Abstract
The chorioamnionitis associated with preterm delivery is often polymicrobial with ureaplasma being the most common isolate. To evaluate interactions between the different pro-inflammatory mediators, we hypothesized that ureaplasma exposure would increase fetal responsiveness to LPS. Fetal sheep were given intra-amniotic injections of media (control) or Ureaplasma parvum serovar 3 either 7d or 70d before preterm delivery. Another group received an intraamniotic injection of E.coli lipo-polysaccharide (LPS) 2d prior to delivery. To test for interactions, intraamniotic U. parvum exposed animals were challenged with intraamniotic LPS and delivered 2d later. All animals were delivered at 124±1d gestation (Term=150d). Compared to the 2d LPS exposure group, the U. parvum 70d+LPS group had: 1) decreased lung pro and anti-inflammatory cytokine expression 2) fewer CD3+ T-lymphocytes, CCL2+, myeloperoxidase+, and PU.1+ cells in the lung. Interestingly, exposure to U. parvum for 7d did not change responses to a subsequent intraamniotic LPS challenge, and exposure to intraamniotic U. parvum alone induced mild lung inflammation. Exposure to U. parvum increased pulmonary TGFβ1 expression but did not change mRNA expression of either the receptor TLR4 or some of the downstream mediators in the lung. Monocytes from fetal blood and lung isolated from U. parvum 70d+LPS but not U. parvum 7d+LPS animals had decreased in vitro responsiveness to LPS. These results are consistent with the novel finding of down-regulation of LPS responses by chronic but not acute fetal exposures to U. parvum. The findings increase our understanding of how chorioamnionitis exposed preterm infants may respond to lung injury and postnatal nosocomial infections.
Keywords: Prematurity, Chorioamnionitis, Fetal inflammatory response syndrome, endotoxin tolerance, lung inflammation
Introduction
Preterm births account for 12% of all deliveries in the U.S, and are the most important determinant of neonatal mortality and morbidity (1). Chorioamnionitis or inflammation in the fetal membranes is associated with about 60% of deliveries before 30 weeks gestation (2). The most common organisms isolated from the amniotic fluid of women with chorioamnionitis are the Ureaplasma species (3). Furthermore, among the preterm infants with chorioamnionitis caused by ureaplasma species, about 60% also had co-infection with other organisms (3). In addition, ureaplasmas were isolated from amniotic fluid from first or second trimester or from washed semen in assisted reproduction (4–6). Therefore preterm infants have frequent and often prolonged exposure to Ureaplasmas. Ureaplasma colonization of the upper genital tract can induce preterm labor in non-human primates and is associated with preterm labor in humans (5, 7). Exposure of preterm infants to Ureaplasma is associated with increased risk for adverse pulmonary, gastrointestinal and neurological outcomes (7–10).
The two species of ureaplasmas which colonize humans are U. urealyticum (serovars 2, 4, 5, 7, 13) and Ureaplasma parvum (serovars 1, 3, 6 and 14)(8). Of these, U. parvum is the most common species isolated from preterm neonates and upper genital tracts of women (11, 12). The ureaplasmas are unusual bacteria in that they have a plasma membrane but lack a peptidoglycan cell wall, utilize urea as the sole source of energy, and are dependent on the host for other metabolic functions (13).
We have reported acute and chronic exposure to ureaplasma species in fetal sheep (14, 15). Intraamniotic injection of U. parvum in sheep induces a robust colonization of fetal chorioamnion and lung, with poor bacterial clearance (14). Exposure to UP in sheep induces a mild fetal inflammatory response, increases pulmonary surfactant pools, and results in mild transient developmental abnormalities in the lung (16, 17). In contrast, intraamniotic injection of LPS induces a robust fetal inflammation in the sheep (18). Interestingly, repeated exposures in vivo to intraamniotic LPS induces endotoxin tolerance and cross tolerance to other toll-like agonists (19, 20). Ureaplasma species signal via TLR1/2/6, and can increase LPS mediated inflammation in vitro (21, 22). Although co-infection of Ureaplasma spp with other organisms is common in chorioamnionitis (3), the interactions between ureaplasma and LPS in the fetus have not been explored.
Based on the in vitro observation that ureaplasma increase LPS mediated inflammation (21, 22), we hypothesized that exposure to ureaplasma would increase LPS induced fetal inflammation. Pregnant ewes were given an intraamniotic injection of U. parvum serovar 3 to induce acute or chronic chorioamnionitis. We subsequently challenged fetal sheep with an intraamniotic injection of LPS 2d prior to preterm delivery at 82% of term gestation. Fetal inflammation was assessed after a single exposure to U. parvum, LPS or after combined exposures.
MATERIAL & METHODS
Animals and treatments
The animals were studied in Western Australia with approval from the animal care and use committees of the Cincinnati Children’s Hospital, OH, and The University of Western Australia. Time-mated Merino ewes with singleton fetuses were randomly assigned to study groups of 5 to 7 animals (Table 1). The pregnant sheep were given ultrasound-guided intraamniotic (IA) injections of a) Ureaplasma parvum serovar 3 (U. parvum), 2×107 CFU or 2mL media (control) into the amniotic fluid 70 d or 7 d prior to delivery, b) E.coli LPS (O55:B5, Sigma-Aldrich, St. Louis MO) 10mg in 2mL saline 2 d prior to delivery, or saline only (control). To evaluate immune modulatory effects of U. parvum on LPS, separate groups of animals received IA LPS 2d before delivery after exposure to IA U. parvum 70d or 7d before delivery. All fetal injections were given with ultrasound guidance and with electrolyte analysis to confirm injection into amniotic fluid (23). All animals were delivered at 124±1d GA, umbilical cord blood was collected for plasma and for circulating leukocytes and fetuses were given lethal intravascular doses of pentobarbital for euthanasia. At autopsy tissue from the right lower lobe of the lung and liver were snap frozen for RNA extraction. For bronchoalveolar lavage fluid, the left lung was inflated with normal saline to total lung capacity followed by withdrawal, and the procedure was repeated three times (23). Bronchoalveolar lavage fluids (BALF) were pooled and used for cell counts and protein measurements (24). BALF cell counts were expressed as total cells recovered from the lavage normalized to body weight. Lung compliance was measured from the deflation limb of an air pressure-volume curve with the chest open (24). The right upper lobe of the lung was inflation fixed with 10% buffered formalin at 30 cm H2O pressure for morphology.
Table 1.
Ureaplasma titers and lung volumes in fetal lambs after intraamniotic exposures
| Groups | N | Birth weight (kg) | Amniotic fluid U. parvum titer (CFU ×104/mL) | Lung/body weight (g/kg) | V40 mL/g lung weight |
|---|---|---|---|---|---|
| Control | 7 | 2.8±0.1 | 0 (0, 0) | 31.3±0.4 | 0.31±0.01 |
| 7d UP | 6 | 2.9±0.1 | 4.2 (3.6, 75.3) | 34.6±2.1 | 0.36±0.05 |
| 70d UP | 6 | 2.6±0.2 | 10.2 (6.8, 128.9) | 33.0±2.2 | 0.98±0.19* |
| 2d LPS | 7 | 2.7±0.1 | 0 (0, 0) | 36.8±2.1* | 0.18±0.02* |
| 7d UP+2d LPS | 7 | 2.9±0.1 | 0.4 (0.07, 1.7) | 37.3±1.0* | 0.23±0.03 |
| 70d UP+2d LPS | 5 | 2.1±0.2 | 6.1 (1.2, 39.6) | 36.1±1.2* | 0.90±0.19* |
Lung, Birth weight and V40 - Means ±SEM. U. parvum titer - median (25%ile, 75%ile).
p<0.05 vs. controls, All animals delivered at 124±1d gestation (term=150d), BALF=Broncho alveolar lavage fluid, V40 = lung volume at 40 cm H2O pressure
Culture of lung and blood monocytes
After exsanguination of the fetus, the lung was chopped thoroughly into fine pieces and incubated in RPMI medium (25). The lung suspension was then gently passed through a 100μm mesh filter and the suspension was washed twice with phosphate buffered saline (PBS). Cells from the suspension were then layered over discontinuous Percoll gradients (1.085 g/ml and 1.046 g/ml) (Amersham Pharmacia Biotech, Inc., Piscataway, NJ) to separate the monocytic cells from the other cells at the interface between the Percoll densities (25). Whole blood diluted 1:1 with saline was layered on a 1.046 g/ml Percoll gradient to recover mononuclear cells. Cells were counted using trypan blue to evaluate viability and then plated in culture dishes using media supplemented with 10% heat-inactivated fetal-calf serum (Sigma-Aldrich, Castle-Hill, Australia). After incubation at 37°C for 2 h, non-adherent cells were removed and plates were washed twice with PBS. Monocytes were challenged with media only or LPS (100ng/mL) for 16h (19). Cell responsiveness as indicated by IL-6 secretion was measured in the media with a sandwich ELISA assay (coating antibody – mouse anti-ovine IL-6 (Chemicon # MAB1004, Billerica MA) and primary antibody rabbit anti-ovine IL-6 (Chemicon #AB1839, Billerica MA) (19).
