy numbers of AAV genomes in the liver, but did not show higher levels in the spleen. Only in the AAV2-treated animals and in one of the long-term AAV8 dogs did the spleen contain more vector genomes than the liver, but these dogs did not have preexisting NAbs against these vectors (Fig. 3). The phenomenon of redirection of AAV into the spleen in the presence of NAbs may, therefore, be specific for NHPs, although more canine studies need to be performed.
Liver enzyme levels
Serum liver enzyme activity in all the animals that had received the various AAV serotypes was measured on day 7 and also on day 12 for the additional dogs treated with AAV2. We did not observe elevations in aspartate aminotransferase (AST) or alanine aminotransferase (ALT) above the normal upper limit except for animal M2442 (AAVrh.8), which showed a mild increase in AST (75 U/liter; the normal upper limit is about 65 U/liter). Two samples (M2448, M2409) could not be evaluated because of hemolysis.
Absence of long-term transgene expression and cytotoxic T lymphocyte responses
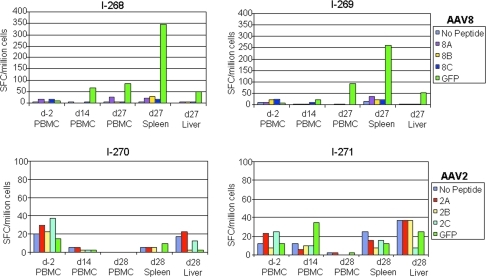
The stability of GFP expression for AAV2 and AAV8 was evaluated. Two dogs each received AAV2 or AAV8 and the liver was examined for GFP expression after 4 weeks. AAV2 did not produce any GFP-positive cells in the liver, as expected, because this vector failed to express at earlier time points. However, GFP expression was also not present with AAV8 at 4 weeks (Fig. 1). The same observation was made in an adult animal injected with AAV8 as described in more detail below (Fig. 6). Although we had not taken a biopsy and, therefore, cannot determine with certainty whether AAV8 generated any transient GFP expression, it appears that in all of the AAV8-treated dogs GFP expression occurred and then was eliminated through a T cell response against the transgene. IFN-γ ELISpot assays from splenocytes showed the presence of GFP-specific T cells in spleen on day 27 after injection of AAV8, but not after injection of AAV2 (Fig. 4). There was also a weaker T cell response against GFP among peripheral blood mononuclear cells (PBMCs) on days 14 and 27 after AAV8 vector treatment and a borderline response on day 27 in lymphocytes from liver (an ELISpot count was considered positive if more than three times the number of spots was observed as in the control without peptide and having a minimum of 55 spots). There was no T cell response against the capsid of either AAV8 or AAV2 at any time point (Fig. 4).
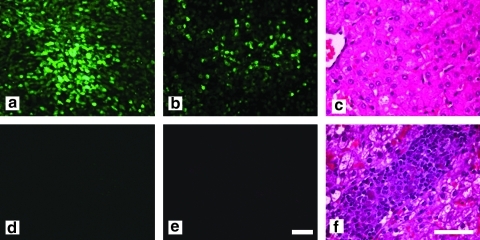
FIG. 6.
GFP expression and histopathology in liver after administration of AAV8.TBG.EGFP to large dogs. (a and b) Animal S473 (16.7 kg; dose, 1.82 × 1013 GC/kg): GFP expression 1 week after vector administration as shown in two different lobes. (d and e) Animal S468 (20 kg; dose, 7.9 × 1012 GC/kg): liver 5 weeks after vector administration; no GFP expression is visible. (c and f) H&E-stained liver sections show large infiltration sites in S468 (f), which are absent in S473 (c). Scale bars: (a, b, d, and e) 100 μm; (c and f) 50 μm.
FIG. 4.
Time course of T cell responses to GFP and capsid in peripheral blood mononuclear cells (PBMCs) and lymphocytes isolated from spleen and liver, determined by IFN-γ ELISpot assay. T cells were stimulated with GFP or peptide pools (A, B, C) corresponding to AAV2 and AAV8 capsid, respectively, and the number of IFN-γ-secreting cells in response to antigen stimulation was determined. T cell responses to GFP can be observed in the two animals that received AAV8 on day 27. SFC, spot-forming cells.
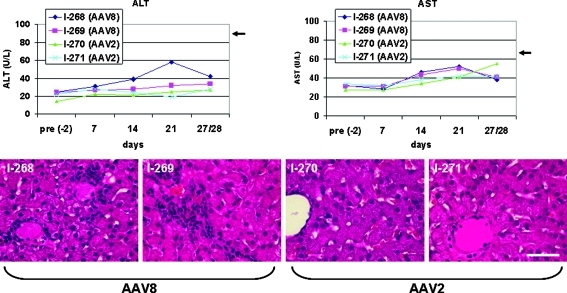
Consistent with the ELISpot data, lymphocytes infiltrating the liver were visible on day 27 in hematoxylin and eosin (H&E)-stained sections from the AAV8-treated dogs but were more or less absent in the AAV2-treated animals (Fig. 5). These infiltrates typically surrounded the central veins and portal areas; however, more severe indicators of liver inflammation such as mitotic or apoptotic hepatocytes were not found.
