Abstract
The labyrinth of the rodent placenta contains villi that are the site of nutrient exchange between mother and fetus. They are covered by three trophoblast cell types that separate the maternal blood sinusoids from fetal capillaries – a single mononuclear cell that is a subtype of trophoblast giant cell (sinusoidal or S-TGC) with endocrine function and two multinucleated syncytiotrophoblast layers, each resulting from cell-cell fusion, that function in nutrient transport. The developmental origins of these cell types have not previously been elucidated. We report here the discovery of cell-layer-restricted genes in the mid-gestation labyrinth (E12.5-14.5) including Ctsq in S-TGCs (also Hand1-positive), Syna in syncytiotrophoblast layer I (SynT-I), and Gcm1, Cebpa and Synb in syncytiotrophoblast layer II (SynT-II). These genes were also expressed in distinct layers in the chorion as early as E8.5, prior to villous formation. Specifically, Hand1 was expressed in apical cells lining maternal blood spaces (Ctsq is not expressed until E12.5), Syna in a layer immediately below, and Gcm1, Cebpa and Synb in basal cells in contact with the allantois. Cebpa and Synb were co-expressed with Gcm1 and were reduced in Gcm1 mutants. By contrast, Hand1 and Syna expression was unaltered in Gcm1 mutants, suggesting that Gcm1-positive cells are not required for the induction of the other chorion layers. These data indicate that the three differentiated trophoblast cell types in the labyrinth arise from distinct and autonomous precursors in the chorion that are patterned before morphogenesis begins.
Keywords: Chorion, Gcm1, Labyrinth, Placenta, Syncytin, Syncytiotrophoblast, Trophoblast, Mouse
INTRODUCTION
The murine placenta is composed of three main structures. The outermost compartment in direct contact with the maternal decidua contains invading trophoblast giant cells and glycogen trophoblast cells. The middle compartment is called the spongiotrophoblast. The inner compartment is termed the labyrinth, named for the tortuous network of maternal and fetal blood spaces, and is the site of nutrient, gas and waste exchange between the fetal and maternal blood supplies. The labyrinth contains both chorion (trophoblast)-derived epithelial cells and allantois (extraembryonic mesoderm)-derived vascular cells. Formation of villi, which initiates labyrinth development, begins on embryonic day (E) 7.5 when expression of the Gcm1 gene appears in small clusters of cells in the extraembryonic ectoderm (chorion) (Basyuk et al., 1999), a flat plate of trophoblast cells that otherwise contains multipotent trophoblast stem (TS) cells (Uy et al., 2002). The occlusion of the ectoplacental cavity around E8.0 then brings cells from the bottom of the ectoplacental cone in contact with the distal side of the chorion, which results in the downregulation of the TS cell markers Cdx2, Eomes and Esrrb within the chorionic trophoblast (Beck et al., 1995; Hancock et al., 1999; Pettersson et al., 1996) and a loss of TS cell potential from chorionic ectoderm (Uy et al., 2002). Shortly after ectoplacental cavity occlusion, the allantois, which grows out from the posterior end of the embryo, makes contact with and attaches to the basal side of the chorion (reviewed by Hemberger and Cross, 2001; Rossant and Cross, 2001). Thereafter, folding of the chorion into simple branches initiates at sites of Gcm1 expression (Anson-Cartwright et al., 2000). Allantoic mesoderm with its associated blood vessels fills into the epithelial branches (Cross, 2000). Gcm1 expression is subsequently confined to the tips of elongating branches (Cross et al., 2006), the site where syncytiotrophoblast differentiation is thought to begin (Hernandez-Verdun, 1974). Continued branching morphogenesis ultimately produces a complex villous structure providing the large surface area that is required for nutrient exchange to support fetal growth past E10.5. The loss of Gcm1 results in failure to initiate branching morphogenesis following chorioallantoic attachment (Anson-Cartwright et al., 2000; Schreiber et al., 2000).
The maternal and fetal blood spaces within the labyrinth are separated by three layers of trophoblast cells (tri-chorial) and by a layer of fetal endothelial cells (Enders, 1965; Hernandez-Verdun, 1974). The trilaminar trophoblast includes a single layer of mononuclear sinusoidal trophoblast giant cells (S-TGCs) that line the maternal blood sinusoids (Coan et al., 2005; Simmons and Cross, 2005; Simmons et al., 2007), and two layers of syncytiotrophoblast, SynT-I and -II, the latter of which is in contact with fetal endothelial cells (Simmons and Cross, 2005; Watson and Cross, 2005). The S-TGCs are secretory in nature, expressing hormones such as placental lactogen II (Campbell et al., 1989; Dai et al., 2000; Deb et al., 1991; Ishida et al., 2004; Lee et al., 2003; Sahgal et al., 2000; Simmons and Cross, 2005; Simmons et al., 2007), and are therefore likely to have a primary endocrine function. S-TGCs are loosely attached to the underlying syncytial layers via desmosomal adhesions and contain fenestrations to allow the SynT-I cells direct access to maternal blood (Coan et al., 2005; Davies and Glasser, 1968; Hernandez-Verdun, 1974). cathepsin Q (Ctsq) has recently been shown to be an exclusive marker of these cells in the mature labyrinth (Simmons et al., 2007). The syncytiotrophoblast cell layers are multinucleated, forming as a result of trophoblast cell-cell fusion, are very thin and function in nutrient transport (Enders, 1965; Hernandez-Verdun, 1974; Jollie, 1964; Snell and Stevens, 1966). The two syncytiotrophoblast layers are tightly adhered to one another through tight junctions, are clearly different in their cellular composition as observed by electron microscopy, and are situated on basement membranes overlying the fetal capillary endothelium (Coan et al., 2005; Davies and Glasser, 1968; Enders, 1965; Hernandez-Verdun, 1974; Jollie, 1964). In addition to the trophoblast cells separating the maternal and fetal blood spaces, histological examination of the mid-gestation labyrinth also reveals abundant ‘pillars’ of spongiotrophoblast that extend inwards, as well as clusters of tightly packed cuboidal cells that resemble the morphology of chorion trophoblast cells at earlier stages. These latter cells are hypothesized to be labyrinth progenitors, but there is little data to directly support this idea.
Mouse mutants have provided significant insights into the details of placental formation, especially into the mechanisms of chorioallantoic attachment (reviewed by Watson and Cross, 2005) and initiation of branching morphogenesis and syncytiotrophoblast differentiation [Gcm1 mutants (Anson-Cartwright et al., 2000; Schreiber et al., 2000)]. However, little molecular detail is known about how the trilaminar trophoblast structure is formed, despite a large number of mutants that manifest labyrinth phenotypes. A major problem to date has been the difficulty in discerning the three trophoblast cell layers at the light microscopy level and the lack of markers that distinguish them. We have previously observed that Gcm1 is not uniformly expressed in all syncytiotrophoblast cells at E14.5, and expression appears to be closer to fetal blood spaces than to maternal blood spaces, suggesting localization to the SynT-II cells (Cross et al., 2006). In this study, we expanded the search for layer-specific markers and used them as tools to define the developmental origins of the three differentiated trophoblast cell layers in the mature labyrinth. Based on our findings, we propose a model for how early patterning and cell specification within the flat chorion lay down the progenitors for the trilaminar trophoblast structure of the labyrinth.
