Abstract
B cell dysfunction represents a central feature in HIV infection and pathogenesis. Our recent studies have shown that peripheral and lymphoid double positive CD21+CD27+ B cells were able to become activated and proliferate at higher rates than other B cell subpopulations. Increased proliferation of tonsillar memory B cells were identified compared to other tissues examined. Here, we demonstrate the decreased proliferation of tonsillar memory (CD21+CD27+) B cells during acute SIV infection also suggests that these cells may play an important role in SIV pathogenesis. Our findings demonstrate that SIV infection may induce selective defective responses in specific tissues, by suppressing memory B cell proliferation in tissues.
Keywords: B-cells, Memory, Proliferation, SIV, Rhesus Macaque, BrdU
INTRODUCTION
Virus-induced immune-cell activation is one of the few widely accepted hallmarks of HIV/SIV pathogenesis and disease progression. HIV/SIV infection has been associated with a wide range of B cell defects, including increased frequencies of activated and terminally differentiated B cells expressing low levels of CD21 [1, 2], polyclonal hypergammaglobulinemia and the presence of immature/transitional CD10+ or exhausted CD27 negative B cells in blood [3–7], exhaustion of tissue-like memory (CD20(hi)/CD27(−)/CD21(lo)) B cells [8] and loss of memory B cell populations [9]. Early loss of B cells in spleen, lymph nodes and peripheral blood (PB) [10–13] in SIV and disruption of gut germinal center during acute HIV infection [14] may be an indicator of the early failure of adaptive immune responses. Although studies have been performed to understand the role of B cells in SIV pathogenesis by artificially depleting B cells [15, 16], the dynamics and mechanism of B cell loss during acute phase of SIV infection are not well characterized. Our recent studies have shown that double positive (DP) CD21+CD27+ B cells are memory cells that are capable of antibody production by polyclonal activation, and without additional help from T cells [17]. Memory CD21+CD27+ B cells were predominant in all lymphoid tissues except for PB. DP CD21+CD27+ B cells were also able to activate and proliferate at higher rates than other B cell subpopulations [17]. In this study, we examined and compared levels of proliferation of T cells and different B cell subsets in lymphoid tissues to correlate their rates of proliferation with plasma viral load.
This study shows that tonsillar CD21+CD27+ B cells are highly proliferating B cells compared to other tissues examined. Furthermore, these data demonstrate differences in proliferative responses of nonhuman primate B memory (CD21+CD27+) cell subsets and suggest that SIV infection may induce early defective responses in specific B cell subsets in specific tissues.
2. MATERIALS AND METHODS
2.1. Animals, virus, BrdU, and tissue sampling
Twenty female and 2 male Indian RMs (Macaca mulatta) between 3–16 years of age, which were initially negative for HIV-2, SIV, type D retrovirus and STLV-1 infection were examined in this study (Table 1). All macaques were given the nucleotide analog BrdU (60mg/kg in sterile saline, Sigma) intraperitoneally 24 hrs prior to euthanasia and tissue collection [17]. Ten female and 2 male RMs were infected either through intravenous, intravaginal or intrarectal route with 10–1000 TCID50 SIVMAC251. EDTA anti-coagulated blood, axillary lymph node (ALN), tonsil, spleen, and intestines (jejunum) were collected at necropsy for functional and/or phenotyping experiments. All RMs were housed at the Tulane National Primate Research Center in accordance with the regulations of the American Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) and all experiments were reviewed and approved by the Tulane Institutional Animal Care and Use Committee.
Table 1.
