Abstract
Withdrawal of progestational support for pregnancy is part of the final common pathways for parturition, but the role of nuclear progesterone receptor (PGR) isoforms in this process is not known. To determine if the PGR-B isoform participates in cervical remodeling at term, cervices were obtained from mice lacking PGR-B (PGR-BKO) and from wild-type (WT) controls before or after birth. PGR-BKO mice gave birth to viable pups at the same time as WT controls during the early morning of Day 19 postbreeding. Morphological analyses indicated that by the day before birth, cervices from PGR-BKO and WT mice had increased in size, with fewer cell nuclei/area as well as diminished collagen content and structure, as evidenced by optical density of picrosirius red-stained sections, compared to cervices from nonpregnant mice. Moreover, increased numbers of resident macrophages, but not neutrophils, were found in the prepartum cervix of PGR-BKO compared to nonpregnant mice, parallel to findings in WT mice. These results suggest that PGR-B does not contribute to the growth or degradation of the extracellular matrix or proinflammatory processes associated with recruitment of macrophages in the cervix leading up to birth. Rather, other receptors may contribute to the progesterone-dependent mechanism that promotes remodeling of the cervix during pregnancy and in the proinflammatory process associated with ripening before parturition.
Keywords: birth, cervix, inflammation, macrophage, neutrophil, parturition, progesterone/progesterone receptor, ripening
A progesterone-mediated receptor mechanism that does not involve the progesterone receptor-B isoform maintains pregnancy and regulates cervical remodeling and parturition.
INTRODUCTION
Progesterone is essential to sustain pregnancy while withdrawal of its trophic actions initiates parturition [1–4]. Classic nuclear progesterone receptors (PGRs) mediate the effects of progestational agents to maintain pregnancy, whereas antagonists of these receptors induce birth. PGR-A and PGR-B are ligand-binding isoforms of the nuclear PGR [5, 6]. Mice lacking both receptor isoforms (PGRKO) or only PGR-A (PGR-AKO) have a variety of reproductive anomalies, including inability to ovulate, uterine hyperplasia, and inflammation, as well as defects in uterine implantation [7, 8]. As a consequence of the essential role for PGR-A in ovulation, PGRKO or PGR-AKO mice are infertile [9]. By contrast, mice lacking the PGR-B isoform (PGR-BKO) are reproductively competent and deliver viable pups [10]. Proliferation and hyperplasia of the uterus are functions attributed to PGR-B through its putative role as a transcriptional activator of progesterone-responsive genes [7, 9]. These PGR-B-mediated activities may be opposed by actions of PGR-A based upon in vitro evidence that PGR-A can function as a dominant repressor of PGR-B actions [11, 12]; however, net genomic effects are likely to be cell and tissue specific as well as PGR-B:PGR-A ratio dependent. Given the variety of progesterone-mediated actions in tissues and specific roles served by PGR isoforms [1, 13], little known is known about PGR isoforms in processes that remodel the cervix with pregnancy or in preparation for parturition.
As pregnancy progresses, progesterone concentrations increase in circulation, achieving a peak in rodents several days before birth while remaining at or near the peak level in peripartum women [14–16]. As term approaches, remodeling of the cervix is characterized by reduced density of cell nuclei, degradation of extracellular matrix collagen content and structure, and immigration of immune cells [17–20]. By the day before birth, immune cells have immigrated into the stroma and perimetrium of the cervix in mice [21, 22] and rats [23, 24]. Evidence in primates also suggests that these characteristics of inflammation are part of the process that remodels the cervix [25–27]. Although PGR-B may not be essential for maintaining pregnancy or for parturition in mice, whether the PGR-B isoform regulates inflammatory processes that remodel the cervix is not known. The present study addressed the hypothesis that the PGR-B isoform mediates recruitment of immune cells into the prepartum cervix but not remodeling of the extracellular matrix in preparation for birth. Findings suggest that a PGR-mediated mechanism other than that related to PGR-B remodels and ripens the cervix for parturition.