FIG. 5.
Top: Liver enzyme values (left, ALT; right, AST) before injection and at 1-week intervals in the animals from the long-term study. No enzyme levels elevated above the normal upper limits were observed although there was a slight increase in AST on day 14 in all dogs and also a transient moderate elevation of ALT in one of the dogs (I268) on day 21. Arrows indicate upper normal levels for ALT and AST. Bottom: Hematoxylin and eosin (H&E)-stained liver sections on day 27, showing infiltrates in the AAV8-treated animals but not the AAV2-treated animals. Scale bar: 50 μm.
There was a 4- to 5-fold reduction in AAV8 genome copy numbers in liver on day 28 (average, 2.16 ± 0.06 GC per diploid genome) compared with copy numbers in the dogs examined on day 7 (average, 9.74 ± 1.62 GC per diploid genome), suggesting again that after initial gene transfer a cellular immune response eliminated some of the GFP-expressing hepatocytes. An alternative explanation for some of the reduction in vector genomes was further growth of the liver in these animals. Interestingly, despite the development of GFP-specific T cells and infiltrating lymphocytes in the AAV8-injected dogs, there was only a moderate elevation in the activity of liver enzymes AST and ALT, but still below the upper normal limit (Fig. 5).
Liver-directed gene transfer with AAV serotypes in adult dogs
Having established AAV8 as the best performing serotype in young dogs, we wanted to evaluate the toxicity of this serotype at higher doses in adult animals and look at potential acute toxicity when delivering AAV8 directly into the hepatic artery by a surgical procedure. An important aspect of acute toxicity is hemodynamic instability at the time of or immediately after gene transfer. No overt manifestations of hypotension have been described after AAV gene transfer in preclinical and clinical trials; however, subclinical evidence of hypotension due to vasodilation or bradycardia has not been systematically evaluated through careful hemodynamic monitoring. To this end, two adult dogs weighing 18 kg (S473) and 20 kg (S468) were infused with AAV8.TBG.EGFP through the hepatic artery. S473 received a dose of 1.82 × 1013 GC/kg and the liver was analyzed 1 week after vector administration. S468 received 7.9 × 1012 GC/kg of the same vector and was sacrificed 5 weeks later.
Level and stability of GFP expression
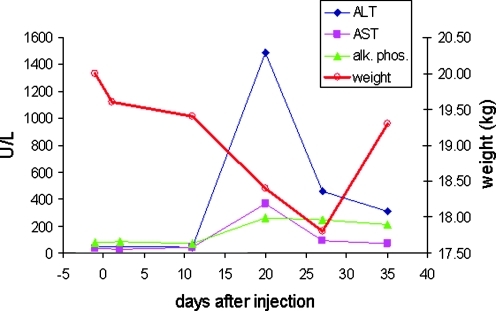
GFP expression in liver 1 week after injection of S473 (Fig. 6) was clearly stronger, with 17.0 ± 11.8% of the liver area positive compared with an average of 2.86 ± 1.3% in the younger animals that received a lower dose. There was no visible GFP expression in liver from S468 examined at the time of sacrifice 5 weeks after injection. Infiltrates in the liver of S468 were not present in the dog that was examined after only 1 week (Fig. 6), consistent with the hypothesis that transient GFP expression was silenced by a T cell response. A further indicator of a T cell response against the transgene in S468 was a transient elevation in liver enzyme activity, with ALT reaching a maximum of 1488 U/liter (normal upper limit is about 90 U/liter) and AST peaking at 370 U/liter (normal upper limit is about 65 U/liter) (Fig. 7). The increase in liver enzymes was accompanied by weight loss from 20 kg to less than 18 kg on day 27. This weight loss was temporary and the dog regained weight by 1 month after vector administration (Fig. 7).
FIG. 7.
Changes in liver enzyme levels and weight in animal S468 after vector administration. Normal upper values for ALT, AST, and alkaline phosphatase (alk. phos.) in dogs are about 90, 65, and 155 U/liter, respectively.
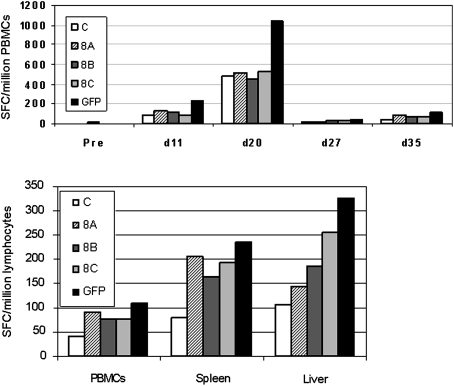
An ELISpot analysis showed the development of GFP-specific T cells on day 20 in PBMCs (1045 spots per 106 cells), although this result did not meet the criterion of being 3-fold over the background value to be considered truly positive (the unstimulated control on day 20 showed 490 spots per 106 cells). However, this criterion was met with T cells isolated from liver on day 35 after injection (325 spots per 106 cells; unstimulated control, 105 spots per 106 cells), demonstrating the presence of GFP-specific T cells in this organ. A borderline value was also observed in splenocytes at this time point (235 spots per 106 cells, unstimulated control, 80 spots per 106 cells). Frequencies of T cells directed against the AAV8 capsid from PBMCs, spleen, and liver were not found to exceed the background counts and were, therefore, not considered positive (Fig. 8).
