Abstract
We demonstrated previously that disruption of the germ cell-specific lactate dehydrogenase C gene (Ldhc) led to male infertility due to defects in sperm function, including a rapid decline in sperm ATP levels, a decrease in progressive motility, and a failure to develop hyperactivated motility. We hypothesized that lack of LDHC disrupts glycolysis by feedback inhibition, either by causing a defect in renewal of the NAD+ cofactor essential for activity of glyceraldehyde 3-phosphate dehydrogenase, sperm (GAPDHS), or an accumulation of pyruvate. To test these hypotheses, nuclear magnetic resonance analysis was used to follow the utilization of labeled substrates in real time. We found that in sperm lacking LDHC, glucose consumption was disrupted, but the NAD:NADH ratio and pyruvate levels were unchanged, and pyruvate was rapidly metabolized to lactate. Moreover, the metabolic disorder induced by treatment with the lactate dehydrogenase (LDH) inhibitor sodium oxamate was different from that caused by lack of LDHC. This supported our earlier conclusion that LDHA, an LDH isozyme present in the principal piece of the flagellum, is responsible for the residual LDH activity in sperm lacking LDHC, but suggested that LDHC has an additional role in the maintenance of energy metabolism in sperm. By coimmunoprecipitation coupled with mass spectrometry, we identified 27 proteins associated with LDHC. A majority of these proteins are implicated in ATP synthesis, utilization, transport, and/or sequestration. This led us to hypothesize that in addition to its role in glycolysis, LDHC is part of a complex involved in ATP homeostasis that is disrupted in sperm lacking LDHC.
Keywords: ATP, flagellum, gamete biology, glycolysis, isozyme, nuclear magnetic resonance, null mutation/knockout, sperm hyperactivation, transgenic/knockout model
Lack of LDHC impairs mouse sperm glycolysis but not pyruvate to lactate conversion.
INTRODUCTION
Aerobic glycolysis is the primary source of ATP in many mammals for sperm motility and capacitation [1, 2]. Glycolysable substrates are essential for motility [3, 4], protein tyrosine phosphorylation and hyperactivation associated with capacitation [4–7], and fertilization [8, 9]. Conversion of pyruvate to lactate with the concomitant oxidation of NADH to NAD+ is essential for the continued production of ATP by glycolysis; this reaction is catalyzed by lactate dehydrogenase (LDH). A unique isozyme of LDH expressed specifically in germ cells [10, 11], LDH type C (LDHC), is abundant in spermatids and spermatozoa [12]. We found that targeted disruption of the Ldhc gene resulted in male infertility due to sperm having a decrease in progressive motility, a failure to develop the hyperactivated motility pattern essential for fertilization, and a rapid decline in ATP levels [13]. A modest amount (17.5%) of LDH activity remained, probably due to the LDHA isozyme variant recently found to be associated with the fibrous sheath [14].
Although these results demonstrated that lack of LDHC in sperm disrupts the glycolytic process and causes a reduction in ATP production, the underlying causes remain to be determined. We hypothesized that lack of LDHC would lead to a decrease in total LDH activity in sperm and induce a feedback inhibition of glycolysis, by causing either a defect in renewal of NAD+ or an accumulation of pyruvate [13]. To test this hypothesis, nuclear magnetic resonance (NMR) spectroscopy was used to follow in real time the utilization of 13C-labeled substrates by sperm. In addition, sodium oxamate, a structural analog of pyruvate that inhibits LDH activity, was used to compare the metabolic phenotype of oxamate-treated WT sperm with that of sperm lacking LDHC. Furthermore, to determine if LDHC has other functions, we used coimmunoprecipitation coupled with mass spectrometry to identify possible LDHC partner proteins involved in ATP metabolism.
MATERIALS AND METHODS
Materials and Reagents
All materials and reagents used were of the highest quality commercially available and purchased from Sigma-Aldrich (St. Louis, MO) or Mallinckrodt Baker (Phillipsburg, NJ) unless designated otherwise. Tyrode (TYH) medium (119.37 mM NaCl, 4.78 mM KCl, 1.71 mM CaCl2.2H2O, 1.19 mM MgSO4.7H2O, 1.19 mM KH2PO4, 25 mM NaHCO3, 5.5 mM glucose) supplemented with 5 mg/ml factor V tissue culture-grade bovine serum albumin (BSA) was prepared fresh and used for in vitro sperm capacitation and hyperactivation assays [15]. Unless indicated otherwise, TYH medium was lactate and pyruvate free. TYH medium was supplemented with 10 mM HEPES (HEPES-TYH) to maintain the pH at ∼7.4 when sperm were incubated in humidified air without CO2 supplementation.
Animal Procedures and Sperm Preparation
All animal procedures were performed in accordance with NIH guidelines and approved by the Animal Care and Use Committee of the National Institute of Environmental Health Sciences. Mice from 12 to 32 wk of age were euthanized by CO2 asphyxiation followed by cervical dislocation. This study was performed with animals from the third and fifth generations backcrossed with wild-type (WT) C57BL/6N mice.
Cauda epididymides collected in 1× PBS (Ca2+/Mg2+-free) were carefully dissected to remove blood vessels and fat, several small cuts were made with iridectomy scissors, and sperm were allowed to swim out in 1 ml PBS for 10 min at room temperature (RT). To remove residual substrates present in the epididymal fluid, sperm were washed by dilution in 13 ml PBS and by gentle centrifugation (10 min at 300 × g) at RT. The sperm pellet was suspended in 1 ml TYH, and the number of spermatozoa determined using a hemocytometer. Sperm were diluted to the appropriate concentration in TYH medium and incubated for selected lengths of time at 37°C in 5% CO2 in humidified air. Sperm viability was determined by dye exclusion using Hoechst 33258 [16]. In experiments involving use of sodium oxamate, the inhibitor was added to TYH medium, and the equivalent molar amount of NaCl was removed to maintain osmotic equivalence.
JC-1 Assay
Sperm mitochondrial membrane potential in WT, Ldhc+/− (HET) and Ldhc−/− (KO) sperm was examined by staining with the supravital fluorescent dye JC-1 (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolyl-carbocyanine chloride). Sperm were incubated at 37°C in 5% CO2 in humidified air in TYH medium containing 5 mM glucose and without (control) or with the mitochondria inhibitor carbonyl cyanide m-chlorophenylhydrazone (CCCP; 10 μM). Fluorescence (excitation 490 nm; emission 530, 590 nm) was monitored in a SpectraMax Gemini EM microplate reader (Molecular Device, Sunnyvale, CA) from the base of the plate. Ratio between red fluorescence (high membrane potential; excitation 490 nm, emission 590 nm) and green fluorescence (low membrane potential; excitation 490 nm, emission 530 nm) was calculated.
