Abstract
Alphavirus transducing systems (ATSs) are important tools for expressing genes of interest (GOI) during infection. ATSs are derived from cDNA clones of mosquito-borne RNA viruses (genus Alphavirus; family Togaviridae). The Alphavirus genus contains about 30 different mosquito-borne virus species. Alphaviruses are enveloped viruses and contain single-stranded RNA genomes (~11.7 Kb). Alphaviruses transcribe a subgenomic mRNA that encodes the structural proteins of the virus required for encapsidation of the genome and maturation of the virus. Alphaviruses are usually highly lytic in vertebrate cells, but persistently infect susceptible mosquito cells with minimal cytopathology. These attributes make them excellent tools for gene expression in mosquito vectors. The most common ATSs in use are derived from Sindbis virus (SINV). The broad species tropism of SINV allows for infection of insect, avian, and mammalian cells8. However, ATSs have been derived from other alphaviruses as well9,10,20. Foreign gene expression is made possible by the insertion of an additional viral subgenomic RNA initiation site or promoter. ATSs in which an exogenous gene sequence is positioned 5' to the viral structural genes is used for stable protein expression in insects. ATSs, in which a gene sequence is positioned 3' to the structural genes, is used to trigger RNAi and silence expression of that gene in the insect.
ATSs have proven to be valuable tools for understanding vector-pathogen interactions, molecular details of viral replication and maintenance infectious cycles3,4,11,19,21. In particular, the expression of fluorescent and bioluminescent reporters has been instrumental tracking the viral infection in the vector and virus transmission5,14-16,18. Additionally, the vector immune response has been described using two strains of SINV engineered to express GFP2,9.
Here, we present a method for the production of SINV containing a fluorescent reporter (GFP) from the cDNA infectious clone. Infectious, full-length RNA is transcribed from the linearized cDNA clone. Infectious RNA is introduced into permissive target cells by electroporation. Transfected cells generate infectious virus particles expressing the GOI. Harvested virus is used to infect mosquitoes, as described here, or other host species (not shown herein). Vector competence is assessed by detecting fluorescence outside the midgut or by monitoring virus transmission7. Use of a fluorescent reporter as the GOI allows for convenient estimation of virus spread throughout a cell culture, for determination of rate of infection, dissemination in exposed mosquitoes, virus transmission from the mosquito and provides a rapid gauge of vector competence.
Protocol
The following protocol is written for production and use of an ATS based on the Sindbis virus MRE16. The ATS is designed to express GFP in the mosquito vector Aedes aegypti. cDNA Infectious clones of a number of alphaviruses (Sindbis virus, Chikungunya virus, O'nyong-nyong virus, western equine encephalitis virus) are currently available that have been designed as ATS's (Table 1). For best results plasmid DNA is amplified in bacteria such as SURE competent bacteria (Stratagene/Agilent Technologies) to avoid unwanted recombination and loss of the viral cDNA. All infectious clone plasmids have a bacteriophage T7 or SP6 promoter at the immediate 5' end of the viral cDNA for transcription of full-length genomic RNA and a unique restriction endonuclease at the 3' end for runoff transcription. For each ATS, users must use the appropriate bacteriophage DNA dependent RNA polymerase and unique restriction endonuclease for in vitro transcription of the viral RNA genome.
1. Generation of Infectious RNA from cDNA Clone.
Linearize the 5μg of the infectious clone plasmid (p5'dsMRE16/GFP) with 20 units of XhoI restriction enzyme in a 100 μL reaction. Incubate for 2-3h at 37°C
Purify linearized DNA using Qiagen QIAprep Spin Miniprep Kit alternate purification protocol, eluting DNA from spin column using 50 μL RNase-free water. Plasmid concentration should be approximately 1.0 μg/μL.
- In an RNase-free 0.2 mL PCR tube, set-up a 50 μL transcription reaction using Ambion MAXIscript Kit per protocol as follows.