FIG. 8.
Development of T cells against transgene and capsid in animal S468 determined by IFN-γ ELISpot. Top: Time course of T cell frequencies in PBMCs against GFP and AAV8 capsid (peptide pools 8A, 8B, 8C; control, C). Bottom: Frequencies of GFP and capsid-specific T cells isolated from spleen and liver compared with PBMCs on day 35 after vector administration.
Vector genome copies were further analyzed in liver and were low at 5 weeks (2.1 ± 0.5 GC per diploid genome) compared with liver examined 1 week after vector administration (59.2 ± 7.0 GC per diploid genome), indicating the elimination of transduced hepatocytes by a cytotoxic T cell response. Direct comparison between these two animals is complicated because they were given slightly different doses.
Changes in vital signs during vector infusion
In contrast to the young animals, the two adult dogs received the vector by infusion into the hepatic artery, an invasive procedure requiring anesthesia. A key aspect of these studies was an assessment of cardiopulmonary toxicity as measured by changes in vital signs (e.g., heart rate, blood pressure, respiratory rate, and O2 saturation). Although this kind of toxicity has not been associated with systemic delivery of AAV, it was the dose-limiting toxicity associated with intravascular administration of adenoviral vectors in subjects with cancer (Reid et al., 2002).
A drop in blood pressure and heart rate was seen during this procedure. To determine whether the vector had played a role in lowering these two parameters we compared the kinetics of vital signs with the time points of vector infusion and administration of anesthetics (shown in Fig. 9 for S473 and Fig. 10 for S468).
In animal S473, body temperature and oxygen saturation (SpO2) showed some fluctuations, with the lowest body temperature at 38.7°C (98.1°F) and the lowest SpO2 at 92%. The dog was mechanically ventilated at times as indicated in Fig. 9 (circles, bottom panel). Systolic blood pressure (measured by Doppler ultrasound) and heart rate dropped during anesthesia. The decrease in both measures started about 50 min before the vector was given. Both values then fell continuously from 155 mmHg and 165 bpm and reached their lowest levels, 60 mmHg and 95 bpm, respectively, during and after the vector infusion (Fig. 9).
Dog S468 received 11 ml of saline before the vector solution was infused. As in the first dog, temperature and SpO2 showed fluctuations with minima at 35.7°C (96.4°F) and 98%, respectively. The heart rate started to fall from 152 bpm shortly after induction of anesthesia and about 1 hour before saline administration, and subsequently the vector was given (Fig. 10). The lowest heart rate (88 bpm) was reached during vector administration at 10:05 a.m. Systolic, mean, and diastolic blood pressure were monitored with an arterial line. After the start of anesthesia, the blood pressure began to fall and fluctuate. When saline was administered, the blood pressure dropped within 5 min (systolic from 98 to 89 mmHg, mean from 77 to 59 mmHg, diastolic from 62 to 29 mmHg) but recovered within another 5 min to the values at the beginning of saline injection. Similarly, when the vector was infused, blood pressure fell again within 5 min (systolic from 100 to 80 mmHg, mean from 78 to 53 mmHg, diastolic from 60 to 42 mmHg) but also increased again within the next 5–10 min (Fig. 10).
As discussed below in more detail, the described kinetics of heart rate and blood pressure suggest that the anesthesia procedure rather than toxicity of the vector had caused these complications.
Discussion
Gene transfer in young dogs
A large number of novel AAV serotypes has been described (Gao et al., 2002, 2004; Vandenberghe et al., 2009), a subset of which had been screened previously for their performance for hepatic gene transfer in mice and NHPs (Wang et al., 2010a,b). Because dogs are an important large animal model for gene therapy studies, studies were extended by testing a selection of the most promising AAV serotypes in 1.5-month-old dogs for transduction efficiency in liver, stability of transgene expression, and immunogenicity. Among the six serotypes evaluated (AAV8, AAV2, AAV6.2, AAVhu.37, AAVrh.64R1, AAVrh.8), AAV8 emerged again as the best performing serotype leading to the highest levels of transgene expression in liver as observed previously in mice and NHPs.