MATERIALS AND METHODS
Animals and tissue preparation
CD1 mice (Charles River) were used for wild-type expression studies. Gcm1 heterozygous mice (Anson-Cartwright et al., 2000) on a CD1 background were crossed to obtain mutant Gcm1−/− conceptuses. Cebpa+/−;Cebpb+/−double heterozygotes (Begay et al., 2004) were crossed to obtain Cebpa−/−, Cebpb−/− and Cebpa−/−;Cebpb−/− double-knockout embryos. Implantation sites were dissected at E6.5 through E14.5, with noon of the day of the vaginal plug defined as E0.5. Genotyping was performed as previously described. Animals were housed under normal light conditions (12 hours light/12 hours dark) with free access to food and water. All animal procedures were carried out in accordance with the University of Calgary Animal Care Committee. For Cebpa;Cebpb mutant mice, all procedures were carried out in accordance with Max Delbrueck Center for Molecular Medicine animal facility standards.
Histology
For histology, whole uteri (E6.5-7.5), isolated implantation sites (E8.5-10.5), or whole dissected placentas (E14.5) were fixed overnight at 4°C in 4% paraformaldehyde (PFA), processed through a sucrose gradient and embedded in OCT compound (Sakura Finetek, Torrence, CA) for preparation of frozen sections. For ultrathin resin histology, implantation sites were fixed overnight in 4% PFA/0.2% glutaraldehyde and embedded in JB-4 epoxy resin according to the manufacturer’s instructions (Electron Microscopy Sciences, Hatfield, PA). Sections (2 μm) were then cut using glass knives on a Leica RM2265 microtome and stained with Tissue Epoxy Stain (Electron Microscopy Sciences) according to the manufacturer’s instructions.
Probes and plasmids
The cDNA probe for Gcm1 has been described (Basyuk et al., 1999). The following cDNAs were generously provided: Esx1 (Dr Richard Behringer, University of Texas, M. D. Anderson Cancer Center, Houston, TX), Dlx3 (Dr Kathleen Mahon, Baylor College of Medicine, Houston, TX) and Nr6a1 (Dr Austin Cooney, Baylor College of Medicine, Houston, TX). cDNA probes for Cebpa, Syna and Synb were generated by RT-PCR using the following primers: Cebpa forward, 5′-CGCTGGTGATCAAACAAGAG-3′ and reverse, 5′-GTCACTGGTCAACTCCAGCA-3′; Syna forward, 5′-TTGCAATCACACCTTTCAGC-3′ and reverse, 5′-TGGTGTCCA-CAGACAGGGTA-3′; Synb forward, 5′-CTTTCCACCACCCATACGTT-3′ and reverse, 5′-TGACCTTGAAGTGGGTAGGG-3′. Amplicons were cloned into pGEM-T easy (Promega, Madison, WI) and verified by sequencing.
In situ hybridization
Frozen sections (8–10 μm) were adhered to Super Frost Plus (VWR International, West Chester, PA) slides and stored at −80°C until used. In situ hybridization was carried out as described (Simmons et al., 2007) with some modifications. Briefly, digoxigenin (DIG) and fluorescein cRNA probes were generated from plasmids according to the manufacturer’s instructions (Roche, Laval, Quebec, Canada). Sections were rehydrated in PBS, post-fixed in 4% PFA, treated with proteinase K (15 μg/ml for 5 minutes at room temperature), acetylated for 10 minutes (acetic anhydride, 0.25%; Sigma Aldrich, Oakville, Ontario, Canada) and hybridized with DIG-labeled probes overnight at 65°C (for double ISH, fluorescein-labeled probes were also added at this stage). Hybridization buffer contained 1× salts (200 mM NaCl, 13 mM Tris, 5 mM sodium phosphate monobasic, 5 mM sodium phosphate dibasic, 5 mM EDTA), 50% formamide, 10% (w/v) dextran sulfate, 1 mg/ml yeast tRNA (Sigma Aldrich), 1× Denhardt’s [1% (w/v) bovine serum albumin, 1% (w/v) Ficoll, 1% (w/v) polyvinylpyrrolidone], and cRNA probe (final dilution of 1:2000 from reaction with 1 μg template DNA). Post-hybridization washes were followed by an RNase treatment [400 mM NaCl, 10 mM Tris (pH 7.5), 5 mM EDTA, 20 μg/ml RNase A]. After blocking, sections were incubated overnight in blocking solution containing anti-DIG antibody (Sigma Aldrich) at 1:2500 dilution. Color was developed using NBT/BCIP according to the manufacturer’s instructions (Promega). For double in situ hybridizations, the anti-DIG antibody conjugated to alkaline phosphatase was inactivated at 65°C in maleic acid buffer for 30 minutes, followed by 30 minutes in 0.1 M glycine (pH 2.2). Sections were blocked again for 1 hour and incubated overnight with anti-fluorescein antibody (1:2500; Roche) at 4°C. After washing, color was developed using INT/BCIP (Roche) until a brown precipitate was visible. In some cases, slides were counterstained with Nuclear Fast Red. For single NBT/BCIP in situ hybridization, slides were dehydrated and cleared in xylene and mounted in Cytoseal Mounting Medium (VWR International, West Chester, PA). For in situ hybridizations containing brown INT/BCIP precipitate (xylene and alcohol soluble), sections were mounted first under Crystal Mount Aqueous Mounting Medium (Sigma Aldrich) to form a barrier, then coverslipped using the xylene-based Cytoseal Mounting Medium. Photographs were taken promptly before fading of the INT/BCIP precipitate occurred.
Trophoblast stem cell cultures
Wild-type trophoblast stem (TS) cells (Rs26 line) were provided by Dr J. Rossant (Tanaka et al., 1998). Gcm1−/− and wild-type TS cell lines were derived from blastocysts isolated from Gcm1+/− intercrosses as previously described (Tanaka et al., 1998). TS cells were cultured and processed as described (Simmons et al., 2007).
Northern blot analysis
Total RNA from TS cell cultures was isolated using QIAshredder and RNeasy columns (Qiagen, Mississauga, Ontario, Canada) following the manufacturer’s instructions. Ten μg of total RNA was separated on a 1.1% formaldehyde agarose gel, blotted onto GeneScreen nylon membrane (Perkin Elmer, Shelton, CT) and UV cross-linked. Random-primed DNA labeling of cDNA probes was carried out with 25 μCi [32P]dCTP and probes were isolated on Sephadex G-50 columns (Amersham Biosciences, Baie d’Urfe, Quebec, Canada). Hybridizations were performed at 60°C overnight in hybridization buffer as described (Church and Gilbert, 1984). Following post-hybridization washes, signals were detected by exposure to BioMax MR film (Kodak, New Haven, CT) at −80°C.