NAD:NADH Level Measurements
The NAD:NADH ratios in sperm were measured using the EnzyChrom NAD:NADH Assay kit (ECND-100; Bioassay Systems, Hayward, CA) following the manufacturer's instructions. Briefly, after incubation sperm were washed in PBS and the pellet was lysed in the buffer appropriate for either NAD or NADH by sonication and heating at 60°C for 5 min. Samples were neutralized with the opposite buffer and centrifuged at 14 000 × g for 5 min. The subtraction of optical density (OD) at time 0 from OD at 30 min at 565 nm was used to determine sample NAD and NADH concentrations from standard curves. Protein levels in each sample were measured with the BCA protein assay kit (Pierce Biotechnology, Indianapolis, IN) and used to normalize the assay results.
Sperm Motility Assessment
Quantitative parameters of sperm motility were determined as previously described [13] using a computer-assisted sperm analysis instrument (software version 12; Hamilton Thorne Research, Beverly, MA). Sperm were counted as motile with any type of movement and as progressively motile when average path velocity > 50 μm/sec and straight line velocity > 50%. Hyperactive sperm were identified with the “sort fraction” function of the Hamilton Thorne analyzer by using these criteria: curvilinear velocity > 240 μm/sec, lateral head displacement > 18 μm, and beat-cross frequency < 40 Hz. Median values of each of the kinematic parameters were obtained for each sample.
ATP Level Measurements
Sperm ATP levels were measured as previously described [2] with slight modifications. After incubation for the indicated length of time at 37°C in 5% CO2 in humidified air, sperm were centrifuged at 1000 × g for 5 min. The pellet was resuspended in buffer (100 mM Tris-HCl, 4 mM EDTA, pH 7.8) and boiled for 5 min. Samples were centrifuged at 10 000 × g for 5 min and aliquots of the supernatant were analyzed in duplicate. ATP was measured using a luciferase bioluminescence assay according to the manufacturer's protocol (ATP Bioluminescence Assay kit CLS II; Roche Applied Science, Indianapolis, IN).
Pyruvate and Lactate Level Measurements
Pyruvate and lactate levels were determined using a commercial kit based on an enzymatic reaction by lactate oxidase and interaction of the product with a probe to produce fluorescence (at excitation/emission = 535/587 nm). After a 4-h incubation in TYH medium (lactate- and pyruvate-free), sperm were centrifuged and the pellet was resuspended in the appropriate assay buffer (BioVision, Mountain View, CA). The concentration of each sample was calculated using a standard curve.
NMR Spectroscopy
Proton decoupled 13C-[1H] NMR spectra were obtained at 25°C on a Varian INOVA 500 NMR spectrometer using a 5-mm broadband probe tuned to 125.869 MHz. A 45° pulse angle with a 2.3 recycle time was employed in order to minimize intensity perturbations due to overpulsing. Data were collected using a 30 000-Hz sweep width and 78 000 data points. Ten percent D2O was added to the samples for locking. Each sample contained 15 million viable spermatozoa in HEPES-TYH without substrates. Samples were shimmed (magnetic field homogeneity optimized) prior to addition of labeled substrate. They were then spiked with either 6 mM of [1-13C]-glucose or [3-13C]-pyruvate (Isotec/Sigma-Aldrich) and NMR acquisition quickly initiated. In the studies utilizing [1-13C]-glucose, data were collected in 1-h blocks of time and followed over a total period of 20 h. In the studies utilizing [3-13C]-pyruvate, data was collected in 10-min blocks of time for 1 h.
Glycolytic Enzyme Activity
Whole sperm were suspended in a nondenaturing buffer (0.1 M Tris-HCl pH 7, 0.1% Triton-X100) and placed on ice for 30 min. The protein concentration was measured using the BCA protein assay kit. Enzymatic activity was assayed in solution by monitoring the NADH or NADPH concentrations at 340 nm with a spectrophotometer over a period of 1–30 min as recommended [17] with minor modifications.
Glucose Uptake
Glucose uptake was determined using radiolabeled [1,2-3H]-2-deoxy-d-glucose [18]. Sperm were incubated 30 min at RT with 0.5 μCi [1,2-3H]-2-deoxy-d-glucose (52 Ci/mmol) in 1 ml modified HEPES-TYH medium without glucose. Uptake of [1,2-3H]-2-deoxy-d-glucose was stopped by addition of 0.2 ml of an ice-cold 0.6 M glucose solution. After 4 washes in cold PBS, sperm were solubilized in 0.2 ml of 4% SDS solution and transferred to scintillation vials containing 1 ml of scintillate. Radioactivity was determined in a scintillation counter. An aliquot (100 μl) of the last PBS wash also was counted to determine the residual amount of [1,2-3H]-2-deoxy-d-glucose present in the extracellular medium and was subtracted as background. Cytochalasin B (20 μM) was used to inhibit glucose uptake, and competition with 5 mM nonlabeled glucose was used as a negative control. This provided an estimate of radioactivity in the extracellular space within the sperm pellet.
Coimmunoprecipitation and Western Blot
Proteins were extracted from freshly isolated sperm using lysis buffer (50 mM Tris pH 8, 150 mM NaCl, 1% Triton X-100) supplemented with EDTA-free 1× protease inhibitor cocktail (Roche Applied Science), and the samples were sonicated on ice 3 times for 5 sec. After centrifugation at 4°C for 5 min at 10 000 × g, the protein concentration in the supernatant was determined using the BCA protein assay kit. Lysate (containing 200 μg protein) from WT mouse sperm was precleared with 100 μl protein G magnetic microbeads (Miltenyi Biotec, Auburn, CA) following manufacturer's instructions, and then incubated with antiserum to LDHC (1/10 000; [19]; IPLDHC) plus 1% BSA or in normal rabbit IgG as a negative control (ab-37415; Abcam, Cambridge, MA; C−) for 2 h at 4°C. Protein G magnetic microbeads (100 μl) were added for 30 min at 4°C. Immunopurification was performed using microcolumns and a microMACS separator. Samples obtained from coimmunoprecipitation were subjected to mass spectrometry (see Supplemental Experimental Procedure; all supplemental data are available online at www.biolreprod.org) after separation on a 4%–20% polyacrylamide gel (Biorad, Hercules, CA) and Coomassie blue staining. Western blots using the Clean-blot IP detection kit from Pierce Biotechnology were performed to validate the mass spectrometry results.
Data Analysis
All results are represented as the mean values of each group ± SEM. The significance of the results was determined using one-way ANOVA followed by the Mann-Whitney U-test. The paired t-test was used when comparing the mean differences between treated and untreated sperm from the same animal. Differences were considered significant at P < 0.05.