GFP expression in the dogs was relatively low; we did not reach the high levels of GFP expression (23.7 and 40.1% of liver area) and correspondingly high numbers of vector genomes (17.4 and 28.9 GC per diploid genome) that we had detected in the best performing NHPs that received AAV8 (Wang et al., 2010a). The highest GFP levels in dogs were found with AAV8 but did not exceed 4.6% of GFP-positive liver area and a maximum of 12 vector GC per diploid genome (Fig. 2). In the dogs, a similar correlation was seen between genome copies in the liver and GFP expression levels as was the case in NHPs, with the exception of one dog (M2450) that had received AAVrh.64R1 and showed a high number of vector genomes (28.75 GC per diploid genome) but only low GFP expression (1.82% GFP-positive area).
Stable transgene expression was not achieved with AAV8 in the dogs. Four weeks after vector administration, no GFP fluorescence was visible, and the presence of GFP-specific T cells and infiltrates in the liver indicated that transient expression of GFP had occurred and then was eliminated by the observed T cell response. The cytotoxic T lymphocyte (CTL) response in our dogs against GFP expressed in liver is therefore similar to T cell reactions in NHPs (and therefore probably humans), contrary to what was observed in mice, in which liver-directed gene transfer with AAV8 normally fails to elicit T cell responses that eliminate transgene expression (reviewed by Mays and Wilson, 2011). However, stable transgene expression in liver has been described in dog models of genetic diseases after receiving AAV expressing the therapeutic transgene (Wang et al., 2000, 2005; Beaty et al., 2002; Jiang et al., 2006). In our study, the transgene was GFP, a foreign protein known to be able to elicit potent immune responses in NHPs, whereas therapeutic transgenes that are almost identical to the endogenous protein typically cause only mild if any T cell responses. However, two hemophilia B dogs with null mutations in the factor IX (FIX) gene did show sustained hepatic FIX expression from AAV (Mount et al., 2002; Niemeyer et al., 2009). Detection of GFP by fluorescence microscopy in tissue sections is likely to be less sensitive than detection of FIX by ELISA, and low levels of GFP RNA were present in the livers of the long-term animals, making it possible that there was residual expression of GFP at levels too low to be observed by microscopic examination. In our previously published experiments with NHPs, GFP expression was obtained in the liver up to day 35 but only when a liver-specific promoter was used. The development of GFP-specific T cells in liver on day 35 did not necessarily prevent the continued expression of GFP in macaques (Wang et al., 2010a). Because only three dogs (two young dogs, one adult dog) were evaluated for sustained GFP expression, and given the variability of hepatic GFP expression observed in NHPs, we may not have examined enough animals to exclude the possibility of long-term expression of GFP in liver.
The animals were injected at an age of 1.5 months, when growth is still occurring. Dilution of episomal vector genomes in the growing liver 4 weeks after vector treatment should therefore also be considered a factor that could diminish transgene expression. Experiments in mice treated with AAV8 at various ages and evaluated at later time points demonstrated a substantial loss of transgene expression in those animals that were injected as newborns but increasingly stable expression when injected at subsequent ages (Cunningham et al., 2008, 2009). Our dogs were not injected as newborns, making it unlikely that liver growth would account for the complete loss of GFP expression. Complete loss of expression was also observed in the adult animal after 5 weeks, further suggesting that liver expansion was not a major factor leading to the absence of GFP at later time points.
The number of AAV8 genomes in the liver of the younger dogs on day 28 was reduced about 4- to 5-fold compared with liver harvested on day 7 (Fig. 3), and was similarly low in the adult animal 5 weeks after vector injection. In the adult animal, this would suggest that a cellular immune response eliminated some of the GFP-expressing hepatocytes. In the younger animals, the drop in vector genome copies could simply be due to dilution of episomal vector genomes in the expanding liver, although a cellular immune response could also play a role. Despite the absence of GFP expression at the late time points, vector genomes were not completely eliminated (average, 2.16 ± 0.06 GC per diploid genome). The persistence of AAV genomes in liver, although potentially at reduced numbers, after elimination of transgene expression through a cytotoxic T cell response has been described previously, but the exact mechanism of this phenomenon remains unknown (Gao et al., 2009; Somanathan et al., 2010; Wang et al., 2010a).
AAV2 failed to produce visible GFP signals in liver on days 7, 12, or 28 except for a few weakly fluorescent cells that could occasionally be found (Fig. 2). In the long-term animals, no T cell elevations against GFP were observed, suggesting that no transient expression of GFP had occurred. The dogs that were sacrificed on day 12 after vector administration did not show any elevation in liver enzymes, again suggesting the absence of GFP expression. Together these data suggest that AAV2 failed to express GFP. This is in contrast to studies in which AAV2 was used successfully in hemophilia dogs for hepatic expression of FVIII or FIX (Wang et al., 2000; Harding et al., 2004; Jiang et al., 2006; Niemeyer et al., 2009). We cannot rule out low levels of GFP expression from AAV2 in liver that went undetected by fluorescence microscopy, and in fact we observed a few transduced hepatocytes in some of the animals along with low levels of GFP RNA. A low number of transduced hepatocytes similar to what we saw in our AAV2-treated dogs could be sufficient to generate the observed levels of secreted proteins in the hemophilia studies.