List of adult Indian Rhesus Macaques examined
| Category | Animal Number | Sex a | Virus | Days of infection | Dosage | Route b |
|---|---|---|---|---|---|---|
| Normal | GN70 | F | Nil | - | - | |
| GN66 | F | Nil | - | - | ||
| GN58 | F | Nil | - | - | ||
| BB01 | F | Nil | - | - | ||
| N483 | F | Nil | - | - | ||
| GN74 | F | Nil | - | - | ||
| CC10 | F | Nil | - | - | ||
| AG71 | F | Nil | - | - | ||
| HI55 | F | Nil | - | - | ||
| DJ78 | F | Nil | - | - | ||
|
| ||||||
| Acute Infection | T108 | F | SIVMAC251 | 8 | 500TCID50 | IV |
| M992 | F | SIVMAC251 | 13 | 500TCID50 | IV | |
| AV91 | M | SIVMAC251 | 10 | 500TCID50 | IV | |
| BA57 | F | SIVMAC251 | 8 | 500TCID50 | IV | |
| HI52 | F | SIVMAC251 | 8 | 500TCID50 | IV | |
|
| ||||||
| Chronic Infection | DV42 | F | SIVMAC251 | 227 | 500TCID50 | IVAG |
| HG56 | F | SIVMAC251 | 154 | 300TCID50 | IV | |
| HG49 | F | SIVMAC251 | 147 | 300TCID50 | IVAG | |
| HG58 | F | SIVMAC251 | 288 | 300TCID50 | IVAG | |
| FE53 | M | SIVMAC251 | 143 | 10TCID50 | IR | |
| EJ26 | F | SIVMAC251 | 308 | 100TCID50 | IV | |
| DR59 | F | SIVMAC251 | 313 | 1000TCID50 | IVAG | |
F and M denote female and male respectively.
IV, IVAG and IR denote intravenous, intravaginal and intrarectal route respectively.
All RMs received BrdU intraperitoneally 24h before sampling.
2.2. Lymphocyte isolation from tissues
Lymphocytes from the PB, intestine, ALN, tonsil, and spleen were isolated as previously described [17–22]. All cells were washed twice and resuspended in complete RPMI-5 medium containing 5% fetal calf serum (FCS) before staining. All lymphocytes were >90% viable by trypan blue dye exclusion method.
2.3. Immunofluorescent staining and flow cytometric analysis
For flow cytometry staining, cells were adjusted to 107 cells/ml and 100ul aliquots or 100ul of whole blood samples were incubated with appropriately diluted concentrations of antibodies for 30 min at 4°C. Whole blood, and spleen samples were then lysed and washed using a whole blood lysis protocol as previously described [19, 23, 24]. BrdU staining was peformed as reported earlier [17, 18]. Cells were kept protected from light at 4°C and acquisition was performed within 24 hrs of staining. Lymphocytes from ALN, jejunum lamina propria lymphocytes (LPL), and tonsil were stained and processed similar to blood tissues with the omission of the whole blood lysing technique [24]. Polychromatic (6–9 parameter) flow cytometric acquisition was performed on a Becton Dickinson LSRII instrument with three lasers (488nm blue laser, 633nm red laser and 407 violet laser) using FITC, PE, PE-Texas red, PE-Cy5, APC, Alexa 700, APC-Cy7, Pacific Blue, and Qdot655 as fluorochrome directly conjugated to antibodies. Single-stained controls for each fluorochrome were used for compensation settings. Monoclonal antibodies CD3 (SP32-2), CD20 (L27), CD21 (B-ly4), CD27 (M-T271), and BrdU FITC (3D4) were obtained from BD Biosciences [17]. CD8 (MHCD0817) and CD4 Qdot655 (T4/19Thy5D7) were obtained from Invitrogen and the NIH Nonhuman Primate Reagent Resource courtesy of Dr. K. Reimann (Harvard University, Cambridge, MA) respectively. CD27 (0323) was obtained from eBioscience.
At least 30,000 events were collected from each sample by gating on lymphocytes and data were analyzed using FlowJo software (TreeStar Inc.) version 9.1.
2.4. Quantitation of plasma viral RNA
Viral RNA in plasma was quantified by bDNA signal amplification assay (Siemens Diagnostics, CA) [25]. The lower limit of detection was 125 SIV RNA copies/ml of plasma.
2.5. Statistics
Results of experimental groups were compared using either a two-tailed Student’s paired t-test or nonparametric Mann-Whitney t test using Prism software (GraphPad software, SanDiego, CA). P values <0.05 were considered significant.
RESULTS AND DISCUSSION
Since we previously showed more DP CD21+CD27+ B cells had the capacity to produce antibodies following polyclonal stimulation (and without T cell help), and higher rates of proliferation than their SP counterparts [17], here we compared differences in proliferation rates in both T and B cell subsets from normal and SIVMAC251 infected RMs. Ten normal and 12 SIVMAC251 infected RMs (Table 1) were inoculated with BrdU 24 hrs prior to sampling to detect cells in S-phase (DNA synthesis) of division. Of the 12 SIV infected RMs examined, five were in acute infection (8–13 days pi) and seven were in the chronic phase of infection (143–313 days pi).