Cytokine mRNA Quantitation
Total RNA was isolated from fetal tissues using a modified Chomzynski method (24) and mRNA quantitation was performed using real-time PCR. The mRNA was reverse transcribed to yield a single-strand cDNA (verso cDNA kit, Thermoscientific UK), which was used as a template with primers and Taqman probes (Applied Biosystems, Carlsbad CA) specific to sheep sequences. The values for each cytokine were normalized to the internal 18S rRNA. Data were expressed as fold increases over control values.
Immunohistochemistry and scoring of inflammation
Inflammatory cells in BALF were counted using a hemocytometer and the differential counts on cytospins were performed using DiffQuick staining (Baxter Health Care, Deerfield IL). For preparation of lung for morphology, formalin was removed from the fixed tissue within 24h by washing in phosphate buffered saline (pH 7.4) and the tissues were transferred to 70% ethanol and embedded in paraffin. After deparaffinization and rehydration of fixed tissue, antigen-retrieval was carried out using citric acid buffer, pH 6.0 with microwave boiling. Endogenous peroxidase activity was blocked with methyl alcohol/hydrogen peroxide. Non-specific interactions were inhibited with 2% goat serum for both primary and secondary antibody incubations. Sections were incubated with anti-CD3 antibody (Dako, Carpinteria CA #A0452, 1:100), anti-PU.1 (Santacruz Biotechnology CA, #sc-225), anti-myeloperoxidase (MPO) antibody (Cell marque, Rocklin CA, catalogue #CMC028) (1:400), guinea-pig polyclonal anti-monocyte chemotactic protein 1 (MCP-1) (1:1000) antibody generated at our institution (Seven Hills Bioreagents, Cincinnati OH). Following incubation with the primary antibody at 4°C overnight, sections were incubated with the appropriate secondary antibody for 30 minutes at room temperature (1:200). Immunostaining was visualized using a Vectastain ABC peroxidase Elite kit to detect the antigen:antibody complexes (Vector Laboratories Inc). Antigen detection was enhanced with nickel-diaminobenzidine, followed by incubation with TRIS-cobalt to give a black precipitate. Nuclei were counterstained with Nuclear Fast Red for photo-microscopy. Blinded scoring of inflammation in the lung was done by counting MCP-1, PU.1, CD3 or MPO positive inflammatory cells in 10 comparable non-overlapping high power fields of each animal (4–6 animals/group).
BALF and plasma protein analysis
TGFβ1 in BALF was measured by acidification of lavage fluid to activate latent TGFβ1 and concentrations were measured using a commercial ELISA kit (#DY240 against human TGFβ1, R&D Systems, Minneapolis MN). Other cytokines were measured using custom ELISA assays (26, 27). The antibodies used were: IL-1β (coating antibody – rabbit anti-ovine IL-1β and primary antibody guinea pig anti-ovine IL-1β (Seven Hills Bioreagents, Cincinnati), IL-8 (coating antibody – mouse anti-ovine IL-8 (Chemicon Billerica MA # MAB10445) and primary antibody rabbit anti-ovine IL-8 (Chemicon Billerica MA # AB1840)), MCP-1 (rabbit anti-sheep MCP-1 coating antibody, and primary antibody guinea pig anti-sheep MCP-1 detection antibody (Seven Hills Bioreagents, Cincinnati OH), The detection antibody in all the assays was an appropriate species-specific HRP-conjugated antibody. Plasma haptoglobin was measured by ELISA (anti-bovine kit, ICL, Newberg OR).
Data analysis
Results are given as mean ± SEM. Comparisons between 3 or more groups were performed by analyses of variance with Student-Newman-Keuls tests used for post-hoc analyses. Comparison of two groups was done by a non-parametric t-test (Mann-Whitney U-test) for data not distributed normally and student t-test for normally distributed data. Statistical significance was accepted at p<0.05.
RESULTS
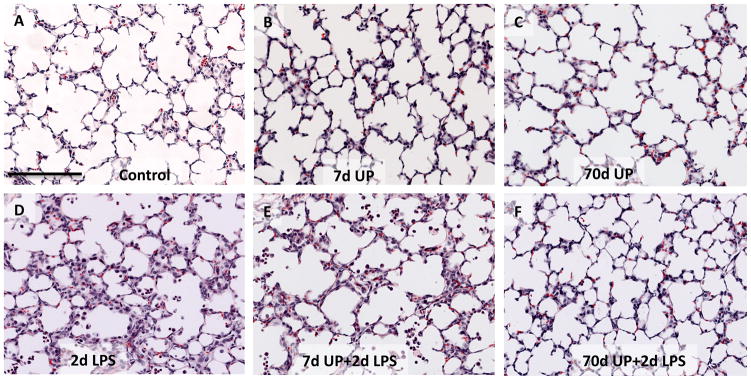
All animals given an intraamniotic injection of U. parvum had positive cultures for U. parvum in the amniotic fluid (Table 1). The amniotic fluid U. parvum titer tended to be lower (statistically not significant) after a 7d exposure compared to 70d exposure, but there was variability within each group. U. parvum did not cause gross developmental abnormalities and the birth weights were similar between infected and uninfected control fetuses (Table 1). Consistent with our previous reports (15), compared to controls, the lung gas volumes increased almost 3-fold after chronic but not acute U. parvum exposure (Table 1) indicating lung maturation. The 2d LPS group had increased lung weight relative to body weight, but decreased lung volume relative to lung weight. Low power lung histology demonstrated that the control, and the U. parvum only exposed animals (both 7d and 70d) had a similar histology (Figure 1A–C). In contrast, 2d LPS exposure recruited inflammatory cells in the airways and lung interstitium (Fig 1D). The histology in animals exposed to acute U. parvum 7d + 2d LPS was indistinguishable from the 2d LPS only (compare 1D and 1E), while chronic U. parvum 70d +2d LPS exposed animals had a lung histology similar to controls (compare 1F and 1A).
Figure 1. Lung histology after intraamniotic exposure to LPS, acute and chronic intraamniotic exposure to U. parvum.
Representative photomicrographs showing hematoxylin and eosin staining of lung sections from the animals with intraamniotic exposures to (A) Controls (B) 7d U. parvum (C) 70d U. parvum (D) 2d LPS (E) 7d U. parvum +2d LPS and (F) 70d U. parvum +2d LPS. Note the similarity of histology in panels A, B, C, and F. Panels D and E demonstrate inflammatory cells in the airways and lung interstitium. (scale bar is 100μm).
Down regulation of LPS induced recruitment of leukocytes in the lung by prior chronic exposure to U. parvum
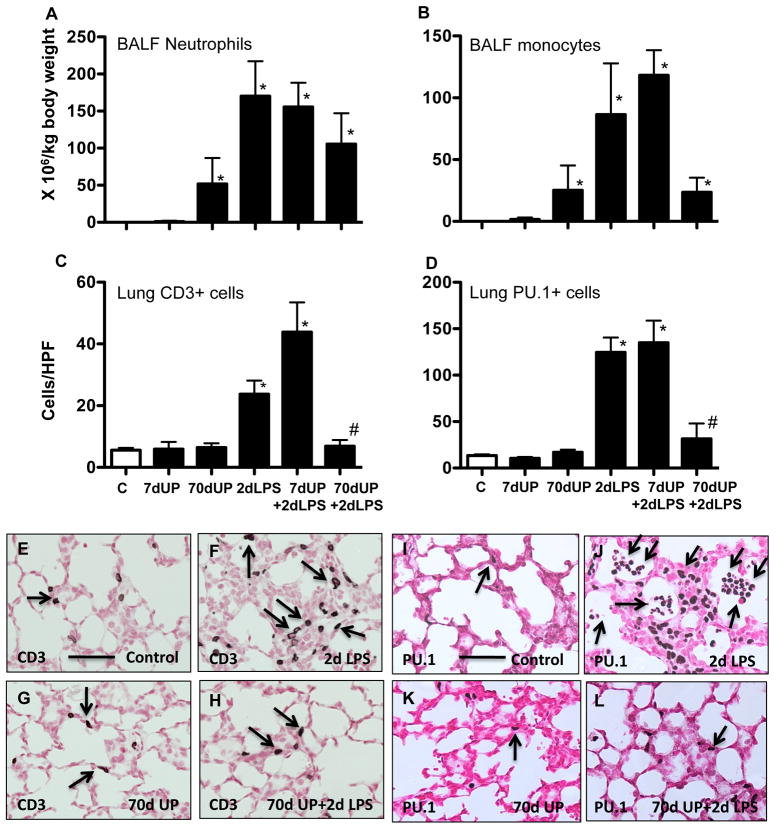
Control fetal lambs had very few neutrophils or monocytes in the pulmonary airspaces (Figure 2A–B). Exposure to IA U. parvum for 7 d caused no significant recruitment of neutrophils, while a chronic exposure (70 d) recruited qualitatively higher numbers of neutrophils and monocytes to the airspaces. IA LPS induced a large recruitment of neutrophils and monocytes. Fetal lambs exposed to either LPS alone or U. parvum 7 d + LPS had similar numbers of inflammatory cells in the airspaces. However, animals exposed to U. parvum 70 d + LPS had qualitatively fewer inflammatory cells in the pulmonary airspaces.