RESULTS
Loss of LDHC Does Not Alter Mitochondrial Activity
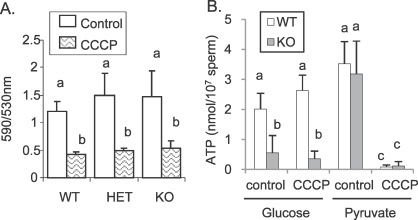
No differences were observed in mitochondrial membrane potential between WT, HET, and KO sperm, and CCCP treatment decreased the 590:530 nm emission ratio in all groups (Fig. 1). Moreover, ATP levels were equivalent in KO and WT sperm incubated with 1 mM pyruvate as a mitochondrial substrate (Fig. 1B), and decreased drastically with CCCP treatment. The same profile also was observed for WT and KO sperm motility (data not shown). These data suggest that the mitochondrial oxidative pathway was not impaired in KO sperm.
FIG. 1.
A) Sperm mitochondrial membrane potential in WT, HET, and KO sperm observed by staining with the supravital fluorescent dye JC-1. Sperm were incubated at 37°C in 5% CO2 in humidified air in TYH medium containing 5 mM glucose with (CCCP) or without (control) 10 μM of the mitochondria inhibitor CCCP. B) ATP levels in WT and KO sperm incubated 2 h with 5 mM glucose as glycolytic substrate or 1 mM pyruvate as mitochondrial substrate with (CCCP) or without (control) 10 μM of the mitochondrial inhibitor CCCP. Values are the mean ± SEM, n = 3. Letters (a, b, c) above the means denote no significant difference if identical or significant difference if not identical.
Loss of LDHC Alters Glycolysis: NMR Experiments
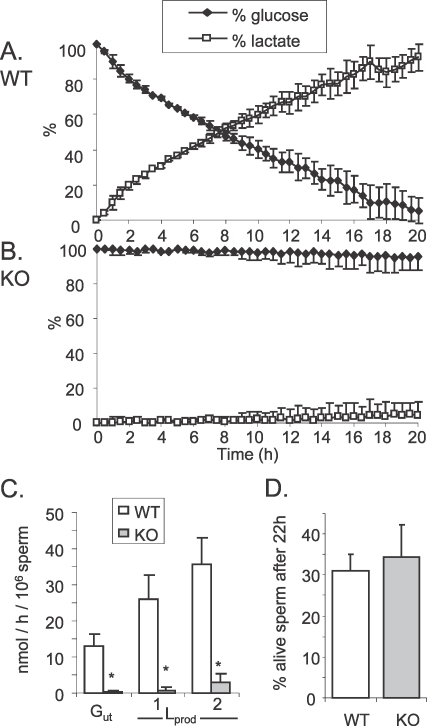
An NMR assay was used to follow in real time the utilization of 13C-labeled glucose by freshly isolated epididymal sperm in HEPES-TYH medium in a system without oxygen supplementation. WT and KO sperm were incubated for 20 h with 6 mM [1-13C]-glucose (Fig. 2) and the fraction converted to lactate was calculated by integration of the peak area. This method was not sufficiently sensitive to detect peaks corresponding to other metabolites. After 20 h incubation, 94.9% ± 7.2% of glucose was metabolized to lactate by WT sperm (Fig. 2A; Supplemental Fig. S1A). As expected, KO sperm produced a low level of lactate, but most of the glucose remained unutilized (Fig. 2B). After 20 h incubation, only a small percentage of the glucose was metabolized to lactate by KO sperm (Supplemental Fig. S1B). Based on these data, glucose utilization (Gut) was calculated in nanomole per hour and per million spermatozoa (nmol per h per million sperm; Fig. 2C). We found that with WT sperm Gut = 12.9 ± 3.4 nmol per h per million sperm, and with KO sperm Gut = 0.4 ± 0.3 nmol per h per million sperm. The lactate production (Lprod nmol per h per million sperm) was calculated as two times the percentage of lactate derived from the intensity of the NMR resonance, because only one of the two lactate molecules derived from [1-13C]glucose will be labeled as [3-13C]lactate. The Lprod equaled 25.9 ± 6.9 for WT and 0.8 ± 0.7 nmol per h per million sperm for KO sperm. These results are consistent with the Lprod found previously [13] using an enzymatic assay (Lprod = 35.7 ± 7.3 for WT and 2.9 ± 2.5 nmol per h per million sperm for KO sperm). No differences in sperm viability were observed between WT and KO sperm after 22 h (Fig. 2D), indicating that the low level of Gut in KO sperm was not due to cell death.
FIG. 2.
[1-13C]-glucose consumption by WT (A) and KO (B) sperm followed in real time by NMR spectroscopy. Six millimolar [1-13C]-glucose was initially added to the sperm preparation and corresponded to 100%. C) Gut and Lprod expressed in nanomole per hour and per million spermatozoa WT and KO sperm. Values for Gut and Lprod (1) were calculated from the NMR assay. Values for Lprod (2) were calculated from an enzymatic assay determining the quantity of lactate secreted by 4 × 106 spermatozoa in 1 ml TYH, 5.5 mM glucose at different times. D) Percentage of WT and KO sperm live after 22 h incubation in TYH medium containing 6 mM glucose, determined by a dye exclusion assay [16]. Values are the mean ± SEM, n = 3, *P < 0.01 compared to WT.
Absence of LDHC Does Not Affect NAD, NADH, or Pyruvate Levels
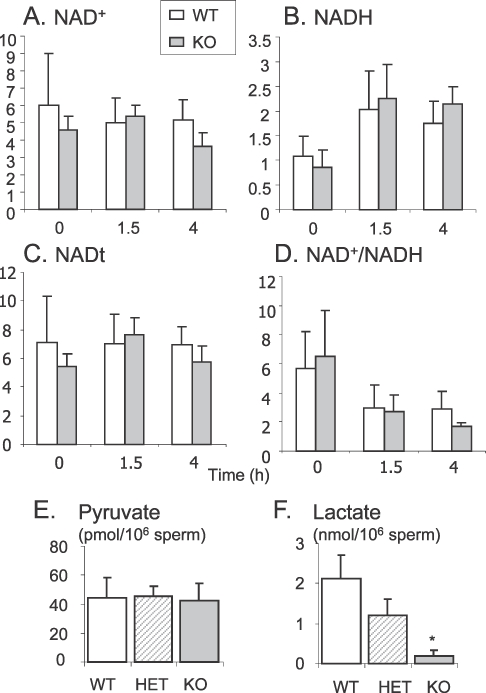
The inhibition of LDHC was hypothesized [13] to lead to an accumulation of its substrate (pyruvate) and cofactor (NADH) and an absence of its products (lactate and NAD+). Therefore, the levels of NAD+ and NADH were measured in WT and KO sperm directly after isolation, and after incubation for 1.5 h and 4 h in TYH containing 5.5 mM glucose (Fig. 3). No significant differences were detected in the levels of NAD+ (Fig. 3A), NADH (Fig. 3B), or NADt (total = NAD+ + NADH; Fig. 3C), or in the NAD+:NADH ratio (Fig. 3D). A decrease in the NAD+:NADH ratio in KO sperm compared to WT sperm was observed after 4 h incubation, but the difference was not statistically significant (P = 0.13). Because variability between animals and assays may have obscured small changes important for regulation of glycolysis, we used an alternative functional approach. Sperm from WT and KO mice were treated with methylene blue, a redox dye capable of oxidizing NADH [20, 21]. However, this treatment did not rescue the phenotype of KO sperm (Supplemental Fig. S2).