RESULTS
The Syna, Synb, Cebpa and Gcm1 genes distinguish the two syncytiotrophoblast cell layers
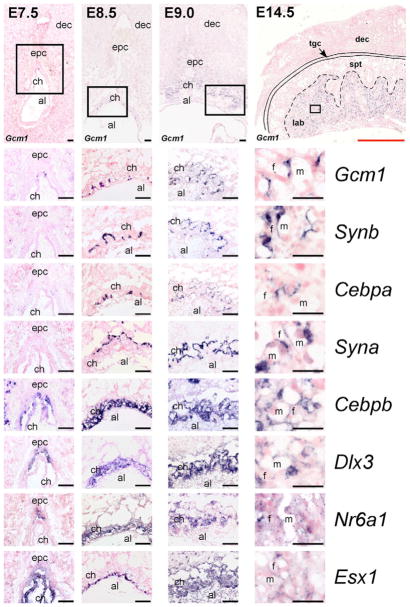
We have previously observed that the Gcm1 gene is not uniformly expressed in the labyrinth and, upon closer examination, that expression is likely to be limited to the SynT-II cell layer specifically (Cross et al., 2006). In order to identify other markers that might distinguish the three differentiated trophoblast cell types in the labyrinth, we investigated the expression of genes encoding Dlx3 (Dlx3), Esx1 (Esx1), Gcnf (Nr6a1), C/EBPα (Cebpa) and the murine endogenous retrovirus envelope proteins syncytin A (Syna) and syncytin B (Synb), all of which have all been used as markers for labyrinth trophoblast (Fig. 1). Expression of both Dlx3 and Nr6a1 was predominantly detected within the ectoplacental cone at E7.5, broadly in the chorion at E8.5, although signal was somewhat stronger in the upper chorion trophoblast cells, and thereafter was broadly in the labyrinth without indication of layer-specificity (Fig. 1). Esx1 was detectable within the chorion at E7.5 and the basal chorion at E8.5, but then more broadly (Fig. 1). Cebpb was detectable in both ectoplacental cone and chorion cells as well as within the decidua at E7.5, throughout the chorion at E8.5 and more broadly throughout trophoblast cell subtypes, such as spongiotrophoblast and labyrinth trophoblast layers, later in gestation (E12.5 and thereafter). Within the developing labyrinth, Cebpb expression was similar to that of Dlx3, Nr6a1 and Esx1 (Fig. 1). In contrast to these genes, expression of Syna, Synb and Cebpa was not detectable at E7.5. However, their expression appeared starting by ~E8.5. We detected both Cebpa and Synb expression in a pattern similar to Gcm1, confined to clusters of trophoblast cells at the chorioallantoic interface and in some cases in cells at the initial branch points, suggesting they are expressed in the same cells as Gcm1 (Fig. 1). By E9.0 and 14.5, Gcm1, Cebpa and Synb were detected in elongated trophoblast cells adjacent to the fetal endothelial cells, consistent with SynT-II expression. By contrast, Syna was detectable at E8.5 in cells at the apical side of the chorion, closer to the maternal blood sinusoids and by E14.5 in cells closer to the maternal blood sinusoids and yet not in S-TGCs (Fig. 1).
Fig. 1. Expression of mouse labyrinth trophoblast markers from E7.5-14.5.
In situ hybridizations for the labyrinth trophoblast markers Gcm1, Synb, Cebpa, Syna, Cebpb, Dlx3, Nr6a1 and Esx1 at E7.5, E8.5, E9.0 and E14.5. Low-magnification images (top row) are of Gcm1 expression. Black boxes indicate the areas shown at high-magnification in the panels beneath. al, allantois; ch, chorion; dec, decidua; epc, ectoplacental cone; f, fetal blood space; lab, labyrinth; m, maternal blood space; spt, spongiotrophoblast; tgc, trophoblast giant cell. Scale bars: black, 100 μm; red, 1.4 mm.
Double in situ hybridization analysis for Syna/Synb as well as for Syna/Gcm1 and Syna/Cebpa (Fig. 2 and data not shown) at E14.5 indicated that these genes show non-overlapping, trophoblast subtype-specific expression. Specifically, Syna expression (Fig. 2B, brown arrow) appeared restricted to SynT-I cells (Fig. 2B, black arrow), whereas expression of Synb (Fig. 2B, purple arrow), Gcm1 and Cebpa (data not shown) was restricted to SynT-II cells. Because clear demarcation of the trilaminar trophoblast subtypes of the labyrinth is difficult at the light microscopy level, double in situ hybridization was also performed using Synb and Ctsq, a marker of S-TGCs (Simmons et al., 2007), to ascertain whether unstained areas indicative of SynT-I cells could be detected between the positive signals for these two genes. Indeed, a clear unstained cell layer was observed between Ctsq+ S-TGCs and Synb+ SynT-II cells (Fig. 2C, black arrow), in the position of cells that express Syna (Fig. 2B, brown arrow).
Fig. 2. Gcm1, Synb and Cebpa are expressed in SynT-II cells and Syna is expressed in SynT-I cells.
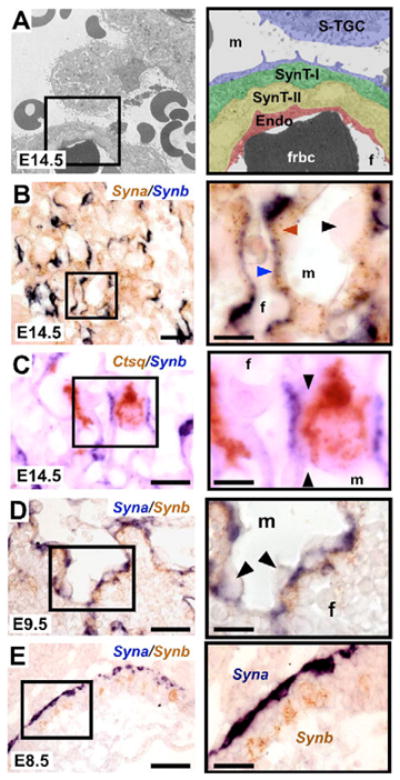
(A) Electron micrograph of the trilaminar trophoblast structure that separates the maternal and fetal blood compartments. The black box indicates the area shown at high-magnification on the right. S-TGC, sinusoidal trophoblast giant cell; SynT-I, syncytiotrophoblast layer I; SynT-II, syncytiotrophoblast layer II; Endo, fetal endothelial cell; m, maternal blood space; f, fetal blood space; frbc, fetal red blood cell. (B) Double in situ hybridization for Syna (brown) and Synb (purple) in the labyrinth at E14.5 indicating that Syna expression is restricted to SynT-I cells and Synb expression is restricted to SynT-II cells. Note that Syna/Gcm1 and Syna/Cebpa double in situ hybridizations produced similar results (data not shown). Black arrowhead indicates unstained S-TGC which lines maternal blood spaces. Brown arrowhead indicates Syna-positive SynT-I cells located closer to maternal blood spaces. Blue arrowhead indicates Synb-positive SynT-II cells located closest to fetal blood spaces. (C) Double in situ hybridization for Ctsq (brown) and Synb (purple) in the labyrinth at E14.5. Black arrows indicate unstained SynT-I cell between Ctsq-positive S-TGC and Synb-positive SynT-II cell. (D) Double in situ hybridization for Syna (purple) and Synb (brown) in the labyrinth at E9.5. Syna and Synb expression do not co-localize. Note that Syna/Gcm1 and Syna/Cebpa double in situ hybridizations showed similar results (data not shown). (E) Double in situ hybridization for Syna (purple) and Synb (brown) in chorion at E8.5. Syna and Synb expression do not co-localize at E8.5 and demarcate distinct trophoblast populations within the chorion. Note that Syna/Gcm1 and Syna/Cebpa double in situ hybridizations showed similar results (data not shown). Scale bars: 100 μm (left); 50 μm (right).
