Abstract
The ability to measure cellular metabolism and understand mitochondrial dysfunction, has enabled scientists worldwide to advance their research in understanding the role of mitochondrial function in obesity, diabetes, aging, cancer, cardiovascular function and safety toxicity.
Cellular metabolism is the process of substrate uptake, such as oxygen, glucose, fatty acids, and glutamine, and subsequent energy conversion through a series of enzymatically controlled oxidation and reduction reactions. These intracellular biochemical reactions result in the production of ATP, the release of heat and chemical byproducts, such as lactate and CO2 into the extracellular environment.
Valuable insight into the physiological state of cells, and the alteration of the state of those cells, can be gained through measuring the rate of oxygen consumed by the cells, an indicator of mitochondrial respiration - the Oxygen Consumption Rate - or OCR. Cells also generate ATP through glycolysis, i.e.: the conversion of glucose to lactate, independent of oxygen. In cultured wells, lactate is the primary source of protons. Measuring the lactic acid produced indirectly via protons released into the extracellular medium surrounding the cells, which causes acidification of the medium provides the Extra-Cellular Acidification Rate - or ECAR.
In this experiment, C2C12 myoblast cells are seeded at a given density in Seahorse cell culture plates. The basal oxygen consumption (OCR) and extracellular acidification (ECAR) rates are measured to establish baseline rates. The cells are then metabolically perturbed by three additions of different compounds (in succession) that shift the bioenergetic profile of the cell.
This assay is derived from a classic experiment to assess mitochondria and serves as a framework with which to build more complex experiments aimed at understanding both physiologic and pathophysiologic function of mitochondria and to predict the ability of cells to respond to stress and/or insults.
Protocol
In this experiment, C2C12 myoblast cells are seeded at a given density in Seahorse cell culture plates. The basal oxygen consumption (OCR) and extracellular acidification (ECAR) rates are measured to establish baseline rates.
1. Cells Injection
The cells are metabolically perturbed by three additions of different compounds (in succession) that shift the bioenergetic profile of the cell. One group will serve as the control, with running media added as control "compounds".
The first injection is oligomycin. Oligomycin inhibits ATP synthesis by blocking the proton channel of the Fo portion ATP synthase (Complex V). In mitochondrial research, it is used to prevent state 3 (phosphorylating) respiration. With cells, it can be used to distinguish the percentage of O2 consumption devoted to ATP synthesis and the percentage of O2 consumption needed to overcome the natural proton leak across the inner mitochondrial membrane.
The second injection is FCCP. FCCP (Carbonyl cyanide-p-trifluoromethoxyphenylhydrazone) is an ionophore that is a mobile ion carrier. It is an uncoupling agent because it disrupts ATP synthesis by transporting hydrogen ions across the mitochondrial membrane instead of the proton channel of ATP synthase (Complex V). This collapse of the mitochondrial membrane potential leads to a rapid consumption of energy and oxygen without the generation of ATP. In this case, both OCR and ECAR will increase, OCR due to uncoupling, and ECAR as the cells attempt to maintain their energy balance by using glycolysis to generate ATP. FCCP treatment can be used to calculate the "spare" respiratory capacity of cells that is defined as the quantitative difference between maximal uncontrolled OCR and the initial basal OCR. It has been proposed that the maintenance of some spare respiratory capacity even under conditions of maximal physiological or pathophysiological stimulus is a major factor defining the vitality and/or survival of cells. The ability of cells to respond to stress under conditions of increased energy demand is in a large part influenced by the bioenergetic capacity of mitochondria. This bioenergetic capacity is deter-mined by several factors, including the ability of the cell to deliver substrate to mitochondria and the functional capacity of enzymes involved in electron transport
In the third injection, rotenone, a Complex I inhibitor, is added to the cells. This will shut down mitochondrial respiration and enable both the mitochondrial and non-mitochondrial fractions contributing to respiration to be calculated. One will observe a decrease in OCR due to impaired mitochondrial function, with a concomitant increase in ECAR as the cell shifts to a more glycolytic state in order to maintain its energy balance Rotenone is a mitochondrial inhibitor that prevents the transfer of electrons from the Fe-S center in Complex I to ubiquinone (Coenzyme Q). This inhibition of Complex I prevents the potential energy in NADH from being converted to usable energy in the form of ATP.
2. Reagents and Materials
Oligomycin, FCCP, and Rotenone Solutions (Seahorse Mito Stress Test Kit)
DMEM Running Media (Seahorse #100965-000)
DMSO (Sigma D8418)
Distilled Water (Gibco 15230-170)
Calibration buffer (Seahorse Bioscience)
3. Growth Medium
500 mL DMEM (Gibco 11965-092)
10% FBS (Hyclone SH90070.03)
5 mL Penn/Strep (Gibco 15140-122)
5 mL Sodium Pyruvate (Sigma S8636)
5 mL Glutamax (Gibco 35050-061)
4. Seeding Protocol
Cells are seeded in XF96 cell cultures plates with 10,000 cells/well in 100 μL of growth medium and placed in 37°C incubator with 10% CO2.