- 22.5 μL RNase-free dH2O
- 5.0 μL Linearized DNA template (1.0 μg/μL)
- 2.5 μL 10mM ATP
- 2.5 μL 10mM CTP
- 2.5 μL 10mM UTP
- 2.5 μL 1mM GTP
- 2.5 μL 10mM cap analog (m7G(5')ppp(5')G)
- 5.0 μL 10X transcription buffer
- 5.0 μL T7 or SP6 polymerase mix
- 50.0 μL Total
Incubate the transcription reaction for 1 hr at 37°C. After incubation, the reaction should be used immediately for electroporation of BHK-21 cells. Preparation of the BHK-21 cells for electroporation should begin during incubation transcription reaction.
2. Generation of Virus from Infectious RNA
Trypsinize two 70-80% confluent 150 cm2 (T-150) flask BHK-21 cells per ATS.
Transfer all cells to a 15 mL conical centrifuge tube.
Pellet the cells by centrifugation at 2000 rpm, 10 min, 4°C in a conventional tabletop centrifuge.
Resuspend cells in sterile PBS without Mg++ and Ca++. Repeat steps 2.3 and 2.4 until cells have been washed three times with PBS.
Resuspend the pelleted cells 0.5 mL MEM containing 10% FBS.
Dilute 10 μL cells with 90 μL MEM with 10% FBS and count on a hemacytometer. If necessary, dilute cells to 2.5-12.5 x 107 cells/mL with MEM containing 10% FBS.
To an ice cold 0.2 cm electroporation cuvette, add 400 μL cell suspension and 20 μL transcription reaction. Wipe condensation from cuvette.
Pulse the cuvette twice: 450V, 1200Ω, 150 μF. We use an Electro Cell Manipulator, Harvard Apparatus Model ECM630.
Place the entire contents of the cuvette into a 25 cm2 tissue culture flask containing 5 mL MEM with 10% FBS.
Incubate flask(s) at 37C with 5% CO2.
Monitor the infection daily using an inverted fluorescent microscope with appropriate filter set (e.g. GFP filter: excitation wavelength = 460-490 nm, emission wavelength = 510-550 nm; or dsRed filter: excitation wavelength = 460-560 nm, emission wavelength = >590 nm).
When fluorescence suggests infection is confluent and/or CPE is visible throughout the flask, collect the contents of the flask, add 30% FBS, aliquot as necessary, and store at -80°C. If desired, cell lysates may be cleared by centrifugation (2000 rpm, 10 min, 4°C) prior to addition of FBS.
Passage virus at least once through a new flask of cells to increase the virus titer for subsequent blood feeding assays.
3. per os Infection of Mosquitoes
Mix together blood (usually purchased de-fibrinated sheep blood) and cell-culture derived virus suspension from step 2.13. Final virus titer in the blood meal typically should be ~1x107 plaque forming units (pfu)/ mL for efficient midgut infection of the mosquito. To stimulate mosquitoes to engorge, adenosine 5'-triphosphate (ATP) can be added to a final concentration of 0.02M.
Mosquitoes may have to be deprived of water and sugar before infection to stimulate them to efficiently blood feed from an artificial feeder. Duration of deprivation should be previously established to minimize mortality. Times will depend on mosquito species, humidity of the insectary etc. As a starting point, remove sugar 24h and water 12h prior to the feed. This protocol uses water-jacketed glass feeders17 with circulating 37°C water. A porcine intestinal membrane (i.e. thoroughly washed sausage casing available at most grocery stores) is placed over the mouth of the feeder and the meal is pipetted into the chamber. Various designs, membranes and protocols are available for different vector types.
Mosquitoes are sorted to select only those mosquitoes engorged with blood. Engorged mosquitoes are transferred to a carton with organdy on the top and the mosquitoes are incubated at 28°C, 75-80% relative humidity until processed for GFP expression.
