Abstract
A LCMS method was developed and validated for the determination of buprenorphine (BUP), norbuprenorphine (NBUP), buprenorphine glucuronide (BUP-Gluc), and norbuprenorphine glucuronide (NBUP-Gluc) in placenta. Quantification was achieved by selected ion monitoring of m/z 468.4 (BUP), 414.3 (NBUP), 644.4 (BUP-Gluc), and 590 (NBUP-Gluc). BUP and NBUP were identified monitoring MS2 fragments m/z 396, 414 and 426 for BUP, and 340, 364 and 382 for NBUP, and glucuronide conjugates monitoring MS3 fragments m/z 396 and 414 for BUP-Gluc, and 340 and 382 for NBUP-Gluc. Linearity was 1–50 ng/g. Intra-day, inter-day and total assay imprecision (% RSD) were <13.4%, and analytical recoveries were 96.2–113.1%. Extraction efficiencies ranged from 40.7–68%, process efficiencies 38.8–70.5%, and matrix effect 1.3–15.4%. Limits of detection were 0.8 ng/g for all compounds. An authentic placenta from an opioid-dependent pregnant woman receiving BUP pharmacotherapy was analyzed. BUP was not detected but metabolite concentrations were NBUP-Gluc 46.6, NBUP 15.7 and BUP-Gluc 3.2 ng/g.
Keywords: Placenta, Buprenorphine, LCMS, Ion trap
Introduction
In 2002, BUP was approved in the United States for office-based treatment of opioid dependence under the Drug Treatment Act of 2000. This permits physicians to write prescriptions for schedule III, IV, and V narcotic medications approved specifically by the Food and Drug Administration for narcotic addiction treatment [1]. As the popularity of BUP increases for opioid dependence treatment, its use has been expanded to high-risk populations such as pregnant women in Europe [2, 3] and Australia [4]. In the US, methadone remains the only approved medication for the treatment of opioid dependence during gestation, although data are available comparing the safety and efficacy of methadone and BUP in this cohort [5]. During pregnancy, the woman, the fetus and the placenta comprise a complex physiological unit. Drugs can affect the fetus directly by crossing the placenta, and/or exerting indirect effects on the mother or placenta.
BUP is a semi-synthetic opioid derived from thebaine, and a highly lipid-soluble base similar in structure to morphine. BUP is well absorbed in the gastrointestinal tract, but has low oral bioavailability due to high first-pass hepatic metabolism. BUP is rapidly N-dealkylated mainly by CYP3A4 in the liver to NBUP [6]. Both compounds BUP and NBUP, are further metabolized by phase II glucuronidation.
Toxicological analysis of the placenta will enable us to better understand placental permeability to drugs and metabolites, and drug accumulation. Placental drug concentrations can be compared to concentrations in other maternal and fetal specimens, increasing our knowledge of fetal exposure and metabolism. BUP treatment during gestation provides a model of controlled drug administration in the maternal and neonatal dyad. If BUP and metabolites can be quantified in the placenta, we can determine if the magnitude of in utero exposure correlates with placenta drug concentrations.
Gas chromatography (GC) coupled to a variety of detectors has been employed for the qualitative and quantitative analysis of BUP and NBUP. Analytical methods for urine [7–13], plasma [8, 10, 14–18], oral fluid [19], hair [13, 20, 21], and feces [12] have been published. To date, enzyme or acidic hydrolysis of buprenorphine and norbuprenorphine glucuronides has been necessary prior to GC testing [7, 9]. Furthermore, derivatization also is usually required but may be difficult to reproduce reliably for NBUP [13]. Liquid chromatography tandem mass spectrometry (LCMSMS) has been applied for BUP and metabolites analysis in a wide variety of matrices including urine [22–32], plasma [17, 27, 33–38], whole blood [24, 27, 29, 39], hair [24, 27, 29, 40–43], sweat [44], meconium [45], and breast milk [46]. Only four articles describe the simultaneous determination of BUP, NBUP, and their glucuronides; one in plasma [37], two in urine [30, 32], and one in both matrices [26]. To our knowledge, this is the first assay for the analysis of BUP, NBUP, BUP-Gluc, and NBUP-Gluc in human placenta. The method will be employed to analyze placenta from pregnant women receiving BUP as pharmacotherapy for opioid dependence.
Experimental section
Chemicals and materials
BUP, NBUP, BUP-Gluc, NBUP-Gluc, buprenorphine-d4 (BUP-d4), and norbuprenorphine-d3 (NBUP-d3) standards and internal standards were obtained from Cerilliant (Austin, TX). Stock BUP-Gluc and NBUP-Gluc quality control (QC) solutions were purchased from ElSohly Laboratories (Oxford, MS). BUP and NBUP QC samples were prepared from a different lot of Cerilliant, when possible, or from a different vial, with preparation on different days than for calibrators. Reagent grade formic and perchloric acid were from Sigma Chemicals (St. Louis, MO) and Acros Organics (Morris Plains, NJ), respectively. All solvents were HPLC grade. Solid phase extraction (SPE) was performed with Strata-XC columns (60 mg/3 mL; Phenomenex, Torrance, CA). Anonymized drug-free placenta were confirmed negative for BUP and metabolites prior to use.