MATERIALS AND METHODS
Animals
Adult mutant and wild-type (WT) mice from the same genetic background (B6x129/SVE) were provided by one of the authors (E.M.A.) from a colony at the Baylor College of Medicine to serve as breeders at Loma Linda University. The initial group of mice consisted of homozygotic PGR-BKO males and heterozygotic females with one PGR-B allele for gene transcription. As previously described, the CRE-loxP gene targeting approach was used in embryonic stem cells to introduce a point mutation into the Pgr gene at the ATG codon encoding Met I [10]. Homozygotic male and female progeny were bred so that time-dated pregnant PGR-BKO females could be used for the present study. To confirm PGR-BKO status, the genotype of every mouse in the present study was determined from a tail tissue clipping (EmBark Scientific, previously known as MendelWorks). Mice were housed in a temperature- and humidity-controlled vivarium room with a 12L:12D photoperiod, lights-on at 0700 h, and food and water provided ad libitum. Animal care followed guidelines set by the National Institutes of Health, and experimental procedures were approved by the Loma Linda University School of Medicine Institutional Animal Care and Use Committee.
Cervical Processing and Analysis
The WT and PGR-BKO mice were killed on the morning of Day 15, 18, or 19 postbreeding (Day 19 = day of birth [∼2–4 h postpartum]). Nonpregnant (NP) mice in estrus or metestrus, based upon assessment of vaginal smears, served as additional controls. The cervix was removed, postfixed overnight, embedded in paraffin, sectioned (thickness, 10 μm), and processed as previously detailed [28, 29]. Briefly, sections of cervix from each mouse were stained with picrosirius red to identify collagen and cross-link structure. Other sections were processed by immunohistochemistry to stain macrophages (BM8 antibody, 1:800; Bachem Americas, Inc.) or neutrophils (7/4 antibody, 1:100; AbD Serotec), then counterstained with hematoxylin to identify cell nuclei. As previously discussed [29], these antibodies are widely used and specific for differentiated immune cells. Cross-reactivity of the 7/4 antisera with naïve monocytes is lost with differentiation into macrophages as cells migrate into tissue. For analyses of collagen content and structure of the extracellular matrix, the optical density of polarized light from type I collagen birefringence in picrosirius red-stained sections of cervix was normalized to the cell nuclei density for each mouse to account for the hypertrophy of tissue in pregnant mouse cervix. To enumerate resident immune cells, an automated image-analysis protocol was used to count BM8-labeled macrophages or 7/4-labeled neutrophils. Briefly, a photomicrograph of the field of view in the microscope was captured, and using Image ProPlus 6 software (Media Cybernetics), cells were enumerated based on criteria related to the color of specific immunohistochemical stain for each pixel. These settings were in the eye-dropper mode (degree of sensitivity = 5, color index = 3, minimum number of pixels = 4) and count/size mode (object pixel connections = 8, smoothing = 0, fill holes checked, area range = 3–330). Photomicrographs were reviewed to assure that each cell count met criteria that colored areas were in association with a single cell nucleus. Macrophage numbers per field were independently confirmed by direct visual counts in a random survey of sections by multiple coauthors blinded to group designations in the experimental design. Three cervical sections per mouse and eight nonoverlapping 10 × 10 grid placements per section (i.e., 152 100 μm2) were analyzed. As before, data were an average of replicate analyses in the three sections normalized to cell nuclei density for each individual to account for hypertrophy and hyperplasia of the reproductive tract tissue, with the mean ± SEM per group determined.
Statistics
Data were normally distributed and individual comparisons made by one-way ANOVA or Student t-test using GraphPad Prism (GraphPad Software). Following ANOVA, Tukey test was used for individual comparisons. In a few instances, Levene test for homogeneity of variance was significant, and data were analyzed using the Kruskal-Wallis test. Data are presented as the mean ± SEM, with P < 0.05 considered to be significant.
RESULTS
Effects of PGR-B Ablation on Parturition
The WT and PGR-BKO mice gave birth in the early morning hours of Day 19 postbreeding. The presence of pups within 2 h of lights-on was comparable to that of other murine strains in our previous reports. An average range of three to eight pups per litter was found postpartum in WT and PGR-BKO mice. Postmortem, the number of implantation sites in the uterus matched the number of pups in each cage. Thus, litter size, lack of fetal mortality, duration of pregnancy, and timing of birth were similar in WT and PGR-BKO mice.