4. Intrathoracic Inoculation of Mosquitoes
Intrathoracic inoculation is an alternative method for infecting the arthropod. This method is used when dissemination through the midgut is not required. (Method described below but the technique is not shown in this visual experiment).
A small number of mosquitoes (5-10) is aspirated from the holding cages into plastic holding tubes. The sealed holding tubes will be placed at 4°C for 15 min to chill the mosquitoes.
2 5 mosquitoes will be spilled from the tube onto a glass petri dish resting on ice. Place the lid on the Petri dish when not in use or if mosquitoes begin to move around. During handling of mosquitoes, care must be taken to count and keep track of the number of mosquitoes being manipulated. As mosquitoes are spilled onto the chill table, the total number will be recorded on a lab counter.
The needle is prepared by melting capillary tubing using Nanoject specific glass and a needle puller.
Setup the Nanoject II microinjector per the manufacturer's instructions for delivery of 69 nL inoculum. Care must be exercised when drawing the inoculum into the needle so that the needle is not damaged.
Using a dissecting microscope, insert the needle into the thorax of the mosquito and activate the Nanoject for inoculation. Care must be exercised when inoculating mosquitoes so that the needle does not completely penetrate the mosquito and exit the other side, resulting in the subsequent death of the mosquito. Check each mosquito to be sure that the inoculum entered before removing it from the needle.
After inoculation, while the mosquito is still impaled on the needle, place it in a paper holding carton through a small hole in the side that is plugged with cotton or a cork stopper at all other times.
When the mosquitoes have inoculated and placed into the carton (up to approximately 50 per carton), carefully tape the cork in place to prevent accidental release of the mosquitoes. Place the cartons in a secure holding bin before further incubation in the insectary at 28°C and 80% relative humidity.
5. Monitoring Infection of Mosquitoes
Place the paper holding carton at 4°C for approximately 15 minutes to immobilize mosquitoes.
Transfer a small number of mosquitoes (5-10 at a time) to a chill table adapted for use on a fluorescent microscope.
Examine and sort mosquitoes based on presence or absence of fluorescence. Additionally, fluorescent intensity may be used as a proxy quantitative measurement of infection. Mosquito tissues such as midguts, salivary glands, fat body can be dissected and monitored for fluorescence.
If appropriate, return selected mosquitoes to paper carton to continue incubation of infected mosquitoes.
6. Transmission Assay (Forced Salivation to Demonstrate Virus Transmission)
Deprive mosquitoes of sugar source for 24 h prior to feed.
Remove legs and wings
To a 50 μL capillary tube that has been heated, pulled and snipped at one end, add 3-5 μL of Cargille Type B immersion oil or 10% serum-sucrose solution.
Insert the proboscis into the tube. If using oil, droplets of saliva will be seen to exude from the proboscis. One μL of a 1% pilocarbine solution in PBS and 0.1% Tween 80 can be applied to the thorax to stimulate.
After 60-90 minutes, remove mosquitoes and store for virus isolation if required.
Remove solution from the tube by centrifugation in a tube containing 100 μL 20% FBS-PBS.
Sterile filter the inoculum through a 0.2 μm syringe filter and then use the filtered the inoculum to infect cultured cells.
BIOSAFETY NOTE: This protocol describes a method for generating, identifying, and monitoring infected mosquitoes. Based on your facilities (i.e. a chill table, containment facility, etc.) and IBC approval, you may be limited to examining them only after removing wings and legs to prevent escape. SINV-based ATS's can be generated and used in a BSL2 environment. The use of ATS's based on other alphaviruses such as Chikungunya virus or western equine encephalitis viruses will require BSL3 facilities.