Instrumentation
LCMS analyses were performed on a Thermo Finnegan LCQ Deca XP Plus ion-trap mass spectrometer, with an electrospray ionization (ESI) source interfaced with a Surveyor autosampler and LC pump (Thermo Electron, San Jose, CA). Specimen homogenization was achieved with a Tissue Tearor (Biospec Products, INC., Bartlesville, OK). Solvent evaporation was carried out on a TurboVap LV evaporator from Zymark (Hopkinton, MA).
Preparation of standard solutions
Solutions containing 10 and 1μg/mL of BUP, NBUP, BUP-Gluc, and NBUP-Gluc were prepared separately in methanol from 100μg/mL stock calibrators. Different working solutions of the four analytes were prepared by appropriate dilution in methanol. The internal standard solution (IStd) at 1μg/mL of BUP-d4 and NBUP-d3 was prepared by dilution of 100μg/mL stock solutions in methanol. Quality control (QC) solutions containing BUP, NBUP, BUP-Gluc, and NBUP-Gluc were prepared in methanol at three different working concentrations across the linear range of the assay.
Calibrator and quality control sample preparation
A six-point calibration curve (1, 2.5, 5, 10, 25, and 50 ng/g) was prepared by adding 50μL of working calibrator and 50μL of IStd solution to 2±0.05 g of blank placenta cut into small pieces. In the same manner, low, medium, and high QC samples containing 1.75, 15 and 40 ng/g were prepared by adding 50μL of working QC solution and 50 μL of IStd solution to 2±0.05 g of blank placenta. After adding 6 mL of 0.1% perchloric acid in water, homogenization was performed for 0.5–1 min at 30,000 rpm followed by centrifugation at 4,000 rpm for 15 min. Supernatant was subjected to SPE.
Specimen preparation
Placenta (2±0.05 g) was weighed, cut into small pieces, and 6 mL of 0.1% perchloric acid and 50μL of IStd solution were added. Homogenization was performed for 0.5–1 min at 30,000 rpm. After centrifugation at 4,000 rpm for 15 min, the supernatant was separated into a clean test tube and subjected to SPE.
Extraction
SPE cartridges were preconditioned with 2 mL of methanol and 2 mL of 0.1% v/v perchloric acid in water. Specimen homogenate was applied followed by wash steps of 2 mL of 2% v/v formic acid in water and 2 mL of methanol. Cartridges were dried for 15 min under vacuum before eluting with 3 mL of methylenechloride:isopropanol:concentrated ammonium hydroxide (60:35:5, v/v/v). Extracts were dried completely under nitrogen at 45°C. Dried extracts were reconstituted with 100μL of a mixture of 85% A and 15% B, mobile phase A (0.1% formic acid in water) and B (0.1% formic acid in acetonitrile), and centrifuged at 1,000 rpm for 2 min. Supernatants were transferred to clean vials, and 20μL were injected into the LCMS.
Liquid chromatography
Chromatographic separation was achieved with a Synergi Polar-RP 80A (75×2 mm, 4μm) column with a 4×2 mm, identically packed guard column (Phenomenex, Torrance, CA) and gradient elution with mobile phase A (0.1% formic acid in water) and B (0.1% formic acid in acetonitrile). The initial mixture (85% A, %15 B), at a 200μL/min flow rate, was maintained for 2.5 min. At 2.51 min, the flow was increased to 300μL/min and B was increased from 15% to 65% over 7.5 min. From 10 to 12 min, B was increased again from 65% to 95%. Flow and mobile phase ratios were returned to initial conditions over 1 min, and the column re-equilibrated for 7 min, for a total run time of 20 min.
Mass spectrometry
Mass spectrometric data were acquired in positive ion mode with the following ESI-MS parameters: sheath gas flow 50; auxiliary gas flow 10; spray voltage 5 kV; and transfer capillary temperature 275°C. Precursor and product ion identification and MSn optimization were established by infusing 1μg/mL of each analyte in methanol directly. Two scan events were performed for each analyte for quantification and identification purposes. The quantification scan event was selected ion monitoring (SIM) of the precursor ion without fragmentation. Glucuronide conjugates were identified by performing MS3, and BUP and NBUP were identified via MS2. MS3 fragmentation was monitored in consecutive reaction monitoring (CRM) mode and MS2 in selected reaction monitoring (SRM) mode. Each free analyte was identified by the presence of four characteristic ions from MH+ fragmentation; BUP and NBUP were identified by the precursor ion and 3 fragment ions. For glucuronidated analytes, MH+ was fragmented, cleaving the glucuronide moiety; the surviving molecule was further fragmented, and two characteristic ions monitored. Table 1 displays monitored ions and collision energies.
