Abstract
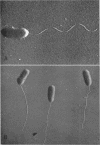
Roberts, F. F., Jr. (University of Maryland, College Park), and R. N. Doetsch. Some singular properties of bacterial flagella, with special reference to monotrichous forms. J. Bacteriol. 91:414–421. 1966.—Heat (60 C for 30 min), 10 m acetamide, and 8 m urea all brought about rapid and complete dissolution of flagella from monotrichous bacteria; hence, these flagella respond similarly to those of peritrichous forms. Chloramphenicol (103 μg/ml) inhibited regeneration of flagella in all peritrichously flagellated cultures; however, monotrichous forms were able to regenerate their flagella in a concentration 102 times that required to inhibit multiplication. Peritrichous bacteria did not synthesize flagella when infected by lytic bacteriophages. In these experiments, the time from infection to lysis was sufficient for uninfected controls to resynthesize their flagella. Monotrichous bacteria, however, in all but one instance, were able to resynthesize their flagella before lysis occurred. A study of flagella resynthesis in a non-nutritive milieu indicated that only a small amount of flagellum precursor is present in any given cell. The effect of temperature on synthesis of flagella indicated that, although some bacteria multiply and are motile at a given temperature, they are unable to resynthesize their flagella at that same temperature. This strongly suggests that initial flagellum synthesis and flagellum regeneration are not necessarily identical processes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ERLANDER S. R., KOFFLER H., FOSTER J. F. Physical properties of flagellin from Proteus vulgaris, a study involving the application of the Archibald sedimentation principle. Arch Biochem Biophys. 1960 Sep;90:139–153. doi: 10.1016/0003-9861(60)90625-1. [DOI] [PubMed] [Google Scholar]
- Gray P. H. A METHOD OF STAINING BACTERIAL FLAGELLA. J Bacteriol. 1926 Oct;12(4):273–274. doi: 10.1128/jb.12.4.273-274.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KERRIDGE D. FLAGELLAR SYNTHESIS IN SALMONELLA TYPHIMURIUM: THE INCORPORATION OF ISOTOPICALLY-LABELLED AMINO ACIDS INTO FLAGELLIN. J Gen Microbiol. 1963 Oct;33:63–76. doi: 10.1099/00221287-33-1-63. [DOI] [PubMed] [Google Scholar]
- KERRIDGE D. Synthesis of flagella by amino acid-requiring mutants of Salmonella typhimurium. J Gen Microbiol. 1959 Aug;21:168–179. doi: 10.1099/00221287-21-1-168. [DOI] [PubMed] [Google Scholar]
- KOFFLER H. Protoplasmic differences between mesophiles and thermophiles. Bacteriol Rev. 1957 Dec;21(4):227–240. doi: 10.1128/br.21.4.227-240.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leifson E. Development of Flagella on Germinating Spores. J Bacteriol. 1931 May;21(5):357–359. doi: 10.1128/jb.21.5.357-359.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QUADLING C., STOCKER B. A. An environmentally-induced transition from the flagellated to the non-flagellated state in Salmonella typhimurium: the fate of parental flagella at cell division. J Gen Microbiol. 1962 Jun;28:257–270. doi: 10.1099/00221287-28-2-257. [DOI] [PubMed] [Google Scholar]
- RENDI R., OCHOA S. Effect of chloramphenicol on protein synthesis in cell-free preparations of Escherichia coli. J Biol Chem. 1962 Dec;237:3711–3713. [PubMed] [Google Scholar]
- RINKER J. N., KOFFLER H. Preliminary evidence that bacterial flagella are not "polysaccharide twirls". J Bacteriol. 1951 Apr;61(4):421–431. doi: 10.1128/jb.61.4.421-431.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOCKER B. A., CAMPBELL J. C. The effect of non-lethal deflagellation on bacterial motility and observations on flagellar regeneration. J Gen Microbiol. 1959 Jun;20(3):670–685. doi: 10.1099/00221287-20-3-670. [DOI] [PubMed] [Google Scholar]
- YEE R. B., GEZON H. M. RIBONUCLEIC ACID OF CHLORAMPHENICOL-TREATED SHIGELLA FLEXNERI. J Gen Microbiol. 1963 Aug;32:299–306. doi: 10.1099/00221287-32-2-299. [DOI] [PubMed] [Google Scholar]