Plasma viral load and CD4+ T cell percentages from both PB and jejunum LPL tissues were quantified in all 10 normal and 12 SIVMAC251 infected RMs. Overall in both the acute and chronically infected RMs there was high plasma viremia proving that all the macaques were infected (Fig. S1). There was no significant difference in CD20+ B cell percentages between normal and acutely infected RMs in all lymphoid and peripheral tissues examined (Fig. S1). However, a significant increase in CD20+ B cells was detected in tonsillar lymphoid tissues compared to PB (P<0.05) (Fig. S1). CD20+ B cell percentages remained high in ALN and splenic lymphoid tissues in chronically infected RMs, but data were not significantly different from uninfected RMs.
CD21 surface expression as well as double positive CD21+CD27+ B cell subsets differs between PB and lymphoid B cell population (Fig. 1). By modeling the rate of BrdU uptake in vivo in normal and SIV infected adult RMs, we were able to compare differences in lymphocyte proliferation rates among B-cell subsets and T cells. In normal RMs, there was a higher proliferation in DP CD21+CD27+ B cells in all-lymphoid tissues (Fig. 2A). Significantly higher rates of DP CD21+CD27+ B cell proliferation were observed in tonsil than compared with any other tissue examined. These high rates of DP CD21+CD27+ B cell proliferation may help to maintain the pool of functional B cell populations in normal RMs. However, 8–13 days after SIV infection, there was a significant reduction in tonsillar DP memory B cell proliferation compared to other tissues, suggesting a rapid and severe localized defect occurs in tonsillar B cells in early infection (Fig. 2A; p<0.01). Decreased tonsillar B cell proliferation also persisted in chronically infected RMs compared to normal RMs (Fig. 2A; P<0.05), but there was a concurrent increased T cell proliferation in all other tissues examined. Jejunum LPL B cell subsets (DP CD21+CD27+ and SP CD21+CD27−) in chronically infected RMs showed increased proliferation compared to normal macaques, whereas the rate of proliferation of SP CD21−CD27+ and DN CD21−CD27− cells was significantly higher compared to normal RMs (Fig. 2A, P<0.01). Combined, these data indicate that in all tissues, DP CD21+CD27+ B cells have much higher rates of proliferation than SP CD21−CD27+ B cells. In addition, DP CD21+CD27+ B cell proliferation increases predominantly due to expansion of preexisting memory cells in most tissues (ALN and Spleen) except tonsil, where the proliferation markedly decreases within days of infection. Progressive depletion of proliferating (Ki67+) B cells has also been reported in lymph node germinal centers at as early as 20 days after SIVMAC239, however, the assay was performed by immunohistochemistry staining [11]. In-contrast, there was a significant increase in T cell proliferation in chronically infected RMs compared to acutely infected and normal RMs in all tissues examined (Fig. 2B, p<0.01). The increased T cell proliferation during chronic infection in tissues is consistent with generalized immune activation [26, 27].
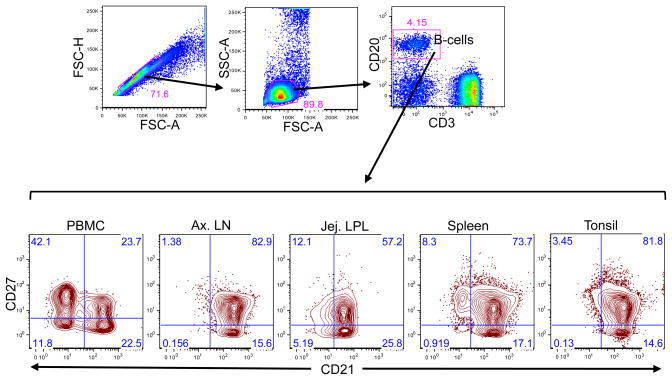
Figure 1.
A representative dot and contour plots showing distribution of CD21 and CD27 phenotype on CD20+ B cells from a normal uninfected healthy rhesus macaque (GN74). Singlets were gated first followed by lymphocytes and finally gating was performed in CD20+ B lymphocytes. All CD20+ B lymphocytes were further analyzed based on their CD21 and CD27 surface molecule expression. Each quadrant shows percentages of specific B cell subset populations.
