Abstract
β-arrestins function as endocytic adaptors and mediate trafficking of a variety of cell-surface receptors, including seven-transmembrane receptors (7TMRs). In the case of 7TMRs, β-arrestins carry out these tasks while simultaneously inhibiting upstream G protein-dependent signaling and promoting alternate downstream signaling pathways. The mechanisms by which β-arrestins interact with a continuously expanding ensemble of protein partners and perform their multiple functions including trafficking and signaling are currently being uncovered. Molecular changes at the level of protein conformation as well as posttranslational modifications of β-arrestins likely form the basis for their dynamic interactions during receptor trafficking and signaling. It is becoming increasingly evident that β-arrestins, originally discovered as 7TMR adaptor proteins, indeed have much broader and more versatile roles in maintaining cellular homeostasis. Here we review the traditional and novel functions of β-arrestins and discuss the molecular attributes that might facilitate it multiple interactions in regulating cell signaling and receptor trafficking.
Regulation of 7-Transmembrane Receptors
β-arrestins 1 and 2 (aka arrestins 2 and 3) along with the visual rod (arrestin1) and cone (arrestin4) arrestins constitute a small family of cytosolic adaptor proteins[1–5]. The initial discovery and cloning of β-arrestins 1 and 2 immediately followed the characterization of the visual rod arrestin [6]. The three proteins were identified as critical mediators of desensitization of their cognate 7TMRs, namely, the β2 adrenergic receptor (β2AR, for β-arrestins) and Rhodopsin (for rod arrestin). A series of in vitro reconstitution experiments involving β2AR complexes and the quenching of the activity of the effector adenylyl cyclase led to the conclusion that 7TMR desensitization minimally involves two steps, namely, seryl-threonyl phosphorylation of agonist-stimulated receptors by the G protein-coupled receptor kinases (GRKs) followed by high affinity interactions of β2AR and non-visual arrestins [7]. The GRK family consists of seven homologs, of which GRKs 1 and 7 phosphorylate the visual opsins, whereas the GRKs 2–6 regulate various members of the huge family of 7TMRs as well as other cell-surface receptors [8]. It is now established that β-arrestins and GRKs serve as universal regulators of the 7TMR family.
7TMRs respond to a wide array of extracellular stimuli that includes hormones, neurotransmitters, lipids, peptides, ions and sensory stimuli and elicit a wide variety of physiological responses, making them important molecules for drug discovery. It is estimated that at least 40% of marketed prescription drugs are either agonists or antagonists acting either directly or indirectly at the 7TMRs [9]. It is noteworthy that the two non-visual arrestins and four ubiquitously expressed GRKs regulate most of the large number of members of the7TMR super-family. Although the various 7TMRs show very different patterns of expression and functions, they are all related by virtue of their highly conserved heptahelical structure.
Upon agonist-stimulation, 7TMRs undergo conformational changes, thus exposing binding sites for heterotrimeric G proteins in their intracellular domains. This leads to exchange of GDP for GTP on the Gα subunit initiating the dissociation of G protein α and βγ dimers that act as signaling units and activate various effectors (e.g. adenylyl cyclase by the α subunit and K+ channels by βγ dimers) [10]. Thus, traditionally, most 7TMR signaling has been considered to be mediated by G protein-receptor coupling. Agonist-occupied 7TMRs become immediate substrates for GRK-mediated phosphorylation, and in turn, the phosphorylated receptors recruit the cytosolic adaptors β-arrestins 1 and 2. β-arrestin-binding blocks further G protein activation by sterically hindering access to the receptor binding domains causing desensitization of 7TMR-G protein signaling (Figure 1). In addition, β-arrestins also scaffold enzymes, such as phosphodiesterases and diacylglycerol kinases, which degrade second messengers generated by G protein coupling, thus providing additional mechanisms for efficient dampening of signaling [11,12]. This scaffolding function of β-arrestins has only been recently appreciated, raising the possibility that it might be a general mechanism by which β-arrestins engage different enzymatic machineries to deplete second messenger pools.
Figure 1. Multifaceted functions of β-arrestins.
Desensitization: Agonist-stimulation of 7TMRs leads to G protein coupling and activation, following which the receptors are rapidly phosphorylated by G protein-coupled receptor kinases (GRKs). Phosphorylated receptors present high affinity binding surfaces to recruit the cytosolic adaptors, β-arrestins. Steric binding by β-arrestin interferes with further G protein coupling leading to the desentization of G protein-dependent signaling. β-arrestins also scaffold the second messenger degrading enzymes (phosphodiesterase 4D, PDE4D that degrades cAMP and diacyl glycerol kinase or DAG-K that converts diacyl glycerol to phosphatidic acid).
Endocytosis: Agonist-stimulation promotes rapid internalization of cell-surface 7TMRs into clathrin-coated vesicles. This internalization is facilitated by β-arrestin binding, which has specific binding domains for clathrin and AP2 interactions. β-arrestin binding to other endocytic proteins is also required for efficient receptor internalization: N ethyl maleimide fusion protein, NSF and ADP ribosylation factor 6, Arf6. The interaction between β-arrestin and the E3 ubiquitin ligase Mdm2 (mouse double minute 2) promotes ubiquitination of β-arrestin, that facilitates robust binding of β-arrestin with both cargo (7TMR) as well as endocytic machinery (clathrin and AP2). Receptor internalization is followed by post-endocytic sorting of internalized receptors for recycling or lysosomal degradation.
Signaling: β-arrestin acquires an active conformation upon forming a complex with agonist-stimulated 7TMRs and scaffolds MAP kinase, MAP kinase kinase, and MAP kinase kinase kinase, leading to the robust activation of MAP kinase, and subsequently targets the activated kinase to distinct subcellular compartments. Such β-arrestin-dependent MAP Kinase activity has been shown to regulate cellular chemotaxis, apoptosis, cancer metastasis and protein translation.
β-arrestins and 7TMR endocytosis
An almost universal property of cell-surface receptors (including 7TMRs) is their ability to internalize upon exposure to a ligand [13–15]. Many 7TMRs internalize via clathrin-coated vesicles, but other mechanisms of internalization also exist. Receptor trafficking is an intricate process that involves dynamic protein-protein interactions as well as activation or deactivation of associated proteins. Many well-characterized 7TMRs utilize β-arrestins as endocytic adaptors, but mechanisms involving alternate adaptors also exist [15]. The idea that β-arrestins are not just signal terminators was fueled by several studies that demonstrated novel roles for β-arrestins in mediating 7TMR endocytosis via clathrin-coated pits (CCPs), [16,17] which are invaginations occurring at the plasma-membrane that are enveloped with clathrin and adaptor-protein2 complexes. The CCPs, aided by the activity of the GTPase Dynamin, pinch off as clathrin-coated vesicles (CCVs) resulting in the internalization of cell-surface receptors. Overexpression of β-arrestin was shown to enhance agonist-stimulated β2AR internalization and rescue the endocytosis of defective mutant receptors [18]. That β-arrestins are essential for endocytosis of 7TMRs has been demonstrated by studies performed in mouse embryonic fibroblast cells isolated from β-arrestin1, 2 double knockout embryos [19]. More recently, this has been corroborated by small interfering RNA (siRNA)-mediated down regulation of β-arrestin isoforms in different cell types [20].
Studies that characterized a direct interaction between β-arrestin1 and clathrin have shed light on the mechanisms by which β-arrestins promote 7TMR endocytosis [15]. Clathrin-binding involves a highly conserved stretch of amino acids toward the C-terminal region of β-arrestins 1 and 2 [17]. A recent x-ray crystal structure of β-arrestin1 with clathrin suggests that additional interacting regions in β-arrestin1 (splice variant, L) might regulate dynamic β-arrestin-clathrin interactions [21,22]. Studies in the past decade have identified additional binding partners of β-arrestin that facilitate receptor internalization. These include interactions with the clathrin adaptor AP2, the N ethyl maleimide-sensitive fusion protein (NSF), the small G protein ARF6, and PI4P kinase, all of which exploit β-arrestin’s adaptor functions in mediating 7TMR endocytosis (Figure 1) [23,24]. Mass spectrometry-based analyses of β-arrestin immunoprecipitates also suggest that a complex network of β-arrestin interacting proteins regulate endocytosis [25]. The interaction of β-arrestins with the clathrin adaptor AP2 involves a peptide motif in the carboxyl terminal region of β-arrestin [26,27]. This region is homologous to the AP2 binding domain in autosomal recessive hypercholesterolemia (ARH) protein, an adaptor that links the low-density lipoprotein (LDL) receptor to AP2 and the clathrin machinery [27]. Upon receptor activation of β-arrestin, this homologous peptide assumes an α-helical conformation triggering β-arrestin-binding with the β2-appendage platform in AP2 thus promoting recruitment of 7TMR-β-arrestin complexes to CCVs [28].
Upon agonist-stimulation, many 7TMRs induce translocation of cytosolic β-arrestins to the plasma membrane, which can occur even in the absence of G protein activation and can be visualized by monitoring Green Fluorescent Protein (GFP)-β-arrestin [29,30]. The translocated β-arrestin forms a complex with the 7TMR, and the stability of these complexes governs the subsequent intracellular trafficking itinerary of the activated receptors [31,32]. Specific molecular determinants, namely the occurrence of a serine-threonine motif in the receptor C-tail, and the pattern of agonist-induced ubiquitination (covalent modification with Ubiquitin or Ub) of β-arrestin determine the stability of 7TMR-β-arrestin complexes [32–34]. 7TMRs such as the angiotensinII 1a (AT1a) and vasopressin 2 (V2) receptors that contain these clustered phosphorylation sites, induce sustained ubiquitination of β-arrestin2, and form tight complexes that cointernalize into endocytic vesicles. These complexes are also associated with sustained mitogen-activated protein kinase (MAPK) activity and function as ‘signalosomes’ (denoting a signaling receptor complex or scaffold associated with endosomes). Receptors that show this pattern of β-arrestin recruitment are referred to as “Class B” receptors [32]. By contrast, 7TMRs such as the β2AR that contain different phosphorylation codes in their cytoplasmic domains than the V2 or AT1a receptors, induce transient ubiquitination of β-arrestin2 and form only transient signaling complexes at the plasma membrane. Such receptors are referred to as “Class A” receptors [32]. Notably, replacing the β2AR-carboxyl tail with that of either the AT1aR or the V2R, or alternatively fusing Ub to the C-terminus of β-arrestin2 converts β2AR-β-arrestin complexes to stable signalosomes [33,35]. The serine-threonine clusters generally occur 9–19 residues downstream of the palmitoylation site found in the Rhodopsin family of 7TMRs and mutation of these clusters to alanines diminishes receptor phosphorylation and stability of β-arrestin recruitment for the neurotensin-1, oxytocin, AT1a and V2 receptors, but not for urotensin II receptor [34,36]. In some 7TMRs, where there are more than one phosphorylation cluster, the sites associated with β-arrestin binding are not necessarily the sites of predominant phosphorylation [37–39]. The stability of β-arrestin-7TMR interaction is also influenced by additional binding determinants present in other intracellular domains of 7TMRs [40–44]. The role of receptor carboxyl tail phosphorylation on β-arrestin recruitment and subsequent β-arrestin functions (desensitization, internalization, signaling) is also dependent upon the particular GRK isoform involved (see later section on Phosphorylation codes set by GRKs and reference [45] for more details).