Figure 2.
Chronic amniotic exposure to U. parvum decreases intraamniotic LPS induced pulmonary recruitment and activation of inflammatory cells. Bronchoalveolar lavage cells were expressed relative to body weight A) Neutrophils, B) Monocytes. Quantitation of (C) CD3+ T-cells and (D) PU.1+ cells per high power field in lung tissue sections. Representative photomicrographs of of lung sections showing immunostaining with (E–H) CD3 and (I–L) PU.1. Arrows point to immunostained inflammatory cells. (scale bar is 50μm)(*p<0.05 vs. control, #p<0.05 vs. 2d LPS).
Very few lymphocytes were detected in the airspaces of animals from any of the groups (data not shown). We therefore evaluated recruitment of CD3+ T-lymphocytes in the fetal lung. Control and U. parvum exposed fetal lambs had a few T-lymphocytes in the lung (Figure 2C,E,G). Exposure to IA LPS recruited T-lymphocytes in the lung, and this recruitment was almost completely blocked by a prior chronic but not acute exposure to U. parvum (compare Figure 2 panels F and H). PU.1 is an ets-domain developmental transcription factor that orchestrates the inflammatory processes in myeloid and non-myeloid cells (28–30). A prior chronic but not acute exposure to U. parvum blocked IA LPS induced PU.1 expressing cells in the fetal lung (Figure 2D, compare panels 2J and 2L).
In vitro responses of lung and blood monocytes to LPS
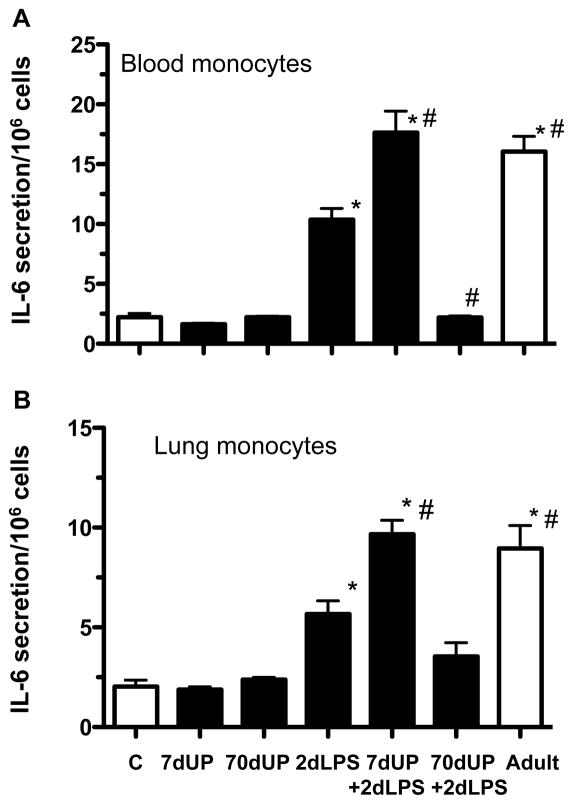
Exposure to chronic U. parvum could decrease LPS responsiveness via secretion of a soluble inhibitor or could induce changes in cell responsiveness to LPS. To evaluate these possibilities, monocytes from both the lung and the peripheral blood were cultured and challenged with LPS in vitro. Consistent with immature LPS responsiveness, fetal monocytes from control lambs had a decreased response to LPS compared to blood monocytes and alveolar macrophages from adult ewes to an in vitro LPS challenge (increase in IL-6 secretion - 2-fold vs. 16 fold for blood monocytes and 2-fold vs. 9 fold for lung monocytes, p<0.05) (Figure 3A–B). Exposure to U. parvum alone did not increase responsiveness to LPS in vitro. However, monocytes from fetal lambs exposed to IA LPS increased IL-6 secretion in response to LPS challenge in vitro, indicating a maturational effect. Interestingly, monocytes from IA LPS+ U. parvum (7 d) further increased IL-6 secretion in response to an in vitro LPS challenge compared to the 2d LPS group, and similar to levels from adult cells. In striking contrast, monocytes from the IA LPS + U. parvum 70 d group had a blunted response to LPS challenge in vitro.
Figure 3. chronic amniotic exposures to U. parvum decreased intraamniotic LPS induced increases in monocyte responses to LPS in vitro.
Monocytes from (A) Blood and (B) Lung were purified over percoll gradients, 106 cells were cultured and challenged with media only or 100ng/mL LPS for 16h. LPS induced secretion of IL-6 in the medium was expressed as fold increase over the media value (C= fetal control, adult=monocytes/macrophages from adult ewes, *p<0.05 vs. fetal control, #p<0.05 vs. 2d LPS).
Down regulation of LPS induced cytokine expression in the lung by prior chronic exposure to U. parvum
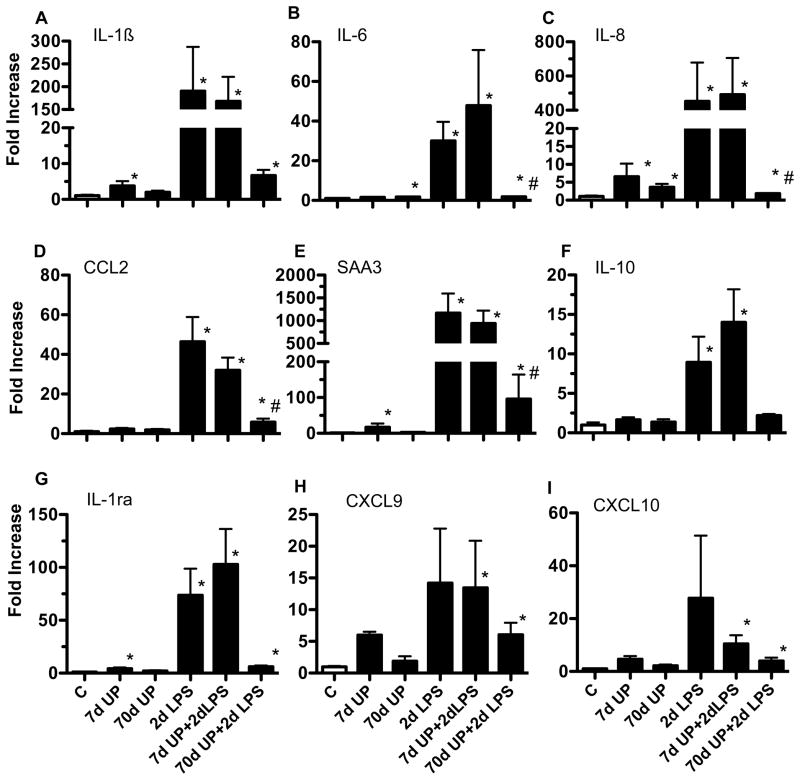
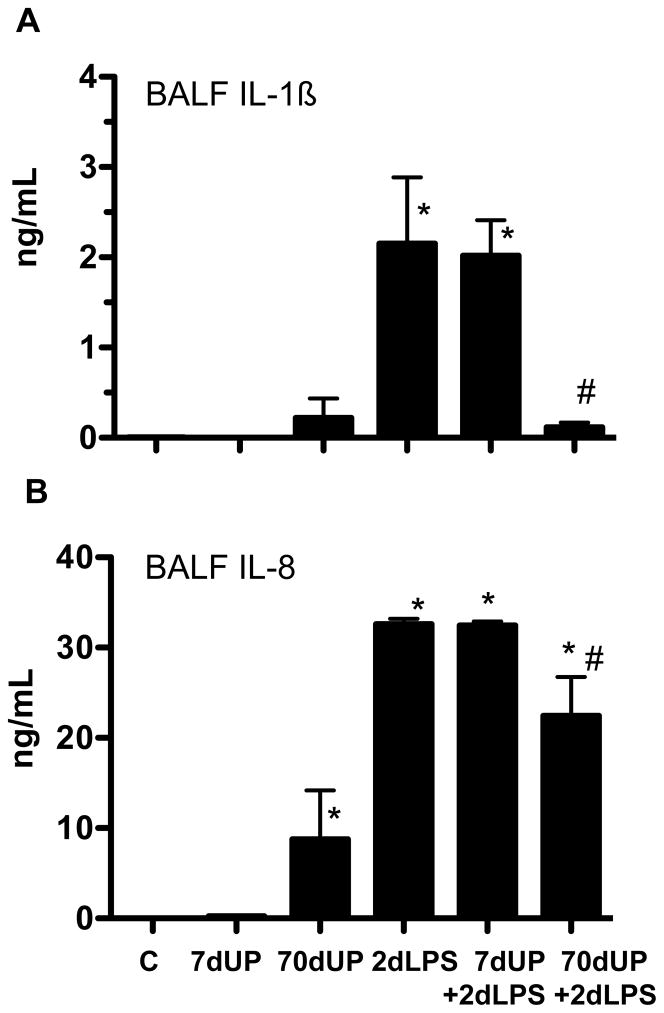