FIG. 3.
NAD+ (A) and NADH (B) levels (μM/μg proteins), were measured in WT and KO sperm after 0, 1.5, and 4 h incubation at 37°C in 5% CO2 in humidified air in TYH, 5.5 mM glucose. Total NADt (NADt = NAD+ + NADH; C) and NAD:NADH ratio (D) were calculated from these values. Pyruvate (E) and lactate (F) levels were measured in sperm extract after 4 h incubation in TYH, 5.5 mM glucose. Values are the mean ± SEM, n = 5 minimum for the NAD+ and NADH assays, n = 4 for pyruvate and lactate assays; *P < 0.01 compared to WT.
Pyruvate and lactate levels were measured by an enzymatic method in sperm extracts from WT, HET, and KO mice, after 4 h incubation in TYH containing 5.5 mM glucose. No differences in pyruvate concentrations (Fig. 3E) were observed, but the lactate level (Fig. 3F) was significantly lower in KO sperm compared to WT and HET sperm.
KO Sperm Metabolize Pyruvate Like WT Sperm
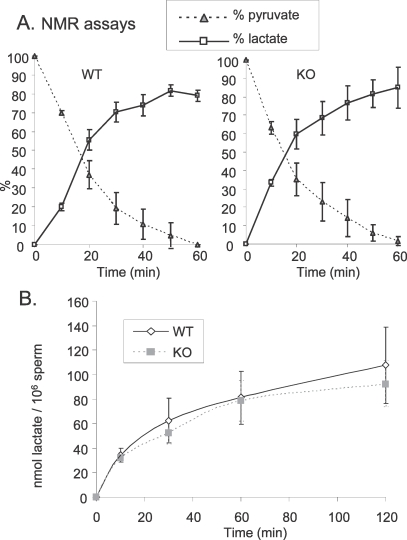
We also followed the utilization of pyruvate by NMR. With WT sperm, we observed that 6 mM [3-13C]-pyruvate was quickly metabolized. After 1 h, all pyruvate was consumed, and around 80% of the pyruvate was converted to lactate (Fig. 4A). Two minor peaks were detected at 25.8 ppm and 23.5 ppm and remain to be identified. Surprisingly, the same profile was found with sperm lacking LDHC (Fig. 4A). We calculated that for WT and KO sperm respectively, 4.9 ± 1.2 and 5.0 ± 1.5 nmol of pyruvate were consumed per min per 106 sperm, and 4.1 ± 1.0 and 4.5 ± 1.4 nmol of lactate per min per million sperm were produced from pyruvate.
FIG. 4.
A) [3-13C]-pyruvate consumption by WT and KO sperm followed in real time by NMR. Initially, 6 mM [3-13C]-pyruvate was added to the sperm preparation and corresponded to 100%. B) Lprod by WT and KO sperm incubated at 37°C in 5% CO2 in humidified air in medium containing 5 mM pyruvate as sole substrate. Lactate levels were measured with an enzymatic assay. Values are the mean ± SEM, n = 3.
Similar results were observed with another approach. WT and KO sperm were incubated in TYH containing 5 mM pyruvate as the sole substrate, and lactate levels were measured in medium at different times (Fig. 4B). WT and KO sperm were able to produce a high quantity of lactate. The Lprod calculated at 10 min was 3.41 ± 0.56 and 3.21 ± 0.31 nmol of lactate per min per million sperm for WT and KO sperm, respectively.
Differences Between Loss of LDHC and Inhibition of LDH Activity
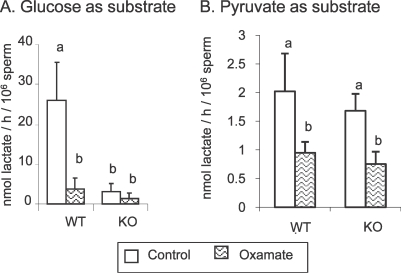
The pyruvate analog sodium oxamate was used as a competitive inhibitor of LDH activity. In sperm extracts, all LDH activity was inhibited by 20 mM sodium oxamate for WT sperm and 5 mM for KO sperm (Supplemental Fig. S3A). Live sperm were incubated for 2 h at 37°C in TYH containing 5 mM glucose plus different concentrations of sodium oxamate (0–100 mM). We determined that 100 mM was the maximum sodium oxamate concentration that could be used without changing the osmolarity of the medium, and that even at this high concentration sperm were as viable, as determined by dye exclusion, as in control medium (data not shown). The endpoint used was inhibition of sperm hyperactivation and the maximum effect observed was at 100 mM (Supplemental Fig. S3B). However, ATP levels and sperm motility were not affected. Treatment with 100 mM sodium oxamate inhibited lactate production: 1) by 85% in WT and 52% in KO sperm when glucose was the substrate (Fig. 5A), and 2) by 53% in WT and 55% in KO sperm when pyruvate was the sole substrate (Fig. 5B). Therefore, this concentration (100 nM sodium oxamate) was chosen to treat WT and KO sperm for 2 h at 37°C in TYH medium containing 5 mM glucose.
FIG. 5.
Effect of 100 mM sodium oxamate treatment on Lprod when WT and KO sperm were incubated at 37°C in 5% CO2 in humidified air in TYH containing 5 mM glucose (A) for 2 h or 1 mM pyruvate (B) for 30 min. Values are the mean ± SEM, n = 3. Letters (a, b) above the means denote no significant difference if identical or significant difference if not identical.
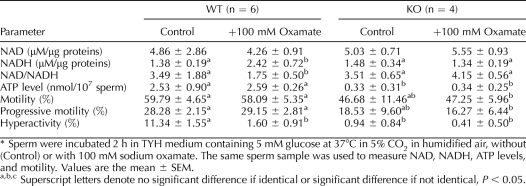
We compared the levels of NAD+, NADH, and ATP, and of sperm motility in treated and untreated WT and KO sperm (Table 1). We observed 1) no differences between untreated and treated KO sperm in motility or in ATP, NAD, and NADH levels; 2) a lower level of ATP and no difference in NAD+ and NADH levels in KO sperm compared to the control WT sperm; 3) that treated WT sperm failed to hyperactivate, as seen for KO sperm; 4) that treated WT sperm differed from KO sperm in that ATP levels in treated WT sperm were comparable to those of control WT sperm; 5) that NADH levels in treated WT sperm were higher, resulting in a lower NAD:NADH ratio.