7. Representative Results
An overview of expression of a marker gene (GFP) using the ATS 5'dsMRE16/GFP is shown in Figure 1. Expression of GFP in BHK-21 and C6/36 (Aedes albopictus) cells is shown in Figure 2 at specific times after infection of cells with 5'dsMRE16/GFP virus at 0.01 multiplicity of infection. Figure 3 is an overview of alphavirus and ATS virus infection in the mosquito when the virus is delivered through an infective blood meal. Figure 4 shows preparation of a blood meal with ~107 pfu/mL of ATS virus followed by oral infection of Aedes aegypti. Whole body view of infected mosquito and selected body parts at 10 days post infection using an epifluorescence microscope (Figure 5). Detection of GFP and dsRED in midguts of infected mosquitoes following infection with ATS 5'dsMRE16 viruses expressing each fluorescent marker gene (Figure 6). Transmission assay using mosquitoes infected with 5'dsMRE16/GFP ATS virus (Figure 7).
Table 1. ATS's currently engineered for gene expression in mosquitoes.
| Alphavirus | ATS | Mosquito | Reference |
| Sindbis Virus | TE/3'2J and TE/5'2J | Aedes aegypti | 12 |
| Ochlerotatus triseriatus | 13 | ||
| 3'dsMRE16 and 5'dsMRE16 | A. aegypti | 5 | |
| Culex tritaeniorhynchus | 5 | ||
| 5'dsTR339 | A. aeg ypti | 2 | |
| Chikungunya virus | 3'dsCHIKV and 5'dsCHIKV | A. aegypti/A. albopictus | 20 |
| O'nyong nyong virus | 5'dsONNV | Anopheles gambiae | 1,9 |
| Western equine encephalitis virus | 5'dsWEEV.McMillan | Culex tarsalis | Stauft et al., unpublished |
Table 2. Advantages/Disadvantages of using ATS's for gene expression in mosquitoes.
| Advantages: |
| Rapid production of high-titer virus |
| Broad host range (SINV) |
| High RNA replication rate |
| High transgene expression levels |
| Relative ease of manipulating genes |
| Stable, persistent gene expression in insects |
| Minimal pathology in insects |
| Disadvantages: |
| Short-term expression mode in vertebrate cells |
| Strong cytopathic effects on vertebrate cells |
| Gene size restrictions (<1Kb, ideally) |
| Some alphaviruses require BSL3 containment |
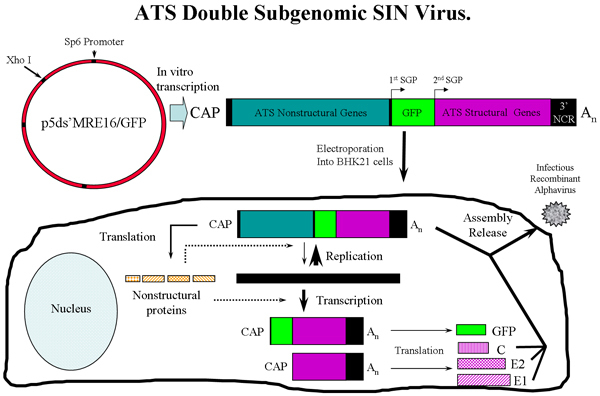 Figure 1: Overview of ATS virus production in BHK-21 cells using p5'dsMRE/GFP SINV expressing GFP. Following in vitro transcription, genomic ATS RNA is electroporated into BHK-21 cells where virus is rescued. The ATS virus can then be used to infect mosquitoes. SGP = subgenomic promoter. Three ATS RNA species are transcribed in the cells, the genomic, subgenomic 1 and subgenomic 2 RNAs. C (capsid), E2 and E1 glycoproteins are assembled with ATS genome to generate infectious virus.
Figure 1: Overview of ATS virus production in BHK-21 cells using p5'dsMRE/GFP SINV expressing GFP. Following in vitro transcription, genomic ATS RNA is electroporated into BHK-21 cells where virus is rescued. The ATS virus can then be used to infect mosquitoes. SGP = subgenomic promoter. Three ATS RNA species are transcribed in the cells, the genomic, subgenomic 1 and subgenomic 2 RNAs. C (capsid), E2 and E1 glycoproteins are assembled with ATS genome to generate infectious virus.