The interaction of β-arrestin2 with the E3 ubiquitin ligase Mdm2 has been shown to be crucial for mediating 7TMR endocytosis. Mdm2 specifically and transiently ubiquitinates β-arrestin2 upon agonist-stimulation of the β2AR [46,47]. The functional effects of Mdm2-mediated β-arrestin2 ubiquitination have been addressed by multiple experimental approaches. Reduction of Mdm2 activity by using dominant negative forms, siRNA mediated knockdown, or Mdm2 null cells all ablate β-arrestin ubiquitination and β2AR internalization [46,47]. Moreover, ubiquitinated β-arrestin shows enhanced clathrin binding, 7TMR interaction as well as scaffolding of MAPKs, whereas nonubiquitinated β-arrestins are impaired in each of these functional interactions. This suggests that the Ub moieties on β-arrestin might serve as efficient binding platforms, which facilitate multiple interactions [48]. Indeed, Ub has been suggested to play such a role in other protein-protein interactions and cargo recognition along the endocytic pathway, because many endocytic proteins contain Ub binding and Ub recognition domains [49]. Interestingly, PalF, an Aspergillus nidulans protein that is distantly related to the arrestins, is also ubiquitinated upon activation of the 7TMR PalH by alkaline pH, suggesting that β-arrestin ubiquitination may be a modification that has been conserved during the evolutionary process [50].
In addition to ubiquitination, β-arrestin2 is SUMOylated (SUMO: small ubiquitin like modifier, that is covalently attached to lysines just as in ubiquitination[51]). The conserved SUMO motif ψ-K-x-E that serves as the recognition domain for the single SUMO-conjugating enzyme UBC-9 makes it possible to predict SUMOylation of substrate proteins in contrast with ubiquitination that does not involve a consensus motif and involves an array of E3 and E2 enzymes [52].
The lysine within the motif LKDE (ψ-K-x-E) in the carboxyl terminal region of bovine β-arrestin2 is suggested to be the major site of modification, and interestingly, this consensus SUMO motif is absent in human and murine β-arrestin [53]. Unlike ubiquitination, the reported SUMOylation of β-arrestin2 does not influence the affinity of 7TMR interaction, but is important for AP-2 interaction and receptor internalization via CCVs [53]. It is noteworthy that consensus SUMO motifs are also present in β-arrestin1 and GRKs as well as β1AR, β2AR, α2AR, but absent in AT1aR and V2R (Table 1). The roles of SUMOylation in β-arrestin1, GRKs and 7TMRs remain to be determined. It is intriguing that two of the well-characterized class B receptors lack these sites, whereas the established class A receptors contain them, which could be yet another factor differentiating trafficking properties of these two classes of 7TMRs.
β-arrestins facilitate trafficking of unconventional 7TMRs
Frizzleds (FZDs) are 7TMRs that are activated by the Wingless/Int-1 (WNT) family of secreted lipo-glycoproteins. WNT/FZD signaling is important for embryonic development, generation of cell polarity, maintenance of stem cell pluripotency and its dysregulation leads to degenerative diseases and cancer. FZDs bind to phosphorylated adaptor proteins called disheveled (DVL), and activation of FZDs leads to stabilization of the transcriptional factor β-catenin, its subsequent nuclear translocation, and transcription of specific genes [54]. β-arrestin2 is recruited to the DVL-FZD complex, and β-arrestin-DVL binding is required for the clathrin-dependent internalization of FZD4 receptor [55]. Additionally, WNT-11 induces the formation of a FZD7/DVL/AP-2 complex that translocates to Ras related gene from brain5 or RAB5-positive endosomes in the Xenopus dorsal marginal zone in a β-arrestin-dependent manner, and this process is critical for planar cell polarity signaling [56].
Another atypical 7TMR that is regulated by β-arrestin is the Smoothened (Smo) receptor that transduces hedgehog (Hh) signaling, which is important for vertebrate development. β-arrestin2 is recruited to Smo after GRK2 phosphorylation of Smo and leads to its clathrin-dependent internalization [57]. At cilia, β-arrestin2 interacts with Kif3a, a subunit of the kinesin-2 motor complex, which is an anterograde molecular motor that transports protein complexes to and within cilia. This interaction between b-arrestin and Kif3a is required for the localization of Smo in primary cilia and for efficient Gli-dependent transcription as well as proper functioning of the centrosome [58,59]. Knockdown of β-arrestin2 in zebra fish results in a phenotype with multiple defects, including defective somite patterning and craniofacial development, which are regulated by Hh [60]. The novel role of β-arrestin2 in Smo signaling is associated with GRK2-mediated phosphorylation of the Smo receptor cytoplasmic domains as demonstrated in HEK-293 cells, and zebra fish embryos [61,62]. Knockdown of zebra fish GRK2/3 results in attenuation of Hh transcriptional activity, impaired muscle development and neural patterning. These findings underscore a central role for the GRK-β-arrestin system in Smo signaling in vertebrates.
β-arrestins regulate endocytosis of non7TMRs and ion channels
Although β-arrestins were identified in the context of 7TMR regulation, there is increasing evidence that they function as adaptors for diverse cell surface receptor molecules. The first example was the demonstration that β-arrestin1 facilitates clathrin-dependent endocytosis of the insulin-like growth factor receptor, IGF-1R [63]. β-arrestin2 can also bind to and enhance endocytosis of the LDL receptor (LDLR) via clathrin-coated vesicles and further influence lipoprotein metabolism [64]. Generally, β-arrestins function as adaptors for phosphorylated forms of receptor and non-receptor proteins. However, their binding to nephrin, a single transmembrane receptor is prevented upon tyrosine phosphorylation of the receptor by Src-family member Yes [65]. Nephrin proteins are essential for proper renal filtration. Nephrin molecules in the glomerular slit diaphragm form a porous structure that results from the interaction between the extracellular domains (which are IgG-like and 35 nm in length) of neighboring nephrin molecules [66]. Internalization of nephrin-β-arrestin2 complexes is preceded by a loss of these homophilic interactions at the extracellular domains, decreased phosphorylation by Yes, and decreased binding to podocin. Loss of cell-surface nephrin molecules disrupts the porous structure of the glomerular slit diaphragm [65]. In contrast to the scenario with nephrins, β-arrestin2 binds to transforming growth factor (TGF)-β type III receptor upon receptor phosphorylation by the TGF-β type II receptor. This leads to endocytosis and downregulation of TGF-β signaling [67]. Akin to the TGF-β III receptor, the Na+/H+ exchanger NHE1 and NHE5 isoforms also internalize and are downregulated in a β-arrestin-dependent manner [68,69]. In addition, β-arrestins are key regulators of the ligand-gated ion channel nicotinic cholinergic receptor [70], cardiac Ca(v)1 voltage-gated channels [71] and the transient receptor potential (TRP) ion channel, TRPV4 [72] (see below).
β-arrestins act as adaptors for ubiquitin ligases and deubiquitinases and control post-endocytic sorting
Ubiquitination of cell-surface receptors has emerged as an important post-translational modification that defines the trafficking itinerary of internalized receptors [73,74]. Ubiquitination is a highly regulated process in which the C-terminal glycine of Ub is appended covalently to the epsilon amino group of a lysine residue in the substrate [75]. Transfer of ubiquitin to substrate requires an enzymatic cascade involving three distinct enzyme activities: first Ub is activated by the enzyme E1, activated Ub is bound and carried by the E2 enzyme, and finally substrate modification proceeds with the help of the third enzyme called E3 ubiquitin ligase (For a detailed review on this please see reference 75). It is estimated that ~600 E3 ligases ubiquitinate thousands of protein substrates with remarkable specificity and timing. Recognition of each substrate is a tightly regulated process, and it appears that E3 ligases have tailored distinct mechanisms for different substrates. For example, they can bind to phosphorylated domains or utilize adaptor proteins. As discussed below, β-arrestins act as indispensable E3 ligase adaptors for 7TMRs and non-7TMRs to mediate ubiquitination, and they may play an equally important role in escorting deubiquitinases (Figure 2). β-arrestins may also be involved in determining the dynamics of ubiquitination/deubiquitination of endocytic proteins that are important for proper steering of internalizing cargo across various vesicular pathways.