Cells will adhere to the XF96 cell culture plate within 1 hour.
Assay cells in XF96 24 hours after seeding.
5. Preparation of Assay Template
Using the Assay Wizard (Appendix I), generate a template with the following group layout:
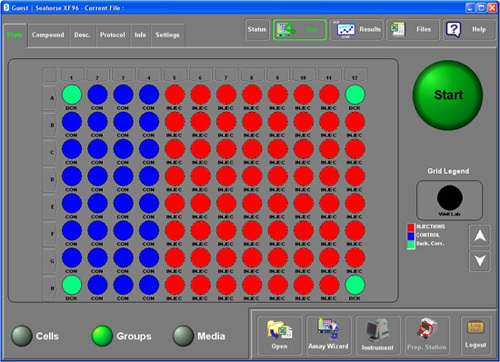 Figure 1. Well grid layout identifying column and group assignments
Figure 1. Well grid layout identifying column and group assignments
6. Compound Preparation
Prepare the following compounds in XF DMEM Assay media as follows: 10 uM Oligomycin, 30.0 uM FCCP, 20.0 μM Rotenone, These concentrations represent the 10X dilution that will be made when the compounds are injected into the well. The working concentrations are: 1 uM Oligomycin, 3.0 uM FCCP, 2.0 μM Rotenone
7. Media Change and Cell Preparation
Place the cell plate on the XF Prep Station
Set the final volume of medium to 160 μL per well.
Incubate in 37°C incubator without CO2 for 60 minutes to allow cells to pre-equilibrate with the assay medium.
8. Loading Sensor Cartridge
- Warm compounds to 37°C prior to loading sensor cartridge and load the compounds into the injector ports as follows:
- Columns 1-4: Load 16, 18, and 20 μL of XF Assay media (DMEM) into ports A, B and C, respectively.
- Columns 5-12: Load 16 μL of Oligomycin into ports A Load 18 μL of FCCP into ports B Load 20 μL of Rotenone into ports C
9. Protocol Commands
| Command | Time (min) | Port |
| Calibrate | ||
| Equilibrate | ||
| Loop Start | 3X | |
| Mix | 3 | |
| Wait | 2 | |
| Measure | 3 | |
| Loop End | ||
| Inject | A | |
| Loop Start | 2X | |
| Mix | 3 | |
| Wait | 2 | |
| Measure | 3 | |
| Inject | B | |
| Loop Start | 2X | |
| Mix | 3 | |
| Wait | 2 | |
| Measure | 3 | |
| Inject | C | |
| Loop Start | 2X | |
| Mix | 3 | |
| Wait | 2 | |
| Measure | 3 | |
| End |
Table 1. Protocol commands
Discussion
This assay is derived from the classic experiment to probe mitochondrial function and serves as a framework with which to build more complex experiments aimed at understanding various changes in cell metabolism, mitochondrial function, and overall bioenergetics.
All compounds used in this experiment should be optimized for the concentration that provides the maximal effect. That is, one must perform separate titration experiments to ascertain these values. This is especially important with FCCP, as the titration curve tends to be quite sharp, and too much FCCP can actually diminish responses in OCR. Typical ranges (final concentrations) to test would be:
0.1 - 1.0 ug/ mL of Oligomycin
0.1 - 5.0 uM FCCP
0.1 - 1.0 uM Rotenone
Note that the responses to each compound above (especially FCCP) will be influenced by the assay media composition (base type, [glucose], [pyruvate], presence/absence of BSA, etc). Further, if the XF assay media composition is changed, optimization will need to be re-performed. The presence and concentration of pyruvate is especially important in obtaining the maximal respiratory capacity due to FCCP. Seahorse Bioscience has observed in a number of cells lines that omission of pyruvate abrogates the ability of cells to respond maximally (above baseline) to FCCP. Typically, concentrations of 1-10 mM pyruvate should be tested to understand the optimal concentration of pyruvate to obtain maximal respiration. Note that [pyruvate] AND [glucose] may need to be "cross-titrated" to obtain the optimal media conditions for the experiment.