The E8.5 chorion shows patterns of Syna-, Gcm1/Synb/Cebpa- and Hand1-expressing cells
In addition to the exclusive patterns at E14.5, Syna and Gcm1/Synb/Cebpa expression patterns were also non-overlapping at earlier stages of development (Fig. 2D,E and data not shown). Gcm1, Synb and Cebpa expression was co-localized in clusters of cells at the basal side of the chorion (Fig. 3A) and in SynT-II cells of the labyrinth (Fig. 1, Fig. 2B–E and data not shown). By contrast, Syna expression was detected initially in cells at the top of the chorion overlying Gcm1/Cebpa/Synb+ cells and was later restricted to SynT-I cells (Fig. 1, Fig. 2B–E). Cells lining the maternal sinusoids, which appear directly above the chorion at E8.5, are lined by cells that presumably become the S-TGC layer. Although S-TGCs express Ctsq (Simmons et al., 2007), this marker is restricted to S-TGCs of the mature labyrinth (after E12.5). S-TGCs in the mature labyrinth express Hand1 (Simmons et al., 2007) and we found that trophoblast cells facing the maternal sinusoids at E8.5 also express Hand1 (Fig. 3A). Hand1 is expressed in several different trophoblast cell subtypes including all TGC subtypes, ectoplacental cone and both apical and basal cells in the chorion. The apical chorion cells were Pl1 (Prl3d1)-negative, indicating that they are not simply parietal TGCs, but are more likely to be early S-TGCs. Importantly, Syna+ cells were almost always separated from maternal sinusoids by at least one layer of cells, predominately Hand1+ (Fig. 3B), although some Syna+ cells without an overlying Hand1+ cell layer were rarely observed. The orientation of Hand1+/Pl1− cells lining the maternal sinusoids, of Syna+ cells along the apical side of the chorion and of Gcm1/Cebpa/Synb+ cells at the leading edge of the E8.5 chorion coincides with the subsequent organization of the trilaminar trophoblast layer in the mature placenta. Electron microscopy (Hernandez-Verdun, 1974) or even histology on ultrathin plastic resin sections (Fig. 3C) also reveals unique populations of chorionic trophoblast (with different morphology) that correspond to the three future layers of the trilaminar structure.
Fig. 3. Early patterning of the mouse chorion.
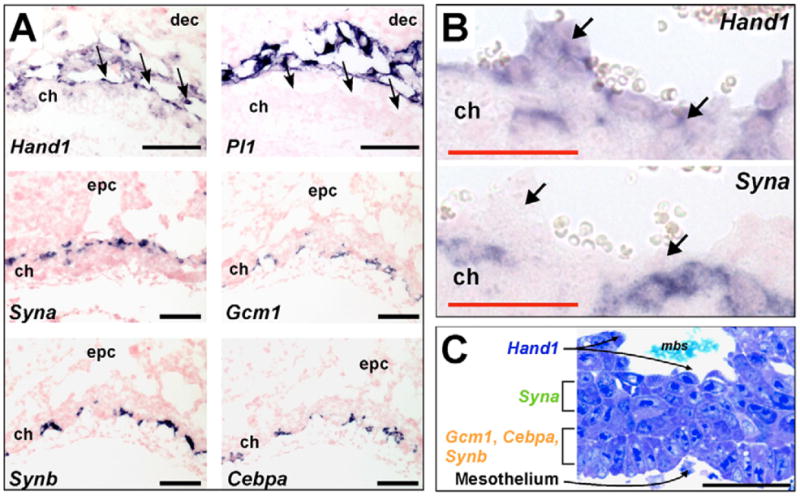
(A) In situ hybridizations for Hand1 and Pl1 at E9.0 indicate that Hand1+/Pl1− cells line the maternal blood sinuses located on the apical side of the chorion. At E8.5, Syna expression is restricted to cells near the top of the chorion, whereas Gcm1, Synb and Cebpa staining (on serial sections) indicates co-localization within a subset of trophoblast located on the basal side of the chorion (confirmed by double in situ hybridization, data not shown). The black arrows indicate Hand1+/Pl1− cells lining maternal sinusoids. (B) Higher magnification images of Hand1 and Syna in situ hybridizations on E8.5 chorion. Note that Syna-positive cells are separated from the maternal blood sinuses by a single layer of cells, typically Hand1-positive. Black arrows indicate the cell layer lining the maternal sinusoids. (C) Ultrathin epoxy section of E8.5 chorion (2 μm) stained with Tissue Epoxy Stain (EMS). Note the unique morphology of the different layers of trophoblast cells in the chorion. Cells that line the maternal sinusoids (Hand1+/Pl1− at E8.5) are positive for Ctsq expression by E12.5 (data not shown). Scale bars: black, 100 μm; red, 50 μm.
Gcm1 regulates Synb and Cebpa but not Syna
Because expression of Gcm1, Cebpa and Synb were co-localized in both the chorion and subsequently in SynT-II cells, we examined the expression of these genes in Gcm1 mutant placentas. Neither Cebpa nor Synb expression was detected at E8.5 (data not shown) or E9.5 (Fig. 4) in Gcm1 mutants, indicating that both genes are downstream of Gcm1. Some limited Cebpa expression was seen in a few Gcm1 mutant placentas by E9.5, although expression was both much reduced and atypically localized (data not shown). The induction of Syna expression was unaffected in Gcm1 mutants, consistent with expression in a different chorionic trophoblast population and no obvious interdependence of these cell layers. However, the morphology and/or organization of Syna+ cells in Gcm1 mutants resembled that of wild-type chorions at earlier developmental stages, consistent with the block to branching morphogenesis known to occur in Gcm1 mutants (Anson-Cartwright et al., 2000; Schreiber et al., 2000). Cebpa;Cebpb compound mutants manifest a similar phenotype to Gcm1 mutants, although some initial branching morphogenesis can occasionally be seen (Begay et al., 2004). Neither Synb nor Gcm1 expression was altered in Cebpa mutants, Cebpb mutants or Cebpa;Cebpb compound mutants (Fig. 4). Therefore, Gcm1 is an upstream regulator of both Cebpa and Synb, but Cebpa (or Cebpb) is not an upstream regulator of Synb.
Fig. 4. Synb and Cebpa are downstream of Gcm1, but Synb is not downstream of Cebpa.
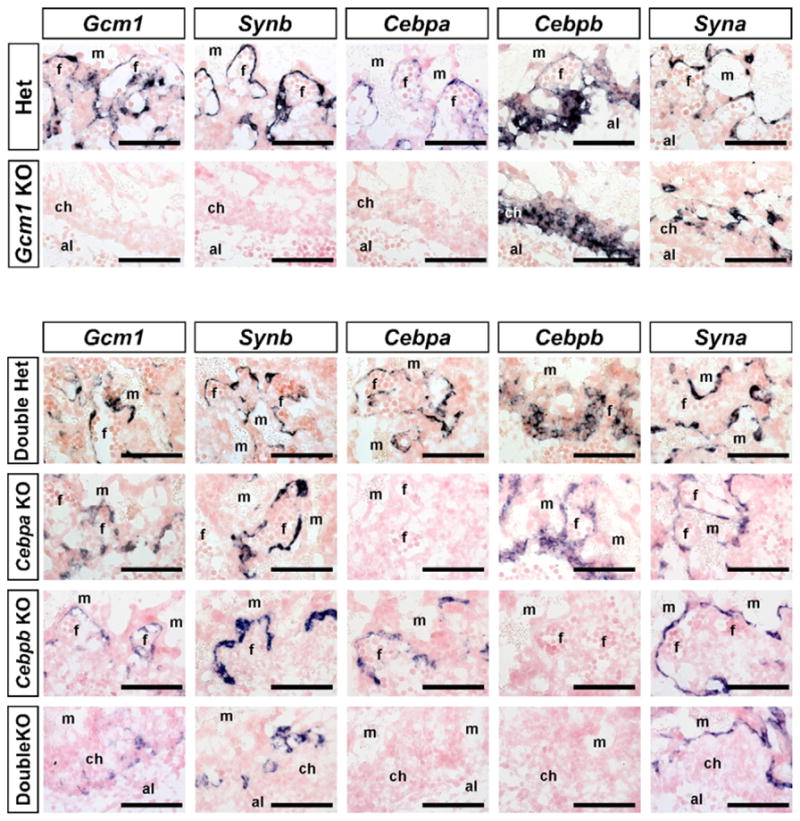
In situ hybridizations for Gcm1, Synb, Cebpa, Cebpb and Syna on 10 μm sections of E9.5 Gcm1+/− (Het) and Gcm1−/− (Gcm1 KO) mouse placentas and Cebpa+/−;Cebpb+/− (Double Het), Cebpa−/− (Cebpa KO), Cebpb−/− (Cebpb KO) and Cebpa−/−;Cebpb−/− (DoubleKO) placentas. al, allantois; ch, chorion; f, fetal blood space; m, maternal blood space. Scale bars: 100 μm.