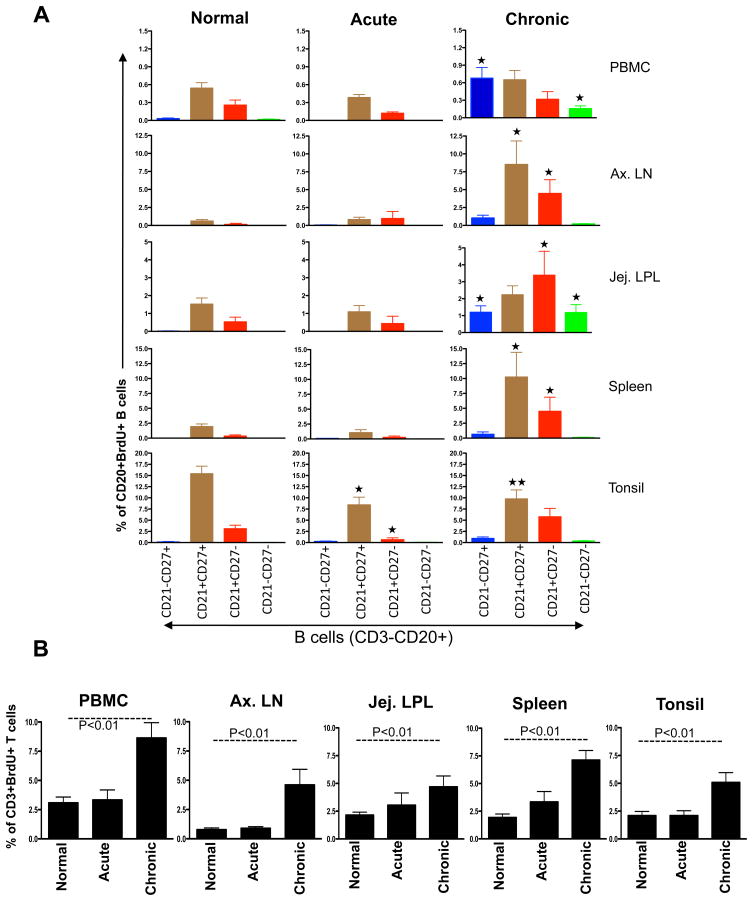
Figure 2.
Bar graphs showing the mean BrdU+ proliferative responses in different CD20+ B cell subsets (A) and CD3+ T cells (B) from different tissues of normal and SIV infected macaques in acute and chronic infection. BrdU was injected intraperitoneally and tissues were collected 24 hrs after inoculation. (A) Higher proliferation of CD21+CD27+ B cell subsets compared to other B cells subsets were observed in all healthy normal rhesus macaques. Interestingly tonsillar CD21+CD27+ B cell proliferation decrease in both acute and chronic SIV infection compared to normal healthy macaques.
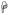 (p<0.01) and
(p<0.01) and
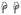 (p<0.05) indicate significant differences between the specified cell subsets and the same subset from normal groups. (B) A significant increase in T cell proliferation in chronically infected RMs compared to acutely infected and normal RMs in all tissues was detected.
(p<0.05) indicate significant differences between the specified cell subsets and the same subset from normal groups. (B) A significant increase in T cell proliferation in chronically infected RMs compared to acutely infected and normal RMs in all tissues was detected.
Decreased proliferation of memory (CD21+CD27+) B cells in tonsil is suggestive of an early B cell defect localized in this tissue which might be a key feature in the failure of functional B cell responses in SIV infection, as these DP CD21+CD27+ B cells are capable of generating more antibodies by polyclonal activation and in the absence of T cell help [28]. These results demonstrate important information about the biology of tissue specific B lymphocyte proliferation in RMs, and suggest the intriguing possibility that tissue specific B cell dysfunction may play an important role in SIV pathogenesis. In summary, these data demonstrate differences in nonhuman primate B cell subsets proliferation in selective tissues, and suggest that SIV infection may induce early selective defective responses in specific tissues compared to T cells.