Effects of PGR-B Ablation on Cervical Remodeling
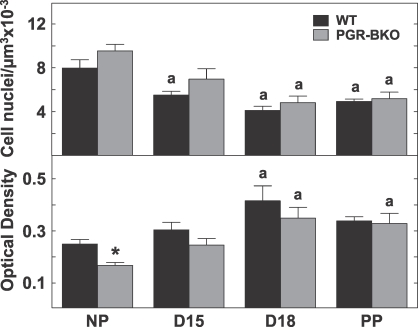
Morphological changes in the cervix with pregnancy were similar in control and mutant mice. The luminal epithelium in the cervix from WT and PGR-BKO mice on Day 15 postbreeding was thicker, with more intracellular space surrounding fewer cell nuclei in the stroma, in each microscopic field of view compared to that in cervical sections from NP mice. The numbers of cell nuclei per area of cervix decreased by Day 15 postbreeding, significantly so in WT mice, and in both WT and PGR-BKO mice on Day 18 postbreeding as well as in postpartum mice relative to NP mice (Fig. 1, top). This decrease in cell nuclei density was accompanied by a reduced content and organization of collagen in the prepartum and postpartum cervix. In NP mice, collagen fibers in the cervix were darkly stained with picrosirius red, and fibrils were tightly packed in a striated arrangement. In sections of cervix from all pregnant mice, whether from WT or PGR-BKO genotypes, the intensity and area of birefringence was reduced, collagen fibrils were loosely packed, and pockets with light stain were evident. The optical density of polarized light, inversely related to collagen content and structure, increased in sections of cervix from all pregnant WT and PGR-BKO mice by Day 18 postbreeding relative to that in NP mice (Fig. 1, bottom). Thus, absence of PGR-B expression did not interfere with growth and remodeling of the extracellular matrix in the peripartum cervix.
FIG. 1.
Top) Number of cell nuclei per area in the cervix in WT (dark, n = 6–10 per group) and PGR-BKO (open, n = 3–6 per group) mice on Day 15 (D15) or Day 18 (D18) of pregnancy or postpartum on Day 19 postbreeding (PP) compared to that in NP mice. Reduced cell nuclei per area indicates hypertrophy of cervix with pregnancy. Bottom) Optical density of picrosirius red-stained sections cervix from WT (n = 6–7 per group) controls and PGR-BKO (n = 3–6 per group) mice before or after birth and from NP controls. Optical density of polarized light was normalized to cell nuclei density and is inversely related to collagen content and structure. Data are presented as the mean ± SEM. aP < 0.05 vs. NP, same strain, by ANOVA, *P < 0.05 vs. WT, same day, by Student t-test.
Effects of PGR-B Ablation on Census of Immune Cells in the Cervix
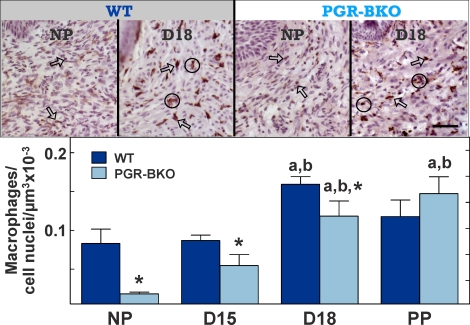
BM8-stained cells were found in the stroma, around blood vessels, in the submucosal epithelium, and to a limited extent, in the epithelium of the cervix of all mice (Fig. 2, top). Dark brown-stained cell bodies and processes were typically associated with a violet counterstained cell nucleus. Brown-colored areas not associated with a violet counterstained cell nucleus (presumably macrophage pseudopodia cell fragments) were not counted. During pregnancy, the luminal epithelium thickened while more macrophages were present in the expanded stroma and between smooth muscle cells in the cervix from WT and PGR-BKO mice (Day 18 postbreeding shown; Fig. 2, top). Thus, the distribution of BM8-stained macrophages was similar in the cervix of WT and PGR-BKO mice and comparable to that of controls in our previous reports for other strains of mice.
