Abstract
The Neuronetics NeuroStar Transcranial Magnetic Stimulation (TMS) System is a class II medical device that produces brief duration, pulsed magnetic fields. These rapidly alternating fields induce electrical currents within localized, targeted regions of the cortex which are associated with various physiological and functional brain changes.1,2,3
In 2007, O'Reardon et al., utilizing the NeuroStar device, published the results of an industry-sponsored, multisite, randomized, sham-stimulation controlled clinical trial in which 301 patients with major depression, who had previously failed to respond to at least one adequate antidepressant treatment trial, underwent either active or sham TMS over the left dorsolateral prefrontal cortex (DLPFC). The patients, who were medication-free at the time of the study, received TMS five times per week over 4-6 weeks.4
The results demonstrated that a sub-population of patients (those who were relatively less resistant to medication, having failed not more than two good pharmacologic trials) showed a statistically significant improvement on the Montgomery-Asberg Depression Scale (MADRS), the Hamilton Depression Rating Scale (HAMD), and various other outcome measures. In October 2008, supported by these and other similar results5,6,7, Neuronetics obtained the first and only Food and Drug Administration (FDA) approval for the clinical treatment of a specific form of medication-refractory depression using a TMS Therapy device (FDA approval K061053).
In this paper, we will explore the specified FDA approved NeuroStar depression treatment protocol (to be administered only under prescription and by a licensed medical profession in either an in- or outpatient setting).
Protocol
1) Preparation
To begin, start up the NeuroStar system, log in, and perform the automated coil test.
Next, access the appropriate patient file from the available list (all patients treated using the NeuroStar system are logged into the central computer)
Finally, ensure the chair settings are not restrictive and lift the chair arm to make it easy for the patient to enter and sit down.
2) Seating and Aligning the Patient
When the patient arrives, seat him/her comfortably in the chair.
Make sure both the patient and the technician insert ear plugs.
Using the adhesive strip, attach the head positioning strap just above the patient's eyebrows. Make sure the central point is located above the nasion.
Recline the chair and adjust the head support as needed.
Lightly attach the top head strap to the Velcro above the patient's nasion.
Activate the laser and ensure it bisects the patient's nasion.
Ask the subject to stare at a point on the wall and adjust the A/P bar until it is level with the eyeballs.
Ensure the patient is fully centered and use the Velcro to secure the side head positioning straps to the treatment chair.
Lightly engage the side head pad to ensure cranial stability during motor threshold determination.
Finally, record the head support system settings using the touch screen interface.
3) Determine Motor Threshold
To determine motor threshold, move the coil down to the patient's head such that the coil is at the vertex and the SenStar tab is aligned above the top of the ear.
Move the A/P bar forward until it aligns with the side of the coil.
Align the center line on the coil with the 0 degree mark on the coil angle indicator and move back the A/P bar.
Once contact and position have been established, ask the subject to enact the hitchhiker position: arm bent 90 degrees at the elbow, fist held loosely, thumb extended outwards.
Begin stimulation and focus on any movement of the thumb.
Gradually increase the MT level from 0.68 to 1.10 until thumb or concordant finger movement is seen.
Once movement is established, begin searching for the motor hotspot. Move the coil around in a grid like pattern, adjusting the MT level as needed. Continue this search until you find a location and power level setting that elicits exactly 5 thumb twitches out of 10 consecutive pulses.
Once the motor hotspot has been determined, move the A/P bar forward until it, once again, aligns with the side of the coil.
Use the touch screen interface to record the A/P position, Coil Angle, SOA position, and Power Level.
Finally, press the Found MT button. We are now ready for treatment.
4) Patient Treatment
To begin, move the coil 5.5 cm anterior to the MT location. This will be the treatment location.
Ensure the coil is making contact with the patient's head and ask the patient to remain still for the duration of the treatment.
The treatment parameters are preloaded into the device. Stimulation will be generated at 120% motor threshold with a pulse sequence of 10 Hz for 4 seconds, followed by a 26 second quiet period. Treatment will last for a total of 37.5 minutes this is a total of 3,000 pulses.
Press the Confirm Pulse Sequence button then press the Start button.
During treatment, remain in the room with the patient and periodically check to ensure the coil is making contact with the patient.
After the pulse sequence is completed, move the coil up and away from the patient's head and move away the side head support pad.
Raise the back of the chair and raise the arm of the chair.
Assist the patient as he/she stands up.
Remove ear plugs and touch the Logout button to end the treatment session.