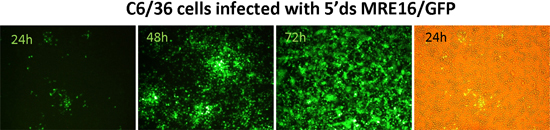 Figure 2: Expression of GFP in mosquito (C6/36) cells following infection with SINV 5'dsMRE16/GFP virus.
Figure 2: Expression of GFP in mosquito (C6/36) cells following infection with SINV 5'dsMRE16/GFP virus.
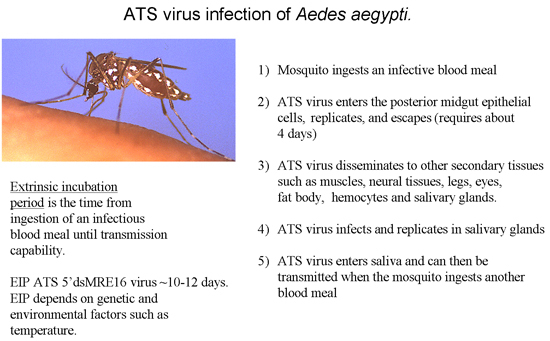 Figure 3: Overview of an ATS virus infection in mosquitoes leading to virus transmission.
Figure 3: Overview of an ATS virus infection in mosquitoes leading to virus transmission.
 Figure 4: ATS SINV 5'dsMRE16 infection by exposing mosquitoes to an infective blood meal.
Figure 4: ATS SINV 5'dsMRE16 infection by exposing mosquitoes to an infective blood meal.
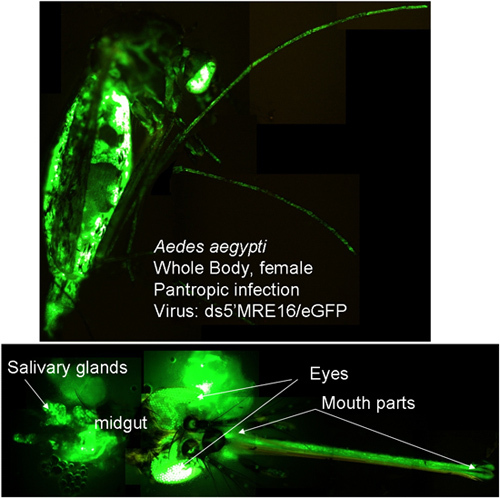 Figure 5: ATS SINV 5'dsMRE16/GFP infection at 10 days post infection. Whole body detection of GFP shows that virus has escaped the midgut and disseminated to secondary tissues. Figure also shows GFP expression in selected body parts that also is indicative of a disseminated infection.
Figure 5: ATS SINV 5'dsMRE16/GFP infection at 10 days post infection. Whole body detection of GFP shows that virus has escaped the midgut and disseminated to secondary tissues. Figure also shows GFP expression in selected body parts that also is indicative of a disseminated infection.
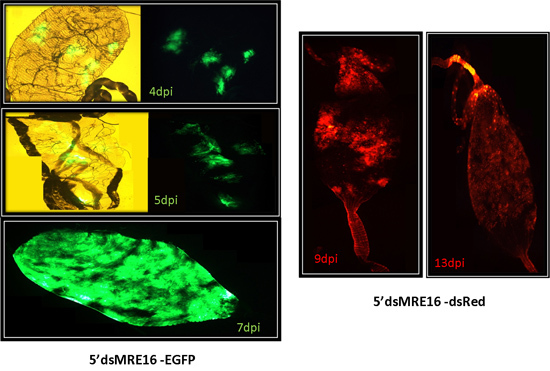 Figure 6: ATS SINV 5'dsMRE16/GFP and 5'dsMRE16/dsRED infection of midguts at specific times post-infection. Midguts usually have distinct foci of infection early after ingesting a blood meal containing virus that spreads throughout the posterior midgut at later times post-infection.
