Abstract
The molecular characterization of muscular dystrophies and myopathies in humans has revealed the complexity of muscle disease and genetic analysis of muscle specification, formation and function in model systems has provided valuable insight into muscle physiology. Therefore, identifying and characterizing molecular mechanisms that underlie muscle damage is critical. The structure of adult Drosophila multi-fiber muscles resemble vertebrate striated muscles 1 and the genetic tractability of Drosophila has made it a great system to analyze dystrophic muscle morphology and characterize the processes affecting muscular function in ageing adult flies 2. Here we present the histological technique for preparing paraffin-embedded and frozen sections of Drosophila thoracic muscles. These preparations allow for the tissue to be stained with classical histological stains and labeled with protein detecting dyes, and specifically cryosections are ideal for immunohistochemical detection of proteins in intact muscles. This allows for analysis of muscle tissue structure, identification of morphological defects, and detection of the expression pattern for muscle/neuron-specific proteins in Drosophila adult muscles. These techniques can also be slightly modified for sectioning of other body parts.
Keywords: Basic Protocols, Issue 46, Drosophila, muscles, histology, paraffin-embedded sections, cryosections
Protocol
1. Preparation
Freshly prepare Carnoy's fixative by combining absolute ethanol, chloroform and glacial acetic acid in proportion 6:3:1 respectively.3 This, as well as all solutions for submerging collars should be kept in glass staining jars (see reagents section for recommendation).
Prepare aluminum foil pockets the correct size for the collars.
Prepare the following solutions in staining jars: 2 X 40% ethanol, 70% ethanol, 2 X 100% ethanol, methylbenzoate (MB), 50/50 v/v MB and paraffin, 2 X paraffin. Place the MB and paraffin containers in an incubator set to 60-65°C.
Warm paraffin to pour into foil pockets to 60-65°C.
2. Fixing Flies in Collars
Attach the collar 4 under the binocular using tape in a vertical position where you can see the entry point for the fly.
Anesthetize flies using carbon dioxide or via hypothermia using an ice block. Be careful not to freeze the flies.
Using forceps, pick up individual flies by grabbing their wings and place into the collar oriented properly (head and thorax on top of the blades and abdomen below the blades). 10-20 flies should easily fit into the collar.
Note: If you are analyzing several genotypes, do not forget to make a note of the collar number and corresponding genotype.
3. Paraffin Sections of Drosophila Thoraxes
Relocate the collar to the Carnoy's solution and fix the tissue at 4°C over night.
After fixation, dehydrate the sample using increasing concentrations of ethanol. For 10 minutes each submerge the collar into 40 % (2 times), 70 % and 100 % (2 times) ethanol at room temperature. Next incubate collar in MB and MB + paraffin solution (1:1) for 30 min in each and then infiltrate the collar in two changes of paraffin for 60 min each at 60-65°C. Rapidly relocate the collar into the foil pocket and fill with melted (60-65°C) paraffin. Place it at room temperature and allow the paraffin to become hard (it is best to leave overnight). Note that the collar can be placed in the foil pocket in different orientations, depending on the orientation of the sections you need (longitudinal or transverse).
Unwrap dry paraffin blocks with collars from the foil and gently separate the collar from the paraffin block. With the help of a sharp blade or scalpel carefully cut out the extra-paraffin from around the fly tissue.
Cut the paraffin block with 7-10 μm section steps on a rotation microtome and allow the cut tissue to float flat in a 37°C water bath. Place the sectioned tissue on polar slides and allow drying over night. These slides can be used for staining with hematoxyline and eosin (Figure 1A-D), toluidine blue, aniline blue or other dies to visualize tissue structures, as well as for antibody staining (Figure 1E-E``).
4. Cryosections of Drosophila Thoraxes
As with paraffin sections, prepare the flies in a collar and fashion an aluminum foil pocket. A freezing cooler will be needed to prepare the samples. Make sure that the cooler is around -60°C, use a little ethanol and dry ice to allow for reaching this temperature. An hour before starting the experiment put the bottle of cryo-embedding medium (Tissue-Tek O.C.T. compound) upside down in a 4°C fridge in order to cool it down and to minimize the formation of air bubbles.
Relocate the collar with flies to the foil pocket that has been pre-cooled for several minutes inside the freezing cooler and rapidly fill with the cryo-embedding compound. Let the sample freeze for 3-10 min. Carefully unwrap the formed block inside the cooler, gently separate the collar from the embedding block and put it at -20°C for at least one day.
Cut the frozen muscles on a cryo-microtome between -15 and -18°C with a section thickness of 10-15 μm. Place on polarized slides and keep at -20°C until ready to do further processing. We suggest fixing the tissue in 4 % formaldehyde PBS solution for 10 min at room temperature prior to antibody staining.