TABLE 1.
Sodium oxamate treatment in WT and KO sperm: consequences on NAD, NADH, ATP levels, and sperm motility.*
KO Sperm Are Able to Take Up and Consume Glucose
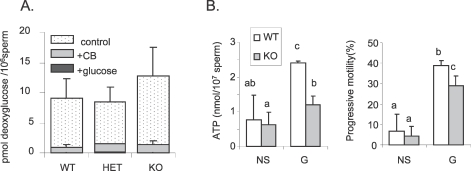
Because LDHC is relatively hydrophobic and thus could associate with the plasma membrane, loss of LDHC might interfere with glucose uptake. Therefore, we measured the uptake of radiolabeled [1,2-3H]-deoxy-glucose. Following 30 min incubation, sperm were carefully washed to remove residual [1,2-3H]-deoxy-glucose. No differences in radioactive content were observed between WT, HET, and KO sperm (Fig. 6A). The levels of radioactivity were barely detectable in sperm following incubation in [1,2-3H]-deoxy-glucose (0.5 μCi/ml = 9.6 μM) containing excess cold glucose (5 mM), and more than 10 times lower with cytochalasin B treatment (inhibitor of facilitative glucose transporters). These results indicate that glucose uptake was not disrupted in sperm from KO mice.
FIG. 6.
A) Levels of radiolabeled [1,2-3H]-2-deoxy-d-glucose detected in sperm extract from WT, HET, and KO mice after a 30-min incubation at RT without (control) or with cytochalasin B treatment (CB) or competition with nonlabeled glucose (glucose). Values are the mean ± SEM, n = 6 minimum for control and cytochalasin B-treated assays, n = 3 for cold glucose competition assay. B) ATP level and progressive motility in WT and KO sperm incubated 1.5 h at 37°C in 5% CO2 in humidified air in TYH with no substrates (NS) or with 1 mM glucose (G). Letters (a, b) above the means denote no significant difference if identical or significant difference (P < 0.05) if not identical; n = 4.
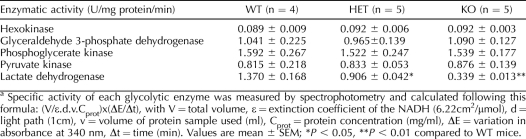
Proteins from Triton X-100 permeabilized WT, HET, and KO sperm were aliquoted and used in parallel assays to measure hexokinase, glyceraldehyde-3-phosphate dehydrogenase, phosphoglycerate kinase, pyruvate kinase, and LDH enzymatic activities. The total LDH activity in extracts of sperm from KO mice was confirmed to be significantly lower than that in extracts of sperm from WT and HET mice. In contrast, no differences in the activities of the other enzymes were observed (Table 2), indicating that the lack of LDHC does not compromise the functional abilities of these other glycolytic enzymes under these conditions.
TABLE 2.
Enzymatic activity of different glycolytic enzymes in WT, HET, and KO sperm.a
In addition, we observed that in medium containing no substrates (NS in Fig. 6B), ATP levels were 3 times lower in WT and 2 times lower in KO sperm than in medium containing glucose (G in Fig. 6B). In assays done in parallel, progressive motility was around six times lower for both WT and KO sperm in the absence of glucose than when glucose was present (Fig. 6B).
Identification of Putative Proteins Associated with LDHC
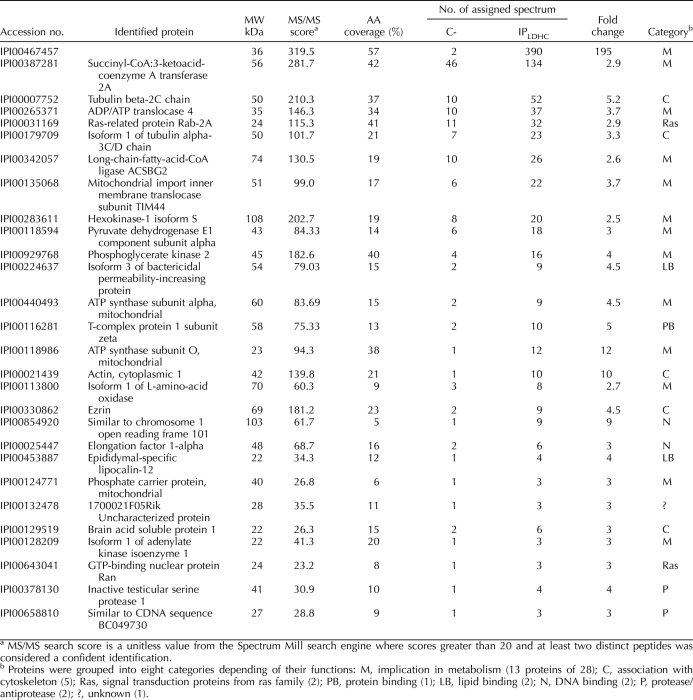
We considered that LDHC might also serve a nonenzymatic role through an association with other proteins. Coimmunoprecipitation followed by mass spectrometry of PAGE slices was used to identify potential LDHC binding partners. As a negative control, the same procedure was performed using normal rabbit IgG. Proteins found in the LDHC immunoprecipitation lane with two or more peptides and a confidence level of more than 95%, but absent or extremely low in the negative control lanes (ratio IPLDHC:negative control ≥2.5), were considered to be directly or indirectly bound to LDHC. Of 173 proteins identified by mass spectrometry, 27 proteins (plus LDHC) met these criteria (Table 3). ADP/ATP translocase 4 (ANT4) was confirmed to be a binding partner by Western blot and inverse coimmunoprecipitation (Supplemental Fig. S4).
TABLE 3.
Putative LDHC interacting proteins out of 173 proteins identified in 3 independent LDHC IPs (ratio IPLDHC / negative control (C-) ≥ 2.5 with a 95% confidence).
DISCUSSION
This study used an NMR approach to follow in real-time Gut by sperm. In WT sperm, a high glucose metabolic flux in the direction of lactate was observed, in agreement with previous data from studies using rat [9] and boar [22] sperm in which a pentose phosphate pathway was undetectable or barely detectable. We confirmed that Lprod in KO sperm was extremely low (around 30 times lower than in WT sperm) and that Gut occurred at a very low level, indicating that the loss of LDHC directly perturbs the process of glycolysis [13]. In addition, preliminary data suggest that LDHC was not essential for production of ATP by oxidative phosphorylation, but further studies will be necessary to confirm these results.