FIG. 2.
Top) Photomicrographs of macrophages in the cervix of NP or Day 18 (D18) WT and PGR-BKO mice. Dark BM8-labeled cells contrast with pale counterstained cell nuclei (brown vs. violet, respectively). Arrows point to examples of individual cells, whereas clusters of macrophages are within circles. Scale bar = 50 μm. Bottom) Number of macrophages (mean ± SEM, n = 3–6 per group per strain) in the cervix from WT and PGR-BKO mice. Data are normalized to cell nuclei to adjust for tissue hypertrophy. D15, Day 15; PP, postpartum. aP < 0.05 vs. NP, same strain, by ANOVA, bP < 0.05 vs. D15, same strain, by ANOVA, *P < 0.05 vs. WT, same day, by Student t-test.
Analyses indicated that more macrophages were present in the cervix by the day before birth in WT and PGR-BKO mice. Numbers of macrophages similarly increased in the cervix of both WT and PGR-BKO mice by Day 18 postbreeding compared to the cervix from NP or Day 15 mice (WT: df = 3, F = 4.02; PGR-BKO: df = 3, F = 20.3; ANOVA, P < 0.05) (Fig. 2, bottom). Fewer macrophages were present in the cervix of PGR-BKO mice compared to WT mice in the NP, Day 15, and Day 18 groups. Postpartum, the census of resident macrophages in the cervix of PGR-BKO mice remained elevated compared to that in NP or Day 15 mice. Thus, in both WT and PGR-BKO mice, a 2-fold increase in resident macrophages was found between Day 15 and the day before birth (Day 18 post breeding) [30].
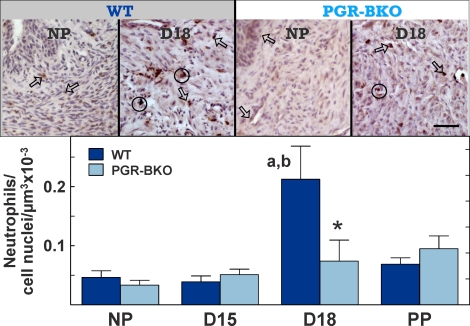
As for macrophages, cells labeled with the 7/4 antibody were similarly distributed in stroma and perimetrium of the cervix; however, residency varied with genotype. In WT mice, the number of neutrophils increased in the cervix by the day before birth (Day 18 postbreeding) compared to that in the cervix from NP or Day 15 mice (df = 3, F = 9.62; ANOVA, P < 0.05) (Fig. 3, bottom). By contrast, neutrophils remained at levels found in NP mice in all PGR-BKO groups. Thus, no prepartum increase in neutrophils occurred in the cervix of prepartum PGR-BKO mice.
FIG. 3.
Top) Photomicrographs of 7/4-labeled neutrophils in the cervix of NP or Day 18 (D18) WT and PGR-BKO mice. Arrows point to examples of individual cells, whereas clusters of macrophages are within circles. Scale bar = 50 μm. Bottom) The census of neutrophils (mean ± SEM, n = 3–6 per group per strain) in the cervix from groups of WT and PGR-BKO mice. Data are normalized to cell nuclei to adjust for tissue hypertrophy. D15, Day 15; PP, postpartum. aP < 0.05 vs. NP, same strain, by ANOVA, bP < 0.05 vs. D15, same strain, by ANOVA, *P < 0.05 vs. WT, same day, by Student t-test.