We previously demonstrated that the fetal lung is a target of inflammation after exposure to intraamniotic (IA) administration of pro-inflammatory agonists (24). We therefore measured expression of pro-inflammatory cytokine, chemokine, acute phase reactant and anti-inflammatory cytokine mRNAs in the fetal lung (Figure 4). Relative to controls, either acute (7 d exposure) or chronic (70 d exposure) to U. parvum caused an inconsistent or modest increase in expression of IL-1β (4-fold), IL-6 (2-fold), IL-8 (7-fold), serum amyloid A3 (17-fold) and IL-1RA (4-fold) expression in the lung. In contrast, exposure to IA LPS for 2 d greatly increased expression of both the pro-inflammatory cytokines/chemokines (IL-1β, IL-6, IL-8, MCP-1 (CCL2), Serum amyloid A3, CXCL9 and CXCL10), and the anti-inflammatory cytokines IL-1RA and IL-10 in the lung. Prior exposure to U. parvum for 7 d did not change responses to LPS. In sharp contrast, chronic exposure to U. parvum, significantly down-regulated LPS responses to near control levels. To further confirm these findings, we measured IL-1β and IL-8 protein in the broncho-alveolar lavage fluid. Chronic exposure to U. parvum alone modestly increased secretion of IL-8 in the lung (Figure 5B). Neither acute nor chronic exposure to U. parvum induced pulmonary IL-1β secretion (Figure 5A). Consistent with the mRNA responses, IA LPS greatly increased IL-1β and IL-8 secretion (Figure 5A–B). Similar to the mRNA effects, chronic but not acute prior exposure to U. parvum decreased LPS mediated induction of IL-1β and IL-8 secretion.
Figure 4. Chronic amniotic exposure to U. parvum decreased intraamniotic LPS induced pulmonary cytokine mRNA expression.
Quantification using real-time PCR assays using sheep specific primers and Taqman probes (A) IL-1β (B) IL-6 (C) IL-8 (D) MCP-1 (E) Serum Amyloid A3 (F) IL-10 and (G) IL-1ra mRNAs. The values for each cytokine were normalized to 18s rRNA. The mean mRNA signals in control animals were given the value of 1 and levels at each time point were expressed relative to controls (*p<0.05 vs. control, #p<0.05 vs. 2d LPS).
Figure 5. chronic amniotic exposures to U. parvum decreased intraamniotic LPS induced lung cytokine proteins.
Cytokine proteins in the bronchoalveolar lavage fluid (BALF) were measured by ELISA (A) IL-1β and (B) IL-8 (*p<0.05 vs. control, #p<0.05 vs. 2d LPS).
Down regulation of LPS induced activation of inflammatory cells in the lung by prior chronic exposure to U. parvum
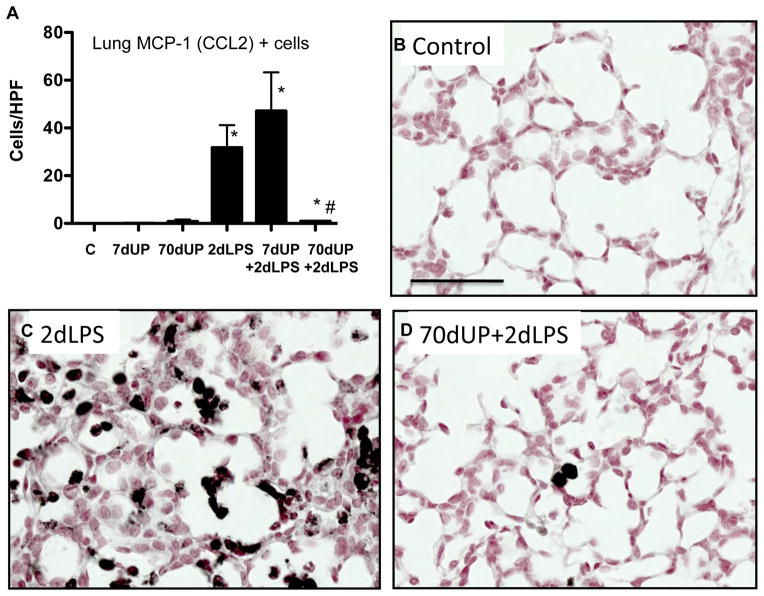
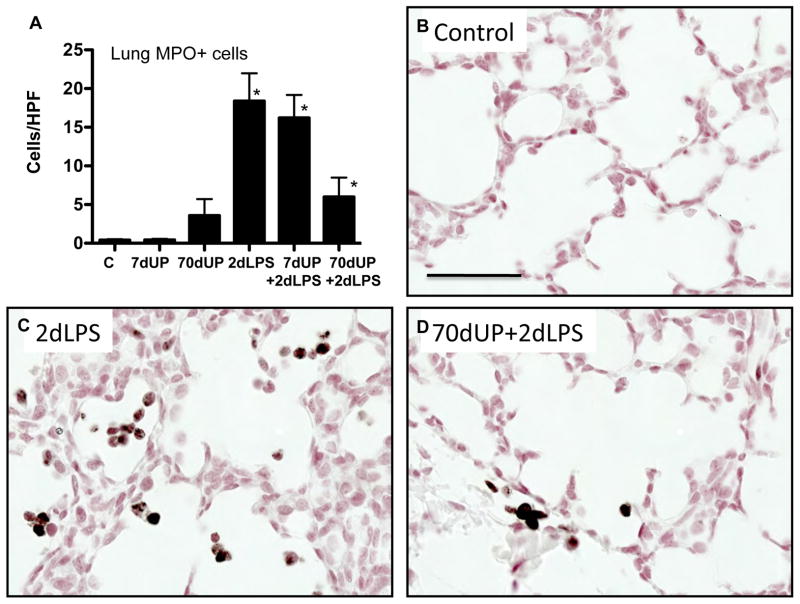
MCP-1 and myeloperoxidase are expressed by activated neutrophils and monocytes in fetal lambs (27, 31). IA LPS induced expression of MCP-1 predominantly in the inflammatory cells with some expression in the pulmonary epithelial cells (Figure 6). Consistent, with the cytokine expression, U. parvum (either acute or chronic exposure) did not increase MCP-1 expressing cells in the lung. Prior acute exposure to U. parvum did not modify LPS effects, but strikingly, chronic exposure to U. parvum significantly reduced LPS induced increases in MCP-1 expressing cells. Similar to MCP-1, U. parvum also did not increase myeloperoxidase (MPO) expressing cells in the lung (Figure 7). Prior chronic but not acute exposure to U. parvum decreased LPS induced increasing in MPO+ cells in the lung.
Figure 6. Chronic amniotic exposure to U. parvum decreased intraamniotic LPS induced MCP-1 expression.
(A) Quantitation of MCP-1 positive cells per high power field. Representative pictures from (B) Controls (C) 2d LPS (D) 70d UP + 2d LPS (Scale bar = 50μm, *p<0.05 vs. control, #p<0.05 vs. 2d LPS).
Figure 7. Chronic amniotic exposure to U. parvum decreased intraamniotic LPS induced myeloperoxidase expression.
(A) Quantitation of MPO positive cells per high power field. Representative pictures from (B) Controls (C) 2d LPS (D) 70d UP + 2d LPS (Scale bar = 50μm, *p<0.05 vs. control).