Table 1.
LCMS parameters, retention times and corresponding internal standards for buprenorphine (BUP) and metabolites norbuprenorphine (NBUP), buprenorphine glucuronide (BUP-Gluc), and norbuprenorphine glucuronide (NBUP-Gluc)
| Analyte | SIMm/z | SRM Transition | CRM Transition | MS2 Collision energy (%) | MS3 Collision energy (%) | Retention time (min) | Internal standard |
|---|---|---|---|---|---|---|---|
| NBUP-Gluc | 590 | – | 590>414>340 | 29 | 32 | 2.6 | NBUP-d3 |
| 590>414>382 | |||||||
| BUP-Gluc | 644.4 | – | 644.4>468>396 | 31 | 36 | 8.6 | NBUP-d3 |
| 644.4>468>414 | |||||||
| NBUP | 414.4 | 414.4>340 | – | 33 | – | 8.9 | NBUP-d3 |
| 414.4>364 | |||||||
| 414.4>382 | |||||||
| NBUP-d3 | 417.3 | 417.3>343 | – | 33 | – | 8.9 | – |
| 417.3>399 | |||||||
| BUP | 468.4 | 468.4>396 | – | 36 | – | 10.8 | BUP-d4 |
| 468.4>414 | |||||||
| 468.4>426 | |||||||
| BUP-d4 | 472.4 | 472.4>400 | – | 36 | – | 10.8 | – |
| 472.4>415 |
SIM selected ion monitoring, SRM selected reaction monitoring, CRM consecutive reaction monitoring
Validation
Validation parameters included linearity, limits of detection (LOD) and quantification (LOQ), imprecision, analytical recovery, extraction efficiency, process efficiency, matrix effect, selectivity, carryover, dilution integrity and stability studies. Linearity was determined by least-squares regression with 1/x weighting. Acceptable linearity was achieved when the coefficient of determination was at least 0.99 and calibrators quantified within ±20% at the LOQ and ±15% at other concentrations. LOD and LOQ were evaluated with decreasing analyte concentrations in drug-fortified placenta. LOD was defined as the lowest concentration with acceptable chromatography, presence of all qualifier ions with signal-to-noise ratios of at least 3, and a retention time (RT) within ±0.2 min of the average calibrator RT. LOQ was the lowest concentration that met LOD criteria and a signal-to-noise ratio of at least 10, imprecision lower than 20%, and analytical recovery between 80–120%.
Imprecision and analytical recovery were determined at three concentrations (1.75, 15, and 40 ng/g) by preparing and analyzing five replicates on four different days (n=20). Imprecision, expressed as % relative standard deviation (% RSD) of the measured values, was expected to be less than 15% except for the low QC, for which 20% was acceptable. The guidelines given by Krouwer and Rabinowitz [47] were followed for the calculation of pooled intra-day, inter-day, and total imprecision. Analytical recovery was evaluated as the percent of target concentration (n=20) that had to be within 85–115%, except for the low QC, for which 80% to 120% was acceptable.
Extraction efficiency for each analyte was measured at each QC concentration. Blank placenta was fortified with QC and internal standard solution before and after SPE. Percent extraction efficiency from placenta was expressed as mean analyte area of samples (n=5) fortified with control solution before extraction divided by mean area of samples (n=5) with control solution added after SPE. Matrix effect was assessed by comparing analyte peak areas in ten unique blank extracted placentas fortified with QC and internal standard solutions after SPE to peak areas of samples at the same nominal concentrations prepared in an 85:15 mixture of mobile phase A and mobile phase B (neat). Matrix suppression or enhancement was calculated as follows: (100×mean peak area of fortified placentas after SPE/mean peak area of neat)–100. Process efficiency examined the overall effect of SPE extraction efficiency and matrix effect on the quantification of analytes of interest. It was determined by comparing mean analyte peak areas of five samples fortified before SPE with mean peak areas of five neat samples prepared in mobile phase at the same concentration.
Interferences from endogenous matrix components were evaluated by analyzing placenta samples from 10 healthy non-drug-consuming subjects only fortified with IStd solution. Endogenous interferences were considered insignificant if analytes were not detected in these 10 placentas. Method specificity was demonstrated by adding high concentrations (500 ng/g) of potentially interfering licit and illicit drugs to low QC samples. The following drugs and metabolites were examined: cocaine, benzoylecgonine, norcocaine, norbenzoylecgonine, ecgonine ethyl ester, ecgonine methyl ester, anhydroecgonine methyl ester, ecgonine, methadone, EMDP, EDDP, methadol, Δ9-tetrahydrocannabinol (THC), 11-hydroxy-THC, 11-nor-9-carboxy-THC, morphine, normorphine, morphine 3-β-glucuronide, morphine 6-β-glucuronide, codeine, norcodeine, 6-acetylmorphine, 6-acetylcodeine, diazepam, lorazepam, oxazepam, alprazolam, imipramine, clomipramine, fluoxetine, norfluoxetine, clonidine, ibuprofen, pentazocine, caffeine, diphenhydramine, chlorpheniramine, brompheniramine, acetylsalicylic acid, acetaminophen, and phencyclidine. In addition, placenta specimens from nine opioid-dependent women maintained on methadone were fortified with BUP and metabolites at low QC concentrations, and analyzed. Many of these women relapsed to heroin and cocaine use during methadone treatment, providing the opportunity to test specificity with drugs and metabolites in authentic specimens. Sufficient specificity was achieved if BUP, NBUP, BUP-Gluc and NBUP-Gluc quantified within ±20% of low QC concentrations.