DISCUSSION
As pregnancy nears term in mammals, the cervix remodels and then ripens as a requirement for parturition and the birth of viable offspring. The present study indicates that the PGR-B isoform does not mediate aspects of remodeling related to growth and hypertrophy of cells in the cervix with pregnancy (i.e., reduced cell nuclei density, reduced extracellular collagen, and by the day before birth, increased presence of immune cells). These morphological characteristics define, in part, remodeling in the rodent cervix [22, 28] and occur when progesterone in circulation is at or near peak (i.e., Days 15–17 postbreeding in mice [2–4 days before birth]) [31, 32]. The characteristics of hypertrophy as well as reduced collagen content and structure in the cervix are dependent upon the actions of progesterone, as evidenced by effects on cervical growth and morphology after prolonged treatment of NP mice with gonadal steroids [28]. Moreover, this phase of remodeling occurs well before the shift to a contractile phenotype by the uterine myometrium sometime late on Day 18 of pregnancy, the day before birth in mice [33]. Findings in the present study suggest that PGR-B is not the sole mediator for progesterone to maintain pregnancy, because PGR-BKO and WT mice had same duration of pregnancy, no evidence of dystocia was found, and labor produced viable pups. These results concerning cervical remodeling and the birth process extend previous findings that neither ovarian nor uterine functions are impaired by the absence of PGR-B [10].
As pregnancy nears term, progesterone withdrawal appears to be critical to the parturition process. This long-standing concept from Csapo and Wiest [4] is consistent with observations that blockade of myometrial activity in most mammals, including rodents, is eliminated with decreased concentrations of progesterone in maternal circulation [1] or, in species that lack a systemic decline, a functional local withdrawal of progesterone [2, 34]. By contrast, mice may be underappreciated as models for prepartum changes in the cervix, because as in primates and guinea pigs when pregnancy nears term, the level of progesterone in circulation is sustained during the period when the cervix remodels. Serum progesterone concentrations are at or near peak between Day 15 and Day 17 of pregnancy in mice when remodeling accelerates. Levels of progesterone in circulation and in the cervix subsequently decline to half those of the peak by the day before birth (Day 18 of pregnancy in mice) [35, 36] but still far exceed those of the estrous cycle peak and the saturation threshold for tissue PGRs [11, 37, 38]. Thus, across species, remodeling and ripening of the cervix occur when progesterone concentrations are well above levels that activate genomic PGR. Possible mechanisms, other than a fall in systemic progesterone concentrations, may mediate a local withdrawal of progesterone actions that, common to all mammals, facilitates cervical hypertrophy, extracellular collagen matrix degradation, and recruitment of immune cells, including changes in steroid metabolism in the cervix [32, 39]. With little known about PGR isoforms in the uterus or cervix of rodents during pregnancy, an alternative local mechanism for progesterone to elicit normal growth, extracellular matrix remodeling, and recruitment of macrophages in the cervix before parturition may be to increase expression and activation of the PGR-A isoform [34, 40, 41] or regulate PGR coactivators [42] via nongenomic actions.
The present study also confirms previous findings in other murine strains that more macrophages are present per area in the cervix by the day before birth than earlier in pregnancy. A significantly lower number of macrophages in the cervix of NP and prepartum pregnant PGR-BKO mice versus WT controls has no effect on cervical remodeling or ripening. In addition, the present study is the first report, to our knowledge, of a coincident increase in the presence of neutrophils in the prepartum cervix of WT mice at term. Findings support previous observations in the cervix of women during parturition that immigrating neutrophils appear to transit from venules into stroma and perimetrium as well as to degranulate and induce collagenolysis [17]. However, in the cervix of prepartum PGR-BKO mice, collagenolytic processes that remodel the extracellular matrix of the cervix in preparation for birth were not affected by the lack of recruitment of neutrophils. These findings do not exclude the possibility that activation of a limited number of resident immune cells may drive increased production of prostaglandins, proinflammatory cytokines, and collagen degradation in the prepartum cervix at term [25, 43, 44] or are important for inflammation-induced preterm cervical remodeling [29, 45]. Based upon the present findings in PGR-BKO mice, the PGR-B isoform is unlikely to be involved in such activational cascades that overcome the progestational protection of peak progesterone in circulation.
In summary, our findings do not support the hypothesis that the PGR-B isoform mediates cellular growth, degradation of extracellular collagen, or recruitment of macrophages as components of an inflammatory process that remodels and ripens the cervix in preparation for birth. Absence of PGR-B in the pregnant dam and fetuses, resulting from mating with a PGR-BKO male, does not interfere with physiological and inflammatory processes associated with pregnancy, cervical remodeling, or parturition. However PGR-B may influence recruitment of macrophages and neutrophils in the prepartum cervix, evidence clearly indicates that a lack of the PGR-B isoform does not interfere with the mechanism that remodels the cervix in preparation for parturition.