Figure 6: ATS SINV 5'dsMRE16/GFP and 5'dsMRE16/dsRED infection of midguts at specific times post-infection. Midguts usually have distinct foci of infection early after ingesting a blood meal containing virus that spreads throughout the posterior midgut at later times post-infection.
 Figure 7: Detection of ATS 5'dsMRE16/GFP in mosquito saliva as early as 8 days post infection. Assay is designed to show transmission of the ATS virus and shows that the 5'dsMRE16/GFP virus can stably express GFP throughout the extrinsic incubation period in the mosquito. The left panel shows a mosquito salivating into a capillary tube, the center panel shows C6/36 cells infected with saliva at 1 day and right panel 3 days post infection.
Figure 7: Detection of ATS 5'dsMRE16/GFP in mosquito saliva as early as 8 days post infection. Assay is designed to show transmission of the ATS virus and shows that the 5'dsMRE16/GFP virus can stably express GFP throughout the extrinsic incubation period in the mosquito. The left panel shows a mosquito salivating into a capillary tube, the center panel shows C6/36 cells infected with saliva at 1 day and right panel 3 days post infection.
Discussion
The methods presented here enable researchers to follow the infection of an alphavirus in mosquito cell culture and the adult female mosquito. The advantages and disadvantages of using ATS viruses are summarized in Table 2. An ATS infectious clone can be genetically manipulated to express any GOI and have been used to express single chain antibodies, suppressors of RNAi, antisense RNAs targeting endogenous genes, and pro-apoptotic proteins. If a reporter is not used, then the imaging portion of this method is not relevant. However, many of the same protocols are necessary for ATS virus rescue, propagation, and mosquito infection. There are limitations in the maximal size of the transgene when inserted into an alphavirus transducing system. The size constraints vary depending on the parental strain used to generate the ATS, but are usually about 1kb although larger reporter genes such as firefly luciferase have been used in constructing ATS for use in mosquitoes. Research labs with a modest amount of virology background should be able to use ATS's following these protocols. A number of research labs have already done so using SINV-based ATS's.
Disclosures
No conflicts of interest declared.
Acknowledgments
This work is supported by the National Institutes of Health (NIH R01 AI46435) and the RMRCE (NIH AI065357).
References
- Brault AC. Infection patterns of o'nyong nyong virus in the malaria-transmitting mosquito Anopheles gambiae. Insect Mol. Biol. 2004;13:625–625. doi: 10.1111/j.0962-1075.2004.00521.x. [DOI] [PubMed] [Google Scholar]
- Campbell CL. Aedes aegypti uses RNA interference in defense against Sindbis virus infection. BMC. Microbiol. 2008;8:47–47. doi: 10.1186/1471-2180-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirimotich CM. Suppression of RNA interference increases alphavirus replication and virus-associated mortality in Aedes aegypti mosquitoes. BMC. Microbiol. 2009;9:49–49. doi: 10.1186/1471-2180-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capurro deLara, M Virus-expressed, recombinant single-chain antibody blocks sporozoite infection of salivary glands in Plasmodium gallinaceum-infected Aedes aegypti. Am. J. Trop. Med. Hyg. 2000;62:427–427. doi: 10.4269/ajtmh.2000.62.427. [DOI] [PubMed] [Google Scholar]
- Foy BD. Development of a new Sindbis virus transducing system and its characterization in three Culicine mosquitoes and two lepidopteran species. Insect Mol. Biol. 2004;13:89–89. doi: 10.1111/j.1365-2583.2004.00464.x. [DOI] [PubMed] [Google Scholar]