Induction and specification of SynT-I precursor cells does not require interaction with SynT-II cells
Whereas Gcm1 expression begins at ~E7.5, expression of Synb and Cebpa was only evident closer to E8.5 (data not shown), consistent with their being downstream of Gcm1. Syna expression, by contrast, was not always detectable in our E8.5 samples, which was likely to result from variation in when mating occurred versus the consistency in sampling times (data not shown). This indicates that Syna expression begins at ~E8.5 or slightly after, clearly after Gcm1, Cebpa and Synb. This raises the possibility that specification of SynT-I and S-TGC precursors within the chorion might require interaction with, or signals from, SynT-II precursors (Gcm1/Cebpa/Synb+ cells). Earlier studies of labyrinth morphogenesis by electron microscopy indicated that SynT-I formation, the fusion of trophoblast cells into the first syncytial layer, was dependent upon interaction with SynT-II cells, now known to be Gcm1/Cebpa/Synb+ (Hernandez-Verdun, 1974). In addition, there is no evidence of trophoblast cell-cell fusion in Gcm1 mutants (Anson-Cartwright et al., 2000). However, gene expression studies in Gcm1 mutants clearly show Hand1+ S-TGC precursors and Syna+ SynT-I precursors in the absence of Gcm1+ cells or branching morphogenesis (Fig. 5). In addition, differentiating Gcm1−/− TS cell cultures also demonstrate the ability to form Syna+ and Ctsq+ cells. Contrary to what we observed in situ, some Cebpa and Synb expression could be detected in cultured Gcm1−/− TS cells, although the expression patterns were significantly altered over the course of differentiation as compared with wild-type TS cells, suggesting that Gcm1 does have a role in regulating Synb and Cepba in vitro, albeit not as the sole regulator (Fig. 5)
Fig. 5. Gcm1/Synb/Cebpa-expressing cells are not required to induce or maintain Syna or Hand1 expression.
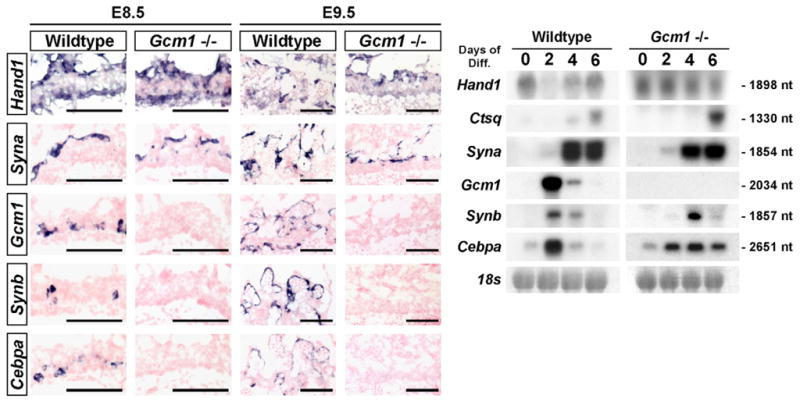
(Left) In situ hybridizations for Hand1, Syna, Gcm1, Cebpa and Synb were performed on E8.5 and E9.5 wild-type and Gcm1−/− mouse placentas. (Right) Northern blot analysis of Hand1, Ctsq, Syna, Gcm1, Cebpa and Synb expression in differentiating TS cultures following the removal of human recombinant FGF4, heparin and embryonic fibroblast-conditioned media. 18s rRNA was used as a loading control.
DISCUSSION
It has long been recognized that the maternal-fetal interface in the labyrinth of rodents is hemotrichorial, meaning that maternal blood passes through sinusoidal spaces lined by trophoblast cells rather than endothelial cells, and that three layers of trophoblast cells separate it from fetal blood vessels (Davies and Glasser, 1968; Enders, 1965; Hernandez-Verdun, 1974; Jollie, 1964). However, very little progress has been made in understanding the developmental origins of the three cell layers. There were several key unanswered questions. (1) How early in development do the three cell layers become distinguishable from the multipotent TS cells of the chorion/extraembryonic ectoderm? (2) Do the three cell types have a common progenitor and, if so, is it simply TS cells of the chorion/extraembryonic ectoderm that are present up to at least the early post-implantation stages (Tanaka et al., 1998; Uy et al., 2002)? (3) How do the two layers of syncytiotrophoblast form so that they remain separate from each other during cell-cell fusion within their respective layers? This is of interest because other hemochorial placental animals, including humans and guinea pigs, contain only one layer of syncytiotrophoblast. (4) What is the sequence of differentiation events and do the cell layers develop autonomously? Our studies here have identified several genes that are differentially expressed in the three trophoblast cell layers and have helped to lead us to answers to these questions.
The expression of several genes has been shown to be localized to the labyrinth including Gcm1 (Anson-Cartwright et al., 2000; Basyuk et al., 1999), Dlx3 (Berghorn et al., 2005; Morasso et al., 1999), Nr6a1 (Morasso et al., 1999; Schreiber et al., 2000; Susens et al., 1997), Tead3 (Anson-Cartwright et al., 2000; Jacquemin et al., 1998), Tcfeb (Steingrimsson et al., 1998; Tompers et al., 2005; Voss et al., 2000), Esx1 (Li et al., 1997; Morasso et al., 1999; Voss et al., 2000), Hand1 (Scott et al., 2000; Simmons et al., 2007) and Syna and Synb (Dupressoir et al., 2005). Of these genes, some show layer-specific expression that has allowed insight into the early developmental origins of the three cell types (see summary in Fig. 6). Specifically, Hand1 is expressed in S-TGCs (Simmons et al., 2007), Syna is restricted to SynT-I cells, and Gcm1, Cebpa and Synb are all expressed in SynT-II cells in the mature labyrinth. These genes begin their expression in the chorion at ~E8.5 and, even from the earliest detection, their patterns are non-overlapping. Specifically, at E8.5, Hand1 is expressed in cells at the apical side of the chorion facing the maternal sinuses; Syna is expressed in cells just below the Hand1-positive cells; and Gcm1/Cebpa/Synb are expressed in clusters of cells on the basal side of the chorion at the interface with the allantois. These molecular data are supported by electron microscopy (Hernandez-Verdun, 1974) and ultrathin epoxy resin histology (this study) that reveal distinct cellular morphologies of the different layers of the chorion at E8.5, well before morphogenesis and syncytial fusion begin. The presence of these chorion cell populations suggests that the chorion is already patterned into distinct cell types by E8.5, establishing the precursors of the S-TGC, SynT-I and SynT-II cell layers in the mature labyrinth.
Fig. 6. Model for mouse labyrinth formation from a pre-patterned chorion.