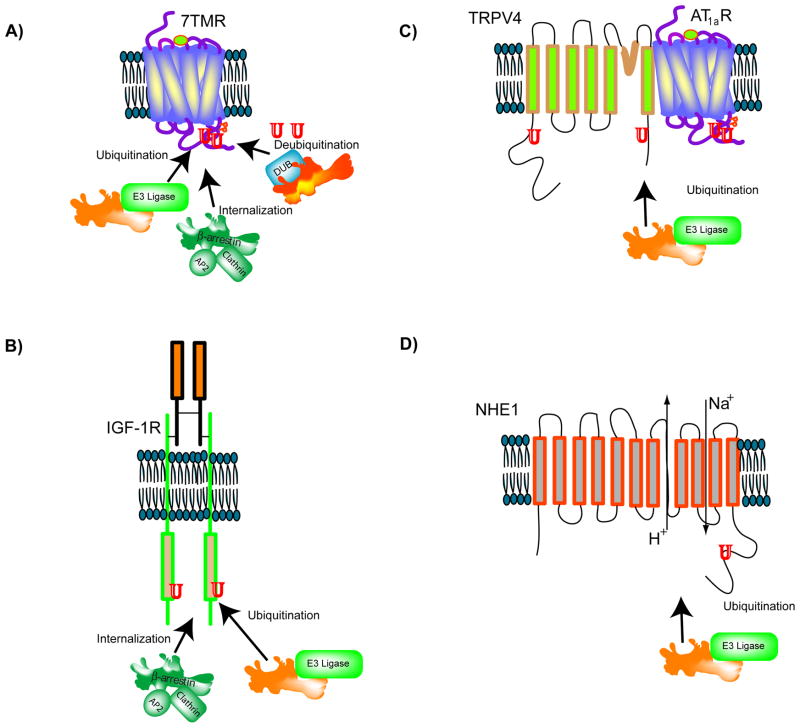
Figure 2. β-arrestins function as versatile adaptors for different cell-surface receptors.
A) For 7TMRs, β-arrestins act as clathrin-AP2 adaptors to facilitate receptor internalization, E3 ubiquitin ligase and deubiquitinase adaptors to facilitate receptor ubiquitination and deubiquitination. B) For the insulin-like growth factor receptor (IGF1R), β-arrestin1 functions as a clathrin adaptor as well as an E3 ligase adaptor, facilitating internalization and ubiquitination of IGF-activated receptors. C) The cell surface Transient Receptor Potential Vanilloid 4 channel (TRPV4) remains complexed with the Angiotensin 1a receptor (AT1aR) and upon AT1aR activation, becomes ubiquitinated in a β-arrestin1 dependent manner. In this process, β-arrestin1 functions as an E3 ubiquitin ligase adaptor. D) The Na+/H+ exchanger channel NHE1 is internalized and ubiquitinated in a β-arrestin-dependent manner.
For mammalian 7TMRs, ubiquitination was first demonstrated for the β2AR and the chemokine CXCR4 receptors [46,76]. Although prior studies had indicated that endocytosis of yeast 7TMRs Ste2 and Ste3 depends on ubiquitination, in the case of most mammalian 7TMRs, ubiquitination is not required for receptors to internalize, but is required for their targeting to lysosomal compartments for degradation [77–80]. β2AR ubiquitination is detected in β-arrestin-null mouse embryonic fibroblasts only after re-expression of β-arrestin2, which acts as the adaptor for recruiting the HECT-domain containing E3 ligase Nedd4 to the activated β2AR complex [46,80]. β-arrestin1 acts as an essential adaptor for the ubiquitination of the insulin-like growth factor 1 receptor (IGF-1R) by the RING-domain containing E3 ligase Mdm2, whereas β-arrestin2 serves the role for the androgen receptor [81,82]. Interestingly Mdm2 ubiquitinates β-arrestin2 itself and when scaffolded by β-arrestin2, it acts as an E3 ligase for GRK2 and phosphodiesterase (PDE)4D [46,83,84].
The adaptor role of β-arrestin1 has recently been shown for TRPV4 and NHE1 ubiquitination, in which the E3 ligases AIP4 and Nedd4-1 are involved, respectively [68,72]. In both cases, ubiquitination leads to internalization and subsequent downregulation of the ubiquitinated species. TRPV4 ubiquitination mediated by β-arrestin1-AIP4 is induced upon ligand-activation of the AT1aR receptor, thus linking a 7TMR and an ion channel and providing a mechanism for ubiquitin-dependent Ca2+ regulation. The E3 ligase adaptor role of β-arrestins is also observed beyond the mammalian system: Kurtz, the Drosophila β-arrestin homolog, binds and escorts the E3 ligase deltex to mediate ubiquitination of the Notch receptor[85]. Ub-dependent post-endocytic sorting of internalized receptors involves recognition of ubiquitinated cargo by the protein complexes termed ESCRTs, whose role has been extensively characterized in yeast [86]. An interaction between β-arrestin1 and STAM-1, a protein subunit of ESCRT-0 has been recently suggested to inhibit lysosomal degradation of CXCR4 [87].
β-arrestin2 also acts as an adaptor for two homologous deubiquitinases (USP20 and 33) that deubiquitinate the β2AR [88]. An increase in USP20/33 expression promotes receptor deubiquitination. This leads to an increase in recycling and resensitization of the β2AR, whereas inhibition of their enzymatic activity stabilizes receptor ubiquitination and promotes lysosomal degradation [88]. Deubiquitination of Protease Activated Receptor 2 (PAR2) involves other USP enzymes, but whether β-arrestins play a role in this process is not known [89].
β-arrestin-related proteins: the α-arrestins
β-arrestin homologs are not expressed in yeast and lower metazoans, but structurally similar β-arrestin-related proteins, called arrestin-related trafficking adaptors (ARTs) with the characteristic ‘arrestin domain’ fold have been shown to function as E3 ligase adaptors in yeast [90–92]. The yeast ARTs are localized at the late Golgi and translocate to the plasma membrane in response to chemical stress or changes in nutrients. At the membrane, they recognize and mediate ubiquitination of specific plasma membrane nutrient transporters, leading to their intracellular trafficking and degradation in the yeast vacuole [92]. The interesting feature that sets these ARTs apart from the mammalian β-arrestins is the presence of characteristic poly-proline motifs that are known to bind to WW domains contained in the HECT E3 ligase Rsp5 (yeast homolog of mammalian Nedd4). Whether the yeast ARTs regulate ubiquitination and trafficking of the 7TMRs, Ste2 and Ste3 remains to be determined.
Mammalian cells express five ART homologs, termed arrestin-domain containing proteins (ARRDC 1–5). In addition, vacuole sorting protein 26 VPS26, which has no sequence identity with β-arrestins, was also shown to share a similar structural fold with mammalian arrestins [93,94]. Vps26 forms part of a retromer subcomplex involved in recognition of cargo at the endosome and facilitates retrograde transport to the Golgi complex. β-arrestins and visual arrestins contain a ‘polar core’ region in their structure, disruption of which is thought to precede conformational changes and activation of arrestins [95]. VPS26 also has a similar polar core, but the structural contacts involve different side chains [93].
Future studies should reveal the exact role of these arrestin-related proteins in conjunction with those of β-arrestins during receptor trafficking and signalling. It is likely that β-arrestins utilize these extra adaptors to drive internalized receptor complexes through distinct vesicle populations. The fact that double knockout of β-arrestins1 and 2 is associated with pre- or perinatal mortality [19] indicates that the arrestin-related proteins do not complement β-arrestin functions in vivo. Thus, although there may be some commonalities between the β-arrestins and α-arrestins, β-arrestins have unique and critical roles in mediating cellular homeostasis and embryonic development.
β-arrestins balance 7TMR trafficking and signal transduction and lead to physiological outcomes
Receptor internalization was originally considered a means for turning off signaling because activated receptors are sequestered away from the cell surface and cannot bind extracellular ligands. However, it is now evident that, in many cases, signal transduction persists after the receptor has internalized, especially in the initiation of β-arrestin-dependent signaling, which coincides with the early stages of receptor endocytosis. β-arrestins 1 and 2 bind various kinases and regulatory proteins, and in many cases, β-arrestins function as receptor-activated scaffolds to coordinate upstream and downstream components of particular signal transduction pathways [8,96]. For example, β-arrestin binds cRAF-1 (upstream MAPK kinase kinase) and ERK2 (downstream MAPK) and recruits MEK1 (MAPK kinase), thus assembling the core components for activating ERK2 (Fig 1) [97,98]. Furthermore, such scaffold assembly is often potentiated by 7TMR activation, thus providing a stimulus-dependent signal transduction event to direct the biological response of the cell. An additional feature of such signaling scaffolds is that upon high affinity interaction of β-arrestin and 7TMR, they become localized on endosomes, (i.e. signalosomes), which bestows compartmentalization on 7TMR signaling [99,100]. Thus, β-arrestin-dependent signals are not only temporally distinct from the initial second-messenger responses generated by G protein signaling, they are also spatially segregated from them as well. Although a direct connection has not been demonstrated between endocytosis and all β-arrestin-dependent signaling pathways characterized thus far, it is likely that the two processes are intimately linked.
In general, a 7TMR agonist is capable of activation of G protein-dependent pathways and subsequent regulation and signaling by GRKs and β-arrestins. In the presence of bound β-arrestin2, phosphorylated β2AR and M2 muscarinic receptor (M2R) display increased affinity to agonists, suggesting that just as with G protein-receptor complexes, β-arrestin-phospho-receptor complexes can exist as ‘active’ ternary complexes [101]. It is now evident that certain ligands can preferentially activate β-arrestins while blocking or minimally activating G proteins or vice versa [102,103]. This ligand-directed signaling is defined as biased agonism, in which receptor-activated signal transduction (and perhaps physiological effects) are channeled through only one form of active coupling (either G protein or β-arrestin) [104,105]. The existence of distinct β-arrestin-biased signaling suggests the prevalence of distinct activated conformation(s) of the receptor for signaling through G proteins or β-arrestins. This necessitates further modification of receptor active-state models to include additional conformation(s) of the receptor and formation of additional ternary complexes [103,104]. Examples of biased-signaling and related physiological effects where a direct relationship to β-arrestin-dependent trafficking was demonstrated are discussed below (for a more extensive discussion and examples, please see reference #105)
Serotonin-stimulated ERK1/2 activation and internalization of 5-HT2ARs requires β-arrestin2 and leads to a head-twitch response in mice (this is an indication that the 7TMR, serotonin receptor is activated [106]. In β-arrestin2 KO mice, these effects are not initiated by serotonin, but another hallucinogenic ligand 2,5-dimethoxy-4-iodoamphetamine (DOI), induces ERK1/2, receptor trafficking and head twitch response in the absence of β-arrestin2. This provides an example of an interesting scenario in which a downstream pathway of receptor activity and its conformation is ligand-specific.