Supplementary Material
(A). Plasma viral RNA levels and CD4+ T cell percentages in peripheral blood and jejunum LPL tissues in normal healthy, SIV acute and chronically infected macaques. Note that SIV acute and chronically infected macaques were euthanized for tissue collection 8–13 and 143–313 days post SIV infection time points respectively. The line in each plot represents average values. Overall in both the acute and chronically infected RMs there was high plasma viremia (103.8 to 108.2 vRNA/ml and there were no significant differences in viral loads between these groups (p=0.53). Uninfected RMs maintained significantly higher percentages of peripheral CD4+ T cells (mean 57.7%) compared to chronically infected RMs (mean 34.3%; p=0.01). In contrast, there were no significant differences in peripheral CD4+ T cell percentages between normal and acutely infected RMs (mean 53.1%). Similarly jejunum LPL CD4+ T cell percentages in normal and acutely infected RMs were similar (mean 48.2% and 54.7% respectively). However, there were significant differences in jejunum LPL CD4+ T cell percentages between normal (range from 29.1 to 39.2%) and chronically infected RMs (range from 0.5–8.5%; p=0.002) (B). Comparison of CD20+ B cell percentages in peripheral blood, Ax. LN, jejunum LPL, Spleen and tonsillar lymphoid tissues from normal healthy, SIV acute and chronically infected macaques.
 indicates significant differences in CD20+ B cell percentages between specified groups.
indicates significant differences in CD20+ B cell percentages between specified groups.
Highlights.
DP CD21+CD27+ B cells were able to become activated and proliferate at higher rates.
Increased proliferation of tonsillar memory B cells were identified.
Decreased proliferation of tonsillar memory B cells during acute SIV infection.
Acknowledgments
We thank Maryjane Dodd, Janell LeBlanc, Linda Green, Maury Duplantis, Nancy Parr, Lara Doyle, and all animal care staff of the department of veterinary medicine for their technical assistance. We also thank Drs. Preston Marx, Gus Kousoulas and Vida Dennis for their support in this study. The work was supported by NIH grants P20 RR020159, R21 AI080395 (BP) and RR000164, R01 AI084793 (RSV), and amfAR grant-106719–40-RGRL (BP).
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moir S, Malaspina A, Pickeral OK, Donoghue ET, Vasquez J, Miller NJ, Krishnan SR, Planta MA, Turney JF, Justement JS, Kottilil S, Dybul M, Mican JM, Kovacs C, Chun TW, Birse CE, Fauci AS. Decreased survival of B cells of HIV-viremic patients mediated by altered expression of receptors of the TNF superfamily. J Exp Med. 2004;200:587–599. [PubMed] [Google Scholar]
- 2.Moir S, Malaspina A, Ogwaro KM, Donoghue ET, Hallahan CW, Ehler LA, Liu S, Adelsberger J, Lapointe R, Hwu P, Baseler M, Orenstein JM, Chun TW, Mican JA, Fauci AS. HIV-1 induces phenotypic and functional perturbations of B cells in chronically infected individuals. Proc Natl Acad Sci U S A. 2001;98:10362–10367. doi: 10.1073/pnas.181347898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malaspina A, Moir S, Ho J, Wang W, Howell ML, O’Shea MA, Roby GA, Rehm CA, Mican JM, Chun TW, Fauci AS. Appearance of immature/transitional B cells in HIV-infected individuals with advanced disease: correlation with increased IL-7. Proc Natl Acad Sci U S A. 2006;103:2262–2267. doi: 10.1073/pnas.0511094103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malaspina A, Moir S, Chaitt DG, Rehm CA, Kottilil S, Falloon J, Fauci AS. Idiopathic CD4+ T lymphocytopenia is associated with increases in immature/transitional B cells and serum levels of IL-7. Blood. 2007;109:2086–2088. doi: 10.1182/blood-2006-06-031385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ho J, Moir S, Malaspina A, Howell ML, Wang W, DiPoto AC, O’Shea MA, Roby GA, Kwan R, Mican JM, Chun TW, Fauci AS. Two overrepresented B cell populations in HIV-infected individuals undergo apoptosis by different mechanisms. Proc Natl Acad Sci U S A. 2006;103:19436–19441. doi: 10.1073/pnas.0609515103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moir S, Fauci AS. Pathogenic mechanisms of B-lymphocyte dysfunction in HIV disease. J Allergy Clin Immunol. 