ACKNOWLEDGMENTS
We thank Lindsey Clyde, John Hough, Allyson Oshiro, and Sarah Hoffman for technical support.
Footnotes
Supported in part by National Institutes of Health HD 054931; Richard Chinnock, M.D., Chair, Department of Pediatrics, and H. Roger Hadley, M.D., Dean, of the Loma Linda University School of Medicine; and the Perinatal Institute of the Loma Linda University Adventist Health Sciences Medical Center.
REFERENCES
- Mesiano S, Wang Y, Norwitz ER. Progesterone receptors in the human pregnancy uterus: do they hold the key to birth timing? Reprod Sci 2011; 18: 6 19 [DOI] [PubMed] [Google Scholar]
- Astle S, Slater DM, Thornton S. The involvement of progesterone in the onset of human labor. Eur J Obstet Gynecol Reprod Biol 2003; 108: 177 181 [DOI] [PubMed] [Google Scholar]
- Mitchell BF, Taggart MJ. Are animal models relevant to key aspects of human parturition? Am J Physiol Regul Integr Comp Physiol 2009; 297: R525 R545 [DOI] [PubMed] [Google Scholar]
- Csapo AI, Wiest WG. An examination of the quantitative relationship between progesterone and the maintenance of pregnancy. Endocrinology 1969; 85: 735 746 [DOI] [PubMed] [Google Scholar]
- Conneely OM, Mulac-Jericevic B, DeMayo F, Lydon JP, O'Malley BW. Reproductive functions of progesterone receptors. Recent Prog Horm Res 2002; 57: 339 355 [DOI] [PubMed] [Google Scholar]
- Sleiter N, Pang Y, Park C, Horton TH, Dong J, Thomas P, Levine JE. Progesterone receptor A (PGR-A) and PGR-B-independent effects of progesterone on gonadotropin-releasing hormone release. Endocrinology 2009; 150: 3833 3844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conneely OM, Mulac-Jericevic B, Lydon JP. Progesterone-dependent regulation of female reproductive activity by two distinct progesterone receptor isoforms. Steroids 2003; 68: 771 778 [DOI] [PubMed] [Google Scholar]
- Conneely OM, Mulac-Jericevic B, Lydon JP, De Mayo FJ. Reproductive functions of the progesterone receptor isoforms: lessons from knock-out mice. Mol Cell Endocrinol 2001; 179: 97 103 [DOI] [PubMed] [Google Scholar]
- Mulac-Jericevic B, Mullinax RA, DeMayo FJ, Lydon JP, Conneely OM. Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science 2000; 289: 1751 1754 [DOI] [PubMed] [Google Scholar]
- Mulac-Jericevic B, Lydon JP, DeMayo FJ, Conneely OM. Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc Natl Acad Sci U S A 2003; 100: 9744 9749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegeto E, Shahbaz MM, Wen DX, Goldman ME, O'Malley BW, McDonnell DP. Human progesterone receptor A form is a cell- and promoter-specific repressor of human progesterone receptor B function. Mol Endocrinol 1993; 7: 1244 1255 [DOI] [PubMed] [Google Scholar]
- Kraus WL, Weis KE, Katzenellenbogen BS. Inhibitory cross-talk between steroid hormone receptors: differential targeting of estrogen receptor in the repression of its transcriptional activity by agonist- and antagonist-occupied progestin receptors. Mol Cell Biol 1995; 15: 1847 1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham JD, Yager ML, Hill HD, Byth K, O'Neill GM, Clarke CL. Altered progesterone receptor isoform expression remodels progestin responsiveness of breast cancer cells. Mol Endocrinology 2005; 19: 2713 2735 [DOI] [PubMed] [Google Scholar]
- Boroditsky RS, Reyes FI, Winter JS, Faiman C. Maternal serum estrogen and progesterone concentrations preceding normal labor. Obstet Gynecol 1978; 51: 686 691 [PubMed] [Google Scholar]