Gene transfer in adult dogs
In the two adult dogs that received AAV8, we observed several complications during anesthesia. In contrast to our experiments in the young dogs, these animals received higher vector doses (1.82 × 1013 GC/kg in S473 and 7.9 × 1012 GC/kg in S468 vs. 3 × 1012 GC/kg in the young dogs), and the vector was infused into the hepatic artery by a surgical procedure instead of by intravenous injection. The higher dose may have contributed to the liver toxicity made evident by elevation of liver enzyme activity in animal S468, whereas the young animals showed no rise in enzyme levels. Importantly, in both adult animals heart rate and blood pressure dropped, raising a question concerning whether the administration of the vector played any role in this phenomenon.
In dog S473, both heart rate and blood pressure started to decrease well before the vector infusion, suggesting no direct involvement of the vector. There was a somewhat steeper decline both in blood pressure and heart rate when the vector was injected (between 10:15 a.m. and 10:20 a.m.), but similar episodes of steep decreases could also be observed before vector infusion for blood pressure (between 9:30a.m. and 9:35 a.m.) and, although less pronounced, for heart rate (between 9:45 a.m. and 9:50 a.m.). In animal S468, the heart rate also started to fall and the blood pressure showed wide fluctuations before the vector was given. An immediate drop in blood pressure was observed when saline was injected first and then a similar drop occurred when the vector was infused, suggesting that the infusion of liquid rather than the vector caused this phenomenon. Although the heart rate had started to decrease with induction of anesthesia, there was a steeper drop when the vector was administered (between 10:00 a.m. and 10:05 a.m.) that was not observed during the preceding saline injection. However, a similar steep decrease was seen at the beginning of anesthesia when 160 mg of propofol had been given, and it seems possible that the two final propofol boluses (between 10:00 a.m. and 10:05 a.m., 55 mg total) out of a series of five boluses (between 9:45 a.m. and 10:05 a.m., 130 mg total) caused this drop.
A decrease in average values for blood pressure (systolic, diastolic, and mean) and heart rate in dogs during anesthesia has been described in a large-scale meta-analysis (Redondo et al., 2007). In this study, propofol was used for induction in 1040 out of a total of 1281 studied cases. The animals in our study received high doses of propofol during the procedure (total, 11.4 mg/kg for S473 and 14.5 mg/kg for S468). Propofol is well known for its ability to cause a decrease in blood pressure in humans (Grounds et al., 1985; Larijani et al., 1989; Muzi et al., 1992; Robinson et al., 1994, 1997) as well as in dogs (Musk et al., 2005). In the latter study, four groups of 20 dogs each received propofol to establish blood propofol concentrations of 2.5, 3.0, 3.5, and 4.0 μg/ml. These concentrations induced anesthesia in 65, 80, 100, and 100% of the dogs from each group, respectively. Occurrences of hypotension, defined as a mean arterial blood pressure under 60 mmHg, were observed within several minutes in dogs from all four groups (1 animal of 20 at 2.5 μg/ml propofol, 6 of 20 at 3.0 μg/ml, 3 of 20 at 3.5 μg/ml, 8 of 20 at 4.0 μg/ml) (Musk et al., 2005). For comparison, in S468 a mean arterial blood pressure of 60 mmHg or lower was reached at four time points, with 53 mmHg as the lowest value (we did not measure mean blood pressure in S473). In addition, isoflurane has also been described to induce lowering of blood pressure in dogs (Hellebrekers, 1986; Cutfield et al., 1988; Merin et al., 1991).
The extent to which intraarterial vector administration yields higher transduction levels in liver, compared with injection into a peripheral vein, cannot be established from our study because of differences in dose and age of the animals. Using AAV2 in mice, peripheral intravenous injection has been reported to be less efficient in hepatocyte transduction than intraarterial or intraportal administration (Mingozzi et al., 2002; Grimm et al., 2003; Ohashi et al., 2005; Berraondo et al., 2006). This was different with AAV8 tested in NHPs, in which intraportal delivery gave comparable transduction levels in liver as injection into a peripheral vein (Nathwani et al., 2007).
Administration of AAV via hepatic artery has been performed seemingly without adverse events in dogs as well as in clinical trials in humans (Arruda et al., 2001; Manno et al., 2006; Hasbrouck and High, 2008). Likewise, no complications were reported in dogs during AAV delivery through the portal vein (Wang et al., 2005; Jiang et al., 2006; Sarkar et al., 2006; Margaritis et al., 2009). The finding that a decrease in blood pressure and heart rate was observed in both adult dogs before the time of vector administration, the fact that both parameters usually drop during anesthesia and especially after propofol administration, and the safety record of AAV administration by surgical procedure suggest that the AAV8 vector did not cause the observed changes in blood pressure and heart rate. However, we cannot rule out an independent contribution of the vector to hypotension/bradycardia masked by the anesthesia effect. This study points to the complexities in assessing key aspects of acute toxicities in animal models and the need for careful evaluation, using methods that will be deployed in clinical studies.