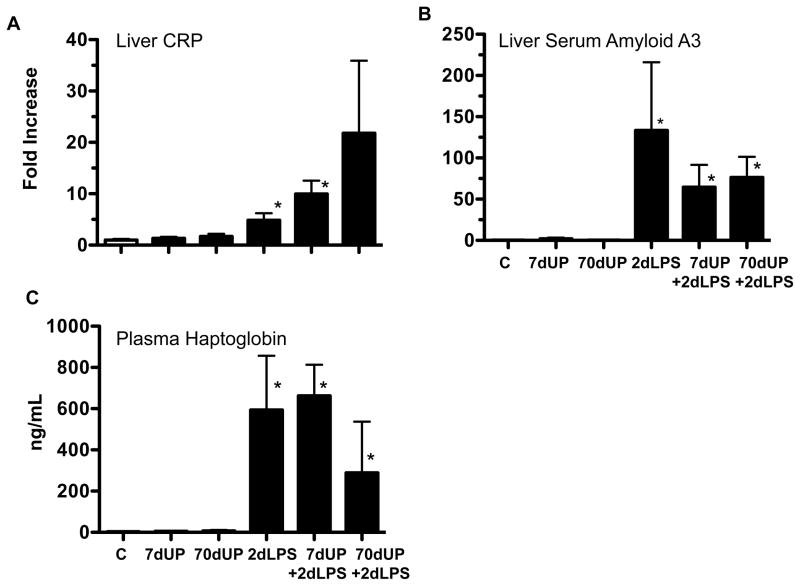
Systemic acute phase response to LPS was not inhibited by prior chronic exposure to U. parvum
Next, we asked if U. parvum modulated IA LPS induced systemic fetal inflammation. We measured the expression of acute phase response genes Serum amyloid A3 and CRP in the fetal liver (Figure 8A–B) and haptoglobin levels in the plasma (Figure 8C). The expression of serum amyoid A3, CRP and plasma haptoglobin were low in control lambs and those exposed to U. parvum alone (Figure 8A–C). Exposure to IA LPS increased expression of the acute phase reactants. Prior acute or chronic exposure to U. parvum had no effect on LPS induction of acute phase reactants.
Figure 8. Chronic amniotic exposure to U. parvum did not decrease intraamniotic LPS induced increases in acute phase reactants.
Quantification using real-time PCR assays using sheep specific primers and Taqman probes for (A) Liver CRP (B) Liver Serum amyloid A3 mRNA expression. The values for each cytokine were normalized to 18s rRNA. The mean mRNA signals in control animals were given the value of 1 and levels at each time point were expressed relative to controls. (C) Haptoglobin levels were measured in the plasma by ELISA (*p<0.05 vs. control).
Components of TLR4 Signaling and TGF-β1 expression
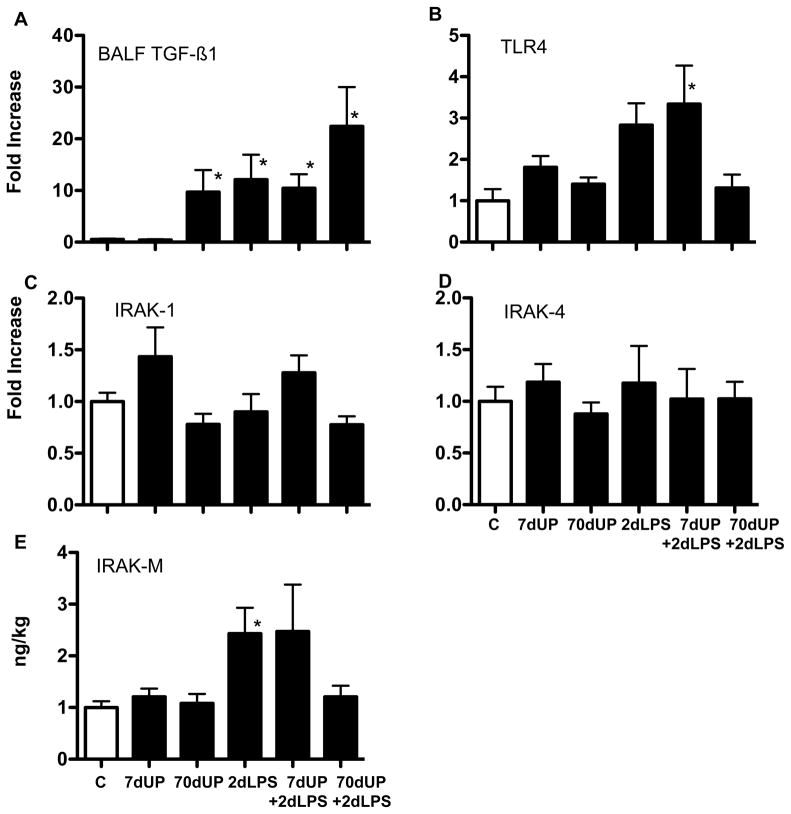
To evaluate some elements that may contribute to decreased LPS responsiveness after prior chronic U. parvum exposure, we measured TGFβ1 expression in the lung because of its known anti-inflammatory properties (32). Control lambs and those exposed to acute U. parvum had low levels of TGFβ1 expression in the airways (Figure 9A). Chronic U. parvum, LPS or acute U. parvum + LPS exposure equivalently increased TGFβ1 secretion. Interestingly, the group chronic U. parvum+LPS had the highest levels of BALF TGFβ1. Next, we quantified expression of several TLR4 signaling molecules. IL-1 receptor associated kinases IRAK-1 and IRAK-4 are positive regulators, while IRAK-M is a negative regulator of TLR4 signaling (33, 34). TLR4 and IRAK-M mRNA expression increased 2.5 to 3 fold in the fetal lung exposed to IA LPS or IA LPS + U. parvum (7 d)(Figure 9B, E), while the expression of IRAK-1 and IRAK-4 mRNA were similar to controls (Figure 9C, D). Compared to controls, chronic exposure to U. parvum or U. parvum +LPS did not change expression of TLR4, IRAK-1, IRAK-4 or IRAK-M mRNAs in the fetal lung.
Figure 9. Chronic amniotic exposures to U. parvum increased pulmonary TGFβ1 secretion but did not change mRNA expression of genes in the TLR4 pathway.
(A) TGFβ1 was measured in BALF by ELISA after acid activation and values expressed per kg body weight. Quantification of mRNAs of select mediators of TLR4 signaling in the lung, using real-time PCR assays with sheep specific primers and Taqman probes (B) TLR4 (C) IRAK-1 (D) IRAK-4 and (E) IRAK-M. The values for each cytokine were normalized to 18s rRNA. The mean mRNA signals in control animals were given the value of 1 and levels at each time point were expressed relative to controls. (*p<0.05 vs. control).
Discussion
We report a profound decrease in responsiveness to LPS after a chronic exposure to U. parvum. Expression of all the measured genes with the sole exception of TGFβ1 did not increase in the lung in response to LPS in fetal lambs chronically exposed to U. parvum. Consistent with previous reports (21), we observed augmentation of LPS responses in vitro in monocytes from the acute U. parvum+LPS group compared to the LPS group or controls. However, LPS responsiveness was blunted in monocytes from the chronic U. parvum+LPS group. Endotoxin tolerance is now understood to be a complex re-programming of inflammatory and non-inflammatory cells to repeated exposures to bacterial products (35). Although a large body of literature on endotoxin tolerance is derived from in vitro studies, historical studies have reported endotoxin tolerance in patients recovering from typhoid fever (36). Also, leukocytes from patients with sepsis display endotoxin tolerance (37). Our findings differ from these observations in several respects: Similar to human chorioamnionitis, intraamniotic injection of U. parvum causes local colonization of the organism in the organs contacting the amniotic fluid e.g. lung, chorioamnion, gut and the skin, but infrequently causes systemic bacteremia (10, 14). Secondly, we found a near global decreased capacity to respond to LPS in the fetal lung after a chronic U. parvum exposure rather than a complex re-programming of inflammation. Thirdly, the down-regulated LPS responsiveness was demonstrated 70d after exposure but not after a 7d exposure. Our study is unique in that, the host is a preterm fetus with developmental immaturity of the immune system.