A recent study reports a completely novel β-arrestin-dependent trafficking and signaling effect in which docosahexaenic acid (DHA) and eicosapentaenoic acid (EPA), the major natural ω-3 fatty acid constituents of dietary supplements such as fish oil, cause anti-inflammatory effects by stimulating the 7TMR GPR120 and recruiting β-arrestins [107]. The GPR120-β-arrestin complex internalizes and sequesters the transforming growth factor –β activated kinase 1 (TAK1) binding protein, TAB1, and blocks the downstream activation of nuclear factor (NF)-kB and Janus kinase (JNK) thus inhibiting the inflammatory responses mediated by toll-like receptor4 (TLR4) and tumor necrosis factor α (TNFα). This blockade of inflammatory cytokine receptor signalling promotes the return of anti-inflammatory macrophages to adipose tissue and restores insulin sensitivity in obese mice [107]. β-arrestin-biased signaling and trafficking that are prevalent for the β2AR, β1AR, AT1aR, and parathyroid hormone1 receptor (PTH1R) in model cells are likely to be linked to distinct physiological effects observed in these cases, namely mitigation of catecholamine-induced cardiomyocyte apoptosis for the βARs and mediation β-arrestin-dependent anabolic bone formation for the PTH1R [105]. These studies suggest the exciting possibility that β-arrestin-dependent signaling via these receptors can be exploited for developing new drugs to prevent heart failure and treat osteoporosis and that β-arrestin-receptor signalosomes could also be targets for therapeutic intervention.
Molecular mechanisms regulating β-arrestin Functions
β-arrestins undergo post-translational modifications, most often in response to 7TMR stimulation (Figure 3), and as discussed below, these molecular changes in β-arrestin could represent its activated conformation(s), and form the basis of its various functional effects in mediating receptor trafficking and signaling.
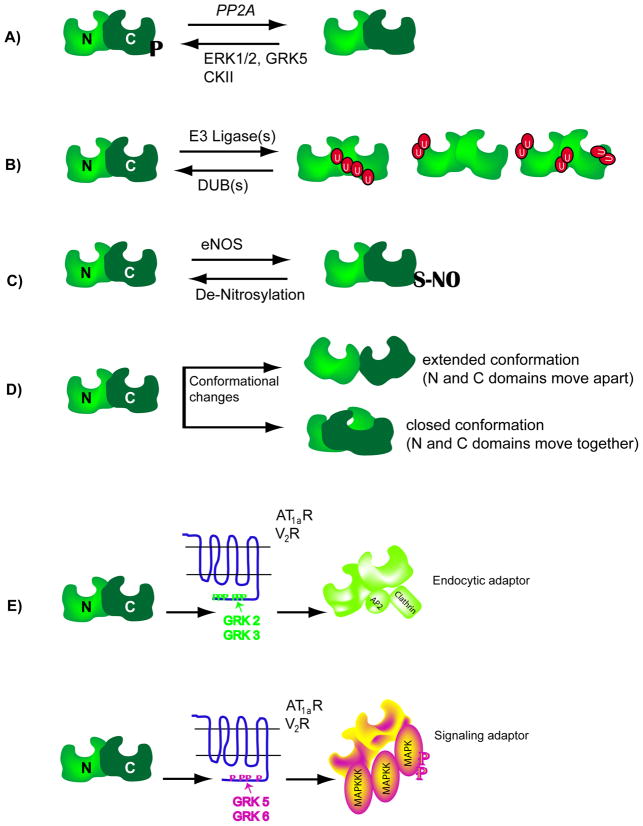
Figure 3. Schematics showing molecular changes in β-arrestin.
A) β-arrestins are phosphorylated at a seryl or threonyl residues in the C-terminal domain (see text for details) and become dephosphorylated upon 7TMR activation. Dephosphorylated β-arrestins interact more efficiently with clathrin than phosphorylated forms. Upon receptor internalization, β-arrestins become rephosphorylated. ERK1/2: extra cellular signal regulated kinase, GRK: G protein-coupled receptor kinase, CKII: casein kinase II, PP2A: protein phosphatase 2A. B) 7TMR activation leads to ubiquitination of β-arrestin2, the site of ubiquitination is unique to a particular 7TMR-β-arrestin pair, thus leading to differential modifications or differential display of ubiquitinated β-arrestins. C) The C-terminal cysteine residue in β-arrestin2 is nitrosylated and this modification promotes its interaction with clathrin. D) Upon binding to 7TMRs, the β-arrestin molecule undergoes conformational rearrangement, which could either push the amino (N) and carboxyl (C) domains apart or pull them together. The conformational changes have been assessed by various biochemical methods (limited proteolysis of purified β-arrestins, Resonance energy transfer between fluorophores attached to the two domains etc). E) When β-arrestin2 is recruited to the AT1aR or V2R that are phosphorylated by GRK2 or GRK3 isoforms, it is able to function as an endocytic adaptor, but not as a signaling adaptor. In contrast, when β-arrestin2 binds to the same receptors that are phosphorylated by GRK5 or GRK6 isoforms, it functions as a signaling adaptor leading to robust activation of MAP kinases ERK 1 and 2. It is suggested that the different GRK isoforms set respective phosphorylation ‘bar codes’ to dictate the downstream activity of β-arrestin.
Phosphorylation of β-arrestin
β-arrestin 1 and 2 are cytosolic phosphoproteins that, upon agonist-stimulated recruitment to the β2AR, become rapidly dephosphorylated (Figure 3A) [108–110]. In β-arrestin1, serine 412 is phosphorylated by ERK1/2 when β2ARs are activated and by GRK5 when 5-HT4 receptors are activated [108,111]. Threonine 383 in rat β-arrestin2 (which is threonine 382 of bovine β-arrestin2) is phosphorylated by casein kinase II [110,112]. Studies conducted with both phospho-mimetic (serine/threonine mutated to aspartate) and phosphorylation-impaired (serine/threonine mutated to alanine) mutants have indicated that dephosphorylation of β-arrestin1 and 2 is important for clathrin interaction and clathrin-dependent-receptor internalization. Such mutations of serine 412 or threonine 383 respectively in β-arrestin1 or 2 do not alter their properties with respect to receptor binding and desensitization. However, when β-arrestin1 is phosphorylated by GRK5, it triggers a unique sequence of events in which the G protein-independent signaling of 5-HT4 receptor to c-Src/ERK1/2 is inhibited [111]. A tyrosine (Y54) residue found only in the β-arrestin1 isoform is phosphorylated by c-Src, and this inhibits interaction with the mu subunit of adaptin2, thus lowering its ability to augment β2AR internalization [113]. β-arrestin2 has a natural substitution at this residue (phenylalanine 54) and has a more productive interaction with AP2 than β-arrestin1.
The exact mechanism of dephosphorylation of β-arrestins remains to be determined; however, the protein phosphatase PP2A has been shown to interact with β-arrestin2 upon Dopamine D2R as well as AT1aR activation [25,114,115], and the PP2A inhibitor okadaic acid increases β-arrestin Serine 412 phosphorylation resulting from insulin treatment [116]. Phosphorylation of β-arrestins appears to be a mechanism of feedback regulation for specific protein interactions during endocytosis in general and may regulate signaling at 7TMRs.
Ubiquitination of β-arrestins
As discussed above, Mdm2-dependent ubiquitination of β-arrestin2 is crucial for rapid agonist-promoted internalization of the β2AR. β-arrestin ubiquitination is also important for forming a high affinity complex with the β2AR [33,48]. Overexpression of Mdm2 augments β-arrestin ubiquitination and stabilizes β-arrestin-β2AR binding, thus promoting their cointernalization and colocalization on endosomes [47]. Comparison of two modified β-arrestins, one which lacks all ubiquitin acceptor sites and the other in which stable ubiquitination is conferred by fusing ubiquitin at the carboxyl terminus, have provided insights as to how β-arrestin ubiquitin might regulate both β2AR endocytosis and ERK1/2 signaling [48]. The stably ubiquitinated β-arrestin2-Ub fusion protein bound the activated β2AR, clathrin and RFP-ERK2 with higher affinity than wild-type β-arrestin and formed stable signalosomes, whereas the nonubiquitinated β-arrestin showed only weak or very transient binding with these partners [48]. Ubiquitination is also targeted to different domains of β-arrestin2 depending on the 7TMR that is being activated [35], thus providing a means of generating an ensemble of ubiquitinated-β-arrestins, differentially adorned with Ub moieties at distinct sites (Figure 3B). Differential ubiquitination could be a consequence of distinct conformational changes induced in β-arrestins when they bind to specific 7TMRs. It might also form the molecular basis by which β-arrestins bind a variety of protein-partners in distinct subcellular compartments, leading to distinct trafficking and signaling pathways.
The roles of β-arrestin ubiquitination in receptor binding, endocytosis and signaling are further illuminated by the discovery that it can be reversed by a specific deubiquitinating enzyme, USP33 [88]. Upon β2AR activation, the recruited β-arrestins are rapidly ubiquitinated and deubiquitinated, and this is facilitated by a high-affinity interaction between β-arrestin2 and USP33. This interaction leads to deubiquitination of β-arrestin and its dissociation from the internalizing β2AR, thus promoting only transient binding with activated ERK1/2. Indeed, depletion of USP33 not only stabilizes β-arrestin ubiquitination, but also promotes its stable interaction with the internalized β2AR on endosomes; it also increases the magnitude and duration of β-arrestin-dependent ERK1/2 activity [47]. By contrast, depletion of the E3 ubiquitin ligase Mdm2 ablates both β-arrestin ubiquitination and β-arrestin-dependent ERK1/2 activity [47]. Thus, the kinetics of β-arrestin ubiquitination and deubiquitination are tightly regulated by these cellular enzymes, which ensures the appropriate duration and magnitude of β-arrestin-biased signaling.
S-Nitrosylation of β-arrestin2
β-arrestin2, interacts with endothelial NO synthase (eNOS), an enzyme that releases NO in blood vessels. β-arrestin2 is S-nitrosylated at a single cysteine (C-terminal residue 410) by eNOS, and S-nitrosylation of β-arrestin2 is promoted by endogenous S-nitrosogluthathione [117]. Interestingly, S-nitrosylation of cysteine 410 enhances the interaction of β-arrestin2 with clathrin and β-adaptin, in striking contrast to the inhibitory effect of S-nitrosylation on the interaction between β-arrestin 2 and eNOS [117]. Although S-nitrosylation of β-arrestin2 was reported to increase β2AR internalization, its effect on β-arrestin-dependent signaling is not known at this time (Figure 3C).