Lack of carryover was demonstrated by injecting IStd-fortified blank placenta immediately after a sample spiked at 100 ng/g of all analytes or two times the upper LOQ. Carryover was considered negligible if the analyte was not detected in the blank placenta or if the measured concentration was below the LOD. Dilution integrity was evaluated by diluting placenta samples (n=2) containing 160 ng/g of each analyte with blank placenta to achieve a 1:4 dilution. Internal standard was added to diluted samples that were extracted as described. Dilution integrity was maintained if samples quantified within ±20% of 40 ng/g.
Analyte stability was investigated under a variety of conditions. Drug stability in the extract stored on the autosampler at 10°C for 48 and 72 h was determined by duplicate QC samples at low, medium, and high concentrations. In addition, stability was tested for drug-fortified placenta stored at room temperature (22°C) for 16 h, in the refrigerator (4°C) for 72 h, and after three freeze–thaw cycles. Stability was considered acceptable if QC samples quantified within ±20% of target.
Identification criteria
The identification criteria were RT within ±0.2 min of the average calibrator RT, presence of the precursor ion, and presence of three MS2 products for BUP and NBUP, or presence of two MS3 products for BUP-Gluc and NBUP-Gluc.
Proof of method
In order to prove method applicability, an authentic placenta from an opioid-dependent pregnant woman under controlled BUP treatment was analyzed. The specimen was collected as part of an Institutional-Review-Board-approved clinical study on treatment of opioid dependence during gestation, with written informed consent provided by participants.
Results and discussion
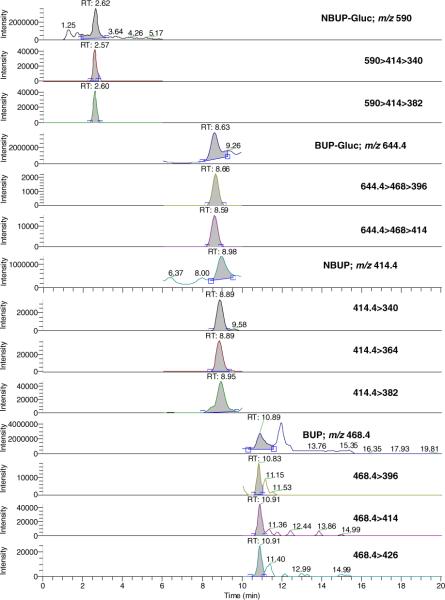
The linearity of analyte-to-IStd peak ratio versus theoretical concentration was verified in placenta using 1/x-weighted linear regression. Curvature was tested on a set of four calibration curves and determination coefficients (r2) were above 0.99 (BUP intercept = 0.063±0.037, slope=0.039±0.001, r2=0.998±0.002; NBUP intercept=0.037±0.019, slope=0.041±0.002, r2=0.997±0.003; BUP-Gluc intercept = 0.085 ± 0.074, slope=0.129±0.007, r2=0.994±0.003; NBUP-Gluc intercept = 0.066±0.096, slope=0.041±0.005, r2=0.995±0.003), with residuals within ±20% at the LOQ and ±15% at other calibrator concentrations. The linear calibration range including six concentrations was 1–50 ng/g with 0.8 ng/g LOD for all compounds. Figure 1 shows a chromatogram of a placenta sample fortified at the low QC concentration. Quantification was performed with the parent ion because with the use of transition ions, the coefficient of determination was less than 0.99 and residuals were higher than ±20% at LOQ and greater than ±15% at other concentrations. Similar accuracy in quantification was reported for BUP and metabolites with an ion-trap mass spectrometer [29], albeit in another matrix utilizing our approach, while other investigators employed transition ions [28, 30, 37, 45], also in other matrices. In these cases, a coefficient of determination of 0.98 and residuals within ±20% in the entire calibration range were accepted [45], or no information about the value of each calibrator against the curve was reported [28, 30, 37].
Fig. 1.