Since Ureaplasma spp are common colonizers of the lower genital tract, there is some debate about the pathogenicity of the organism in pregnancy. However, several studies have unequivocally demonstrated the association of female upper genital ureaplasma colonization with preterm deliveries, fetal inflammation and adverse neonatal outcomes (8, 9, 13). Furthermore, Ureaplasma species can induce inflammatory cells to produce pro-inflammatory cytokines via TLR2/6 in vitro (21, 22). Consistent with its low virulence, we observed a persistent colonization but low-grade lung inflammation both at 7d and 70d after intra-amniotic injection of U. parvum. Lung inflammation after intraamniotic Ureaplasma exposure is likely via direct colonization, since we previously reported a 2-log order higher Ureaplasma titer in the fetal lung fluid compared to the amniotic fluid (14). Ureaplasmas are known to produce biofilms, which could trap inflammatory products thereby mechanically block inflammatory signaling (38). However, this is an unlikely explanation for the profound hypo responsiveness to LPS in our experiments, since LPS responses were preserved when U. parvum exposure was for 7d. Further, both lung and blood monocytes from fetal lambs exposed to U. parvum for 70d but not 7d demonstrated decreased responsiveness to LPS in vitro. Whether these responses are dependent on the degree of prematurity is not known. Also, the precise mechanism/s by which a chronic exposure to U. parvum can decrease LPS responses remain to be identified.
Exposure to chronic but not acute U. parvum infection decreased both the influx of pulmonary inflammatory cells and expression of activation markers induced by intraamniotic LPS. Therefore, a question is whether the decreased expression of activation markers reflected decreased inflammatory cells in the fetal lung. The inflammatory cell composition in the lung 2 days after intraamniotic LPS is roughly 60% neutrophils and 35% monocytes with few lymphocytes. Compared to effects of LPS alone, a prior exposure to chronic U. parvum reduced the neutrophil influx by about 30% and monocytes by 60% (these reductions were not statistically significant). In contrast, the reductions in expression of cytokines, PU.1+, MCP-1+, and MPO+ cells were much larger, and the monocytes from fetal lambs with chronic U. parvum + LPS were poorly responsive to an in vitro LPS challenge. Taken together, these findings suggest that chronic exposure to U. parvum decreased activation and possibly recruitment of leukocytes to the fetal lung in response to LPS.
Endotoxin tolerance causes a complex re-programming of inflammatory responses (35). Pro-inflammatory cytokine expression is down regulated, while there is no change or even an increase in the expression of anti-inflammatory genes, antimicrobial genes, genes mediating phagocytosis (35). Indeed, microarray analysis of tolerant vs. non-tolerant monocytes demonstrate a host of genes that are down regulated, while other genes are not down regulated (39). The net result of endotoxin tolerance appears to prevent host organ injury while maintaining antimicrobial functions. However, almost all the information regarding endotoxin tolerance is derived from gene expression in functionally mature monocytes or macrophages. Since endotoxin tolerance is an adaptive host response, the characteristics of endotoxin tolerance might vary depending on the inflammatory context. We previously reported that preterm sheep fetuses exposed to repeated doses of intraamniotic LPS demonstrate endotoxin tolerance and cross-tolerance to other toll-like agonists (19, 20). The genes reported not to be down regulated after endotoxin tolerance include IL-10, IL-1ra, TGFβ1, serum amyloid and others (39). While IL-10 and IL-1ra were also down regulated in the chronic U. parvum +LPS animals in our study, TGFβ1 increased relative to controls. Another class of genes not down regulated during endotoxin tolerance is the TRIF mediated interferon inducible genes (40). Further, interferon signaling negatively regulates TLR4 signaling and can abrogate endotoxin tolerance in blood monocytes (41, 42). In the present experiment however, the pattern of expression of the interferon inducible genes CXCL9 (MIG) and CXCL10 (IP10) mRNA expression in the lung after chronic U. parvum + LPS exposure was similar to the other pro-inflammatory cytokines. These results suggest that chronic exposure to U. parvum induced down regulation of LPS responses were more global in nature than previously reported in endotoxin tolerance.
Both intracellular negative regulators and extracellular soluble factors have been implicated in the mechanism of endotoxin tolerance. Extracellular/humoral factors that potentially mediate endotoxin signaling include steroid hormones (43), HSP-70 (44), and IL-10 (45). The plasma cortisol levels (data not shown) and lung expression of IL-10 mRNA did not increase in these fetal lambs. However, TGFβ1 expression in the airways was higher in the lambs with down-regulated LPS responses compared to controls. These data are consistent with the known anti-inflammatory properties of TGFβ1 (32). We previously reported increased expression of TGFβ1 and the downstream mediator phospho-SMAD2 after exposure to intraamniotic LPS (46). Both TGFβ1 and p-SMAD2 are detected in multiple lung cell types in the epithelium and the interstitium of fetal lung. The expression of mRNAs for the receptor TLR4, or the downstream transcription factors IRAK-1, IRAK-4, or IRAK-M in the lung did not change. Similarly, a growing list of intracellular mediators including MyD88s (47), IRAK-M (34), SIGIRR (48), SOCS-1 (49) and others have been proposed as mediators of endotoxin tolerance. We previously reported that TLR4 mRNA is expressed ubiquitously in most of the lung cell types in the preterm fetus (50). In the absence of cell-type expression data for downstream mediators of TLR signaling, a caveat in the interpretation of changes in expression is that whole lung homogenates used for quantification of mRNAs may dilute the changes in the relatively non-abundant recruited inflammatory cells.
Regulatory T-cells (Tregs) expressing FOXP3 are abundant in the fetal circulation and down regulate inflammatory responses (51). However in our study, acute or chronic exposure to U. parvum did not change FOXP3+ cells in the mediastinal lymph node or the lung (data not shown). Another mechanism for endotoxin tolerance is chromatin remodeling, changes in histone acetylation and methylation induced gene silencing (39, 41). While the precise molecular pathways of down regulated endotoxin responses in chorioamnionitis remain to be determined, increased TGFβ1 expression was demonstrated in the lungs of fetal lambs with down-regulated LPS responses.
In contrast to sepsis, exposure of the fetus to bacterial components in chorioamnionitis is via the epithelia of the airway, the chorioamnion and the gastrointestinal tract but not the vascular compartment. The resulting systemic inflammatory response is a mild increase in cytokines and acute phase reactants rather than the cytokine storm associated with sepsis in the adult (52, 53). Liver expression of acute phase reactant genes CRP and serum amyloid A3 and plasma haptoglobin increased after intraamniotic LPS as expected, but the expression was not down regulated in lambs exposed to chronic U. parvum +LPS. These findings suggest that liver, the major source of acute-phase reactants, was not subject to down-regulated LPS responses after exposure to chronic U. parvum. However the blood monocytes from these chronic U. parvum + LPS animals were poorly responsive to LPS. Taken together, these findings suggest that LPS responsiveness in the fetus differed in different organs.
There are several clinical implications of our findings. Although Ureaplasma spp are the organisms most commonly associated with chorioamnionitis, about 60% of cases with chorioamnionitis have co-infection with ureaplasma and other microorganisms (3). Our findings suggest the possibility of decreased fetal inflammation in response to Gram-negative organisms with concomitant ureaplasma exposure. On the other hand, analogous to adults with sepsis (37), diminished innate immune responses could cause adverse fetal outcomes. Similarly, post natal nosocomial sepsis occurs in about 25% of very low birth weight preterm neonates (54). Our data suggests the possibility that ureaplasma chorioamnionitis could diminish innate immune responses and thereby increase the susceptibility of preterm infant to nosocomial sepsis. In summary, the experiments demonstrate a novel finding that a chronic exposure to intraamniotic U. parvum decreases both in vivo and in vitro lung endotoxin responsiveness in a preterm fetus.
Acknowledgments
Grants: Funded by NIH HD57869 (to SGK) and HL97064 (to AHJ and SGK).
The authors wish to thank Prof. Jane Pillow for helping with animal use, Dr. Kathy Heel and Ms. Tracey Lee-Pullen, Center for Microscopy Characterization and Analysis, Univ. of Western Australia, Perth for consultation on flow cytometry, and to Amy Whitescarver, Manuel Alvarez Jr., Avedis Kazanjian, Relana Nowacki, Richard Dalton, Joe Derwort, Carryn McLean, Jennifer Henderson, Andrea Lee, Shaofu Li, and Masatoshi Saito for their expert technical assistance.
Footnotes
Disclosures:
None of the authors have a commercial interest in any entity related to subject of the manuscript or have a conflict of interest relative to the manuscript.