Conformational changes in β-arrestin
β-arrestins 1 and 2 interact with numerous receptors and non-receptor proteins and many of these interactions are regulated by 7TMR stimulation. It is likely that the basal conformation of free form of β-arrestins is changed to an ‘active’ conformation upon recruitment to agonist-occupied 7TMRs (Figure 3D) [8,95,118]. The X-ray structures of bovine visual arrestin and β-arrestin1 in the basal inactive state indicate that arrestin is an elongated molecule with two domains (N- and C- domain), connected through a 12-residue linker region [119,120]. A notable feature is that of a hydrogen-bonded polar core, embedded between the N- and C-domains, which is disrupted by the phosphate moieties on the activated receptors, resulting in a conformational rearrangement within the β-arrestin molecule [121].
Consistent with this structural information, conformational changes have been demonstrated for β-arrestins 1 and 2 in the presence of a phosphopeptide that mimics the GRK-phosphorylated carboxyl tail of the V2R [122,123]. Addition of the phosphopeptide leads to the exposure of a buried tryptic cleavage site (arginine 393 in β-arrestin1 and arginine 394 in β-arrestin2) as well as to the release of the buried carboxyl terminus of β-arrestin, which contains the previously mapped sites for clathrin interaction [124]. In this “activated” conformation induced by the phosphopeptide, β-arrestin binding to clathrin is much more robust than in the presence of a nonphosphorylated peptide.
Recently conformational changes in β-arrestins have been demonstrated by monitoring intramolecular Bioluminescence Resonance Energy Transfer (BRET) between a donor enzyme, luciferase, which is fused at the amino terminus of β-arrestin2 and an acceptor fluorophore, yellow fluorescent protein (YFP) appended to the carboxyl terminus of the same β-arrestin molecule. Any intramolecular reorientation of β-arrestin causes a change in the amount of BRET, reflective of a conformational change in β-arrestin [125]. Recent studies with the above BRET biosensor have indicated a net decrease in BRET elicited by β-arrestin-biased mutant receptors or β-arrestin-biased ligands and an increase in BRET by the unbiased ligands or receptors[126] These conformational changes likely stabilize β-arrestins in particular orientations that expose or bury domains important in partner protein interactions. Because each receptor-β-arrestin pair may be bound in a particular manner, the conformational changes induced could be unique for a particular 7TMR-β-arrestin pair. Specific conformations of β-arrestins induced by different 7TMR binding can also determine the kinetics and stability of interaction of β-arrestin with the deubiquitinating enzyme USP33 [47].
Phosphorylation codes set by GRKs
GRK-mediated phosphorylation has been considered as a generic and global process that affects all available phosphorylation sites on the receptors, leading to β-arrestin binding. However recent analyses of GRK functions utilizing siRNA-mediated knockdown of individual GRKs have suggested that receptor phosphorylation is a more specific and targeted process. Moreover, the downstream effects of receptor phosphorylation by a particular GRK isoform are specialized and distinct. For example, if the AT1aR or V2R is phosphorylated by GRKs 2 or 3, β-arrestin-dependent ERK1/2 signaling is diminished, whereas receptor endocytosis and the magnitude of β-arrestin recruitment remain the same [127,128]. Furthermore, phosphorylation by GRKs 5 and 6 does not promote robust internalization and β-arrestin-binding, but is required for β-arrestin-dependent ERK1/2 activation [127,128]. This reciprocal regulation of β-arrestin-dependent processes by GRK-mediated receptor phosphorylation suggests that specific GRKs target specific phosphorylation sites on 7TMRs, and that the β-arrestin conformation recruited to such differentially modified receptors could be different leading to distinct functional outputs. In this framework, the differential patterns of receptor phosphorylation establish a ‘bar code’ for β-arrestin recruitment and activity (Figure 3E) [127,128].
Although the exact sites of phosphorylation targeted by each GRK are yet to be determined, several other studies support the bar code hypothesis. GRKs 2 and 3 target phosphosites within the third intracellular loop of the D2 dopamine receptor, and this is important for post-endocytic recycling of the receptor[129]. Phosphorylation of a subset of serines in the carboxyl tail of the follicle-stimulating hormone receptor (FSHR) is required for receptor desensitization, but not for β-arrestin-dependent ERK1/2 signaling [130]. Mutagenesis studies of distinct sets of serines and threonines on the β2AR carboxyl tail showed striking differences with respect to β-arrestin recruitment and receptor internalization [131]. In the somatin subtype 2A (SST2A) receptor, internalization and desensitization involves different phosphorylation sites [132]. In the thyrotropin-releasing hormone receptor (TRHR), the proximal (but not distal) phosphosites on the C tail evoke β-arrestin-dependent internalization and desensitization after its recruitment [39]. For the chemokine receptor CCR7, two endogenous agonists lead to phosphorylation of the receptor by different GRK isoforms: CCL19 leads to GRK3- and 6-mediated phosphorylation. By contrast, CCL21 engages only GRK6, leading to differential consequences namely, robust β-arrestin binding and desensitization only by CCL19 but β-arrestin-dependent ERK1/2 activation by both agonists [133]. Using tandem mass spectrometry, phosphosite-specific antibodies and selective knockdown of GRK isoforms, distinct phosphorylation sites were mapped on the CXCR4 [134]. This study also revealed that distinct sites of phosphorylation differentially regulated β-arrestin recruitment and ERK1/2 activation. Thus, the downstream effects of β-arrestin binding to the activated receptor may involve reading a bar code preset by distinct GRK-mediated phosphorylation events. Furthermore, GRK-mediated regulation could be differentially effective based on the tissue-specific expression of each GRK isoform, thus providing yet another level of specificity for β-arrestin-dependent internalization and signaling.
Concluding remarks
Over the past two decades our understanding about and appreciation of the multifaceted functions of β-arrestins have greatly evolved. Nonetheless, many aspects of the functional roles of β-arrestins remain poorly understood. How does β-arrestin bind the growing ensemble of interactors? How are these novel interactions choreographed during trafficking? How are the additional interactions/functions favored over its traditional roles in receptor binding? These are some outstanding questions that need to be addressed from a molecular and structural standpoint in the future.
Even though mapping of binding domains of several partners has been shown to involve distinct structural regions in β-arrestin, simultaneous binding of multiple partners is not likely. It is more likely that different β-arrestin molecules engage in different interactions, and these can occur simultaneously in different subcellular compartments. Furthermore, many β-arrestin-partner interactions could be dynamic in nature, precisely timed, and regulated by various molecular changes in β-arrestins such as ubiquitination. Whether ubiquitination of β-arrestin is a cause or consequence of β-arrestin’s conformational change is a debatable issue. Perhaps the conformational changes in β-arrestin lead to distinct ubiquitin modifications of β-arrestins by specific E3 ligases, which could provide unique protein-interaction surfaces based on the nature and length of polyubiquitin chain modifications on β-arrestin. On the other hand, the covalent attachment of ubiquitin or ubiquitin chains may further shift domains within β-arrestins causing changes in overall conformation. Nonetheless, these molecular features confer utmost specificity in space and time to β-arrestin-dependent trafficking and signaling mechanisms.