Typical results of this experiment are presented below in a graph showing OCR vs. time and another showing ECAR vs. time:
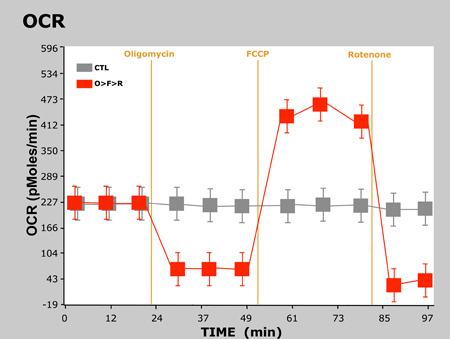 Figure 2. OCR vs. Time
Figure 2. OCR vs. Time
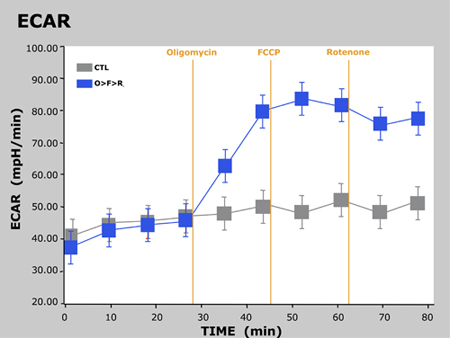 Figure 3. ECAR vs. Time
Figure 3. ECAR vs. Time
Here we observed the expected responses in OCR and ECAR as the cells are treated with each successive compound. For oligomycin, OCR decreases as a result of blocking ATP synthesis at mitochondrial Complex V. Since the cells are unable to synthesize ATP via OXPHOS, they revert to glycolysis to meet their demand for ATP, thus we observe an increase in ECAR. As shown previously, FCCP acts as an uncoupling agent. Since the cells must now overcome the proton leak across the inner mitochondrial membrane, OCR increases significantly as more O2 is consumed to pump the excess protons back across the mitochondrial membrane. Finally, rotenone inhibits mitochondrial Complex I and Complex III, respectively, which causes the flow of electrons to cease in the electron transport chain, and thus the consumption of O2 is drastically reduced.
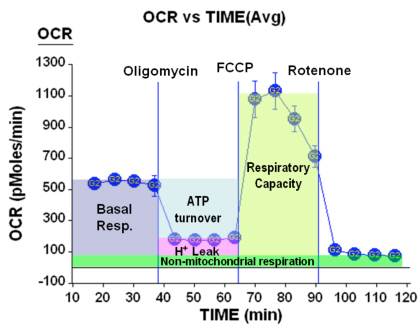 Figure 4. Respiration parameters
Figure 4. Respiration parameters
Beyond the expected changes in respiration and ECAR, a number of respiratory parameters may be obtained from this data. This is summarized in the figure above:
Here we see that we may obtain information about the basal respiration of the cells, the percent of O2 consumption devoted to ATP production as well as the amount devoted to maintaining the proton gradient (due to H+ leak). Further, we may obtain the maximal respiratory rate under conditions of uncoupled respiration (sometimes referred to as spare respiratory capacity) and finally, we can determine the amount of O2 consumption not due to mitochondrial processes.
A rapidly growing number of studies are employing this mitochondrial profile to assess cellular bioenergetics, identify mitochondrial dysfunction and to predict the ability of cells to respond to stress and/or insults. For more information and details about this experimental method and the idea of spare respiratory capacity, please see refer to the following publications 1-8.
Disclosures
Min Wu, Per Bo Jensen, George W. Rogers, and David A. Ferrick are employees of Seahorse Bioscience, which produces reagents and instrumentation featured in this article.
References
- Choi WS, Gerencser AA, Nicholls DG. Bioenergetic analysis of isolated cerebrocortical nerve terminals on a microgram scale: spare respiratory capacity and stochastic mitochondrial failure. J Neurochem. 2009;109:1179–1191. doi: 10.1111/j.1471-4159.2009.06055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill BG, Dranka BP, Zou L, Chatham JC, Darley-Usmar V. Importance of the bioenergetic reserve capacity in response to cardiomyocyte stress induced by 4-hydroxynonenal. Biochem J. 2009;424:99–107. doi: 10.1042/BJ20090934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Cao L, Chen J, Song S, Lee IH, Quijano C, Liu H, Keyvanfar K, Chen H. Bmi1 regulates mitochondrial function and the DNA damage response pathway. Nature. 2009:459–7245. doi: 10.1038/nature08040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmgren S, Nicholls DG, Taneera J, Bacos K, Koeck T, Tamaddon A, Wibom R, Groop L, Ling C, Mulder H, Sharoyko VV. Tight coupling between glucose and mitochondrial metabolism in clonal beta-cells is required for robust insulin secretion. J Biol Chem. 2009;284:32395–32404. doi: 10.1074/jbc.M109.026708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dranka BP, Hill BG, Darley-Usmar VM. Mitochondrial reserve capacity in endothelial cells: The impact of nitric oxide and reactive oxygen species. Free Radic Biol Med. 2010;48:905–914. doi: 10.1016/j.freeradbiomed.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez J, Hill BG, Benavides GA, Dranka BP, Darley-Usmar VM. Role of cellular bioenergetics in smooth muscle cell proliferation induced by platelet-derived growth factor. Biochem J. 2010;428:255–267. doi: 10.1042/BJ20100090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morán M, Rivera H, Sánchez-Aragó M, Blázquez A, Merinero B, Ugalde C, Arenas J, Cuezva JM, Martín MA. Mitochondrial bioenergetics and dynamics interplay in complex I-deficient fibroblasts. Biochim Biophys Acta. 2010:1802–185. doi: 10.1016/j.bbadis.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Cárdenas C, Miller RA, Smith I, Bui T, Molgó J. Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell. 2010:142–142. doi: 10.1016/j.cell.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]