2008;122:12–19. doi: 10.1016/j.jaci.2008.04.034. quiz 20–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moir S, Fauci AS. B cells in HIV infection and disease. Nat Rev Immunol. 2009;9:235–245. doi: 10.1038/nri2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moir S, Ho J, Malaspina A, Wang W, DiPoto AC, O’Shea MA, Roby G, Kottilil S, Arthos J, Proschan MA, Chun TW, Fauci AS. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J Exp Med. 2008;205:1797–1805. doi: 10.1084/jem.20072683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Milito A, Morch C, Sonnerborg A, Chiodi F. Loss of memory (CD27) B lymphocytes in HIV-1 infection. Aids. 2001;15:957–964. doi: 10.1097/00002030-200105250-00003. [DOI] [PubMed] [Google Scholar]
- 10.Peruchon S, Chaoul N, Burelout C, Delache B, Brochard P, Laurent P, Cognasse F, Prevot S, Garraud O, Le Grand R, Richard Y. Tissue-specific B-cell dysfunction and generalized memory B-cell loss during acute SIV infection. PLoS One. 2009;4:e5966. doi: 10.1371/journal.pone.0005966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang ZQ, Casimiro DR, Schleif WA, Chen M, Citron M, Davies ME, Burns J, Liang X, Fu TM, Handt L, Emini EA, Shiver JW. Early depletion of proliferating B cells of germinal center in rapidly progressive simian immunodeficiency virus infection. Virology. 2007;361:455–464. doi: 10.1016/j.virol.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Kuhrt D, Faith SA, Leone A, Rohankedkar M, Sodora DL, Picker LJ, Cole KS. Evidence of early B-cell dysregulation in simian immunodeficiency virus infection: rapid depletion of naive and memory B-cell subsets with delayed reconstitution of the naive B-cell population. J Virol. 2010;84:2466–2476. doi: 10.1128/JVI.01966-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dykhuizen M, Mitchen JL, Montefiori DC, Thomson J, Acker L, Lardy H, Pauza CD. Determinants of disease in the simian immunodeficiency virus-infected rhesus macaque: characterizing animals with low antibody responses and rapid progression. J Gen Virol. 1998;79(Pt 10):2461–2467. doi: 10.1099/0022-1317-79-10-2461. [DOI] [PubMed] [Google Scholar]
- 14.Levesque MC, Moody MA, Hwang KK, Marshall DJ, Whitesides JF, Amos JD, Gurley TC, Allgood S, Haynes BB, Vandergrift NA, Plonk S, Parker DC, Cohen MS, Tomaras GD, Goepfert PA, Shaw GM, Schmitz JE, Eron JJ, Shaheen NJ, Hicks CB, Liao HX, Markowitz M, Kelsoe G, Margolis DM, Haynes BF. Polyclonal B cell differentiation and loss of gastrointestinal tract germinal centers in the earliest stages of HIV-1 infection. PLoS Med. 2009;6:e1000107. doi: 10.1371/journal.pmed.1000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller CJ, Genesca M, Abel K, Montefiori D, Forthal D, Bost K, Li J, Favre D, McCune JM. Antiviral antibodies are necessary for control of simian immunodeficiency virus replication. J Virol. 2007;81:5024–5035. doi: 10.1128/JVI.02444-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmitz JE, Kuroda MJ, Santra S, Simon MA, Lifton MA, Lin W, Khunkhun R, Piatak M, Lifson JD, Grosschupff G, Gelman RS, Racz P, Tenner-Racz K, Mansfield KA, Letvin NL, Montefiori DC, Reimann KA. Effect of humoral immune responses on controlling viremia during primary infection of rhesus monkeys with simian immunodeficiency virus. J Virol. 2003;77:2165–2173. doi: 10.1128/JVI.77.3.2165-2173.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Das A, Xu H, Wang X, Yau CL, Veazey RS, Pahar B. Double positive CD21+CD27+ B cells are highly proliferating memory cells and their distribution differs in mucosal and peripheral tissues. PLoS One. 2011;6:e16524. doi: 10.1371/journal.pone.0016524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X, Das A, Lackner AA, Veazey RS, Pahar B. Intestinal double-positive CD4+CD8+ T cells of neonatal rhesus macaques are proliferating, activated memory cells and primary targets for SIVMAC251 infection. Blood. 2008;112:4981–4990. doi: 10.1182/blood-2008-05-160077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pahar B, Cantu MA, Zhao W, Kuroda MJ, Veazey RS, Montefiori DC, Clements JD, Aye PP, Lackner AA, Lovgren-Bengtsson K, Sestak K. Single epitope mucosal vaccine delivered via immuno-stimulating complexes induces low level of immunity against simian-HIV. Vaccine. 2006;24:6839–6849. doi: 10.1016/j.vaccine.2006.06.050. [DOI] [PubMed] [Google Scholar]