Our initial hypothesis was that the lack of LDHC led to an accumulation of pyruvate and a decrease in NAD+ renewal, thereby causing an imbalance in the NAD:NADH ratio that would inhibit glyceraldehyde 3-phosphate dehydrogenase, sperm (GAPDHS) function and concomitantly ATP production. Although there was a decrease in the NAD:NADH ratio in KO sperm compared to WT sperm after 4 h incubation, it was not statistically significant (P = 0.13). Moreover, no differences were observed in NAD+ and NADH levels between WT and KO sperm after incubation for 1.5 h, when ATP levels were already lower in KO sperm. However, treatment with methylene blue to oxidize NADH did not rescue the phenotype of KO sperm, suggesting that the down-regulation does not occur because of limitation of NAD+ renewal and inhibition of the GAPDHS reaction. Also, the low level of Lprod by KO sperm was not accompanied by an accumulation of pyruvate.
Following pyruvate utilization by NMR spectroscopy and using an enzymatic approach to assay the production of lactate from pyruvate, we found that KO sperm were able to convert pyruvate into lactate at the same rate as WT sperm, showing that sperm lacking LDHC still contain appreciable levels of LDH activity. We assumed previously that approximately 17.5% of the LDH activity in sperm was due to the action of the LDHA isozyme [13] and our data suggest that LDHA is able to compensate partially for the absence of LDHC in terms of processing a high concentration of external pyruvate. Could the compartmentalization make the external pyruvate more accessible to LDHA than the internal pyruvate (produced during the glycolytic reactions)? Previous studies have shown that 1) the conversion of pyruvate to lactate under anaerobic conditions in skeletal muscle is performed by LDHA [23]; 2) direct interaction between GAPDH and LDHA occurs in cardiac and skeletal muscle [24] suggests that substrate channeling prevented the escape of NADH coenzyme into the bulk phase between both enzymes [25, 26]; 3) LDHC is loosely associated with the fibrous sheath (which makes LDHC more available to process external pyruvate) and both LDHA and GAPDHS are tightly bound [14, 27]; 4) of the five Ldha splice variants in the mouse, LDHA_V2 has an N-terminal extension specific to the testis and is a strong candidate for the LDHA isozyme present in the fibrous sheath (NCBI accession number NP_001129541; http://www.ncbi.nlm.nih.gov/protein/257743039). This led us to suggest that LDHA was responsible for some or most of the LDH activity in KO sperm associated with the renewal of NAD+ for the GAPDHS reaction [13]. However, a conditional Ldha KO in male germ cells will be needed to test this hypothesis.
We expected WT sperm treated with the LDH competitive inhibitor sodium oxamate to lack LDH activity and to have a phenotype similar to KO sperm. Indeed, the treated WT and KO sperm both failed to develop a hyperactivated motility pattern. However, whereas KO sperm had low ATP levels and no differences in the NAD:NADH ratio, the ATP level in treated WT sperm was normal and the NADH level was elevated, resulting in a decrease in the NAD:NADH ratio. These results strongly suggested that the molecular mechanism preventing hyperactivation was different between oxamate-treated WT sperm and sperm lacking LDHC. We suggested previously [13] that the motility seen initially in KO sperm was due to ATP produced by LDHA. In that case, the inhibition of LDHA by sodium oxamate treatment should accentuate the phenotype of KO sperm. However, the lack of differences between treated and untreated KO sperm strongly suggested that glycolysis in KO sperm was not disrupted solely because of a lack of LDH activity.
KO sperm possessed the other enzymatic activities necessary for glucose metabolism, and glucose uptake was not different between WT, HET, and KO sperm. However, the NMR results indicated that glucose was consumed very slowly by KO sperm, with peaks detected only for glucose and lactate. The lack of accumulation of hexose phosphate intermediates suggests that the glycolytic flow was reduced considerably by a decrease in the first phase of glycolysis. However, it should be noted that the identification of other metabolites present at low concentrations is limited by the inherently low sensitivity of the NMR technique.
If the payoff phase of glycolysis is blocked, production of hexose phosphate should not only limit ATP production, but also waste ATP [28]. Therefore, the ATP levels in KO sperm would be expected to decrease more rapidly in medium containing glucose than in medium lacking glucose. However, the opposite was observed. ATP levels and progressive motility decreased significantly in both KO and WT sperm in medium lacking glucose compared to when they were incubated in medium containing glucose. These data suggest that a low level of glycolysis in KO sperm is still present and needed to maintain a degree of motility. They also support our hypothesis that the inhibition of glycolysis occurs in earlier steps of glycolysis to avoid futile production of hexose phosphate and waste of ATP.
The efficient conversion of pyruvate to lactate by KO sperm was unexpected. One possible explanation is that the efficient conversion of pyruvate to lactate in KO sperm is related to differences in the kinetic characteristics of LDHA and LDHC. Earlier studies reported that LDHC had a higher affinity for pyruvate (lower Km) than LDHA [29]. In addition, LDHC was found to be susceptible to inhibition by pyruvate at lower concentrations than are optimal for LDHA. It is possible that at lower concentrations the pyruvate in WT sperm is reduced to lactate, whereas pyruvate would reach higher levels in KO sperm before being metabolized by LDHA. The NADH in KO sperm might also be shunted to other NADH-dependent enzymes, compensating for the changes in metabolism in KO sperm [30]. However, the relationships between Km and the saturation characteristics of purified enzymes in vitro [29] and when they are present in intact sperm are unknown.
Another possibility was that LDHC has noncatalytic functions essential for regulation of glycolysis in sperm that other LDH isozymes are unable to provide. A systematic proteomic approach, combining coimmunoprecipitation of native protein complexes, separation on one-dimensional SDS-PAGE, and mass spectrometry, was used to identify proteins that directly or indirectly associated with LDHC. This led to the identification of 27 putative LDHC-interacting proteins, 13 of which were implicated in energy metabolism and 5 of which were cytoskeleton associated. Interestingly, four of the proteins associated with ATP metabolism are specifically expressed in male germ cells: ANT4 [31–33], hexokinase-1 isoform S (HK1S) [34], phosphoglycerate kinase 2 (PGK2) [35], and pyruvate dehydrogenase E1 alpha 2 (PDHE1A2) [36].