Acknowledgments
The authors thank Julie Johnston and Arbans Sandhu (Penn Vector Core) for producing the vectors, Julio Sanmiguel for quantitative RNA analysis, Di Wu and Hongwei Yu for histology work, and Patricia O'Donnell and Caryn A. Reynolds for expert care of the dogs. This work was supported by grants from the NIDDK (P30 DK47757, DK54481), NCRR (P40 RR02512), and GlaxoSmithKline.
Author Disclosure Statement
J.M.W. is a consultant to ReGenX Holdings and is a founder of, holds equity in, and receives a grant from affiliates of ReGenX Holdings. He is an inventor on patents licensed to various biopharmaceutical companies, including affiliates of ReGenX Holdings. L.H.V. is an inventor on patents licensed to various biopharmaceutical companies, including ReGenX.
References
- Arruda V.R. Fields P.A. Milner R., et al. Lack of germline transmission of vector sequences following systemic administration of recombinant AAV-2 vector in males. Mol. Ther. 2001;4:586–592. doi: 10.1006/mthe.2001.0491. [DOI] [PubMed] [Google Scholar]
- Beaty R.M. Jackson M. Peterson D., et al. Delivery of glucose-6-phosphatase in a canine model for glycogen storage disease, type Ia, with adeno-associated virus (AAV) vectors. Gene Ther. 2002;9:1015–1022. doi: 10.1038/sj.gt.3301728. [DOI] [PubMed] [Google Scholar]
- Bell P. Wang L. Lebherz C., et al. No evidence for tumorigenesis of AAV vectors in a large-scale study in mice. Mol. Ther. 2005;12:299–306. doi: 10.1016/j.ymthe.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Berraondo P. Crettaz J. Ochoa L., et al. Intrahepatic injection of recombinant adeno-associated virus serotype 2 overcomes gender-related differences in liver transduction. Hum. Gene Ther. 2006;17:601–610. doi: 10.1089/hum.2006.17.601. [DOI] [PubMed] [Google Scholar]
- Calcedo R. Vandenberghe L.H. Gao G., et al. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J. Infect. Dis. 2009;199:381–390. doi: 10.1086/595830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal M. Haskins M. Large animal models and gene therapy. Eur. J. Hum. Genet. 2006;14:266–272. doi: 10.1038/sj.ejhg.5201535. [DOI] [PubMed] [Google Scholar]
- Cunningham S.C. Dane A.P. Spinoulas A., et al. Gene delivery to the juvenile mouse liver using AAV2/8 vectors. Mol. Ther. 2008;16:1081–1088. doi: 10.1038/mt.2008.72. [DOI] [PubMed] [Google Scholar]
- Cunningham S.C. Spinoulas A. Carpenter K.H., et al. AAV2/8-mediated correction of OTC deficiency is robust in adult but not neonatal Spfash mice. Mol. Ther. 2009;17:1340–1346. doi: 10.1038/mt.2009.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutfield G.R. Francis C.M. Foex P., et al. Isoflurane and large coronary artery haemodynamics: A study in dogs. Br. J. Anaesth. 1988;60:784–790. doi: 10.1093/bja/60.7.784. [DOI] [PubMed] [Google Scholar]
- Davidoff A.M. Gray J.T. Ng C.Y., et al. Comparison of the ability of adeno-associated viral vectors pseudotyped with serotype 2, 5, and 8 capsid proteins to mediate efficient transduction of the liver in murine and nonhuman primate models. Mol. Ther. 2005;11:875–888. doi: 10.1016/j.ymthe.2004.12.022. [DOI] [PubMed] [Google Scholar]
- Gao G. Vandenberghe L.H. Alvira M.R., et al. Clades of adeno-associated viruses are widely disseminated in human tissues. J. Virol. 2004;78:6381–6388. doi: 10.1128/JVI.78.12.6381-6388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G. Lu Y. Calcedo R., et al. Biology of AAV serotype vectors in liver-directed gene transfer to nonhuman primates. Mol. Ther. 2006;13:77–87. doi: 10.1016/j.ymthe.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Gao G. Wang Q. Calcedo R., et al. Adeno-associated virus-mediated gene transfer to nonhuman primate liver can elicit destructive transgene-specific T cell responses. Hum. Gene Ther. 2009;20:930–942. doi: 10.1089/hum.2009.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G.P. Alvira M.R. Wang L., et al. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc. Natl. Acad. Sci. U.S.A. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm D. Zhou S. Nakai H., et al. Preclinical in vivo evaluation of pseudotyped adeno-associated virus vectors for liver gene therapy. Blood. 2003;102:2412–2419. doi: 10.1182/blood-2003-02-0495. [DOI] [PubMed] [Google Scholar]
- Grounds R.M. Twigley A.J. Carli F., et al. The haemodynamic effects of intravenous induction: Comparison of the effects of thiopentone and propofol. Anaesthesia. 1985;40:735–740. doi: 10.1111/j.1365-2044.1985.tb10996.x. [DOI] [PubMed] [Google Scholar]