- 20.Veazey RS, Lifson JD, Pandrea I, Purcell J, Piatak M, Jr, Lackner AA. Simian immunodeficiency virus infection in neonatal macaques. J Virol. 2003;77:8783–8792. doi: 10.1128/JVI.77.16.8783-8792.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Veazey RS, Rosenzweig M, Shvetz DE, Pauley DR, DeMaria M, Chalifoux LV, Johnson RP, Lackner AA. Characterization of gut-associated lymphoid tissue (GALT) of normal rhesus macaques. Clin Immunol Immunopathol. 1997;82:230–242. doi: 10.1006/clin.1996.4318. [DOI] [PubMed] [Google Scholar]
- 22.Pahar B, Lackner AA, Piatak M, Jr, Lifson JD, Wang X, Das A, Ling B, Montefiori DC, Veazey RS. Control of viremia and maintenance of intestinal CD4(+) memory T cells in SHIV(162P3) infected macaques after pathogenic SIV(MAC251) challenge. Virology. 2009;387:273–284. doi: 10.1016/j.virol.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X, Rasmussen T, Pahar B, Poonia B, Alvarez X, Lackner AA, Veazey RS. Massive infection and loss of CD4+ T cells occurs in the intestinal tract of neonatal rhesus macaques in acute SIV infection. Blood. 2007;109:1174–1181. doi: 10.1182/blood-2006-04-015172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pahar B, Lackner AA, Veazey RS. Intestinal double-positive CD4+CD8+ T cells are highly activated memory cells with an increased capacity to produce cytokines. Eur J Immunol. 2006;36:583–592. doi: 10.1002/eji.200535520. [DOI] [PubMed] [Google Scholar]
- 25.Pahar B, Wang X, Dufour J, Lackner AA, Veazey RS. Virus-specific T cell responses in macaques acutely infected with SHIV(sf162p3) Virology. 2007;363:36–47. doi: 10.1016/j.virol.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaur A, Di Mascio M, Barabasz A, Rosenzweig M, McClure HM, Perelson AS, Ribeiro RM, Johnson RP. Dynamics of T- and B-lymphocyte turnover in a natural host of simian immunodeficiency virus. J Virol. 2008;82:1084–1093. doi: 10.1128/JVI.02197-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Boer RJ, Mohri H, Ho DD, Perelson AS. Turnover rates of B cells, T cells, and NK cells in simian immunodeficiency virus-infected and uninfected rhesus macaques. J Immunol. 2003;170:2479–2487. doi: 10.4049/jimmunol.170.5.2479. [DOI] [PubMed] [Google Scholar]
- 28.Das A, Xu H, Wang X, Yau CL, Veazey RS, Pahar B. Double-Positive CD21+CD27+ B Cells Are Highly Proliferating Memory Cells and Their Distribution Differs in Mucosal and Peripheral Tissues. PLoS One. 2011;6:e16524. doi: 10.1371/journal.pone.0016524. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A). Plasma viral RNA levels and CD4+ T cell percentages in peripheral blood and jejunum LPL tissues in normal healthy, SIV acute and chronically infected macaques. Note that SIV acute and chronically infected macaques were euthanized for tissue collection 8–13 and 143–313 days post SIV infection time points respectively. The line in each plot represents average values. Overall in both the acute and chronically infected RMs there was high plasma viremia (103.8 to 108.2 vRNA/ml and there were no significant differences in viral loads between these groups (p=0.53). Uninfected RMs maintained significantly higher percentages of peripheral CD4+ T cells (mean 57.7%) compared to chronically infected RMs (mean 34.3%; p=0.01). In contrast, there were no significant differences in peripheral CD4+ T cell percentages between normal and acutely infected RMs (mean 53.1%). Similarly jejunum LPL CD4+ T cell percentages in normal and acutely infected RMs were similar (mean 48.2% and 54.7% respectively). However, there were significant differences in jejunum LPL CD4+ T cell percentages between normal (range from 29.1 to 39.2%) and chronically infected RMs (range from 0.5–8.5%; p=0.002) (B). Comparison of CD20+ B cell percentages in peripheral blood, Ax. LN, jejunum LPL, Spleen and tonsillar lymphoid tissues from normal healthy, SIV acute and chronically infected macaques.
 indicates significant differences in CD20+ B cell percentages between specified groups.
indicates significant differences in CD20+ B cell percentages between specified groups.