The discovery of β-arrestin-biased ligands has opened up a new approach for treating various diseases, which might lead to the formulation of drugs with higher efficacy, specificity and lesser side effects than the 7TMR-targeted medications currently available. Another developing idea is that the ‘active’ conformational states of β-arrestins are favored by distinct patterns of phosphorylation in 7TMRs (the bar code hypothesis), which enables transformation of recruited β-arrestins into different forms of active β-arrestins. This facilitates regulation of a wide-variety of cellular responses, including endocytic trafficking, signaling in various cellular compartments, gene transcription, protein translation, and processes such as chemotaxis and apoptosis. The analogy between β-arrestins and related arrestin-domain-containing adaptors in intracellular trafficking suggests that the endocytic adaptor role of β-arrestins might be evolutionarily conserved. However, the mammalian β-arrestins have acquired additional specialization with respect to the regulation of the super-family of 7TMRs that make them particularly well suited for targeting specific pathways in drug development.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lefkowitz RJ. Historical review: a brief history and personal retrospective of seven-transmembrane receptors. Trends Pharmacol Sci. 2004;25 (8):413–422. doi: 10.1016/j.tips.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Yamaki K, et al. Molecular cloning of the S-antigen cDNA from bovine retina. Biochem Biophys Res Commun. 1987;142 (3):904–910. doi: 10.1016/0006-291x(87)91499-9. [DOI] [PubMed] [Google Scholar]
- 3.Lohse MJ, et al. beta-Arrestin: a protein that regulates beta-adrenergic receptor function. Science. 1990;248 (4962):1547–1550. doi: 10.1126/science.2163110. [DOI] [PubMed] [Google Scholar]
- 4.Attramadal H, et al. Beta-arrestin2, a novel member of the arrestin/beta-arrestin gene family. J Biol Chem. 1992;267 (25):17882–17890. [PubMed] [Google Scholar]
- 5.Smith WC, et al. Cloning and functional characterization of salamander rod and cone arrestins. Invest Ophthalmol Vis Sci. 2000;41 (9):2445–2455. [PubMed] [Google Scholar]
- 6.Kuhn H, Wilden U. Deactivation of photoactivated rhodopsin by rhodopsin-kinase and arrestin. J Recept Res. 1987;7 (1–4):283–298. doi: 10.3109/10799898709054990. [DOI] [PubMed] [Google Scholar]
- 7.Benovic JL, et al. Functional desensitization of the isolated beta-adrenergic receptor by the beta-adrenergic receptor kinase: potential role of an analog of the retinal protein arrestin (48-kDa protein) Proc Natl Acad Sci U S A. 1987;84 (24):8879–8882. doi: 10.1073/pnas.84.24.8879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science. 2005;308 (5721):512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- 9.Wise A, et al. Target validation of G-protein coupled receptors. Drug Discov Today. 2002;7 (4):235–246. doi: 10.1016/s1359-6446(01)02131-6. [DOI] [PubMed] [Google Scholar]
- 10.Neves SR, et al. G protein pathways. Science. 2002;296 (5573):1636–1639. doi: 10.1126/science.1071550. [DOI] [PubMed] [Google Scholar]
- 11.Perry SJ, et al. Targeting of cyclic AMP degradation to beta 2-adrenergic receptors by beta-arrestins. Science. 2002;298 (5594):834–836. doi: 10.1126/science.1074683. [DOI] [PubMed] [Google Scholar]
- 12.Nelson CD, et al. Targeting of diacylglycerol degradation to M1 muscarinic receptors by beta-arrestins. Science. 2007;315 (5812):663–666. doi: 10.1126/science.1134562. [DOI] [PubMed] [Google Scholar]
- 13.Brown MS, et al. Receptor-mediated uptake of lipoprotein-cholesterol and its utilization for steroid synthesis in the adrenal cortex. Recent Prog Horm Res. 1979;35:215–257. doi: 10.1016/b978-0-12-571135-7.50009-6. [DOI] [PubMed] [Google Scholar]
- 14.Drake MT, et al. Trafficking of G protein-coupled receptors. Circ Res. 2006;99 (6):570–582. doi: 10.1161/01.RES.0000242563.47507.ce. [DOI] [PubMed] [Google Scholar]
- 15.Moore CA, et al. Regulation of receptor trafficking by GRKs and arrestins. Annu Rev Physiol. 2007;69:451–482. doi: 10.1146/annurev.physiol.69.022405.154712. [DOI] [PubMed] [Google Scholar]
- 16.Zhang J, et al. Dynamin and beta-arrestin reveal distinct mechanisms for G protein-coupled receptor internalization. J Biol Chem. 1996;271 (31):18302–18305. doi: 10.1074/jbc.271.31.18302. [DOI] [PubMed] [Google Scholar]
- 17.Goodman OB, Jr, et al. Beta-arrestin acts as a clathrin adaptor in endocytosis of the beta2-adrenergic receptor. Nature. 1996;383 (6599):447–450. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- 18.Ferguson SS, et al. Role of beta-arrestin in mediating agonist-promoted G protein-coupled receptor internalization. Science. 1996;271 (5247):363–366. doi: 10.1126/science.271.5247.363. [DOI] [PubMed] [Google Scholar]
- 19.Kohout TA, et al. beta-Arrestin 1 and 2 differentially regulate heptahelical receptor signaling and trafficking. Proc Natl Acad Sci U S A. 2001;98 (4):1601–1606. doi: 10.1073/pnas.041608198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahn S, et al. Desensitization, internalization, and signaling functions of beta-arrestins demonstrated by RNA interference. Proc Natl Acad Sci U S A. 2003;100 (4):1740–1744. doi: 10.1073/pnas.262789099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kern RC, et al. Arrestin2/clathrin interaction is regulated by key N- and C-terminal regions in arrestin2. Biochemistry. 2009;48 (30):7190–7200. doi: 10.1021/bi900369c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang DS, et al. Structure of an arrestin2-clathrin complex reveals a novel clathrin binding domain that modulates receptor trafficking. J Biol Chem. 2009;284 (43):29860–29872. doi: 10.1074/jbc.M109.023366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lefkowitz RJ, et al. New roles for beta-arrestins in cell signaling: not just for seven-transmembrane receptors. Mol Cell. 2006;24 (5):643–652. doi: 10.1016/j.molcel.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 24.Nelson CD, et al. Beta-arrestin scaffolding of phosphatidylinositol 4-phosphate 5-kinase Ialpha promotes agonist-stimulated sequestration of the beta2-adrenergic receptor. J Biol Chem. 2008;283 (30):21093–21101. doi: 10.1074/jbc.M800431200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao K, et al. Functional specialization of beta-arrestin interactions revealed by proteomic analysis. Proc Natl Acad Sci U S A. 2007;104 (29):12011–12016. doi: 10.1073/pnas.0704849104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laporte SA, et al. The interaction of beta-arrestin with the AP-2 adaptor is required for the clustering of beta 2-adrenergic receptor into clathrin-coated pits. J Biol Chem. 2000;275 (30):23120–23126. doi: 10.1074/jbc.M002581200. [DOI] [PubMed] [Google Scholar]
- 27.Mishra SK, et al. Functional dissection of an AP-2 beta2 appendage-binding sequence within the autosomal recessive hypercholesterolemia protein. J Biol Chem. 2005;280 (19):19270–19280. doi: 10.1074/jbc.M501029200. [DOI] [PubMed] [Google Scholar]
- 28.Edeling MA, et al. Molecular switches involving the AP-2 beta2 appendage regulate endocytic cargo selection and clathrin coat assembly. Dev Cell. 2006;10 (3):329–342. doi: 10.1016/j.devcel.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 29.Oakley RH, et al. The cellular distribution of fluorescently labeled arrestins provides a robust, sensitive, and universal assay for screening G protein-coupled receptors. Assay Drug Dev Technol. 2002;1 (1 Pt 1):21–30. doi: 10.1089/154065802761001275. [DOI] [PubMed] [Google Scholar]
- 30.DeWire SM, et al. Beta-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- 31.Oakley RH, et al. Association of beta-arrestin with G protein-coupled receptors during clathrin-mediated endocytosis dictates the profile of receptor resensitization. J Biol Chem. 1999;274 (45):32248–32257. doi: 10.1074/jbc.274.45.32248. [DOI] [PubMed] [Google Scholar]
- 32.Oakley RH, et al. Differential affinities of visual arrestin, beta arrestin1, and beta arrestin2 for G protein-coupled receptors delineate two major classes of receptors. J Biol Chem. 2000;275 (22):17201–17210. doi: 10.1074/jbc.M910348199. [DOI] [PubMed] [Google Scholar]
- 33.Shenoy SK, Lefkowitz RJ. Trafficking patterns of beta-arrestin and G protein-coupled receptors determined by the kinetics of beta-arrestin deubiquitination. J Biol Chem. 2003;278 (16):14498–14506. doi: 10.1074/jbc.M209626200. [DOI] [PubMed] [Google Scholar]
- 34.Oakley RH, et al. Molecular determinants underlying the formation of stable intracellular G protein-coupled receptor-beta-arrestin complexes after receptor endocytosis*. J Biol Chem. 2001;276 (22):19452–19460. doi: 10.1074/jbc.M101450200. [DOI] [PubMed] [Google Scholar]
- 35.Shenoy SK, Lefkowitz RJ. Receptor-specific ubiquitination of beta-arrestin directs assembly and targeting of seven-transmembrane receptor signalosomes. J Biol Chem. 2005;280 (15):15315–15324. doi: 10.1074/jbc.M412418200. [DOI] [PubMed] [Google Scholar]
- 36.Proulx CD, et al. Involvement of a cytoplasmic-tail serine cluster in urotensin II receptor internalization. Biochem J. 2005;385 (Pt 1):115–123. doi: 10.1042/BJ20040807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neuschafer-Rube F, et al. Identification of a Ser/Thr cluster in the C-terminal domain of the human prostaglandin receptor EP4 that is essential for agonist-induced beta-arrestin1 recruitment but differs from the apparent principal phosphorylation site. Biochem J. 2004;379 (Pt 3):573–585. doi: 10.1042/BJ20031820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Milasta S, et al. The sustainability of interactions between the orexin-1 receptor and beta-arrestin-2 is defined by a single C-terminal cluster of hydroxy amino acids and modulates the kinetics of ERK MAPK regulation. Biochem J. 2005;387 (Pt 3):573–584. doi: 10.1042/BJ20041745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones BW, Hinkle PM. Arrestin binds to different phosphorylated regions of the thyrotropin-releasing hormone receptor with distinct functional consequences. Mol Pharmacol. 2008;74 (1):195–202. doi: 10.1124/mol.108.045948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marion S, et al. A beta-arrestin binding determinant common to the second intracellular loops of rhodopsin family G protein-coupled receptors. J Biol Chem. 2006;281 (5):2932–2938. doi: 10.1074/jbc.M508074200. [DOI] [PubMed] [Google Scholar]