5. Lipids Detection in Drosophila Muscles
Lipid droplets can be detected with oil red O stain on cryosections using a protocol adopted from Sieber and Thummel 5.
After fixation, wash slides with water twice for 5 min, equilibrated in propylene glycol for 10 min and incubate for 3 h in oil red O stain at room temperature. Then wash samples 2 times for 5 min in propylene glycol and 30 min in PBS. Mount in 30 % glycerol.
6. Representative Results:
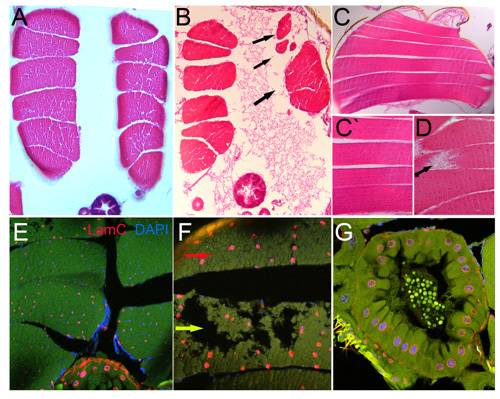 Figure 1. Parrafin-embedded sections Hematoxyline and eosine stained transverse (A-B) and longitudinal (C-D) sections of indirect flight muscles. A and C shows normal structured muscles. Abnormal by size and morphology muscles are represented on B and D respectively (black arrows). Transverse section of Drosophila thorax stained with anti-LamC, nuclear envelope marker and DAPI, nuclear stain (E-F). Enlarged view of normal (red arrow) and deteriorated (yellow arrow) muscles (F). G represents section of Drosophila intestinal tract stained with LamC and DAPI.
Figure 1. Parrafin-embedded sections Hematoxyline and eosine stained transverse (A-B) and longitudinal (C-D) sections of indirect flight muscles. A and C shows normal structured muscles. Abnormal by size and morphology muscles are represented on B and D respectively (black arrows). Transverse section of Drosophila thorax stained with anti-LamC, nuclear envelope marker and DAPI, nuclear stain (E-F). Enlarged view of normal (red arrow) and deteriorated (yellow arrow) muscles (F). G represents section of Drosophila intestinal tract stained with LamC and DAPI.
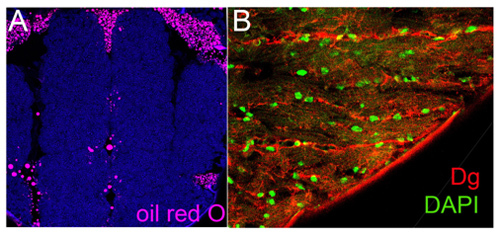 Figure 2. Frozen sectionsA. Transverse frozen sections of Drosophila thorax stained with oil red O, lipid droplets label. B. Longitudinal frozen sections of indirect flight muscles stained with anti-Dg, muscle sarcolemma marker and DAPI.
Figure 2. Frozen sectionsA. Transverse frozen sections of Drosophila thorax stained with oil red O, lipid droplets label. B. Longitudinal frozen sections of indirect flight muscles stained with anti-Dg, muscle sarcolemma marker and DAPI.
Disclosures
No conflicts of interest declared.
Acknowledgments
We thank Prof. Eichele for allowing us to use the cryo-microtome. Work was funded by Max-Planck-Gesselschaft.
References
- Miller A. Cold Spring Harbor: CSHL Press; 1950. The internal anatomy and histology of the imago of Drosophila melanogaster. [Google Scholar]
- Shcherbata HR. Dissecting muscle and neuronal disorders in a Drosophila model of muscular dystrophy. The EMBO journal. 2007;26:481–481. doi: 10.1038/sj.emboj.7601503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucherenko MM. Genetic modifier screens reveal new components that interact with the Drosophila dystroglycan-dystrophin complex. PloS one. 2008;3:e2418–e2418. doi: 10.1371/journal.pone.0002418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puchtler H, Waldrop FS, Conner HM, Terry MS. Carnoy fixation: practical and theoretical considerations. Histochemie. 1968;16:361–36. doi: 10.1007/BF00306359. [DOI] [PubMed] [Google Scholar]
- Ashburner M. Drosophila - A Laboratory Manual. CSHL Press; 1989. [Google Scholar]
- Sieber MH, Thummel CS. The DHR96 nuclear receptor controls triacylglycerol homeostasis in Drosophila. Cell metabolism. 2009;10:481–481. doi: 10.1016/j.cmet.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]