- Burden HW, Lawrence IE., Jr Experimental studies on the acetylcholinesterase-positive nerves in the ovary of the rat. Anat Rec 1978; 190: 233 241 [DOI] [PubMed] [Google Scholar]
- Bridges RS. A quantitative analysis of the roles of dosage, sequence, and duration of estradiol and progesterone exposure in the regulation of maternal behavior in the rat. Endocrinology 1984; 114: 930 940 [DOI] [PubMed] [Google Scholar]
- Junqueira LC, Zugaib M, Montes GS, Toledo OM, Krisztan RM, Shigihara KM. Morphologic and histochemical evidence for the occurrence of collagenolysis and for the role of neutrophilic polymorphonuclear leukocytes during cervical dilation. Am J Obstet Gynecol 1980; 138: 273 281 [DOI] [PubMed] [Google Scholar]
- Kirby LS, Kirby MA, Warren JW, Tran LT, Yellon SM. Increased innervation and ripening of the prepartum murine cervix. J Soc Gynecol Investig 2005; 12: 578 585 [DOI] [PubMed] [Google Scholar]
- Ledingham MA, Thomson AJ, Jordan F, Young A, Crawford M, Norman JE. Cell adhesion molecule expression in the cervix and myometrium during pregnancy and parturition. Obstet Gynecol 2001; 97: 235 242 [DOI] [PubMed] [Google Scholar]
- Luque EH, Munoz de Toro MM, Ramos JG, Rodriguez HA, Sherwood OD. Role of relaxin and estrogen in the control of eosinophilic invasion and collagen remodeling in rat cervical tissue at term. Biol Reprod 1998; 59: 795 800 [DOI] [PubMed] [Google Scholar]
- Mackler AM, Iezza G, Akin MR, McMillan P, Yellon SM. Macrophage trafficking in the uterus and cervix precedes parturition in the mouse. Biol Reprod 1999; 61: 879 883 [DOI] [PubMed] [Google Scholar]
- Yellon SM, Mackler AM, Kirby MA. The role of leukocyte traffic and activation in parturition. J Soc Gynecol Investig 2003; 10: 323 338 [DOI] [PubMed] [Google Scholar]
- Boyd JW, Lechuga TJ, Ebner CA, Kirby MA, Yellon SM. Cervix remodeling and parturition in the rat: lack of a role for hypogastric innervation. Reproduction 2009; 137: 739 748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clyde LA, Lechuga TJ, Ebner CA, Burns AE, Kirby MA, Yellon SM. Transection of the pelvic or vagus nerve forestalls ripening of the cervix and delays birth in rats. Biol Reprod 2011; 84: 587 594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Word RA, Li XH, Hnat M, Carrick K. Dynamics of cervical remodeling during pregnancy and parturition: mechanisms and current concepts. Semin Reprod Med 2007; 25: 69 79 [DOI] [PubMed] [Google Scholar]
- Sakamoto Y, Moran P, Bulmer JN, Searle RF, Robson SC. Macrophages and not granulocytes are involved in cervical ripening. J Reprod Immunol 2005; 66: 161 173 [DOI] [PubMed] [Google Scholar]
- Stjernholm-Vladic Y, Stygar D, Mansson C, Masironi B, Akerberg S, Wang H, Ekman-Ordeberg G, Sahlin L. Factors involved in the inflammatory events of cervical ripening in humans. Reprod Biol Endocrinol 2004; 2: 74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellon SM, Burns AE, See JL, Lechuga TJ, Kirby MA. Progesterone withdrawal promotes remodeling processes in the nonpregnant mouse cervix. Biol Reprod 2009; 81: 1 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellon SM, Ebner CA, Elovitz MA. Medroxyprogesterone acetate modulates remodeling, immune cell census, and nerve fibers in the cervix of a mouse model for inflammation-induced preterm birth. Reprod Sci 2009; 16: 257 264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magyar DM, Elsner CW, Fridshal D, Eliot J, Klein A, Glatz T, Lowe KC, Nathanielsz PW, Buster JE. Time-trend analysis of plasma 11-desoxycorticosterone, corticosterone, cortisol, and aldosterone in fetal and maternal sheep during the last 18 days of gestation. J Steroid Biochem 1981; 14: 1091 1099 [DOI] [PubMed] [Google Scholar]