- Foy BD, Olson KE. Alphavirus transducing systems. Adv. Exp. Med. Biol. 2008;627:19–19. doi: 10.1007/978-0-387-78225-6_2. [DOI] [PubMed] [Google Scholar]
- Franz AW. Engineering RNA interference-based resistance to dengue virus type 2 in genetically modified Aedes aegypti. Proc. Natl. Acad. Sci. U. S. A. 2006;103:4198–4198. doi: 10.1073/pnas.0600479103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn CS. Infectious Sindbis virus transient expression vectors for studying antigen processing and presentation. Proc. Natl. Acad. Sci. U. S. A. 1992;89:2679–2679. doi: 10.1073/pnas.89.7.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene KM. RNA interference acts as a natural antiviral response to O'nyong-nyong virus (Alphavirus; Togaviridae) infection of Anopheles gambiae. Proc. Natl. Acad. Sci. U. S. A. 2004;101:17240–17240. doi: 10.1073/pnas.0406983101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundstrom K. Expression of mammalian membrane proteins in mammalian cells using Semliki Forest virus vectors. Methods Mol. Biol. 2010;601:149–149. doi: 10.1007/978-1-60761-344-2_10. [DOI] [PubMed] [Google Scholar]
- Myles KM. Alphavirus-derived small RNAs modulate pathogenesis in disease vector mosquitoes. Proc. Natl. Acad. Sci. U. S. A. 1993;105:19938–19938. doi: 10.1073/pnas.0803408105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson KE. Genetically engineered resistance to dengue-2 virus transmission in mosquitoes. Science. 1996;272:884–884. doi: 10.1126/science.272.5263.884. [DOI] [PubMed] [Google Scholar]
- Olson KE. The expression of chloramphenicol acetyltransferase in Aedes albopictus (C6/36) cells and Aedes triseriatus mosquitoes using a double subgenomic recombinant Sindbis virus. Insect Biochem. Mol. Biol. 1994;24:39–39. doi: 10.1016/0965-1748(94)90121-x. [DOI] [PubMed] [Google Scholar]
- Olson KE. Development of a Sindbis virus expression system that efficiently expresses green fluorescent protein in midguts of Aedes aegypti following per os infection. Insect Mol. Biol. 2000;9:57–57. doi: 10.1046/j.1365-2583.2000.00162.x. [DOI] [PubMed] [Google Scholar]
- Parikh GR, Oliver JD, Bartholomay LC, C L. A haemocyte tropism for an arbovirus. J. Gen. Virol. 2009;90:292–292. doi: 10.1099/vir.0.005116-0. [DOI] [PubMed] [Google Scholar]
- Pierro DJ. Development of an orally infectious Sindbis virus transducing system that efficiently disseminates and expresses green fluorescent protein in Aedes aegypti. Insect Mol. Biol. 2003;12:107–107. doi: 10.1046/j.1365-2583.2003.00392.x. [DOI] [PubMed] [Google Scholar]
- Rutledge LCWard, A R, Gould DJ. Studies on the feeding response of mosquitoes to nutritive solutions in a new membrane feeder. Mosq. News. 1964;24:407–407. [Google Scholar]
- Sanders HR. Sindbis virus induces transport processes and alters expression of innate immunity pathway genes in the midgut of the disease vector Aedes aegypti. Insect Biochem. Mol. Biol. 2005;35:1293–1293. doi: 10.1016/j.ibmb.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Uhlirova M. Use of Sindbis virus-mediated RNA interference to demonstrate a conserved role of Broad-Complex in insect metamorphosis. Proc. Natl. Acad. Sci. U. S. A. 2003;100:15607–15607. doi: 10.1073/pnas.2136837100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlandingham DL. Development and characterization of a double subgenomic chikungunya virus infectious clone to express heterologous genes in Aedes aegypti mosquitoes. Insect Biochem. Mol. Biol. 2005;35:1162–1162. doi: 10.1016/j.ibmb.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Wang H. Effects of inducing or inhibiting apoptosis on Sindbis virus replication in mosquito cells. J. Gen. Virol. 2008;89:2651–2651. doi: 10.1099/vir.0.2008/005314-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