References
- 1.Mathews TJ, Minino AM, Osterman MJ, Strobino DM, Guyer B. Annual summary of vital statistics: 2008. Pediatrics. 2011;127:146–157. doi: 10.1542/peds.2010-3175. [DOI] [PubMed] [Google Scholar]
- 2.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342:1500–1507. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 3.Onderdonk AB, Delaney ML, DuBois AM, Allred EN, Leviton A. Detection of bacteria in placental tissues obtained from extremely low gestational age neonates. Am J Obstet Gynecol. 2008;198:110, e111–117. doi: 10.1016/j.ajog.2007.05.044. [DOI] [PubMed] [Google Scholar]
- 4.Perni SC, Vardhana S, Korneeva I, Tuttle SL, Paraskevas LR, Chasen ST, Kalish RB, Witkin SS. Mycoplasma hominis and Ureaplasma urealyticum in midtrimester amniotic fluid: association with amniotic fluid cytokine levels and pregnancy outcome. Am J Obstet Gynecol. 2004;191:1382–1386. doi: 10.1016/j.ajog.2004.05.070. [DOI] [PubMed] [Google Scholar]
- 5.Gerber S, Vial Y, Hohlfeld P, Witkin SS. Detection of Ureaplasma urealyticum in second-trimester amniotic fluid by polymerase chain reaction correlates with subsequent preterm labor and delivery. J Infect Dis. 2003;187:518–521. doi: 10.1086/368205. [DOI] [PubMed] [Google Scholar]
- 6.Knox CL, Allan JA, Allan JM, Edirisinghe WR, Stenzel D, Lawrence FA, Purdie DM, Timms P. Ureaplasma parvum and Ureaplasma urealyticum are detected in semen after washing before assisted reproductive technology procedures. Fertil Steril. 2003;80:921–929. doi: 10.1016/s0015-0282(03)01125-7. [DOI] [PubMed] [Google Scholar]
- 7.Novy MJ, Duffy L, Axthelm MK, Sadowsky DW, Witkin SS, Gravett MG, Cassell GH, Waites KB. Ureaplasma parvum or Mycoplasma hominis as sole pathogens cause chorioamnionitis, preterm delivery, and fetal pneumonia in rhesus macaques. Reprod Sci. 2009;16:56–70. doi: 10.1177/1933719108325508. [DOI] [PubMed] [Google Scholar]
- 8.Schelonka RL, Waites KB. Ureaplasma infection and neonatal lung disease. Semin Perinatol. 2007;31:2–9. doi: 10.1053/j.semperi.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Viscardi RM. Ureaplasma species: role in diseases of prematurity. Clin Perinatol. 2010;37:393–409. doi: 10.1016/j.clp.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Viscardi RM, Hashmi N, Gross GW, Sun CC, Rodriguez A, Fairchild KD. Incidence of invasive ureaplasma in VLBW infants: relationship to severe intraventricular hemorrhage. J Perinatol. 2008;28:759–765. doi: 10.1038/jp.2008.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knox CL, Cave DG, Farrell DJ, Eastment HT, Timms P. The role of Ureaplasma urealyticum in adverse pregnancy outcome. Aust N Z J Obstet Gynaecol. 1997;37:45–51. doi: 10.1111/j.1479-828x.1997.tb02216.x. [DOI] [PubMed] [Google Scholar]
- 12.Kim M, Kim G, Romero R, Shim SS, Kim EC, Yoon BH. Biovar diversity of Ureaplasma urealyticum in amniotic fluid: distribution, intrauterine inflammatory response and pregnancy outcomes. J Perinat Med. 2003;31:146–152. doi: 10.1515/JPM.2003.020. [DOI] [PubMed] [Google Scholar]
- 13.Glass JI, Lefkowitz EJ, Glass JS, Heiner CR, Chen EY, Cassell GH. The complete sequence of the mucosal pathogen Ureaplasma urealyticum. Nature. 2000;407:757–762. doi: 10.1038/35037619. [DOI] [PubMed] [Google Scholar]
- 14.Knox CL, Dando SJ, Nitsos I, Kallapur SG, Jobe AH, Payton D, Moss TJ, Newnham JP. The severity of chorioamnionitis in pregnant sheep is associated with in vivo variation of the surface-exposed multiple-banded antigen/gene of Ureaplasma parvum. Biol Reprod. 2010;83:415–426. doi: 10.1095/biolreprod.109.083121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moss TJ, Knox CL, Kallapur SG, Nitsos I, Theodoropoulos C, Newnham JP, Ikegami M, Jobe AH. Experimental amniotic fluid infection in sheep: effects of Ureaplasma parvum serovars 3 and 6 on preterm or term fetal sheep. Am J Obstet Gynecol. 2008;198:122, e121–128. doi: 10.1016/j.ajog.2007.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collins JJ, Kallapur SG, Knox CL, Nitsos I, Polglase GR, Pillow JJ, Kuypers E, Newnham JP, Jobe AH, Kramer BW. Inflammation in fetal sheep from intra-amniotic injection of Ureaplasma parvum. Am J Physiol Lung Cell Mol Physiol. 2010;299:L852–860. doi: 10.1152/ajplung.00183.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polglase GR, Dalton RG, Nitsos I, Knox CL, Pillow JJ, Jobe AH, Moss TJ, Newnham JP, Kallapur SG. Pulmonary vascular and alveolar development in preterm lambs chronically colonized with Ureaplasma parvum. Am J Physiol Lung Cell Mol Physiol. 2010;299:L232–241. doi: 10.1152/ajplung.00369.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kallapur SG, Moss TJM, Ikegami M, Jasman RL, Newnham JP, Jobe AH. Recruited Inflammatory Cells Mediate Endotoxin Induced Lung Maturation in Preterm Fetal Lambs. Am J Respir Crit Care Med. 2005;172:1315–1321. doi: 10.1164/rccm.200506-1007OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kallapur SG, Jobe AH, Ball MK, Nitsos I, Moss TJ, Hillman NH, Newnham JP, Kramer BW. Pulmonary and systemic endotoxin tolerance in preterm fetal sheep exposed to chorioamnionitis. J Immunol. 2007;179:8491–8499. doi: 10.4049/jimmunol.179.12.8491. [DOI] [PubMed] [Google Scholar]
- 20.Kramer BW, Kallapur SG, Moss TJ, Nitsos I, Newnham JP, Jobe AH. Intra-amniotic LPS modulation of TLR signaling in lung and blood monocytes of fetal sheep. Innate Immun. 2009;15:101–107. doi: 10.1177/1753425908100455. [DOI] [PubMed] [Google Scholar]
- 21.Manimtim WM, Hasday JD, Hester L, Fairchild KD, Lovchik JC, Viscardi RM. Ureaplasma urealyticum modulates endotoxin-induced cytokine release by human monocytes derived from preterm and term newborns and adults. Infect Immun. 2001;69:3906–3915. doi: 10.1128/IAI.69.6.3906-3915.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shimizu T, Kida Y, Kuwano K. Ureaplasma parvum lipoproteins, including MB antigen, activate NF-{kappa}B through TLR1, TLR2 and TLR6. Microbiology. 2008;154:1318–1325. doi: 10.1099/mic.0.2007/016212-0. [DOI] [PubMed] [Google Scholar]
- 23.Jobe AH, Newnham JP, Willet KE, Moss TJ, Ervin MG, Padbury JF, Sly PD, Ikegami M. Endotoxin-induced lung maturation in preterm lambs is not mediated by cortisol. Am J Respir Crit Care Med. 2000;162:1656–1661. doi: 10.1164/ajrccm.162.5.2003044. [DOI] [PubMed] [Google Scholar]
- 24.Kallapur SG, Willet KE, Jobe AH, Ikegami M, Bachurski CJ. Intra-amniotic endotoxin: chorioamnionitis precedes lung maturation in preterm lambs. Am J Physiol Lung Cell Mol Physiol. 2001;280:L527–L536. doi: 10.1152/ajplung.2001.280.3.L527. [DOI] [PubMed] [Google Scholar]
- 25.Kramer BW, Joshi SN, Moss TJM, Newnham JP, Sindelaar R, Jobe AH, Kallapur SG. Endotoxin-induced maturation of monocytes in preterm sheep lung. Am J Physiol Lung Cell Mol Physiol. 2007;293:L345–L353. doi: 10.1152/ajplung.00003.2007. [DOI] [PubMed] [Google Scholar]
- 26.Kallapur SG, Moss TJ, Auten RL, Jr, Nitsos I, Pillow JJ, Kramer BW, Maeda DY, Newnham JP, Ikegami M, Jobe AH. IL-8 signaling does not mediate intra-amniotic LPS-induced inflammation and maturation in preterm fetal lamb lung. Am J Physiol Lung Cell Mol Physiol. 2009;297:L512–519. doi: 10.1152/ajplung.00105.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shah TA, Hillman NH, Nitsos I, Polglase GR, Pillow JJ, Newnham JP, Jobe AH, Kallapur SG. Pulmonary and systemic expression of monocyte chemotactic proteins in preterm sheep fetuses exposed to lipopolysaccharide-induced chorioamnionitis. Pediatr Res. 2010;68:210–215. doi: 10.1203/PDR.0b013e3181e9c556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shibata Y, Berclaz PY, Chroneos ZC, Yoshida M, Whitsett JA, Trapnell BC. GM-CSF regulates alveolar macrophage differentiation and innate immunity in the lung through PU.1. Immunity. 2001;15:557–567. doi: 10.1016/s1074-7613(01)00218-7. [DOI] [PubMed] [Google Scholar]