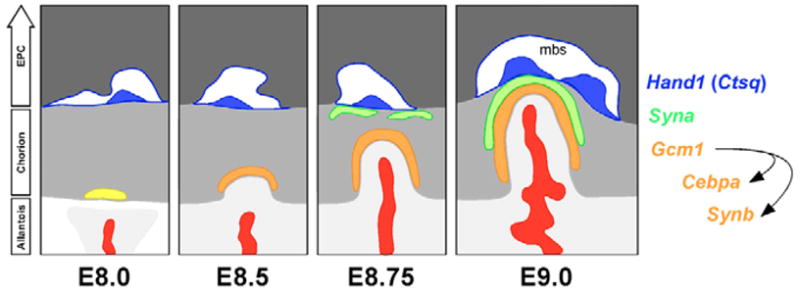
Note that Ctsq expression within S-TGCs is not fully evident until E12.5. No exclusive marker has been identified for S-TGC precursors, although cells lining the early maternal sinusoids are Hand1+/Pl1−. However, Hand1 expression is not restricted to cells lining the maternal sinusoids in the way that Ctsq is restricted to S-TGCs in mid-gestation; Hand1 is expressed in all TGC subtypes and within the ectoplacental cone and chorion (not shown). It is the combined Hand1+/Pl1− nature of the cells lining the maternal sinusoids, together with their location, that implies they are S-TGC progenitors. For simplicity, cell bodies of Hand1+/Pl1− cells are shown only along the top of the chorion. Light gray, allantois; gray, chorion; dark gray, ectoplacental cone (EPC); red, extraembryonic mesoderm-derivatives; yellow, Gcm1+ chorionic trophoblast cells; orange, Gcm1/Synb/Cebpa+ chorionic trophoblast cells; green, Syna+ trophoblast cells; blue, developing sinusoidal giant cells (S-TGCs) which will subsequently express Ctsq; mbs, maternal blood sinus.
The patterns for the layer-specific genes imply that the three differentiated cell types have distinct precursors. Gcm1 is the only one of the marker genes that is expressed prior to E8.5, but it is unlikely to be a marker of a common progenitor because Gcm1-expressing cells are post-mitotic in the chorion at E8.5 (Cross et al., 2006) and ectopic Gcm1 expression in TS cells promotes cell cycle exit (Hughes et al., 2004). It has been hypothesized that the basal-most layer of the chorion at E8.5 is derived from the extraembryonic ectoderm at E7.5, whereas the upper layers of the chorion originate from the basal ectoplacental cone based on similarity of cell ultrastructure and reaction to various fixatives apparent by electron microscopy (Hernandez-Verdun, 1974). These layers come together after the collapse of the ectoplacental cavity around E8.0-8.5. The expression patterns of Dlx3, Nr6a1 and Esx1 also attest to the divergent developmental origins of cells at the apical and basal sides of the E8.5 chorion. At E7.5, Dlx3 and Nr6a1 are expressed in ectoplacental cone cells, whereas Esx1 is expressed in the chorion, and by E8.5 they have distinct apical (Dlx3 and Nr6a1) and basal (Esx1) bias.
Consistent with the lack of a common precursor cell type in the chorion, the ability to derive TS cell lines from the chorion declines after ~E8.5 (Uy et al., 2002). However, it is clear that there must be significant ongoing cell contribution to labyrinth growth because the number of trophoblast cells in the mature labyrinth is likely to exceed the number of cells in the chorion at E8.5. In addition, cell proliferation does occur in the chorion (Cross et al., 2006). The Syna- and Gcm1/Cebpa/Synb-expressing cell layers at E8.5 are separated by several layers of cells that do not express any of the marker genes. These cells could represent a population of fusion-competent trophoblast cells, perhaps differentially expressing the receptors for the syncytins, and/or be a reserve of still-proliferating progenitors (either multipotent or layer-restricted). Proliferative cells are known to be located closer to the chorioallantoic interface, adjacent to Gcm1-positive cells (Cross et al., 2006). A possible marker of these cells is Rhox4b (Ehox), a gene expressed in extraembryonic ectoderm early in development and later in clusters of proliferating trophoblast preferentially located near the chorionic plate (A. Davies, D.R.C.N., D.G.S., E. Mariusdottir, J. G. Matyas and J.C.C., unpublished). The Syna-negative Gcm1/Cebpa/Synb-negative cells in the E8.5 chorion are small, tightly packed and cuboidal. Cells with this same morphology do persist into mid-gestation in clusters throughout the labyrinth. Interestingly, even as early as E9, they are no longer present between the Syna-positive and the Gcm1/Cebpa/Synb-positive cell layers. This might simply be the result of the invaginating cell shape change and movements of the Gcm1-positive cells (Cross et al., 2006) that push up towards the apical side of the chorion (Hernandez-Verdun, 1974).
An explanation for how the two layers of syncytiotrophoblast cells could form next to each other and remain distinct, despite each undergoing cell-cell fusion, is apparent given the fact that Syna and Synb show exclusive, non-overlapping expression. Syncytin A and B are murine endogenous retrovirus envelope proteins that can promote cell-cell fusion in transfected cell cultures. Importantly, they do not promote fusion in all cell types and indeed they have fusogenic activity in different cell lines (Dupressoir et al., 2005). These data indicate that they utilize different receptors and/or cell machinery to promote cell-cell fusion. Differential use of the fusogenic proteins within each syncytial layer is a possible way to ensure the formation of two independent syncytial layers. For this model to work, the different Syncytin receptors may or may not have unique localization patterns.
Our data now outline a fairly comprehensive view of the sequence of events leading to differentiation of the three trophoblast cell layers in the labyrinth (Fig. 6). Events start with the onset of expression of Gcm1 in clusters of cells in the extraembryonic ectoderm at ~E7.5 that persists in the basal chorion at E8.5, when Cebpa and Synb expression appears in the same cells. The temporal pattern as well as the analysis of Gcm1 and Cebpa;Cebpb compound-mutant placentas indicate that Gcm1 induces Cebpa and Synb. Although C/EBPα is a transcription factor, it is not required for Synb expression and therefore its target genes are unclear. It is still unclear whether Gcm1 regulates the Cebpa and Synb genes directly or indirectly. However, Gcm1 is a transcriptional activator (Akiyama et al., 1996; Chang et al., 2005; Schreiber et al., 1997; Schubert et al., 2004) and both Cebpa and Synb contain Gcm1 binding sites within their regulatory regions. Within 10 kb upstream of the Cebpa transcriptional start site, there are seven predicted Gcm1 binding sites at −9256, −7558, −6335, −5842, −5275, −5231 and −1861, whereas within the same region upstream of Synb, there are eight at −6496, −3178, −2656, −2634, −1864, −1767, −866 and −769. It is important to note that although expression of Cebpa and Synb was undetectable in Gcm1 mutants at E8.5, there was a small number of Cebpa-positive cells by E9.5 and the genes were also detectable in Gcm1 mutant TS cells, albeit in abnormal patterns. This suggests that Gcm1 is not essential for Cebpa and Synb transcription per se, but clearly is for their full expression and in the correct pattern.
Only after the establishment of the Gcm1/Cebpa/Synb pattern in the basal chorion, does Syna expression become detectable in the apical chorion, immediately below the Hand1-expressing cells that line the maternal blood spaces (Fig. 6). Neither the induction nor the maintenance of Syna or Hand1 requires the presence of Gcm1/Cebpa/Synb-expressing cells, as both the Syna and Hand1 patterns are properly specified in Gcm1 and Cebpa;Cebpb mutant placentas. However, fusion of the Syna-positive cells to form SynT-I cells does appear to require some sort of interaction with Gcm1/Cepba/Synb-positive cells as suggested by two lines of evidence. First, syncytiotrophoblast formation is not present in Gcm1 mutant placentas (Anson-Cartwright et al., 2000). Second, fusion of trophoblast cells into the SynT-I syncytium occurs only after the SynT-II cells make contact (Hernandez-Verdun, 1974). Gcm1 mutants do not survive past ~E10.5 and, therefore, it is unclear whether the Hand1-positive cells in them would be able to differentiate into S-TGCs. Interestingly, Cyr61 mutant placentas, which contain few fetal vessels within the labyrinth, have a single layer of syncytiotrophoblast in areas containing only maternal sinuses (Mo et al., 2002). This implies that single layers of SynT can form. However, the cell lineage origin of the single SynT layer is unknown. Cyr61 placentas do contain areas where both maternal and fetal vessels are separated by a normal trilaminar trophoblast barrier. It is unclear whether the single layer of SynT observed in Cyr61 mutants is continuous with, and potentially originates from, SynT located in areas where there is a normal trilaminar barrier between fetal and maternal blood compartments. As there is some normal branching morphogenesis in these mice, albeit much reduced, it could provide the necessary signals to induce the lateral fusion of SynT-I cells. What is clear from the Cyr61 mutants is that in the absence of any branching morphogenesis, as is the case with Gcm1 mutants, cell-cell fusion in either layer is blocked.