- Fata JE, Chaudhary V, Khokha R. Cellular turnover in the mammary gland is correlated with systemic levels of progesterone and not 17beta-estradiol during the estrous cycle. Biol Reprod 2001; 65: 680 688 [DOI] [PubMed] [Google Scholar]
- Mahendroo MS, Porter A, Russell DW, Word RA. The parturition defect in steroid 5alpha-reductase type 1 knockout mice is due to impaired cervical ripening. Mol Endocrinol 1999; 13: 981 992 [DOI] [PubMed] [Google Scholar]
- Mackler AM, Ducsay CA, Veldhuis JD, Yellon SM. Maturation of spontaneous and agonist-induced uterine contractions in the peripartum mouse uterus. Biol Reprod 1999; 61: 873 878 [DOI] [PubMed] [Google Scholar]
- Zakar T, Hertelendy F. Progesterone withdrawal: key to parturition. Am J Obstet Gynecol 2007; 196: 289 296 [DOI] [PubMed] [Google Scholar]
- Sasaki S, Nagata K, Kobayashi Y. Regulation of the estrous cycle by neutrophil infiltration into the vagina. Biochem Biophys Res Commun 2009; 382: 35 40 [DOI] [PubMed] [Google Scholar]
- Robertson SA, Christiaens I, Dorian CL, Zaragoza DB, Care AS, Banks AM, Olson DM. Interleukin-6 is an essential determinant of on-time parturition in the mouse. Endocrinology 2010; 151: 3996 4006 [DOI] [PubMed] [Google Scholar]
- Wilson L Jr. Parsons M. Endocrinology of human gestation. : Adashi EY, Rock A, Rosenwakr Z. (eds.), Reproductive Endocrinology, Surgery and Technology. Philadelphia: Lippincott-Raven; 1996: 458 [Google Scholar]
- Condon JC, Hardy DB, Kovaric K, Mendelson CR. Upregulation of the progesterone receptor (PR)-C isoform in laboring myometrium by activation of NF-{kappa}B may contribute to the onset of labor through inhibition of PR function. Mol Endocrinol 2006; 20: 764 775 [DOI] [PubMed] [Google Scholar]
- Andersson S, Minjarez D, Yost NP, Word RA. Estrogen and progesterone metabolism in the cervix during pregnancy and parturition. J Clin Endocrinol Metab 2008; 93: 2366 2374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez HA, Kass L, Varayoud J, Ramos JG, Ortega HH, Durando M, Munoz de Toro M, Luque EH. Collagen remodeling in the guinea-pig uterine cervix at term is associated with a decrease in progesterone receptor expression. Mol Hum Reprod 2003; 9: 807 813 [DOI] [PubMed] [Google Scholar]
- Chapman NR, Kennelly MM, Harper KA, Europe-Finner GN, Robson SC. Examining the spatio-temporal expression of mRNA encoding the membrane-bound progesterone receptor-alpha isoform in human cervix and myometrium during pregnancy and labor. Mol Hum Reprod 2006; 12: 19 24 [DOI] [PubMed] [Google Scholar]
- Mani S, Portillo W. Activation of progestin receptors in female reproductive behavior: interactions with neurotransmitters. Front Neuroendocrinol 2010; 31: 157 171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiaens I, Zaragoza DB, Guilbert L, Robertson SA, Mitchell BF, Olson DM. Inflammatory processes in preterm and term parturition. J Reprod Immunol 2008; 79: 50 57 [DOI] [PubMed] [Google Scholar]
- Yellon SM, Ebner CA, Sugimoto Y. Parturition and recruitment of macrophages in cervix of mice lacking the prostaglandin F receptor. Biol Reprod 2008; 78: 438 444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry SM, Romero R, Gomez R, Puder KS, Ghezzi F, Cotton DB, Bianchi DW. Premature parturition is characterized by in utero activation of the fetal immune system. Am J Obstet Gynecol 1995; 173: 1315 1320 [DOI] [PubMed] [Google Scholar]