- 29.Kramer BW, Joshi SN, Moss TJ, Newnham JP, Sindelar R, Jobe AH, Kallapur SG. Endotoxin-induced maturation of monocytes in preterm fetal sheep lung. Am J Physiol Lung Cell Mol Physiol. 2007;293:L345–353. doi: 10.1152/ajplung.00003.2007. [DOI] [PubMed] [Google Scholar]
- 30.Natoli G, Ghisletti S, Barozzi I. The genomic landscapes of inflammation. Genes Dev. 2011;25:101–106. doi: 10.1101/gad.2018811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheah FC, Pillow JJ, Kramer BW, Polglase GR, Nitsos I, Newnham JP, Jobe AH, Kallapur SG. Airway inflammatory cell responses to intra-amniotic lipopolysaccharide in a sheep model of chorioamnionitis. Am J Physiol Lung Cell Mol Physiol. 2009;296:L384–393. doi: 10.1152/ajplung.90547.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diebold RJ, Eis MJ, Yin M, Ormsby I, Boivin GP, Darrow BJ, Saffitz JE, Doetschman T. Early-onset multifocal inflammation in the transforming growth factor beta 1-null mouse is lymphocyte mediated. Proc Natl Acad Sci U S A. 1995;92:12215–12219. doi: 10.1073/pnas.92.26.12215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fan H, Cook JA. Molecular mechanisms of endotoxin tolerance. Journal of endotoxin research. 2004;10:71–84. doi: 10.1179/096805104225003997. [DOI] [PubMed] [Google Scholar]
- 34.Kobayashi K, Hernandez LD, Galan JE, Janeway CA, Jr, Medzhitov R, Flavell RA. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell. 2002;110:191–202. doi: 10.1016/s0092-8674(02)00827-9. [DOI] [PubMed] [Google Scholar]
- 35.Biswas SK, Lopez-Collazo E. Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol. 2009;30:475–487. doi: 10.1016/j.it.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 36.Lehman JS, Jr, Bassily S. Endotoxin tolerance in patients with chronic bacteremia and bacteriuria due to Salmonella. J Infect Dis. 1971;124:318–321. doi: 10.1093/infdis/124.3.318. [DOI] [PubMed] [Google Scholar]
- 37.Monneret G, Venet F, Pachot A, Lepape A. Monitoring immune dysfunctions in the septic patient: a new skin for the old ceremony. Mol Med. 2008;14:64–78. doi: 10.2119/2007-00102.Monneret. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garcia-Castillo M, Morosini MI, Galvez M, Baquero F, del Campo R, Meseguer MA. Differences in biofilm development and antibiotic susceptibility among clinical Ureaplasma urealyticum and Ureaplasma parvum isolates. J Antimicrob Chemother. 2008;62:1027–1030. doi: 10.1093/jac/dkn337. [DOI] [PubMed] [Google Scholar]
- 39.Foster SL, Hargreaves DC, Medzhitov R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature. 2007;447:972–978. doi: 10.1038/nature05836. [DOI] [PubMed] [Google Scholar]
- 40.Biswas SK, Bist P, Dhillon MK, Kajiji T, Del Fresno C, Yamamoto M, Lopez-Collazo E, Akira S, Tergaonkar V. Role for MyD88-independent, TRIF pathway in lipid A/TLR4-induced endotoxin tolerance. J Immunol. 2007;179:4083–4092. doi: 10.4049/jimmunol.179.6.4083. [DOI] [PubMed] [Google Scholar]
- 41.Chen J, Ivashkiv LB. IFN-gamma abrogates endotoxin tolerance by facilitating Toll-like receptor-induced chromatin remodeling. Proc Natl Acad Sci U S A. 2010;107:19438–19443. doi: 10.1073/pnas.1007816107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bafica A, Feng CG, Santiago HC, Aliberti J, Cheever A, Thomas KE, Taylor GA, Vogel SN, Sher A. The IFN-inducible GTPase LRG47 (Irgm1) negatively regulates TLR4-triggered proinflammatory cytokine production and prevents endotoxemia. J Immunol. 2007;179:5514–5522. doi: 10.4049/jimmunol.179.8.5514. [DOI] [PubMed] [Google Scholar]
- 43.McKay LI. Molecular control of immune/inflammatory responses: interactions between nuclear factor-kappa B and steroid receptor-signaling pathways. Endocr Rev. 1999;20:435–459. doi: 10.1210/edrv.20.4.0375. [DOI] [PubMed] [Google Scholar]
- 44.Aneja R, Odoms K, Dunsmore K, Shanley TP, Wong HR. Extracellular heat shock protein-70 induces endotoxin tolerance in THP-1 cells. J Immunol. 2006;177:7184–7192. doi: 10.4049/jimmunol.177.10.7184. [DOI] [PubMed] [Google Scholar]
- 45.Varma TK, Durham M, Murphey ED, Cui W, Huang Z, Lin CY, Toliver-Kinsky T, Sherwood ER. Endotoxin priming improves clearance of Pseudomonas aeruginosa in wild-type and interleukin-10 knockout mice. Infect Immun. 2005;73:7340–7347. doi: 10.1128/IAI.73.11.7340-7347.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kunzmann S, Speer CP, Jobe AH, Kramer BW. Antenatal inflammation induced TGF-beta1 but suppressed CTGF in preterm lungs. Am J Physiol Lung Cell Mol Physiol. 2007;292:L223–231. doi: 10.1152/ajplung.00159.2006. [DOI] [PubMed] [Google Scholar]
- 47.Burns K, Janssens S, Brissoni B, Olivos N, Beyaert R, Tschopp J. Inhibition of interleukin 1 receptor/Toll-like receptor signaling through the alternatively spliced, short form of MyD88 is due to its failure to recruit IRAK-4. J Exp Med. 2003;197:263–268. doi: 10.1084/jem.20021790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wald D, Qin J, Zhao Z, Qian Y, Naramura M, Tian L, Towne J, Sims JE, Stark GR, Li X. SIGIRR, a negative regulator of Toll-like receptor-interleukin 1 receptor signaling. Nat Immunol. 2003;4:920–927. doi: 10.1038/ni968. [DOI] [PubMed] [Google Scholar]
- 49.Nakagawa R, Naka T, Tsutsui H, Fujimoto M, Kimura A, Abe T, Seki E, Sato S, Takeuchi O, Takeda K, Akira S, Yamanishi K, Kawase I, Nakanishi K, Kishimoto T. SOCS-1 participates in negative regulation of LPS responses. Immunity. 2002;17:677–687. doi: 10.1016/s1074-7613(02)00449-1. [DOI] [PubMed] [Google Scholar]
- 50.Hillman NH, Moss TJ, Nitsos I, Kramer BW, Bachurski CJ, Ikegami M, Jobe AH, Kallapur SG. Toll-like receptors and agonist responses in the developing fetal sheep lung. Pediatr Res. 2008;63:388–393. doi: 10.1203/PDR.0b013e3181647b3a. [DOI] [PubMed] [Google Scholar]
- 51.Mold JE, Venkatasubrahmanyam S, Burt TD, Michaelsson J, Rivera JM, Galkina SA, Weinberg K, Stoddart CA, McCune JM. Fetal and adult hematopoietic stem cells give rise to distinct T cell lineages in humans. Science. 2010;330:1695–1699. doi: 10.1126/science.1196509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kallapur SG, Nitsos I, Moss TJ, Polglase GR, Pillow JJ, Cheah FC, Kramer BW, Newnham JP, Ikegami M, Jobe AH. IL-1 mediates pulmonary and systemic inflammatory responses to chorioamnionitis induced by lipopolysaccharide. Am J Respir Crit Care Med. 2009;179:955–961. doi: 10.1164/rccm.200811-1728OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matsuda N, Hattori Y. Systemic inflammatory response syndrome (SIRS): molecular pathophysiology and gene therapy. Journal of pharmacological sciences. 2006;101:189–198. doi: 10.1254/jphs.crj06010x. [DOI] [PubMed] [Google Scholar]
- 54.Stoll BJ, Hansen N. Infections in VLBW infants: studies from the NICHD Neonatal Research Network. Semin Perinatol. 2003;27:293–301. doi: 10.1016/s0146-0005(03)00046-6. [DOI] [PubMed] [Google Scholar]