LCMS and LCMSMS chromatograms of a placenta sample spiked at 1.75 ng/g (low quality control) buprenorphine (BUP) and metabolites norbuprenorphine (NBUP), buprenorphine glucuronide (BUP-Gluc), and norbuprenorphine glucuronide (NBUP-Gluc)
Imprecision and analytical recovery were satisfactory for all tested concentrations (Table 2). Extraction efficiencies ranged from 40.7% to 68%; the lowest were for BUP and NBUP (40.8–55.6%) and the highest for glucuronides (63.6–68%). No significant matrix effect was detected for any compound. Matrix effect, suppression or enhancement, ranged from 1.3 to 15.4% with variation between 10 different placentas <16.7%. Process efficiencies ranged from 38.8 to 70.5%. As for extraction efficiencies, the lowest values were obtained for BUP and NBUP (38.8–46.8%), and the highest values were for BUP-Gluc and NBUP-Gluc (54.5–70.5%). Process and extraction efficiency showed similar values because no significant matrix effect was detected. All these data are shown in Table 3.
Table 2.
Imprecision and analytical recovery for buprenorphine (BUP) and metabolites norbuprenorphine (NBUP), buprenorphine glucuronide (BUP-Gluc), and norbuprenorphine glucuronide (NBUP-Gluc) in human placenta by LCMS
| Analyte | Concentration (ng/g) | Total mean (n=20, ng/g) | Imprecision (n=20, %RSD) |
Analytical recovery (n=20, % of target) | ||
|---|---|---|---|---|---|---|
| Pooled intra-day | Inter-day | Total | ||||
| BUP | 1.75 | 1.7 | 10.1 | 5.7 | 11.6 | 98.5 |
| 15 | 16.2 | 7 | 0 | 7 | 108 | |
| 40 | 38.5 | 7.2 | 6.9 | 10 | 96.2 | |
| NBUP | 1.75 | 1.8 | 9.6 | 4.2 | 10.5 | 105 |
| 15 | 15.6 | 8.9 | 4.5 | 9.9 | 104.1 | |
| 40 | 39.3 | 8.6 | 3.3 | 9.2 | 98.1 | |
| BUP-Gluc | 1.75 | 1.8 | 6.3 | 9.4 | 11.3 | 105.6 |
| 15 | 16.9 | 5 | 0 | 5 | 113.1 | |
| 40 | 42.6 | 8.7 | 4.2 | 9.7 | 107.9 | |
| NBUP-Gluc | 1.75 | 1.7 | 8.9 | 10 | 13.4 | 99 |
| 15 | 16.3 | 7.9 | 0 | 7.9 | 108.9 | |
| 40 | 43 | 8.2 | 0 | 8.2 | 107.5 | |
Table 3.
Extraction efficiency, process efficiency and matrix effect for buprenorphine (BUP) and metabolites norbuprenorphine (NBUP), buprenorphine glucuronide (BUP-Gluc), and norbuprenorphine glucuronide (NBUP-Gluc) in human placenta by LCMS
| Analyte | Concentration (ng/g) | Extraction efficiency (%, n=5) | Process efficiency (%, n=5) | Matrix effect (n=10) |
|
|---|---|---|---|---|---|
| Effect (%) | % RSD | ||||
| BUP | 1.75 | 55.6 | 45.6 | 3.9 | 6.7 |
| 15 | 40.8 | 38.8 | 1.3 | 10.8 | |
| 40 | 47.9 | 43.2 | 2.1 | 16 | |
| NBUP | 1.75 | 47.6 | 46.3 | 8 | 9.6 |
| 15 | 45.9 | 46.8 | 10.4 | 6.7 | |
| 40 | 46.5 | 45.6 | −1.7 | 9.3 | |
| BUP-Gluc | 1.75 | 63.6 | 68.7 | 15.3 | 15.3 |
| 15 | 64 | 62 | −0.5 | 5.5 | |
| 40 | 66.5 | 64.4 | 4.6 | 8.9 | |
| NBUP-Gluc | 1.75 | 66.2 | 70.5 | 11 | 11.7 |
| 15 | 65.7 | 55.6 | −14 | 13.1 | |
| 40 | 68 | 54.5 | −15.4 | 16.7 | |
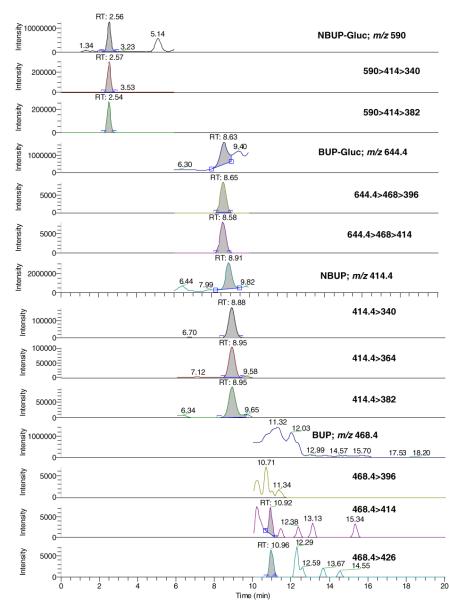
Under the described conditions, there was no interference with any extractable endogenous compound in placenta (Figure 2). Method selectivity was demonstrated by adding high concentrations (500 ng/g) of 41 potentially interfering licit and illicit drugs to low QC samples. All test samples quantified within ±20% of target, indicating no interference with the four analytes of interest. In addition, nine placentas from women under methadone treatment were also fortified at the low QC, and all samples quantified within ±20%. No analyte was detected in a blank sample injected immediately following analysis of a 100 ng/g sample, indicating no carryover at this concentration. The ability of the method to accurately quantify specimens containing high concentrations of analytes was evaluated by diluting 160 ng/g samples (n=2) with blank placenta; 1.5 g of blank placenta was added to 0.5 g of fortified sample in order to achieve a 1:4 dilution. Samples quantified within 18.2% of 40 ng/g, confirming dilution integrity.