- Harding T.C. Koprivnikar K.E. Tu G.H., et al. Intravenous administration of an AAV-2 vector for the expression of factor IX in mice and a dog model of hemophilia B. Gene Ther. 2004;11:204–213. doi: 10.1038/sj.gt.3302142. [DOI] [PubMed] [Google Scholar]
- Hasbrouck N.C. High K.A. AAV-mediated gene transfer for the treatment of hemophilia B: Problems and prospects. Gene Ther. 2008;15:870–875. doi: 10.1038/gt.2008.71. [DOI] [PubMed] [Google Scholar]
- Haskins M. Gene therapy for lysosomal storage diseases (LSDs) in large animal models. ILAR J. 2009;50:112–121. doi: 10.1093/ilar.50.2.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellebrekers L.J. Comparison of isoflurane and halothane as inhalation anaesthetics in the dog. Vet. Q. 1986;8:183–188. doi: 10.1080/01652176.1986.9694041. [DOI] [PubMed] [Google Scholar]
- High K. Gene transfer for hemophilia: Can therapeutic efficacy in large animals be safely translated to patients? J. Thromb. Haemost. 2005;3:1682–1691. doi: 10.1111/j.1538-7836.2005.01460.x. [DOI] [PubMed] [Google Scholar]
- Ill C.R. Yang C.Q. Bidlingmaier S.M., et al. Optimization of the human factor VIII complementary DNA expression plasmid for gene therapy of hemophilia A. Blood Coagul. Fibrinolysis. 1997;8(Suppl. 2):S23–S30. [PubMed] [Google Scholar]
- Jiang H. Lillicrap D. Patarroyo-White S., et al. Multiyear therapeutic benefit of AAV serotypes 2, 6, and 8 delivering factor VIII to hemophilia A mice and dogs. Blood. 2006;108:107–115. doi: 10.1182/blood-2005-12-5115. [DOI] [PubMed] [Google Scholar]
- Larijani G.E. Gratz I. Afshar M. Jacobi A.G. Clinical pharmacology of propofol: An intravenous anesthetic agent. DICP. 1989;23:743–749. doi: 10.1177/106002808902301001. [DOI] [PubMed] [Google Scholar]
- Lebherz C. Gao G. Louboutin J.P., et al. Gene therapy with novel adeno-associated virus vectors substantially diminishes atherosclerosis in a murine model of familial hypercholesterolemia. J. Gene Med. 2004;6:663–672. doi: 10.1002/jgm.554. [DOI] [PubMed] [Google Scholar]
- Livak K.J. Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Manno C.S. Pierce G.F. Arruda V.R., et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat. Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- Margaritis P. Roy E. Aljamali M.N., et al. Successful treatment of canine hemophilia by continuous expression of canine FVIIa. Blood. 2009;113:3682–3689. doi: 10.1182/blood-2008-07-168377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mays L.E. Wilson J.M. The complex and evolving story of T cell activation to AAV vector-encoded transgene products. Mol. Ther. 2011;19:16–27. doi: 10.1038/mt.2010.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merin R.G. Bernard J.M. Doursout M.F., et al. Comparison of the effects of isoflurane and desflurane on cardiovascular dynamics and regional blood flow in the chronically instrumented dog. Anesthesiology. 1991;74:568–574. doi: 10.1097/00000542-199103000-00027. [DOI] [PubMed] [Google Scholar]
- Mingozzi F. Schuttrumpf J. Arruda V.R., et al. Improved hepatic gene transfer by using an adeno-associated virus serotype 5 vector. J. Virol. 2002;76:10497–10502. doi: 10.1128/JVI.76.20.10497-10502.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingozzi F. Maus M.V. Hui D.J., et al. CD8+ T-cell responses to adeno-associated virus capsid in humans. Nat. Med. 2007;13:419–422. doi: 10.1038/nm1549. [DOI] [PubMed] [Google Scholar]
- Mount J.D. Herzog R.W. Tillson D.M., et al. Sustained phenotypic correction of hemophilia B dogs with a factor IX null mutation by liver-directed gene therapy. Blood. 2002;99:2670–2676. doi: 10.1182/blood.v99.8.2670. [DOI] [PubMed] [Google Scholar]
- Musk G.C. Pang D.S. Beths T. Flaherty D.A. Target-controlled infusion of propofol in dogs—evaluation of four targets for induction of anaesthesia. Vet. Rec. 2005;157:766–770. doi: 10.1136/vr.157.24.766. [DOI] [PubMed] [Google Scholar]
- Muzi M. Berens R.A. Kampine J.P. Ebert T.J. Venodilation contributes to propofol-mediated hypotension in humans. Anesth. Analg. 1992;74:877–883. doi: 10.1213/00000539-199206000-00017. [DOI] [PubMed] [Google Scholar]