ANT subfamily proteins usually are associated with the inner mitochondrial membrane and involved in the exchange of ADP and ATP between cytosol and mitochondria, as well as with mitochondria-dependent apoptosis [37]. ANT4 is expressed mainly in male germ cells and at highest levels in spermatocytes. A knockout of the gene (Slc25a31) for ANT4 in mice led to disruption of spermatogenesis with an early meiotic arrest and an increase of apoptosis [32, 38]. However, ANT4 also was found in the sperm principal piece in most human sperm and only occasionally in the midpiece where the sperm mitochondria are located [33]. This led to the suggestions that ANT4 acts as an ATP reservoir or ATP carrier in the sperm flagellum. HK1S and PGK2 are important sperm-specific glycolytic enzymes. HK1S uses ATP in the first phase of glycolysis to convert glucose into glucose-6-phosphate, whereas PGK2 catalyses the first reaction in the second phase of glycolysis to produce ATP. HK1S is the only hexokinase detected in mouse sperm, and a recent study demonstrated that the N-terminal 24-amino acid spermatogenic cell-specific region of HK1S binds to a testis-specific isoform of phosphofructokinase (PFKMS) [39]. A lack of PGK2 induces male infertility and the phenotype of PGK2-null sperm is similar to the LDHC-null sperm [35]. However, the HK1S and PFKMS enzymatic activities were not different in permeabilized LDHC-null sperm and WT sperm. PDHE1A2 is a testis-specific subunit of the PDH complex, which converts pyruvate into acetyl-coA. Like ANT proteins, the PDH complex usually is associated with mitochondria. However, studies in the hamster showed that a PDHA2 orthologue is located in the sperm flagellum, phosphorylated during sperm capacitation, and essential for sperm hyperactivation [36]. However, cross-contamination with mitochondria is possible and further investigations will be needed in order to validate these preliminary data.
These observations suggest that the in vivo macromolecular organization of enzymes is crucial in the regulation of glycolysis in the sperm flagellum, with LDHC being an integral component of a complex containing an ATP carrier protein (ANT4) that redistributes ATP from PGK2 to HK1S and thereby regulates the initial phase of glycolysis. This is reminiscent of an intriguing model proposed for the compartmentalization of glycolysis in glycosomes of flagellated trypanosomes to protect them from toxic accumulation of intermediates [40]. The glycosome model suggests that rather than allosteric events or feedback inhibition occurring if glycolysis is blocked downstream, compartmentalization keeps ATP from being made directly available to HK or to phosphofructokinase, thereby avoiding use of ATP in nonproductive glucose phosphorylation and an accumulation of hexose phosphates.
Additional studies will be necessary to determine if the results seen in this study are because LDHC is required for the integrity of a macromolecular complex. Nevertheless, LDHC has been conserved throughout evolution from marsupial to placental mammals, suggesting that its novel characteristics have conferred an advantage essential for the maintenance of glycolysis in mammalian sperm.
Supplementary Material
ACKNOWLEDGMENTS
We thank Drs. Christopher Geyer, Polina Danshina, and Huanghui Tang for critically reading this manuscript and for their constructive suggestions.
Footnotes
Supported in part by the Intramural Research Program of the National Institutes of Health (NIH), National Institute of Environmental Health Sciences (NIEHS) ES070076 (E.M.E.) and by NIH HD05863 (E.G.). A preliminary description of these studies was presented in a poster at the 42nd Annual Meeting of the Society for the Study of Reproduction, 18–22, July 2009, Pittsburgh, Pennsylvania.
REFERENCES
- Miki K. Energy metabolism and sperm function. Soc Reprod Fertil Suppl 2007; 65: 309 325 [PubMed] [Google Scholar]
- Miki K, Qu W, Goulding EH, Willis WD, Bunch DO, Strader LF, Perreault SD, Eddy EM, O'Brien DA. Glyceraldehyde 3-phosphate dehydrogenase-S, a sperm-specific glycolytic enzyme, is required for sperm motility and male fertility. Proc Natl Acad Sci U S A 2004; 101: 16501 16506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai C, Okuno M. Glycolysis plays a major role for adenosine triphosphate supplementation in mouse sperm flagellar movement. Biol Reprod 2004; 71: 540 547 [DOI] [PubMed] [Google Scholar]
- Williams AC, Ford WC. The role of glucose in supporting motility and capacitation in human spermatozoa. J Androl 2001; 22: 680 695 [PubMed] [Google Scholar]
- Travis AJ, Jorgez CJ, Merdiushev T, Jones BH, Dess DM, Diaz-Cueto L, Storey BT, Kopf GS, Moss SB. Functional relationships between capacitation-dependent cell signaling and compartmentalized metabolic pathways in murine spermatozoa. J Biol Chem 2001; 276: 7630 7636 [DOI] [PubMed] [Google Scholar]
- Urner F, Sakkas D. Protein phosphorylation in mammalian spermatozoa. Reproduction 2003; 125: 17 26 [DOI] [PubMed] [Google Scholar]
- Urner F, Leppens-Luisier G, Sakkas D. Protein tyrosine phosphorylation in sperm during gamete interaction in the mouse: the influence of glucose. Biol Reprod 2001; 64: 1350 1357 [DOI] [PubMed] [Google Scholar]
- Urner F, Sakkas D. Glucose participates in sperm-oocyte fusion in the mouse. Biol Reprod 1996; 55: 917 922 [DOI] [PubMed] [Google Scholar]
- Bone W, Jones NG, Kamp G, Yeung CH, Cooper TG. Effect of ornidazole on fertility of male rats: inhibition of a glycolysis-related motility pattern and zona binding required for fertilization in vitro. J Reprod Fertil 2000; 118: 127 135 [DOI] [PubMed] [Google Scholar]
- Goldberg E. Reproductive implications of LDH-C4 and other testis-specific isozymes. Exp Clin Immunogenet 1985; 2: 120 124 [PubMed] [Google Scholar]
- Coonrod S, Vitale A, Duan C, Bristol-Gould S, Herr J, Goldberg E. Testis-specific lactate dehydrogenase (LDH-C4); Ldh3) in murine oocytes and preimplantation embryos. J Androl 2006; 27: 502 509 [DOI] [PubMed] [Google Scholar]
- Li SS, O'Brien DA, Hou EW, Versola J, Rockett DL, Eddy EM. Differential activity and synthesis of lactate dehydrogenase isozymes A (muscle), B (heart) and C (testis) in mouse spermatogenic cells. Biol Reprod 1989; 40: 173 180 [DOI] [PubMed] [Google Scholar]