Fig. 2.
LCMS and LCMSMS chromatograms of a blank placenta
No significant loss or deterioration was observed for any analyte when samples were stored on the autosampler for 48 or 72 h; low QC quantified within ±20%, and medium and high QC within ±15% of target. Table 4 summarizes stability data of analytes in fortified placenta, reported as the percent difference between mean fresh control concentrations and mean concentrations following storage. Analytes were stable under all storage conditions with percent difference from fresh controls in absolute values ranging from 0.02 to 18.7%.
Table 4.
The stability of buprenorphine (BUP) and metabolites norbuprenorphine (NBUP), buprenorphine glucuronide (BUP-Gluc), and norbuprenor-phine glucuronide (NBUP-Gluc) in human placenta under diffrent storage conditions
| Compound | Fresh (n=3) | RT (n=3) |
4C (n=3) |
F/T (n=3) |
|||
|---|---|---|---|---|---|---|---|
| Mean (ng/g) | Mean (ng/g) | Diff % | Mean (ng/g) | Diff % | Mean (ng/g) | Diff % | |
| BUP | 1.8 | 2 | 8.2 | 1.9 | 5.4 | 2 | 8.1 |
| 16.9 | 17.6 | 3.7 | 16.4 | −3.2 | 16.5 | −2.7 | |
| 42.2 | 42.4 | 0.7 | 42.4 | 0.6 | 41.2 | −2.3 | |
| NBUP | 1.5 | 1.6 | 3.3 | 1.7 | 15.2 | 1.5 | 1.3 |
| 16.1 | 13.7 | −14.9 | 15.4 | −4.4 | 16.2 | 0.7 | |
| 40.2 | 34.9 | −13.1 | 39.8 | −0.9 | 36.4 | −9.4 | |
| BUP-Gluc | 2 | 2 | 2.7 | 1.9 | −3.4 | 1.8 | −8 |
| 17.8 | 17.3 | −3 | 16.8 | −5.7 | 17.5 | −2.1 | |
| 45.1 | 46.3 | 2.5 | 44 | −2.6 | 41.5 | −8 | |
| NBUP-Gluc | 1.9 | 1.9 | 3 | 1.8 | −7.6 | 1.7 | −12.8 |
| 17.6 | 15.3 | −13.2 | 14.4 | −18.1 | 15.3 | −12.9 | |
| 43.2 | 43.2 | 0 | 40.8 | −5.6 | 35.1 | −18.7 | |
RT(room temperature);4C (4°);F/T (three freeze/thaw cycles);Diff %(percentage difference from fresh controls)
Besides chromatographic retention time, identification criteria included the presence of 1 precursor and 3 MS2 fragments for BUP and NBUP, and one precursor and two MS3 fragments for BUP-Gluc and NBUP-Gluc (Table 1), respectively, yielding 6.5 identification points [48]. According to the EU Official Agency a minimum of three identification points should be required [48].
This validated method was employed in the analysis of a placenta specimen from a woman treated with up to 18 mg/day BUP (mean daily dose 12.3 mg/day) for 151 days. The cumulative dose over this period was 1,850 mg. Interestingly, BUP was not detected, but metabolite concentrations were NBUP-Gluc 46.6, NBUP 15.7, and BUP-Gluc 3.2 ng/g (Figure 3).
Fig. 3.
LCMS and LCMSMS chromatograms of an authentic placenta positive for norbuprenorphine (15.7 ng/g), buprenorphine glucuronide (3.2 ng/g) and norbuprenorphine glucuronide (46.6 ng/g). No buprenorphine was detected. The cumulative dose over 151 days treatment period was 1,850 mg
Conclusion
For the first time, a novel LCMS method simultaneously quantifies BUP and its main metabolites' concentrations in human placenta with good selectivity and sensitivity. This method will be employed to analyze placentas collected in a clinical study investigating BUP as pharmacotherapy for opioid dependence during pregnancy.
Acknowledgments
This research was supported by the National Institutes of Health, Intramural Research Program, National Institute on Drug Abuse, and funding for Marta Concheiro, Ph.D. from the Ministerio de Educación y Ciencia of Spain (Grant number EX-2007-1194). The authors would like to thank Drs. Fred Askin, Gerrun March, and Leigh Ruane from Johns Hopkins Bayview Medical Center (Baltimore, MD) for their help in providing placenta samples.