- Nathwani A.C. Gray J.T. McIntosh J., et al. Safe and efficient transduction of the liver after peripheral vein infusion of self-complementary AAV vector results in stable therapeutic expression of human FIX in nonhuman primates. Blood. 2007;109:1414–1421. doi: 10.1182/blood-2006-03-010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemeyer G.P. Herzog R.W. Mount J., et al. Long-term correction of inhibitor-prone hemophilia B dogs treated with liver-directed AAV2-mediated factor IX gene therapy. Blood. 2009;113:797–806. doi: 10.1182/blood-2008-10-181479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi K. Nakai H. Couto L.B. Kay M.A. Modified infusion procedures affect recombinant adeno-associated virus vector type 2 transduction in the liver. Hum. Gene Ther. 2005;16:299–306. doi: 10.1089/hum.2005.16.299. [DOI] [PubMed] [Google Scholar]
- Redondo J.I. Rubio M. Soler G., et al. Normal values and incidence of cardiorespiratory complications in dogs during general anaesthesia: A review of 1281 cases. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2007;54:470–477. doi: 10.1111/j.1439-0442.2007.00987.x. [DOI] [PubMed] [Google Scholar]
- Reid T. Warren R. Kirn D. Intravascular adenoviral agents in cancer patients: Lessons from clinical trials. Cancer Gene Ther. 2002;9:979–986. doi: 10.1038/sj.cgt.7700539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson B.J. Buyck H.C. Galletly D.C. Effect of propofol on heart rate, arterial pressure and digital plethysmograph variability. Br. J. Anaesth. 1994;73:167–173. doi: 10.1093/bja/73.2.167. [DOI] [PubMed] [Google Scholar]
- Robinson B.J. Ebert T.J. O'Brien T.J., et al. Mechanisms whereby propofol mediates peripheral vasodilation in humans: Sympathoinhibition or direct vascular relaxation? Anesthesiology. 1997;86:64–72. doi: 10.1097/00000542-199701000-00010. [DOI] [PubMed] [Google Scholar]
- Sarkar R. Tetreault R. Gao G., et al. Total correction of hemophilia A mice with canine FVIII using an AAV 8 serotype. Blood. 2004;103:1253–1260. doi: 10.1182/blood-2003-08-2954. [DOI] [PubMed] [Google Scholar]
- Sarkar R. Mucci M. Addya S., et al. Long-term efficacy of adeno-associated virus serotypes 8 and 9 in hemophilia a dogs and mice. Hum. Gene Ther. 2006;17:427–439. doi: 10.1089/hum.2006.17.427. [DOI] [PubMed] [Google Scholar]
- Somanathan S. Breous E. Bell P. Wilson J.M. AAV vectors avoid inflammatory signals necessary to render transduced hepatocyte targets for destructive T cells. Mol. Ther. 2010;18:977–982. doi: 10.1038/mt.2010.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C.E. Storm T.A. Huang Z. Kay M.A. Rapid uncoating of vector genomes is the key to efficient liver transduction with pseudotyped adeno-associated virus vectors. J. Virol. 2004;78:3110–3122. doi: 10.1128/JVI.78.6.3110-3122.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe L.H. Breous E. Nam H.J., et al. Naturally occurring singleton residues in AAV capsid impact vector performance and illustrate structural constraints. Gene Ther. 2009;16:1416–1428. doi: 10.1038/gt.2009.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandendriessche T. Thorrez L. Acosta-Sanchez A., et al. Efficacy and safety of adeno-associated viral vectors based on serotype 8 and 9 vs. lentiviral vectors for hemophilia B gene therapy. J. Thromb. Haemost. 2007;5:16–24. doi: 10.1111/j.1538-7836.2006.02220.x. [DOI] [PubMed] [Google Scholar]
- Wang L. Nichols T.C. Read M.S., et al. Sustained expression of therapeutic level of factor IX in hemophilia B dogs by AAV-mediated gene therapy in liver. Mol. Ther. 2000;1:154–158. doi: 10.1006/mthe.2000.0031. [DOI] [PubMed] [Google Scholar]
- Wang L. Calcedo R. Nichols T.C., et al. Sustained correction of disease in naive and AAV2-pretreated hemophilia B dogs: AAV2/8-mediated, liver-directed gene therapy. Blood. 2005;105:3079–3086. doi: 10.1182/blood-2004-10-3867. [DOI] [PubMed] [Google Scholar]
- Wang L. Figueredo J. Calcedo R., et al. Cross-presentation of adeno-associated virus serotype 2 capsids activates cytotoxic T cells but does not render hepatocytes effective cytolytic targets. Hum. Gene Ther. 2007;18:185–194. doi: 10.1089/hum.2007.001. [DOI] [PubMed] [Google Scholar]
- Wang L. Calcedo R. Wang H., et al. The pleiotropic effects of natural AAV infections on liver-directed gene transfer in macaques. Mol. Ther. 2010a;18:126–134. doi: 10.1038/mt.2009.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. Wang H. Bell P., et al. Systematic evaluation of AAV vectors for liver directed gene transfer in murine models. Mol. Ther. 2010b;18:118–125. doi: 10.1038/mt.2009.246. [DOI] [PMC free article] [PubMed] [Google Scholar]