To date over 125 mouse mutants have been generated that manifest defects in placental development and function, and the majority of placental phenotypes involve very poorly characterized defects in the labyrinth (reviewed by Watson and Cross, 2005). Markers for S-TGC (Hand1, Ctsq), SynT-I (Syna) and SynT-II (Gcm1, Cebpa, Synb) should allow for greater insights into which cell types are affected, increasing our understanding of labyrinth morphogenesis. However, it is worth pointing out that there are some limitations to the markers that we have to date. First, Ctsq expression is not evident throughout the S-TGC population until after E12.5 (Simmons et al., 2007). Hand1 is expressed in the presumptive S-TGC precursors as early as E8.5, in the apical chorion and in S-TGCs in the mature labyrinth, but Hand1 is not a specific marker of this lineage because it is expressed in all TGC subtypes as well as the upper ectoplacental cone and some cells within the chorion itself. Second, expression of the SynT layer-specific genes Gcm1, Cebpa, Synb and Syna, is not uniformly detected in each of their respective populations by mid-gestation and is ultimately downregulated between E14.5 and E16.5. This makes these markers of limited use in studying late-stage placentas. Because these genes are thought not to be just markers, but also functional for syncytiotrophoblast differentiation, their downregulation is consistent with the slowed expansion of the labyrinth layer towards the end of gestation.
In summary, the labyrinth is the most complex compartment of the rodent placenta in terms of its functions, the number of mouse mutants with defects in it, and the number of different cell types and cellular interactions that are present. The current studies will significantly advance our understanding of the labyrinth through provision of new markers and methods for assessing its structure and through insights into how the trilaminar trophoblast structure that forms the maternal-fetal interface forms.
Acknowledgments
We thank Colleen Geary-Joo and Fran Snider for excellent technical support. The work was supported by grants from the Alberta Heritage Foundation for Medical Research (AHFMR) and the Canadian Institutes for Health Research. D.G.S. was supported by fellowships from the Lalor Foundation and the AHFMR. D.R.C.N. was supported by fellowships from the AHFMR and CIHR. J.C.C. is an AHFMR Scientist.
References
- Akiyama Y, Hosoya T, Poole AM, Hotta Y. The gcm-motif: a novel DNA-binding motif conserved in Drosophila and mammals. Proc Natl Acad Sci USA. 1996;93:14912–14916. doi: 10.1073/pnas.93.25.14912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anson-Cartwright L, Dawson K, Holmyard D, Fisher SJ, Lazzarini RA, Cross JC. The glial cells missing-1 protein is essential for branching morphogenesis in the chorioallantoic placenta. Nat Genet. 2000;25:311–314. doi: 10.1038/77076. [DOI] [PubMed] [Google Scholar]
- Basyuk E, Cross JC, Corbin J, Nakayama H, Hunter P, Nait-Oumesmar B, Lazzarini RA. Murine Gcm1 gene is expressed in a subset of placental trophoblast cells. Dev Dyn. 1999;214:303–311. doi: 10.1002/(SICI)1097-0177(199904)214:4<303::AID-AJA3>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Beck F, Erler T, Russell A, James R. Expression of Cdx-2 in the mouse embryo and placenta: possible role in patterning of the extra-embryonic membranes. Dev Dyn. 1995;204:219–227. doi: 10.1002/aja.1002040302. [DOI] [PubMed] [Google Scholar]
- Begay V, Smink J, Leutz A. Essential requirement of CCAAT/enhancer binding proteins in embryogenesis. Mol Cell Biol. 2004;24:9744–9751. doi: 10.1128/MCB.24.22.9744-9751.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghorn KA, Clark PA, Encarnacion B, Deregis CJ, Folger JK, Morasso MI, Soares MJ, Wolfe MW, Roberson MS. Developmental expression of the homeobox protein Distal-less 3 and its relationship to progesterone production in mouse placenta. J Endocrinol. 2005;186:315–323. doi: 10.1677/joe.1.06217. [DOI] [PubMed] [Google Scholar]
- Campbell WJ, Deb S, Kwok SC, Joslin JA, Soares MJ. Differential expression of placental lactogen-II and prolactin-like protein-A in the rat chorioallantoic placenta. Endocrinology. 1989;125:1565–1574. doi: 10.1210/endo-125-3-1565. [DOI] [PubMed] [Google Scholar]
- Chang CW, Chuang HC, Yu C, Yao TP, Chen H. Stimulation of GCMa transcriptional activity by cyclic AMP/protein kinase A signaling is attributed to CBP-mediated acetylation of GCMa. Mol Cell Biol. 2005;25:8401–8414. doi: 10.1128/MCB.25.19.8401-8414.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church GM, Gilbert W. Genomic sequencing. Proc Natl Acad Sci USA. 1984;81:1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coan PM, Ferguson-Smith AC, Burton GJ. Ultrastructural changes in the interhaemal membrane and junctional zone of the murine chorioallantoic placenta across gestation. J Anat. 2005;207:783–796. doi: 10.1111/j.1469-7580.2005.00488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross JC. Genetic insights into trophoblast differentiation and placental morphogenesis. Semin Cell Dev Biol. 2000;11:105–113. doi: 10.1006/scdb.2000.0156. [DOI] [PubMed] [Google Scholar]
- Cross JC, Nakano H, Natale DR, Simmons DG, Watson ED. Branching morphogenesis during development of placental villi. Differentiation. 2006;74:393–401. doi: 10.1111/j.1432-0436.2006.00103.x. [DOI] [PubMed] [Google Scholar]
- Dai G, Wang D, Liu B, Kasik JW, Muller H, White RA, Hummel GS, Soares MJ. Three novel paralogs of the rodent prolactin gene family. J Endocrinol. 2000;166:63–75. doi: 10.1677/joe.0.1660063. [DOI] [PubMed] [Google Scholar]
- Davies J, Glasser SR. Histological and fine structural observations on the placenta of the rat. Acta Anat Basel. 1968;69:542–608. doi: 10.1159/000143100. [DOI] [PubMed] [Google Scholar]
- Deb S, Faria TN, Roby KF, Larsen D, Kwok SC, Talamantes F, Soares MJ. Identification and characterization of a new member of the prolactin family, placental lactogen-I variant. J Biol Chem. 1991;266:1605–1610. [PubMed] [Google Scholar]
- Dupressoir A, Marceau G, Vernochet C, Benit L, Kanellopoulos C, Sapin V, Heidmann T. Syncytin-A and syncytin-B, two fusogenic placenta-specific murine envelope genes of retroviral origin conserved in Muridae. Proc Natl Acad Sci USA. 2005;102:725–730. doi: 10.1073/pnas.0406509102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders AC. A comparative study of the fine structure of the trophoblast in several hemochorial placentas. Am J Anat. 1965;116:29–67. doi: 10.1002/aja.1001160103. [DOI] [PubMed] [Google Scholar]