5) Representative Results
TMS is typically applied daily for a period of 4-6 weeks. Recent data suggests some patients may be prone to symptomatic relapse after 4-6 months.8 If this occurs, practitioners may want to consider maintenance sessions to prolong effect. This treatment has been effective in approximately 60% of the patients treated.
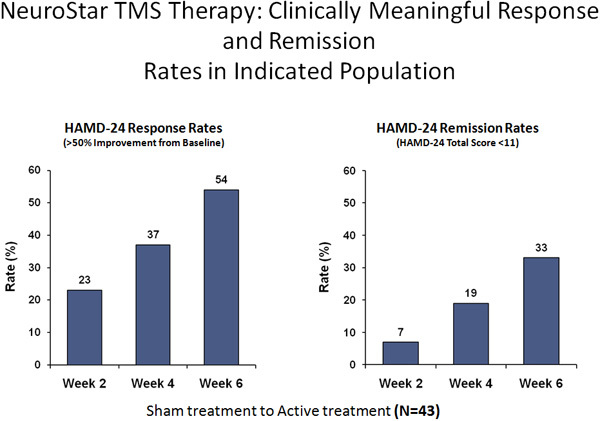
Discussion
When considering TMS as a potential therapeutic intervention for depression, it is important to recognize the FDA approved protocol is highly specified. To be concrete, FDA approval is limited to 10 Hz suprathreshold stimulation applied daily for 4-6 weeks using the NeuroStar device upon patients who have failed to achieve satisfactory improvement from one, but no more than one, adequate antidepressant medication trials during the current depressive episode. At this point, utilization of any alternate stimulation pattern, alternate time course, alternate device, or alternate patient population is considered off-label.
As results continue to be generated in support of the efficacy of TMS treatment5,6,7, new data is revealing the possibility of symptomatic relapse occurring 4-6 months after treatment cessation. As of this publication, there are no approved maintenance protocols. Therefore, issues concerning additional treatment and/or effect maintenance must be considered and monitored closely by individual practitioners.
Disclosures
The video production of this article was sponsored by Neuronetics, which produces instruments used in these studies.
References
- Pascual-Leone A, Davey M, Wassermann EM, Rothwell J, Puri B. Handbook of Transcranial Magnetic Stimulation. London: Edward Arnold; 2002. [Google Scholar]
- Walsh V, Pascual-Leone A. Transcranial Magnetic Stimulation: A Neurochronometrics of Mind. Cambridge: MIT Press; 2005. [Google Scholar]
- Horvath JC, Perez J, Forrow L, Fregni F, Pascual-Leone A. Transcranial Magnetic Stimulation: An Historical Evaluation and Future Prognosis of Therapeutically Relevant Ethical Concerns. doi: 10.1136/jme.2010.039966. Forthcoming. [DOI] [PubMed] [Google Scholar]
- O'Reardon JP, Solvason HB, Janicak PG, Sampson S, Isenberg KE, Nahas Z, McDonald WM, Avery D, Fitzgerald PB, Loo C, Demitrack MA, George MS, Sackheim HA. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized control trial. Biological Psychiatry. 2007;62:1208–1216. doi: 10.1016/j.biopsych.2007.01.018. [DOI] [PubMed] [Google Scholar]
- George MS, Lisanby SH, Avery D, McDonald WM, Durkalski V, Pavlicova M, Anderson B, Nahas Z, Bulow P, Zarkowski P, Holtzheimer PD, Schwartz T, Sackheim HA. Daily prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized study. Arch Gen Psychiatry. 2010;67:507–516. doi: 10.1001/archgenpsychiatry.2010.46. [DOI] [PubMed] [Google Scholar]
- Lisanbym SH, Husain MM. Daily Left Prefrontal Repetitive Transcranial Magnetic Stimulation (rTMS) in the Acute Treatment of Major Depression: Clinical Predictors of Outcome in a Multisite, Randomized Controlled Clinical Trial. Neuropsychopharmacology. 2008;34:522–534. doi: 10.1038/npp.2008.118. [DOI] [PubMed] [Google Scholar]
- Thase M, Demitrack MA. Evaluating clinical significance of treatment outcomes in studies of resistant major depression. Biological Psychiatry. 2008;63:138–138. [Google Scholar]
- Demirtas-Tatlidede A, Mechanic-Hamilton D, Press DZ, Pearlman C, Stern WM, Thall M, Pascual-Leone A. An open-label, prospective study of repetitive transcranial magnetic stimulation (rTMS) in the long-term treatment of refractory depression: reproducibility and duration of the antidepressant effect in medication-free patients. Journal of Clinical Psychiatry. 2008;69:930–934. doi: 10.4088/jcp.v69n0607. [DOI] [PubMed] [Google Scholar]