- 41.Nakamura K, et al. Seven non-contiguous intracellular residues of the lutropin/choriogonadotropin receptor dictate the rate of agonist-induced internalization and its sensitivity to non-visual arrestins. J Biol Chem. 2000;275 (1):241–247. doi: 10.1074/jbc.275.1.241. [DOI] [PubMed] [Google Scholar]
- 42.Kim OJ, et al. The role of phosphorylation in D1 dopamine receptor desensitization: evidence for a novel mechanism of arrestin association. J Biol Chem. 2004;279 (9):7999–8010. doi: 10.1074/jbc.M308281200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ouedraogo M, et al. Distinct motifs of neuropeptide Y receptors differentially regulate trafficking and desensitization. Traffic. 2008;9 (3):305–324. doi: 10.1111/j.1600-0854.2007.00691.x. [DOI] [PubMed] [Google Scholar]
- 44.Lan H, et al. An intracellular loop 2 amino acid residue determines differential binding of arrestin to the dopamine D2 and D3 receptors. Mol Pharmacol. 2009;75 (1):19–26. doi: 10.1124/mol.108.050542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tobin AB, et al. Location, location, location...site-specific GPCR phosphorylation offers a mechanism for cell-type-specific signalling. Trends Pharmacol Sci. 2008;29 (8):413–420. doi: 10.1016/j.tips.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shenoy SK, et al. Regulation of receptor fate by ubiquitination of activated beta 2-adrenergic receptor and beta-arrestin. Science. 2001;294 (5545):1307–1313. doi: 10.1126/science.1063866. [DOI] [PubMed] [Google Scholar]
- 47.Shenoy SK, et al. Beta-arrestin-dependent signaling and trafficking of 7-transmembrane receptors is reciprocally regulated by the deubiquitinase USP33 and the E3 ligase Mdm2. Proc Natl Acad Sci U S A. 2009;106 (16):6650–6655. doi: 10.1073/pnas.0901083106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shenoy SK, et al. Ubiquitination of beta-arrestin links seven-transmembrane receptor endocytosis and ERK activation. J Biol Chem. 2007;282 (40):29549–29562. doi: 10.1074/jbc.M700852200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hicke L, et al. Ubiquitin-binding domains. Nat Rev Mol Cell Biol. 2005;6 (8):610–621. doi: 10.1038/nrm1701. [DOI] [PubMed] [Google Scholar]
- 50.Penalva MA, et al. Ambient pH gene regulation in fungi: making connections. Trends Microbiol. 2008;16 (6):291–300. doi: 10.1016/j.tim.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 51.Johnson ES. Protein modification by SUMO. Annu Rev Biochem. 2004;73:355–382. doi: 10.1146/annurev.biochem.73.011303.074118. [DOI] [PubMed] [Google Scholar]
- 52.Muller S, et al. SUMO, ubiquitin’s mysterious cousin. Nat Rev Mol Cell Biol. 2001;2 (3):202–210. doi: 10.1038/35056591. [DOI] [PubMed] [Google Scholar]
- 53.Wyatt D, et al. Small ubiquitin-like modifier modification of arrestin-3 regulates receptor trafficking. J Biol Chem. 2011;286 (5):3884–3893. doi: 10.1074/jbc.M110.152116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schulte G, et al. beta-Arrestins - scaffolds and signalling elements essential for WNT/Frizzled signalling pathways? Br J Pharmacol. 2010;159 (5):1051–1058. doi: 10.1111/j.1476-5381.2009.00466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen W, et al. Dishevelled 2 recruits beta-arrestin 2 to mediate Wnt5A-stimulated endocytosis of Frizzled 4. Science. 2003;301 (5638):1391–1394. doi: 10.1126/science.1082808. [DOI] [PubMed] [Google Scholar]
- 56.Kim GH, Han JK. Essential role for beta-arrestin 2 in the regulation of Xenopus convergent extension movements. Embo J. 2007;26 (10):2513–2526. doi: 10.1038/sj.emboj.7601688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen W, et al. Activity-dependent internalization of smoothened mediated by beta-arrestin 2 and GRK2. Science. 2004;306 (5705):2257–2260. doi: 10.1126/science.1104135. [DOI] [PubMed] [Google Scholar]
- 58.Kovacs JJ, et al. Beta-arrestin-mediated localization of smoothened to the primary cilium. Science. 2008;320 (5884):1777–1781. doi: 10.1126/science.1157983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Molla-Herman A, et al. Targeting of beta-arrestin2 to the centrosome and primary cilium: role in cell proliferation control. PLoS One. 2008;3 (11):e3728. doi: 10.1371/journal.pone.0003728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wilbanks AM, et al. Beta-arrestin 2 regulates zebrafish development through the hedgehog signaling pathway. Science. 2004;306 (5705):2264–2267. doi: 10.1126/science.1104193. [DOI] [PubMed] [Google Scholar]
- 61.Meloni AR, et al. Smoothened signal transduction is promoted by G protein-coupled receptor kinase 2. Mol Cell Biol. 2006;26 (20):7550–7560. doi: 10.1128/MCB.00546-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Philipp M, et al. Smoothened signaling in vertebrates is facilitated by a G protein-coupled receptor kinase. Mol Biol Cell. 2008;19 (12):5478–5489. doi: 10.1091/mbc.E08-05-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lin FT, et al. beta-arrestins regulate mitogenic signaling and clathrin-mediated endocytosis of the insulin-like growth factor I receptor. J Biol Chem. 1998;273 (48):31640–31643. doi: 10.1074/jbc.273.48.31640. [DOI] [PubMed] [Google Scholar]
- 64.Wu JH, et al. The adaptor protein beta-arrestin2 enhances endocytosis of the low density lipoprotein receptor. J Biol Chem. 2003;278 (45):44238–44245. doi: 10.1074/jbc.M309450200. [DOI] [PubMed] [Google Scholar]
- 65.Quack I, et al. beta-Arrestin2 mediates nephrin endocytosis and impairs slit diaphragm integrity. Proc Natl Acad Sci U S A. 2006;103 (38):14110–14115. doi: 10.1073/pnas.0602587103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Patrakka J, Tryggvason K. Nephrin--a unique structural and signaling protein of the kidney filter. Trends Mol Med. 2007;13 (9):396–403. doi: 10.1016/j.molmed.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 67.Chen W, et al. Beta-arrestin 2 mediates endocytosis of type III TGF-beta receptor and down-regulation of its signaling. Science. 2003;301 (5638):1394–1397. doi: 10.1126/science.1083195. [DOI] [PubMed] [Google Scholar]
- 68.Simonin A, Fuster D. Nedd4–1 and beta-arrestin-1 are key regulators of Na+/H+ exchanger 1 ubiquitylation, endocytosis, and function. J Biol Chem. 2010;285 (49):38293–38303. doi: 10.1074/jbc.M110.115089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Szabo EZ, et al. beta-Arrestins bind and decrease cell-surface abundance of the Na+/H+ exchanger NHE5 isoform. Proc Natl Acad Sci U S A. 2005;102 (8):2790–2795. doi: 10.1073/pnas.0407444102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dasgupta P, et al. Nicotine induces cell proliferation by beta-arrestin-mediated activation of Src and Rb-Raf-1 pathways. J Clin Invest. 2006;116 (8):2208–2217. doi: 10.1172/JCI28164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lipsky R, et al. beta-Adrenergic receptor activation induces internalization of cardiac Cav1.2 channel complexes through a beta-Arrestin 1-mediated pathway. J Biol Chem. 2008;283 (25):17221–17226. doi: 10.1074/jbc.C800061200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 72.Shukla AK, et al. Arresting a transient receptor potential (TRP) channel: beta-arrestin 1 mediates ubiquitination and functional down-regulation of TRPV4. J Biol Chem. 2010;285 (39):30115–30125. doi: 10.1074/jbc.M110.141549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mukhopadhyay D, Riezman H. Proteasome-independent functions of ubiquitin in endocytosis and signaling. Science. 2007;315 (5809):201–205. doi: 10.1126/science.1127085. [DOI] [PubMed] [Google Scholar]
- 74.Shenoy SK. Seven-transmembrane receptors and ubiquitination. Circ Res. 2007;100 (8):1142–1154. doi: 10.1161/01.RES.0000261939.88744.5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 76.Marchese A, Benovic JL. Agonist-promoted ubiquitination of the G protein-coupled receptor CXCR4 mediates lysosomal sorting. J Biol Chem. 2001;276 (49):45509–45512. doi: 10.1074/jbc.C100527200. [DOI] [PubMed] [Google Scholar]
- 77.Hicke L, Dunn R. Regulation of membrane protein transport by ubiquitin and ubiquitin-binding proteins. Annu Rev Cell Dev Biol. 2003;19:141–172. doi: 10.1146/annurev.cellbio.19.110701.154617. [DOI] [PubMed] [Google Scholar]
- 78.Marchese A, et al. G protein-coupled receptor sorting to endosomes and lysosomes. Annu Rev Pharmacol Toxicol. 2008;48:601–629. doi: 10.1146/annurev.pharmtox.48.113006.094646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hislop JN, von Zastrow M. Role of ubiquitination in endocytic trafficking of G-protein-coupled receptors. Traffic. 2010;12 (2):137–148. doi: 10.1111/j.1600-0854.2010.01121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shenoy SK, et al. Nedd4 mediates agonist-dependent ubiquitination, lysosomal targeting, and degradation of the beta2-adrenergic receptor. J Biol Chem. 2008;283 (32):22166–22176. doi: 10.1074/jbc.M709668200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Girnita L, et al. {beta}-Arrestin is crucial for ubiquitination and down-regulation of the insulin-like growth factor-1 receptor by acting as adaptor for the MDM2 E3 ligase. J Biol Chem. 2005;280 (26):24412–24419. doi: 10.1074/jbc.M501129200. [DOI] [PubMed] [Google Scholar]
- 82.Lakshmikanthan V, et al. Identification of betaArrestin2 as a corepressor of androgen receptor signaling in prostate cancer. Proc Natl Acad Sci U S A. 2009;106 (23):9379–9384. doi: 10.1073/pnas.0900258106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li X, et al. Mdm2 directs the ubiquitination of beta-arrestin-sequestered cAMP phosphodiesterase-4D5. J Biol Chem. 2009;284 (24):16170–16182. doi: 10.1074/jbc.M109.008078. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 84.Salcedo A, et al. Mdm2 is involved in the ubiquitination and degradation of G-protein-coupled receptor kinase 2. Embo J. 2006;25 (20):4752–4762. doi: 10.1038/sj.emboj.7601351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mukherjee A, et al. Regulation of Notch signalling by non-visual beta-arrestin. Nat Cell Biol. 2005;7 (12):1191–1201. doi: 10.1038/ncb1327. [DOI] [PubMed] [Google Scholar]