- Hancock SN, Agulnik SI, Silver LM, Papaioannou VE. Mapping and expression analysis of the mouse ortholog of Xenopus Eomesodermin. Mech Dev. 1999;81:205–208. doi: 10.1016/s0925-4773(98)00244-5. [DOI] [PubMed] [Google Scholar]
- Hemberger M, Cross JC. Genes governing placental development. Trends Endocrinol Metab. 2001;12:162–168. doi: 10.1016/s1043-2760(01)00375-7. [DOI] [PubMed] [Google Scholar]
- Hernandez-Verdun D. Morphogenesis of the syncytium in the mouse placenta. Ultrastructural study. Cell Tissue Res. 1974;148:381–396. doi: 10.1007/BF00224265. [DOI] [PubMed] [Google Scholar]
- Hughes M, Dobric N, Scott IC, Su L, Starovic M, St-Pierre B, Egan SE, Kingdom JC, Cross JC. The Hand1, Stra13 and Gcm1 transcription factors override FGF signaling to promote terminal differentiation of trophoblast stem cells. Dev Biol. 2004;271:362–371. doi: 10.1016/j.ydbio.2004.03.029. [DOI] [PubMed] [Google Scholar]
- Ishida M, Ono K, Taguchi S, Ohashi S, Naito J, Horiguchi K, Harigaya T. Cathepsin gene expression in mouse placenta during the latter half of pregnancy. J Reprod Dev. 2004;50:515–523. doi: 10.1262/jrd.50.515. [DOI] [PubMed] [Google Scholar]
- Jacquemin P, Sapin V, Alsat E, Evain-Brion D, Dolle P, Davidson I. Differential expression of the TEF family of transcription factors in the murine placenta and during differentiation of primary human trophoblasts in vitro. Dev Dyn. 1998;212:423–436. doi: 10.1002/(SICI)1097-0177(199807)212:3<423::AID-AJA10>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Jollie WP. Fine structural changes in placental labyrinth of the rat with increasing gestational age. J Ultrastruct Res. 1964;10:27–47. doi: 10.1016/s0022-5320(64)90018-8. [DOI] [PubMed] [Google Scholar]
- Lee CK, Moon DH, Shin CS, Kim H, Yoon YD, Kang HS, Lee BJ, Kang SG. Circadian expression of Mel1a and PL-II genes in placenta: effects of melatonin on the PL-II gene expression in the rat placenta. Mol Cell Endocrinol. 2003;200:57–66. doi: 10.1016/s0303-7207(02)00414-8. [DOI] [PubMed] [Google Scholar]
- Li Y, Lemaire P, Behringer RR. Esx1, a novel X chromosome-linked homeobox gene expressed in mouse extraembryonic tissues and male germ cells. Dev Biol. 1997;188:85–95. doi: 10.1006/dbio.1997.8640. [DOI] [PubMed] [Google Scholar]
- Mo F, Muntean AG, Chen C, Stolz DB, Watkins SC, Lau LF. CYR61 (CCN1) is essential for placental development and vascular integrity. Mol Cell Biol. 2002;22:8709–8720. doi: 10.1128/MCB.22.24.8709-8720.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morasso MI, Grinberg A, Robinson G, Sargent TD, Mahon KA. Placental failure in mice lacking the homeobox gene Dlx3. Proc Natl Acad Sci USA. 1999;96:162–167. doi: 10.1073/pnas.96.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson K, Svensson K, Mattsson R, Carlsson B, Ohlsson R, Berkenstam A. Expression of a novel member of estrogen response element-binding nuclear receptors is restricted to the early stages of chorion formation during mouse embryogenesis. Mech Dev. 1996;54:211–223. doi: 10.1016/0925-4773(95)00479-3. [DOI] [PubMed] [Google Scholar]
- Rossant J, Cross JC. Placental development: lessons from mouse mutants. Nat Rev Genet. 2001;2:538–548. doi: 10.1038/35080570. [DOI] [PubMed] [Google Scholar]
- Sahgal N, Knipp GT, Liu B, Chapman BM, Dai G, Soares MJ. Identification of two new nonclassical members of the rat prolactin family. J Mol Endocrinol. 2000;24:95–108. doi: 10.1677/jme.0.0240095. [DOI] [PubMed] [Google Scholar]
- Schreiber J, Sock E, Wegner M. The regulator of early gliogenesis glial cells missing is a transcription factor with a novel type of DNA-binding domain. Proc Natl Acad Sci USA. 1997;94:4739–4744. doi: 10.1073/pnas.94.9.4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber J, Riethmacher-Sonnenberg E, Riethmacher D, Tuerk EE, Enderich J, Bosl MR, Wegner M. Placental failure in mice lacking the mammalian homolog of glial cells missing, GCMa. Mol Cell Biol. 2000;20:2466–2474. doi: 10.1128/mcb.20.7.2466-2474.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert SW, Kardash E, Khan MA, Cheusova T, Kilian K, Wegner M, Hashemolhosseini S. Interaction, cooperative promoter modulation, and renal colocalization of GCMa and Pitx2. J Biol Chem. 2004;279:50358–50365. doi: 10.1074/jbc.M404587200. [DOI] [PubMed] [Google Scholar]
- Scott IC, Anson-Cartwright L, Riley P, Reda D, Cross JC. The HAND1 basic helix-loop-helix transcription factor regulates trophoblast differentiation via multiple mechanisms. Mol Cell Biol. 2000;20:530–541. doi: 10.1128/mcb.20.2.530-541.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons DG, Cross JC. Determinants of trophoblast lineage and cell subtype specification in the mouse placenta. Dev Biol. 2005;284:12–24. doi: 10.1016/j.ydbio.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Simmons DG, Fortier AL, Cross JC. Diverse subtypes and developmental origins of trophoblast giant cells in the mouse placenta. Dev Biol. 2007;304:567–578. doi: 10.1016/j.ydbio.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Snell GD, Stevens LC. Early embryology. In: Green EL, editor. Biology of the Laboratory Mouse. New York: McGraw-Hill; 1966. pp. 205–245. [Google Scholar]
- Steingrimsson E, Tessarollo L, Reid SW, Jenkins NA, Copeland NG. The bHLH-Zip transcription factor Tfeb is essential for placental vascularization. Development. 1998;125:4607–4616. doi: 10.1242/dev.125.23.4607. [DOI] [PubMed] [Google Scholar]
- Susens U, Aguiluz JB, Evans RM, Borgmeyer U. The germ cell nuclear factor mGCNF is expressed in the developing nervous system. Dev Neurosci. 1997;19:410–420. doi: 10.1159/000111238. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Kunath T, Hadjantonakis AK, Nagy A, Rossant J. Promotion of trophoblast stem cell proliferation by FGF4. Science. 1998;282:2072–2075. doi: 10.1126/science.282.5396.2072. [DOI] [PubMed] [Google Scholar]
- Tompers DM, Foreman RK, Wang Q, Kumanova M, Labosky PA. Foxd3 is required in the trophoblast progenitor cell lineage of the mouse embryo. Dev Biol. 2005;285:126–137. doi: 10.1016/j.ydbio.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Uy GD, Downs KM, Gardner RL. Inhibition of trophoblast stem cell potential in chorionic ectoderm coincides with occlusion of the ectoplacental cavity in the mouse. Development. 2002;129:3913–3924. doi: 10.1242/dev.129.16.3913. [DOI] [PubMed] [Google Scholar]
- Voss AK, Thomas T, Gruss P. Mice lacking HSP90beta fail to develop a placental labyrinth. Development. 2000;127:1–11. doi: 10.1242/dev.127.1.1. [DOI] [PubMed] [Google Scholar]
- Watson ED, Cross JC. Development of structures and transport functions in the mouse placenta. Physiology Bethesda. 2005;20:180–193. doi: 10.1152/physiol.00001.2005. [DOI] [PubMed] [Google Scholar]