References
- 1.Drug Addiction Treatment Act of 2000. 2000. p. 111. STAT. 1101. [Google Scholar]
- 2.Lejeune C, Simmat-Durand L, Gourarier L, et al. Drug Alcohol Depend. 2006;82:250–257. doi: 10.1016/j.drugalcdep.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Fischer G, Johnson RE, Eder H, et al. Addiction. 2000;95(2):239–244. doi: 10.1046/j.1360-0443.2000.95223910.x. [DOI] [PubMed] [Google Scholar]
- 4.Dunlop AJ, Panjari M, O'Sullivan H, et al. Clinical guidelines for the use of buprenorphine in pregnancy. Turning Point Alcohol and Drug Centre; Fitzroy: 2003. [Google Scholar]
- 5.Jones HE, Johnson RE, Jasinski DR, et al. Drug Alcohol Depend. 2005;79:1–10. doi: 10.1016/j.drugalcdep.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi K, Tamamoto T, Chiba K, et al. Drug Metab Dispos. 1998;26:818–821. [PubMed] [Google Scholar]
- 7.Bottcher M, Beck O. J Anal Toxicol. 2005;29:769–76. doi: 10.1093/jat/29.8.769. [DOI] [PubMed] [Google Scholar]
- 8.Blom Y, Bondesson U. J Chromatogr. 1985;338:89–98. doi: 10.1016/0378-4347(85)80073-6. [DOI] [PubMed] [Google Scholar]
- 9.George S, George C, Chauhan M. Forensic Sci Int. 2004;143:121–125. doi: 10.1016/j.forsciint.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 10.Lange WR, Fudala PJ, Dax EM, et al. Drug Alcohol Depend. 1990;26:19–28. doi: 10.1016/0376-8716(90)90078-s. [DOI] [PubMed] [Google Scholar]
- 11.Lisi AM, Kazlauskas R, Trout GJ. J Chromatogr B Biomed Sci Appl. 1997;692:67–77. doi: 10.1016/s0378-4347(96)00496-3. [DOI] [PubMed] [Google Scholar]
- 12.Cone EJ, Gorodetzky CW, Yousefnejad D, et al. J Chromatogr. 1985;337:291–300. doi: 10.1016/0378-4347(85)80042-6. [DOI] [PubMed] [Google Scholar]
- 13.Vincent F, Bessard J, Vacheron J, et al. J Anal Toxicol. 1999;23:270–279. doi: 10.1093/jat/23.4.270. [DOI] [PubMed] [Google Scholar]
- 14.Ohtani M, Shibuya F, Kotaki H, et al. J Chromatogr. 1989;487:469–475. doi: 10.1016/s0378-4347(00)83057-1. [DOI] [PubMed] [Google Scholar]
- 15.Kuhlman JJ, Magluilo J, Cone E, et al. J Anal Toxicol. 1996;20:229–235. doi: 10.1093/jat/20.4.229. [DOI] [PubMed] [Google Scholar]
- 16.Everhart ET, Cheung P, Shwonek P, et al. Clin Chem. 1997;43:2292–2302. [PubMed] [Google Scholar]
- 17.Moody DE, Laycock JD, Spanbauer AC, et al. J Anal Toxicol. 1997;21:406–411. doi: 10.1093/jat/21.6.406. [DOI] [PubMed] [Google Scholar]
- 18.Tiong GKL, Olley JE. Naunyn-Schmiedeberg's Arch Pharmacol. 1988;338:202–206. doi: 10.1007/BF00174871. [DOI] [PubMed] [Google Scholar]
- 19.Gunnar T, Ariniemi K, Lillsunde P. J Mass Spectrom. 2005;40:739–753. doi: 10.1002/jms.846. [DOI] [PubMed] [Google Scholar]
- 20.De Giovanni N, Fucci N, Scarlata S, et al. Clinical Chemistry and Laboratory Medicine. 2005;43:1377–1379. doi: 10.1515/CCLM.2005.235. [DOI] [PubMed] [Google Scholar]
- 21.Vinner E, Vignau J, Thibault D, et al. Forensic Science International. 2003;133:57–62. doi: 10.1016/s0379-0738(03)00050-1. [DOI] [PubMed] [Google Scholar]
- 22.Hegstad S, Khiabani HZ, Oiestad EL, et al. J Anal Toxicol. 2007;31:214–219. doi: 10.1093/jat/31.4.214. [DOI] [PubMed] [Google Scholar]
- 23.Kronstrand R, Selden TG, Josefsson M. J Anal Toxicol. 2003;27:464–70. doi: 10.1093/jat/27.7.464. [DOI] [PubMed] [Google Scholar]
- 24.Cirimele V, Etienne S, Villain M, et al. Forensic Sci Int. 2004;143:153–156. doi: 10.1016/j.forsciint.2004.02.035. [DOI] [PubMed] [Google Scholar]