- 86.Saksena S, et al. ESCRTing proteins in the endocytic pathway. Trends Biochem Sci. 2007;32 (12):561–573. doi: 10.1016/j.tibs.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 87.Malik R, Marchese A. Arrestin-2 interacts with the endosomal sorting complex required for transport machinery to modulate endosomal sorting of CXCR4. Mol Biol Cell. 2010;21 (14):2529–2541. doi: 10.1091/mbc.E10-02-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Berthouze M, et al. The deubiquitinases USP33 and USP20 coordinate beta2 adrenergic receptor recycling and resensitization. Embo J. 2009;28 (12):1684–1696. doi: 10.1038/emboj.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Murphy JE, et al. Endosomes: a legitimate platform for the signaling train. Proc Natl Acad Sci U S A. 2009;106 (42):17615–17622. doi: 10.1073/pnas.0906541106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Aubry L, et al. The arrestin fold: variations on a theme. Curr Genomics. 2009;10 (2):133–142. doi: 10.2174/138920209787847014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nikko E, et al. Arrestin-like proteins mediate ubiquitination and endocytosis of the yeast metal transporter Smf1. EMBO Rep. 2008;9 (12):1216–1221. doi: 10.1038/embor.2008.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lin CH, et al. Arrestin-related ubiquitin-ligase adaptors regulate endocytosis and protein turnover at the cell surface. Cell. 2008;135 (4):714–725. doi: 10.1016/j.cell.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 93.Collins BM, et al. Structure of Vps26B and mapping of its interaction with the retromer protein complex. Traffic. 2008;9 (3):366–379. doi: 10.1111/j.1600-0854.2007.00688.x. [DOI] [PubMed] [Google Scholar]
- 94.Shi H, et al. The retromer subunit Vps26 has an arrestin fold and binds Vps35 through its C-terminal domain. Nat Struct Mol Biol. 2006;13 (6):540–548. doi: 10.1038/nsmb1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gurevich VV, Gurevich EV. The molecular acrobatics of arrestin activation. Trends Pharmacol Sci. 2004;25 (2):105–111. doi: 10.1016/j.tips.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 96.Miller WE, Lefkowitz RJ. Expanding roles for beta-arrestins as scaffolds and adapters in GPCR signaling and trafficking. Curr Opin Cell Biol. 2001;13 (2):139–145. doi: 10.1016/s0955-0674(00)00190-3. [DOI] [PubMed] [Google Scholar]
- 97.DeFea KA, et al. beta-arrestin-dependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2. J Cell Biol. 2000;148 (6):1267–1281. doi: 10.1083/jcb.148.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Luttrell LM, et al. Activation and targeting of extracellular signal-regulated kinases by beta-arrestin scaffolds. Proc Natl Acad Sci U S A. 2001;98 (5):2449–2454. doi: 10.1073/pnas.041604898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.DeWire SM, et al. Beta-arrestin-mediated signaling regulates protein synthesis. J Biol Chem. 2008;283 (16):10611–10620. doi: 10.1074/jbc.M710515200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.DeFea KA. Beta-arrestins as regulators of signal termination and transduction: how do they determine what to scaffold? Cell Signal. 2011;23 (4):621–629. doi: 10.1016/j.cellsig.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 101.Gurevich VV, et al. Agonist-receptor-arrestin, an alternative ternary complex with high agonist affinity. J Biol Chem. 1997;272 (46):28849–28852. doi: 10.1074/jbc.272.46.28849. [DOI] [PubMed] [Google Scholar]
- 102.Violin JD, Lefkowitz RJ. Beta-arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol Sci. 2007;28 (8):416–422. doi: 10.1016/j.tips.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 103.Kenakin T. Collateral efficacy in drug discovery: taking advantage of the good (allosteric) nature of 7TM receptors. Trends Pharmacol Sci. 2007;28 (8):407–415. doi: 10.1016/j.tips.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 104.Rajagopal S, et al. Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nat Rev Drug Discov. 2010;9 (5):373–386. doi: 10.1038/nrd3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Whalen EJ, et al. Therapeutic potential of beta-arrestin- and G protein-biased agonists. Trends Mol Med. 2010;17 (3):126–139. doi: 10.1016/j.molmed.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schmid CL, et al. Agonist-directed signaling of the serotonin 2A receptor depends on beta-arrestin-2 interactions in vivo. Proc Natl Acad Sci U S A. 2008;105 (3):1079–1084. doi: 10.1073/pnas.0708862105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Oh da Y, et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142 (5):687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lin FT, et al. Clathrin-mediated endocytosis of the beta-adrenergic receptor is regulated by phosphorylation/dephosphorylation of beta-arrestin1. J Biol Chem. 1997;272 (49):31051–31057. doi: 10.1074/jbc.272.49.31051. [DOI] [PubMed] [Google Scholar]
- 109.Lin FT, et al. Feedback regulation of beta-arrestin1 function by extracellular signal-regulated kinases. J Biol Chem. 1999;274 (23):15971–15974. doi: 10.1074/jbc.274.23.15971. [DOI] [PubMed] [Google Scholar]
- 110.Lin FT, et al. Phosphorylation of beta-arrestin2 regulates its function in internalization of beta(2)-adrenergic receptors. Biochemistry. 2002;41 (34):10692–10699. doi: 10.1021/bi025705n. [DOI] [PubMed] [Google Scholar]
- 111.Barthet G, et al. Beta-arrestin1 phosphorylation by GRK5 regulates G protein-independent 5-HT4 receptor signalling. Embo J. 2009;28 (18):2706–2718. doi: 10.1038/emboj.2009.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kim YM, et al. Regulation of arrestin-3 phosphorylation by casein kinase II. J Biol Chem. 2002;277 (19):16837–16846. doi: 10.1074/jbc.M201379200. [DOI] [PubMed] [Google Scholar]
- 113.Marion S, et al. N-terminal tyrosine modulation of the endocytic adaptor function of the beta-arrestins. J Biol Chem. 2007;282 (26):18937–18944. doi: 10.1074/jbc.M700090200. [DOI] [PubMed] [Google Scholar]
- 114.Beaulieu JM, et al. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122 (2):261–273. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 115.Xiao K, et al. Global phosphorylation analysis of beta-arrestin-mediated signaling downstream of a seven transmembrane receptor (7TMR) Proc Natl Acad Sci U S A. 2010;107 (34):15299–15304. doi: 10.1073/pnas.1008461107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hupfeld CJ, et al. Insulin-induced beta-arrestin1 Ser-412 phosphorylation is a mechanism for desensitization of ERK activation by Galphai-coupled receptors. J Biol Chem. 2005;280 (2):1016–1023. doi: 10.1074/jbc.M403674200. [DOI] [PubMed] [Google Scholar]
- 117.Ozawa K, et al. S-nitrosylation of beta-arrestin regulates beta-adrenergic receptor trafficking. Mol Cell. 2008;31 (3):395–405. doi: 10.1016/j.molcel.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Palczewski K, et al. Phosphorylated rhodopsin and heparin induce similar conformational changes in arrestin. J Biol Chem. 1991;266 (28):18649–18654. [PubMed] [Google Scholar]
- 119.Hirsch JA, et al. The 2.8 A crystal structure of visual arrestin: a model for arrestin’s regulation. Cell. 1999;97 (2):257–269. doi: 10.1016/s0092-8674(00)80735-7. [DOI] [PubMed] [Google Scholar]
- 120.Han M, et al. Crystal structure of beta-arrestin at 1.9 A: possible mechanism of receptor binding and membrane Translocation. Structure. 2001;9 (9):869–880. doi: 10.1016/s0969-2126(01)00644-x. [DOI] [PubMed] [Google Scholar]
- 121.Vishnivetskiy SA, et al. An additional phosphate-binding element in arrestin molecule. Implications for the mechanism of arrestin activation. J Biol Chem. 2000;275 (52):41049–41057. doi: 10.1074/jbc.M007159200. [DOI] [PubMed] [Google Scholar]
- 122.Xiao K, et al. Activation-dependent conformational changes in {beta}-arrestin 2. J Biol Chem. 2004;279 (53):55744–55753. doi: 10.1074/jbc.M409785200. [DOI] [PubMed] [Google Scholar]
- 123.Nobles KN, et al. The active conformation of beta-arrestin1: direct evidence for the phosphate sensor in the N-domain and conformational differences in the active states of beta-arrestins1 and -2. J Biol Chem. 2007;282 (29):21370–21381. doi: 10.1074/jbc.M611483200. [DOI] [PubMed] [Google Scholar]
- 124.Krupnick JG, et al. Arrestin/clathrin interaction. Localization of the clathrin binding domain of nonvisual arrestins to the carboxy terminus. J Biol Chem. 1997;272 (23):15011–15016. doi: 10.1074/jbc.272.23.15011. [DOI] [PubMed] [Google Scholar]
- 125.Charest PG, et al. Monitoring agonist-promoted conformational changes of beta-arrestin in living cells by intramolecular BRET. EMBO Rep. 2005;6 (4):334–340. doi: 10.1038/sj.embor.7400373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shukla AK, et al. Distinct conformational changes in beta-arrestin report biased agonism at seven-transmembrane receptors. Proc Natl Acad Sci U S A. 2008;105 (29):9988–9993. doi: 10.1073/pnas.0804246105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kim J, et al. Functional antagonism of different G protein-coupled receptor kinases for beta-arrestin-mediated angiotensin II receptor signaling. Proc Natl Acad Sci U S A. 2005;102 (5):1442–1447. doi: 10.1073/pnas.0409532102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ren XR, et al. Different G protein-coupled receptor kinases govern G protein and beta-arrestin-mediated signaling of V2 vasopressin receptor. Proc Natl Acad Sci U S A. 2005;102 (5):1448–1453. doi: 10.1073/pnas.0409534102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Namkung Y, et al. G protein-coupled receptor kinase-mediated phosphorylation regulates post-endocytic trafficking of the D2 dopamine receptor. J Biol Chem. 2009;284 (22):15038–15051. doi: 10.1074/jbc.M900388200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kara E, et al. A phosphorylation cluster of five serine and threonine residues in the C-terminus of the follicle-stimulating hormone receptor is important for desensitization but not for beta-arrestin-mediated ERK activation. Mol Endocrinol. 2006;20 (11):3014–3026. doi: 10.1210/me.2006-0098. [DOI] [PubMed] [Google Scholar]
- 131.Krasel C, et al. Dual role of the beta2-adrenergic receptor C terminus for the binding of beta-arrestin and receptor internalization. J Biol Chem. 2008;283 (46):31840–31848. doi: 10.1074/jbc.M806086200. [DOI] [PubMed] [Google Scholar]
- 132.Liu Q, et al. Distinct phosphorylation sites in the SST2A somatostatin receptor control internalization, desensitization, and arrestin binding. Mol Pharmacol. 2008;73 (2):292–304. doi: 10.1124/mol.107.038570. [DOI] [PubMed] [Google Scholar]
- 133.Zidar DA, et al. Selective engagement of G protein coupled receptor kinases (GRKs) encodes distinct functions of biased ligands. Proc Natl Acad Sci U S A. 2009;106 (24):9649–9654. doi: 10.1073/pnas.0904361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Busillo JM, et al. Site-specific phosphorylation of CXCR4 is dynamically regulated by multiple kinases and results in differential modulation of CXCR4 signaling. J Biol Chem. 2010;285 (10):7805–7817. doi: 10.1074/jbc.M109.091173. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.