- Odet F, Duan C, Willis WD, Goulding EH, Kung A, Eddy EM, Goldberg E. Expression of the gene for mouse lactate dehydrogenase C (Ldhc) is required for male fertility. Biol Reprod 2008; 79: 26 32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krisfalusi M, Miki K, Magyar PL, O'Brien DA. Multiple glycolytic enzymes are tightly bound to the fibrous sheath of mouse spermatozoa. Biol Reprod 2006; 75: 270 278 [DOI] [PubMed] [Google Scholar]
- Lee MA, Storey BT. Bicarbonate is essential for fertilization of mouse eggs: mouse sperm require it to undergo the acrosome reaction. Biol Reprod 1986; 34: 349 356 [DOI] [PubMed] [Google Scholar]
- Cross NL, Morales P, Overstreet JW, Hanson FW. Simple methods for detecting acrosome-reacted human sperm. Gamete Res 1986; 15: 213 226 [Google Scholar]
- Bergmeyer HU. Determination of enzyme activities. : Bergmeyer HU. (ed.), Methods of Enzymatic Analysis, vol. 1. Deerfield Beach, FL: Verlag Chemie International; 1974: 121 131 [Google Scholar]
- McLean DJ, Jones LG, Jr, , Froman DP. Reduced glucose transport in sperm from roosters (Gallus domesticus) with heritable subfertility. Biol Reprod 1997; 57: 791 795 [DOI] [PubMed] [Google Scholar]
- Beyler SA, Wheat TE, Goldberg E. Binding of antibodies against antigenic domains of murine lactate dehydrogenase-C4 to human and mouse spermatozoa. Biol Reprod 1985; 32: 1201 1210 [DOI] [PubMed] [Google Scholar]
- Witters WL, Foley CW. Effect of selected inhibitors and methylene-blue on a possible phosphogluconate pathway in washed bull sperm. J Anim Sci 1976; 43: 159 163 [DOI] [PubMed] [Google Scholar]
- Madison LL, Lochner A, Wulff J. Diabetes Ethanol-induced hypoglycemia. II Mechanism of suppression of hepatic gluconeogenesis. 1967; 16: 252 258 [DOI] [PubMed] [Google Scholar]
- Marin S, Chiang K, Bassilian S, Lee WN, Boros LG, Fernandez-Novell JM, Centelles JJ, Medrano A, Rodriguez-Gil JE, Cascante M. Metabolic strategy of boar spermatozoa revealed by a metabolomics characterization. FEBS Lett 2003; 554: 342 346 [DOI] [PubMed] [Google Scholar]
- Markert CL. Lactate dehydrogenase: biochemistry and function of lactate dehydrogenase. Cell Biochem Funct 1984; 2: 131 134 [DOI] [PubMed] [Google Scholar]
- Svedruzic ZM, Spivey HO. Interaction between mammalian glyceraldehyde-3-phosphate dehydrogenase from heart and muscle. Proteins 2006; 63: 501 511 [DOI] [PubMed] [Google Scholar]
- Spivey HO. Evidence of NADH channeling between dehydrogenases. J Theor Biol 1991; 152: 103 107 [DOI] [PubMed] [Google Scholar]
- Spivey HO, Ovadi J. Substrate channeling. Methods 1999; 19: 306 321 [DOI] [PubMed] [Google Scholar]
- Westhoff D, Kamp G. Glyceraldehyde 3-phosphate dehydrogenase is bound to the fibrous sheath of mammalian spermatozoa. J Cell Sci 1997; 110: 1821 1829 [DOI] [PubMed] [Google Scholar]
- Ford WC. Glycolysis and sperm motility: does a spoonful of sugar help the flagellum go round? Hum Reprod Update 2006; 12: 269 274 [DOI] [PubMed] [Google Scholar]
- Battellino LJ, Blanco A. Catalytic properties of the lactate dehydrogenase isozyme “X” from mouse sperm. J Exp Zool 1970; 174: 173 186 [DOI] [PubMed] [Google Scholar]
- Guppy M, Hochachka PW. Role of dehydrogenase competition in metabolic regulation. The case of lactate and alpha-glycerophosphate dehydrogenases. J Biol Chem 1978; 253: 8465 8469 [PubMed] [Google Scholar]
- Rodic N, Oka M, Hamazaki T, Murawski MR, Jorgensen M, Maatouk DM, Resnick JL, Li E, Terada N. DNA methylation is required for silencing of Ant4, an adenine nucleotide translocase selectively expressed in mouse embryonic stem cells and germ cells. Stem Cells 2005; 23: 1314 1323 [DOI] [PubMed] [Google Scholar]
- Brower JV, Rodic N, Seki T, Jorgensen M, Fliess N, Yachnis AT, McCarrey JR, Oh SP, Terada N. Evolutionarily conserved mammalian adenine nucleotide translocase 4 is essential for spermatogenesis. J Biol Chem 2007; 282: 29658 29666 [DOI] [PubMed] [Google Scholar]
- Kim YH, Haidl G, Schaefer M, Egner U, Mandal A, Herr JC. Compartmentalization of a unique ADP/ATP carrier protein SFEC (Sperm Flagellar Energy Carrier, AAC4) with glycolytic enzymes in the fibrous sheath of the human sperm flagellar principal piece. Dev Biol 2007; 302: 463 476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori C, Nakamura N, Welch JE, Gotoh H, Goulding EH, Fujioka M, Eddy EM. Mouse spermatogenic cell-specific type 1 hexokinase (mHK1-s) transcripts are expressed by alternative splicing from the mHk1 gene and the HK1-S protein is localized mainly in the sperm tail. Mol Reprod Dev 1998; 49: 374 385 [DOI] [PubMed] [Google Scholar]
- Danshina PV, Geyer CB, Dai Q, Goulding EH, Willis WD, Kitto GB, McCarrey JR, Eddy EM, O'Brien DA. Phosphoglycerage kinase 2 (PGK2) is essential for sperm function and male fertility in mice. Biol Reprod 2010; 82: 136 145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V, Rangaraj N, Shivaji S. Activity of pyruvate dehydrogenase A (PDHA) in hamster spermatozoa correlates positively with hyperactivation and is associated with sperm capacitation. Biol Reprod 2006; 75: 767 777 [DOI] [PubMed] [Google Scholar]
- Gallerne C, Touat Z, Chen ZX, Martel C, Mayola E, Sharaf el dein O, Buron N, Le Bras M, Jacotot E, Borgne-Sanchez A, Lemoine A, Lemaire C, et al. The fourth isoform of the adenine nucleotide translocator inhibits mitochondrial apoptosis in cancer cells. Int J Biochem Cell Biol 2010; 42: 623 629 [DOI] [PubMed] [Google Scholar]
- Brower JV, Lim CH, Jorgensen M, Oh SP, Terada N. Adenine nucleotide translocase 4 deficiency to early meiotic arrest of murine male germ cells. Reproduction 2009; 138: 463 470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura N, Mori C, Eddy EM. Molecular complex of three testis-specific isozymes associated with the mouse sperm fibrous sheath: hexokinase 1, phosphofructokinase M, and glutathione S-transferase mu class 5. Biol Reprod 2010; 82: 504 515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haanstra JR, van Tuijl A, Kessler P, Reijnders W, Michels PA, Westerhoff HV, Parsons M, Bakker BM. Compartmentation prevents a lethal turbo-explosion of glycolysis in trypanosomes. Proc Natl Acad Sci U S A 2008; 105: 17718 17723 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.