- 25.Cirimele V, Kintz P, Lohner S, et al. J Anal Toxicol. 2003;27:103–105. doi: 10.1093/jat/27.2.103. [DOI] [PubMed] [Google Scholar]
- 26.Huang W, Moody DE, McCance-Katz EF. Ther Drug Monit. 2006;28:245–251. doi: 10.1097/01.ftd.0000197094.92559.b4. [DOI] [PubMed] [Google Scholar]
- 27.Tracqui A, Kintz P, Mangin P. Journal of Forensic Sciences. 1997;42(1):111–114. [PubMed] [Google Scholar]
- 28.Miller EI, Torrance HJ, Oliver JS. J Anal Toxicol. 2006;30:115–119. doi: 10.1093/jat/30.2.115. [DOI] [PubMed] [Google Scholar]
- 29.Favretto D, Frison G, Vogliardi S, et al. Rapid Communications in Mass Spectrometry. 2006;20:1257–1265. doi: 10.1002/rcm.2444. [DOI] [PubMed] [Google Scholar]
- 30.Liu AC, Lin TY, Su LW, et al. Talanta. 2008;75:198–204. doi: 10.1016/j.talanta.2007.10.050. [DOI] [PubMed] [Google Scholar]
- 30.Hull MJ, Bierer MF, Griggs DA, et al. J Anal Toxicol. 2008;32:516–521. doi: 10.1093/jat/32.7.516. [DOI] [PubMed] [Google Scholar]
- 32.Kacinko S, Concheiro-Guisan M, Shakleya D, et al. Anal Bioanal Chem. 2008;392:903–911. doi: 10.1007/s00216-008-2326-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodriguez-Rosas ME, Lofwall MR, Strain EC, et al. Journal of Chromatography B. Analytical Technologies in the Biomedical and Life Sciences. 2007;850:538–543. doi: 10.1016/j.jchromb.2006.11.025. [DOI] [PubMed] [Google Scholar]
- 34.Musshoff F, Trafkowski J, Kuepper U, et al. J Mass Spectrom. 2006;41:633–640. doi: 10.1002/jms.1021. [DOI] [PubMed] [Google Scholar]
- 35.Ceccato A, Klinkenberg R, Hubert P, et al. J Pharm Biomed Anal. 2003;32:619–631. doi: 10.1016/s0731-7085(03)00169-9. [DOI] [PubMed] [Google Scholar]
- 36.Polettini A, Huestis MA. J Chromatogr B Biomed Sci Appl. 2001;754:447–459. doi: 10.1016/s0378-4347(01)00029-9. [DOI] [PubMed] [Google Scholar]
- 37.Murphy CM, Huestis MA. J Mass Spectrom. 2005;40:70–74. doi: 10.1002/jms.776. [DOI] [PubMed] [Google Scholar]
- 38.Scislowski M, Piekoszewski W, Kamenczak A, et al. J Anal Toxicol. 2005;29:249–253. doi: 10.1093/jat/29.4.249. [DOI] [PubMed] [Google Scholar]
- 39.Hoja H, Marquet P, Verneuil B, et al. J Anal Toxicol. 1997;21:160–165. doi: 10.1093/jat/21.2.160. [DOI] [PubMed] [Google Scholar]
- 40.Wilkins DG, Rollins DE, Valdez AS, et al. J Anal Toxicol. 1999;23:409–415. doi: 10.1093/jat/23.6.409. [DOI] [PubMed] [Google Scholar]
- 41.Kintz P. J Anal Toxicol. 1993;17:443–444. doi: 10.1093/jat/17.7.443. [DOI] [PubMed] [Google Scholar]
- 42.Goodwin RS, Wilkins DG, Averin O, et al. Clin Chem. 2007;53:2136–2143. doi: 10.1373/clinchem.2007.091413. [DOI] [PubMed] [Google Scholar]
- 43.Thieme D, Sachs H, Thevis M. J Mass Spectrom. 2008;43:974–979. doi: 10.1002/jms.1433. [DOI] [PubMed] [Google Scholar]
- 44.Kintz P, Tracqui A, Mangin P, et al. J Anal Toxicol. 1996;20:393–397. doi: 10.1093/jat/20.6.393. [DOI] [PubMed] [Google Scholar]
- 45.Kacinko SL, Shakleya DM, Huestis MA. Analytical Chemistry. 2008;80:246–252. doi: 10.1021/ac701627q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grimm D, Pauly E, Poschl J, et al. Ther Drug Monit. 2005;27:526–530. doi: 10.1097/01.ftd.0000164612.83932.be. [DOI] [PubMed] [Google Scholar]
- 47.Krouwer JS, Rabinowitz R. Clin Chem. 1984;30:290–292. [PubMed] [Google Scholar]
- 48.European Union Decision Off J Eur Commun. 2002;221:8–36. 2002/657/EC (17/8/2002) [Google Scholar]